Abstract
Abstract. Lavender (Lavandula angustifolia) oil, chiefly composed of linalyl acetate (51%) and linalool (35%), is considered to be one of the mildest of known plant essential oils and has a history in wound healing. Concerns are building about the potential for irritant or allergenic skin reactions with the use of lavender oil. This study has demonstrated that lavender oil is cytotoxic to human skin cells in vitro (endothelial cells and fibroblasts) at a concentration of 0.25% (v/v) in all cell types tested (HMEC‐1, HNDF and 153BR). The major components of the oil, linalyl acetate and linalool, were also assayed under similar conditions for their cytotoxicity. The activity of linalool reflected that of the whole oil, indicating that linalool may be the active component of lavender oil. Linalyl acetate cytotoxicity was higher than that of the oil itself, suggesting suppression of its activity by an unknown factor in the oil. Membrane damage is proposed as the possible mechanism of action.
INTRODUCTION
Essential oils are fragrant volatile oils obtained from plants. The oils are complex mixtures of several chemical compounds including terpenes, alcohols, aldehydes and phenols. Lavender oil, obtained from the flowers of Lavandula angustifolia (Family: Lamiaceae) by steam distillation, is chiefly composed of linalyl acetate (3,7‐dimethyl‐1,6‐octadien‐3yl acetate), linalool (3,7‐dimethylocta‐1,6‐dien‐3‐ol), lavandulol, 1,8‐cineole, lavandulyl acetate and camphor (Lis‐Balchin 1995). Whole lavender oil, and its major components linalool and linalyl acetate are used in aromatherapy, and in the flavouring and fragrance industries. Unlike many other essential oils used in aromatherapy, lavender oil is often applied undiluted to the skin. The work of Jager et al. (1992) suggested that essential oils and their components are rapidly absorbed through the skin. Linalool and linalyl acetate were shown to be rapidly detected in plasma after topical application with massage, reaching peak levels after approximately 19 min (Jager et al. 1992).
Whilst in vivo testing is often considered the norm for cytotoxicity testing, several in vitro alternatives have been developed and have found favour as viable alