Abstract
Abstract. The present study was initiated to investigate strain differences in oral mucosal radiosensitivity in mice with regard to induction of clinically manifest ulceration. Mouse ventral tongue epithelium was used as an established animal model for radiobiological studies of radiation‐induced mucositis. Mice from two different strains, C3H/Neu (n = 40) from the Dresden colony, and B6D2F1 (n = 50) from the Harlan/Winkelmann UK colony were subjected to irradiation of tongue mucosa. Graded single doses were applied to a 3 × 3 mm2 test field in the centre of the lower tongue with 25 kV X‐rays in order to generate full dose‐effect curves for acute mucosal ulceration, as a clinically relevant reaction. For both groups, dose‐effect curves were computed by logit analysis; comparison of the curves was by maximum‐likelihood χ2 test. In addition, the time course of ulceration, i.e. latent time and individual ulcer duration, was analysed. In both mouse strains, a well‐defined dose effect was observed. The ED50 values, i.e. the doses at which ulceration is expected in 50% of the animals irradiated, and their standard deviation σ, calculated by logit analysis, can be used to describe radiosensitivity. The ED50 was 11.0 ± 3.4 Gy (95% confidence interval (7.2; 15.4), P for dose dependence: 0.014) and 13.4 ± 3.6 Gy (95% confidence interval (10.6; 16.1), P for dose dependence: 0.0002) in C3H and BDF1 mice, respectively. Hence, oral mucosa in BDF1 mice was found to be marginally more radioresistant (P = 0.1). The latent time to ulceration, i.e. the time between irradiation and first diagnosis of ulcer, was 11.6 ± 0.2 days (mean ± SEM, n = 18) in C3H mice and 5.6 ± 0.1 days (n = 27) in BDF1 mice (P = 0.0001). Both were independent of dose (P C3H = 0.94, P BDF1 = 0.33) and hence were calculated for all responding animals of the respective strain. Ulcer duration was 2.8 ± 0.2 days and 2.4 ± 0.1 days in C3H and B6 mice, respectively, and was also independent of dose (P C3H = 0.25, P BDF1 = 0.99), but was dependent on the mouse strain (P = 0.036). In conclusion, no statistically significant difference in oral mucosal radiosensitivity was observed between the mouse strains. This, however, may be attributed to the small number of animals used per dose group. The time course data, with a shorter latency and ulcer duration in BDF1 mice, are in good accordance with the higher proliferation rates reported for oral mucosa in this mouse strain.
Introduction
In radiotherapy patients, a large variation in the severity of side‐effects after treatment with identical doses is seen. This has been attributed to genetic differences. In cells of donors with a number of genetic disorders, such as ataxia telangiectasia or Nijmegen breakage syndrome, increased radiation effects have been found (e.g. Weichselbaum et al. 1980; Deschavanne et al. 1984; Cleaver 1989; Shiloh 1994; Mitchell & Scott 1997; Tupler et al. 1997). These may, or may not, translate into increased radiosensitivity of the individuals. In cells from donors without known genetic defects, predominantly fibroblasts and lymphocytes, a large variation in in vitro radiosensitivity was observed, which was compared with the clinical responses to radiotherapy (Geara et al. 1993; Brock et al. 1995; Barber et al. 2000; Peacock et al. 2000; Scott 2000). However, with the assays used so far, no convincing correlation of this in vitro reactivity with the clinical radiosensitivity, expressed as the severity of side‐effects, was found (Barber et al. 2000; Peacock et al. 2000).
In contrast, in experimental studies in animals from different strains a genetic basis could be identified for a variety of normal tissue effects of radiation, e.g. in haematopoietic tissue (Vacek et al. 1975; Mori et al. 1994), germinal epithelium (Bianchi et al. 1985; Gasinska & Godewicz 1983), skin (van den Aardweg et al. 1990), urinary bladder (Lundbeck & Stewart 1989), lung (Dileto & Travis 1996; Franko et al. 1996) and heart (Lauk 1986). In mouse oral mucosa, in contrast, no significant difference in radiation sensitivity was observed between C3H/Neu and NMRI mice (Dörr, unpublished data). The latter were used in mouse lip mucosal studies. Recently, in histological studies (Wardley et al. 1998), B6D2F1 mice have not shown a major decrease in cell numbers with doses even as high as 20 Gy. In contrast, similar studies in C3H/Neu mice revealed almost complete cell depletion after doses in the range of 13‐20 Gy (Dörr & Kummermehr 1990). The latter was in good accordance with the induction of clinically manifest ulceration.
The present study was therefore initiated in order to compare the clinical response of oral mucosa to single‐dose irradiation between the two mouse strains in one single laboratory. Graded single doses were applied to generate full dose‐effect curves. Moreover, the time course in responding animals, i.e. latent time to ulceration and ulcer duration, were analysed.
Materials and methods
Animals and housing
In the C3H experiments, female mice of the C3H/Neu strain, bred in the colony of the Medical Faculty Carl Gustav Carus, Technical University of Dresden, were used. This strain is based on the original strain of the colony of GSF Research Center, Neuherberg, where initial experiments had been carried out (Moses & Kummermehr 1986; Dörr & Kummermehr 1990; 1991). The animals were bred and housed under specified pathogen‐free (SPF) conditions with controlled conditions of humidity (30‐50%) and temperature (21‐24 °C). A 12‐h light/12‐h dark rhythm with lights on between 06.00 and 18.00 h was maintained.
B6D2F1 (BDF1) mice were purchased from the UK colony of Harlan/Winkelmann, Borchen, Germany. These are an F1 cross between the C57B6 and DBA2 mouse strains. The animals were allowed to adapt to the novel environment for 14 days before the onset of the experiment.
All mice were kept in size 3 Macrolon® cages, at a maximum of 10 animals per cage, on sawdust bedding (Sniff 3/4; Altrogge, Lage, Germany). Standard mouse diet (Altromin 1326; Altrogge) and filtered city tap water from standard perspex drinking bottles were provided ad libitum.
Irradiation technique
Technique and set‐up for local administration of radiation to the lower tongue surface were reported in detail elsewhere (2000a, 2000b). In brief, a DARPAC 150‐MC device (Forward Raytech Ltd, UK), which was operated at 25 kV with a tube current of 20 mA, was used for local irradiation of tongue epithelium. The beam filter was 0.3 mm Al. The dose rate at the focus‐to‐skin distance of 15 cm was 3.78 Gy/min. The dose rate was checked regularly during the standard procedures for radiotherapy units by the medical physicists of the Department of Radiotherapy and Radiation Oncology, University Hospital Dresden, and found to be constant. Hence, definition of the dose was done by adjustment of the irradiation time.
For irradiation, the mice were immobilized with Pentobarbitone sodium (Narcoren®; Rhone Merieux, Laupheim, Germany) at a dose of about 60 mg/kg, administered intraperitoneally. The animals were placed in the central bore (diameter 2.5 cm) of a prewarmed aluminium block (∼35 °C) in a supine position. The tongue was guided through a hole (diameter: 3 mm) in the roof of the block by means of a forceps, and the upper tongue surface was fixed to the block by double adhesive tape. Subsequently, the head was supported by a polystyrene wedge in order to avoid local hypoxia by impaired blood flow due to traction at the base of the tongue.
The treatment area was defined by a 3 × 3 mm2 window in an aluminium plate (thickness 1 mm), which was positioned over the central portion of the ventral tongue.
Experimental design
In C3H mice, with known radiosensitivity, single doses of 6, 9, 11, 13, and 16 Gy were applied to eight animals at each dose. BDF1 mice were irradiated with 7 (n = 8), 10 (n = 8), 13 (n = 9), 15 (n = 8), 18 (n = 9) and 21 Gy (n = 8).
Scoring of the tongue response was performed daily from day 4 after irradiation until complete re‐epithelialization of all ulcers was found (day 16). For this, the animals were immobilized by ultra short‐term anaesthesia with Methohexitone (Brevimytal®; Lilly, Bad Homberg, Germany) at a dose of ∼40 mg/kg intraperitoneally (Dörr & Weber‐Frisch 1999), and the tongue was fixed carefully with a forceps. Scoring was performed under a cold light source. Frequency of responding animals, latent time and duration of the individual ulcer were assessed.
For all statistical procedures, the Statistical Analysis System (SAS) was used (SAS Institute 1990a). Analysis of dose‐effect relationships was done by probit analysis assuming a normal log distribution (logit analysis), which results in ED50 values and their standard deviation σ (SAS Institute 1990b). In addition, P values for the effect of dose on ulcer induction were calculated. The latter method is based on the slope of the regression line of the probit analysis. For the comparison of dose‐effect relationships, a maximum‐likelihood χ2 test was applied, without the assumption of a threshold dose. The general linear models procedure (GLM) of SAS was applied for analyses of variance (SAS Institute 1990c).
Results
For both mouse strains, well‐defined dose‐effect curves were derived (Fig. 1). The ED50 value for single‐dose irradiation in C3H mice was 11.0 ± 3.4 Gy (Table 1), with 95% confidence limits of 7.2 and 15.4 Gy. This is in good agreement with results from previous studies with the same animal model (Dörr 1994; Dörr & Weber‐Frisch 1995a; Dörr & Weber‐Frisch 1995b; Dörr et al. 2000a). The P value for dose‐dependence of ulcer incidence was 0.0141.
Figure 1.
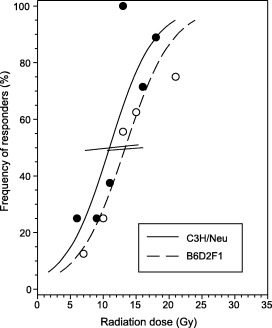
Dose effect for ulcer induction in C3H/Neu and B6D2F1 mice. Dose effect, i.e. relative number of animals developing ventral tongue ulcers, after single dose irradiation with graded doses in the two different mouse strains. Error bars represent 95% confidence intervals of the ED 50 values calculated by probit analysis.
Table 1.
Mouse strain differences in radiation‐induced oral mucositis. Dose‐effect analyses were done by probit analysis, resulting in ED 50 ‐values and their standard deviation σ. Comparison of dose‐effect curves was performed by maximum‐likelihood χ 2 test. Mean latencies and ulcer durations were calculated from all responders in the respective experiment, independent of dose
| Parameter | C3H/Neu | B6D2F1 | P C3H versus B6 |
|---|---|---|---|
| ED50 ± σ (Gy) | 11.0 ± 3.4 | 13.4 ± 3.6 | 0.1000 |
| Latency ± SEM (days) | 11.6 ± 0.2 | 5.6 ± 0.1 | 0.0001 |
| Duration ± SEM (days) | 2.8 ± 0.2 | 2.4 ± 0.1 | 0.0360 |
In BDF1 mice, a higher ED50 of 13.4 ± 3.6 Gy (95% confidence interval (10.6; 16.1) was calculated; P for dose dependence was 0.0002. The difference in the ED50 for C3H and BDF1 mice was not statistically significant, although there was a trend (P = 0.1) (see Fig. 1).
In both groups, latent time to ulceration, defined as the time interval between irradiation and first diagnosis of ulcer, was independent of dose (P C3H = 0.94, P BDF‐1 = 0.33). Similarly, no dose‐dependence was found for ulcer duration in the dose range used (P C3H = 0.25, P BDF1 = 0.99). Hence, it appeared justified to calculate these parameters for all responders of the respective strain. Mean latent times (mean ± SEM) were 11.6 ± 0.2 days (n = 18) for C3H mice, and 5.6 ± 0.1 days (n = 27) for BDF1 mice (P = 0.0001). The ulcers on average lasted for 2.8 ± 0.2 days (mean ± SEM, n = 18) in C3H mice and for 2.4 ± 0.1 days (n = 27) in BDF1 mice (P = 0.036).
The treatment was well‐tolerated, similar to previous studies (Dörr et al. 1993; Dörr & Weber‐Frisch 1995a, 1995b; Dörr et al. 2000a), and no acute morbidity other than tongue ulceration was observed.
Discussion
In oral epithelium, a typical turnover tissue, cell loss via differentiation and mechanical stress at the surface is precisely counteracted by cell production in the germinal layer. This equilibrium is a prerequisite for the stability of cell numbers. Radiation exposure of such tissues, inducing impairment of proliferation, results in insufficient cellular supply to the postmitotic cell layers. On the other hand, cell loss is grossly independent of radiation exposure, but is dependent on the natural lifespan of the cells and therefore continues at an almost normal rate after irradiation (1994, 1996). Cells present at the time of irradiation undergo near‐normal differentiation (Dörr et al. 1996; Liu et al. 1996). The radiation‐induced imbalance between cell production and loss eventually results in almost complete cellular depletion after high doses. In skin and mucosae, the clinical reaction eventually manifests as denudation and ulceration, associated with a significant impairment of the epithelial barrier.
In recent histological studies in oral mucosa in B6D2F1 mice (Wardley et al. 1998), only minor mucosal cell depletion was observed after single doses as high as 20 Gy given to the snouts of the animals. In contrast, earlier histological studies in C3H/Neu mice demonstrated major cell loss after single dose irradiation with doses higher than 10 Gy (Dörr & Kummermehr 1991).
In the present study, the difference in the clinical response, i.e. acute mucosal ulceration, after single‐dose irradiation was investigated in B6D2F1 versus C3H/Neu mice. No gross difference between the two mouse strains in the clinical radiosensitivity of oral mucosa, in contrast to histological changes, could be identified. This may be explained by the experimental protocol, i.e. the small number of animals used in the individual dose groups. Moreover, a broad dose range was used for BDF1 mice, which was chosen because of previously unknown sensitivity to ulcer induction. In a more extended set of data, a slight but significant increase in radiosensitivity may be expected. However, these data are in clear contradiction to the substantial difference in the histological manifestation of the radiation response.
The histological studies have been performed independently in two laboratories (BDF1: Manchester, UK; C3H: Neuherberg, Germany). However, cell numbers are a stable and objective endpoint, and even small variations in the mode of analysis do not result in major deviations of the results. Hence, It is unlikely that this is the reason for the differences observed between histological studies and investigations on ulcer induction as a functional endpoint.
In the present functional studies, the radiation response was scored by one experienced investigator (K.S.) in all animals, hence, interobserver differences, known to be a major factor in mucositis scoring in cancer patients, can be excluded as a reason for the discrepancies.
Recently, it has been demonstrated in head‐and‐neck cancer patients that the clinical score of mucositis only weakly correlates with histological changes in cell numbers (Dörr et al. 2002). Even at severe grades of response, a substantial number of cells are present.
Obviously, the clinical diagnosis is strongly influenced by the appearance of the pseudomembrane covering the lesion, consisting of cell detritus and fibrin (Dörr & Kummermehr 1991), rather than by actual cell numbers. Similar to findings in patients, the histological response in the mouse precedes the clinical response, with a nadir in cell counts at day 8 after a dose of 20 Gy (Dörr & Kummermehr 1991) and a mean latent time to clinical diagnosis of mucositis of 11 days. This indicates that clinical mucositis must be regarded a consequence of cell depletion and is not directly associated with this histological response. Obviously, there is a strain difference in the manifestation of pseudo‐membranous mucositis in relation to cell depletion, with a more direct correlation in C3H, in contrast to major deviations in BDF1 mice. However, comparison of histological changes in both mouse strains in one laboratory and by one investigator is required to validate this hypothesis.
A clear difference between the mouse strains is seen in latent time to ulceration. From a biological point of view, the latent time is based on the lifespan of the cells, which is closely related to the tissue turnover time, and the ability of the damaged cells to undergo residual proliferation, usually depicted as abortive divisions.
The turnover time of the nucleated cell layers of lower (ventral) tongue epithelium in C3H mice, based on stathmokinetic measurements as well as on S‐phase labelling studies, has been estimated as about 5 days (Dörr & Kummermehr 1991; Dörr et al. 1994; Dörr & Weber‐Frisch 1995a, 1995b; Dörr et al. 1996). This is close to the situation in patients (Dörr et al. 1995). Residual proliferation is responsible for the prolongation of the time to nadir cell counts (day 8). The time of clinical diagnosis of ulceration in addition is determined by the development of the pseudomembrane. Similarly, in patients the clinical response is not seen before 9 days after a dose of 20 Gy has been administered (Van der Schueren et al. 1990).
In BDF1 mice, a significantly higher proliferation rate has been described in control epithelium (Wardley et al. 1998; Thomson et al. 1999). This translates into a shorter turnover of about 3 days, assuming duration of the S‐phase of 8 h. Similarly, metaphase arrest measurements indicate this turnover time. This is in good accordance with a shortened time to ulcer diagnosis.
Dependence of ulcer duration on radiation dose would be expected, as less surviving stem cells, functionally defined as those cells able to regenerate the tissue, require more time to restore total cell numbers, based on dose‐independent proliferation rates. In the present as well as in previous experiments (Dörr & Kummermehr 1990; Dörr et al. 1993; Dörr 1994; Dörr & Weber‐Frisch 1995a, 1995b; 2000a, 2000b), however, ulcer duration was independent of dose. This suggests higher proliferation rates after higher doses, i.e. after a greater reduction in stem cell numbers. In fact, various functional studies with varying dose intensities have demonstrated both an earlier onset of stem cell repopulation and a higher proliferative activity with higher doses (Dörr & Weber‐Frisch 1995a, 1995b; Dörr 1994; Dörr & Kummermehr 1990). However, these analyses used tissue radiosensitivity as an endpoint, which is regarded as an indirect measure of stem cell numbers. It must be assumed that the increased stem cell proliferation also translates into increased production of differentiating, non‐stem cells (Dörr & Obeyesekere 2001). There is no direct evidence for this, as stem cells cannot be identified directly.
It has been shown that treatment interruptions in fractionated irradiation regimens results in effective production of differentiating cells (Dörr et al. 1994). The number of differentiating cells produced was dependent on dose, which supports the hypothesis mentioned above.
Alternatively, in the dose range used the differences in stem cell survival may be insufficient to translate into clinically definable differences in ulcer duration. Stem cell survival, based on estimated survival parameters for squamous epithelial cells of α = 0.2 Gy−1 and β = 0.02 Gy−2 (Dörr 1997), is 0.14 for the lowest dose used of 6 Gy, and 9 × 10−5 for 16 Gy or 4 × 10−7 for 21 Gy. A recent meta‐analysis of all ulcer duration data available for the C3H/Neu strain of the Dresden colony (Dörr, unpublished data) yielded a minor but statistically significant increase in ulcer duration with dose.
In conclusion, significant differences between the mouse strains tested are observed for the radiation response of oral mucosa. As a consequence, in animal experimental studies, sufficient control experiments must be performed, using exactly the same mouse strain. Even historical control data may give misleading conclusions. For example, a significant shift in oral mucosal radiosensitivity in C3H/Neu mice, from ED50 values of 14‐15 Gy to 10‐11 Gy was seen in Neuherberg in 1992 (Dörr 1997). This shift was associated with embryo transfer measures undertaken to eliminate mouse hepatitis virus from the colony. However, the basis of the observed changes remained unclear. It is therefore recommended to repeat single‐dose studies on a regular basis in parallel to ongoing investigations, in order to guarantee stable radiosensitivity of the tissue studied in the mouse strain used.
Acknowledgements
All experiments were performed according to the current animal welfare legislation with permission of Regierungspräsidium Dresden. The authors are grateful to Mrs Dorothee Pfitzmann for skilful technical assistance.
References
- Barber JB, Burrill W, Spreadborough AR, Levine E, Warren C, Kiltie AE, Roberts SA, Scott D (2000) Relationship between in vitro chromosomal radiosensitivity of peripheral blood lymphocytes and the expression of normal tissue damage following radiotherapy for breast cancer. Radiother. Oncol. 55, 179. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Delic JI, Hurtado de Catalfo G, Hendry JH (1985) Strain differences in the radiosensitivity of mouse spermatogonia. Int. J. Radiat. Biol. 48, 579. [DOI] [PubMed] [Google Scholar]
- Brock WA, Tucker SL, Geara FB, Turesson I, Wike J, Nyman J, Peters LJ (1995) Fibroblast radiosensitivity versus acute and late normal skin responses in patients treated for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 30, 1371. [DOI] [PubMed] [Google Scholar]
- Cleaver JE (1989) How many human genetic disorders affect cellular radiosensitivity? Cancer Cells 1, 108. [PubMed] [Google Scholar]
- Deschavanne PJ, Debieu D, Fertil B, Malaise EP (1986) Re‐evaluation of in vitro radiosensitivity of human fibroblasts of different genetic origins. Int. J. Radiat. Biol. 50, 279. [DOI] [PubMed] [Google Scholar]
- Dileto CL, Travis EL (1996) Fibroblast radiosensitivity in vitro and lung fibrosis in vivo: comparison between a fibrosis‐prone and fibrosis‐resistant mouse strain. Radiat. Res. 146, 61. [PubMed] [Google Scholar]
- Dörr W (1994) Repopulation in mouse oral mucosa: treatment splits. Radiother. Oncol. 33, 139. [DOI] [PubMed] [Google Scholar]
- Dörr W (1997) Three As of repopulation during fractionated irradiation of squamous epithelia: Asymmetry loss, Acceleration of stem‐cell divisions and Abortive divisions. Int. J. Radiat. Biol. 72, 635. [DOI] [PubMed] [Google Scholar]
- Dörr W, Brankovic K, Hartmann B (2000a) Repopulation in mouse oral mucosa: changes in the effect of dose fractionation. Int. J. Radiat. Biol. 76, 383. [DOI] [PubMed] [Google Scholar]
- Dörr W, Breitner A, Kummermehr J (1993) Capacity and kinetics of SLD repair in mouse tongue epithelium. Radiother. Oncol. 27, 36. [DOI] [PubMed] [Google Scholar]
- Dörr W, Emmendörfer H, Haide E, Kummermehr J (1994) Proliferation equivalent of ‘accelerated repopulation’ in mouse oral mucosa. Int. J. Radiat. Biol. 66, 157. [DOI] [PubMed] [Google Scholar]
- Dörr W, Emmendörfer H, Weber‐Frisch M (1996) Tissue kinetics in mouse tongue mucosa during daily fractionated radiotherapy. Cell Prolif. 29, 495. [DOI] [PubMed] [Google Scholar]
- Dörr W, Hamilton CS, Boyd T, Reed B, Denham JW (2002) Radiation‐induced changes in cellularity and proliferation in human oral mucosa. Int. J. Radiat. Oncol. Biol. Phys. 52, 911. [DOI] [PubMed] [Google Scholar]
- Dörr W, Jakubec A, Kummermehr J, Herrmann T, Dölling‐Jochem. I, Eckelt U (1995) Effects of stimulated repopulation on oral mucositis during conventional radiotherapy. Radiother. Oncol. 37, 100. [DOI] [PubMed] [Google Scholar]
- Dörr W, Kummermehr J (1990) Accelerated repopulation of mouse tongue epithelium during fractionated irradiations or following single doses. Radiother. Oncol. 17, 249. [DOI] [PubMed] [Google Scholar]
- Dörr W, Kummermehr J (1991) Proliferation kinetics of mouse tongue epithelium under normal conditions and following single dose irradiation. Virchows Arch. B. 60, 281. [DOI] [PubMed] [Google Scholar]
- Dörr W, Noack R, Spekl K, Farrell CL (2000b) Amelioration of radiation‐induced oral mucositis by keratinocyte growth factor (rhKGF): experimental studies. Int. J. Radiat. Oncol. Biol. Phys. 46, 729. [Google Scholar]
- Dörr W, Obeyesekere MN (2001) A mathematical model for cell density and proliferation in squamous epithelium after single dose irradiation. Int. J. Radiat. Biol. 77, 497. [DOI] [PubMed] [Google Scholar]
- Dörr W, Weber‐Frisch M (1995a) Effect of changing weekly dose on accelerated repopulation during fractionated irradiation of mouse tongue mucosa. Int. J. Radiat. Biol. 67, 577. [DOI] [PubMed] [Google Scholar]
- Dörr W, Weber‐Frisch M (1995b) Repopulation response of mouse oral mucosa during unconventional radiotherapy protocols. Radiother. Oncol. 37, 230. [DOI] [PubMed] [Google Scholar]
- Dörr W, Weber‐Frisch M (1999) Short‐term immobilisation of mice by methohexitone. Lab. Anim. 33, 35. [DOI] [PubMed] [Google Scholar]
- Franko AJ, Sharplin J, Ward WF, Taylor JM (1996) Evidence for two patterns of inheritance of sensitivity to induction of lung fibrosis in mice by radiation, one of which involves two genes. Radiat Res. 146, 68. [PubMed] [Google Scholar]
- Gasinska A, Godowicz B (1983) Effect of x‐rays on spermatogenesis in inbred strains of mice. Folia Histochem. Cytochem. (Krakow) 21, 219. [PubMed] [Google Scholar]
- Geara FB, Peters LJ, Ang KK, Wike JL, Brock WA (1993) Prospective comparison of in vitro normal cell radiosensitivity and normal tissue reactions in radiotherapy patients. Int. J. Radiat. Oncol. Biol. Phys. 27, 1173. [DOI] [PubMed] [Google Scholar]
- Lauk S (1986) Strain differences in the radiation response of the rat heart. Radiother. Oncol. 5, 333. [DOI] [PubMed] [Google Scholar]
- Liu K, Kasper M, Trott KR (1996) Changes in keratinocyte differentiation during accelerated repopulation of the irradiated mouse epidermis. Int. J. Radiat. Biol. 69, 763. [DOI] [PubMed] [Google Scholar]
- Lundbeck F, Stewart FA (1989) Acute changes in the bladder reservoir function after treatment alone or in combination with chemotherapy: a matter of mouse strain. Scand. J. Urol. Nephrol. 125, 141. [PubMed] [Google Scholar]
- Mitchell EL, Scott D (1997) G2 chromosomal radiosensitivity in fibroblasts of ataxia‐telangiectasia heterozygotes and a Li‐Fraumeni syndrome patient with radioresistant cells. Int. J. Radiat. Biol. 72, 435. [DOI] [PubMed] [Google Scholar]
- Mori N, Okumoto M, Yonezawa M, Nishikawa R, Takamori Y, Esaki K (1994) Factors related to resistance to hematopoietic death in mice. J. Radiat. Res. (Tokyo) 35, 1. [DOI] [PubMed] [Google Scholar]
- Moses R, Kummermehr J (1986) Radiation response of the mouse tongue epithelium. Br. J. Cancer 53 (Suppl. VII), 12. [PMC free article] [PubMed] [Google Scholar]
- Peacock J, Ashton A, Bliss J, Bush C, Eady J, Jackson C, Owen R, Regan J, Yarnold J (2000) Cellular radiosensitivity and complication risk after curative radiotherapy. Radiother. Oncol. 55, 173. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc, Cary NC (1990a). SAS/STAT User's Guide, Version 6. [Google Scholar]
- SAS Institute Inc, , Cary NC (1990b). SAS/STAT User's Guide, Version 6, pp. 892‐906. [Google Scholar]
- SAS Institute Inc, , Cary NC (1990c). SAS/STAT User's Guide, Version 6, pp. 1324‐1350. [Google Scholar]
- Shiloh Y (1997) Ataxia‐telangiectasia and the Nijmegen breakage syndrome: related disorders but genes apart. Annu. Rev. Genet. 31, 635. [DOI] [PubMed] [Google Scholar]
- Thomson PJ, McGurk M, Potten CS, Walton GM, Appleton DR (1999) Tritiated thymidine and bromodeoxyuridine double‐labelling studies on growth factors and oral epithelial proliferation in the mouse. Arch. Oral. Biol. 44, 721. [DOI] [PubMed] [Google Scholar]
- Tupler R, Marseglia GL, Stefanini M, Prosperi E, Chessa L, Nardo T, Marchi A, Maraschio P (1997) A variant of the Nijmegen breakage syndrome with unusual cytogenetic features and intermediate cellular radiosensitivity. J. Med. Genet. 34, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacek A, Rakova A, Rotkovska D (1975) On the role of haemopoietic tissue in interstrain differences in radiosensitivity of mice. Folia Biol. (Praha) 21, 103. [PubMed] [Google Scholar]
- Van den Aardweg GJ, Arnold M, Hopewell JW (1990) A comparison of the radiation response of the epidermis in two strains of pig. Radiat. Res. 124, 283. [PubMed] [Google Scholar]
- Van der Schueren E, Van den Bogaert W, Vanuytsel L, Van Limbergen E (1990) Radiotherapy by multiple fractions per day (MFD) in head and neck cancer: Acute reactions of skin and mucosa. Int. J. Radiat. Oncol. Biol. Phys. 19, 301. [DOI] [PubMed] [Google Scholar]
- Wardley AM, Booth D, Roberts SA, Scarffe JH, Potten CS (1998) A quantitative histometric murine in vivo model of radiation‐induced oral mucositis. Arch. Oral. Biol. 43, 567. [DOI] [PubMed] [Google Scholar]
- Weichselbaum RR, Nove J, Little JB (1980) X‐ray sensitivity of fifty‐three human diploid fibroblast cell strains from patients with characterized genetic disorders. Cancer Res. 40, 920. [PubMed] [Google Scholar]


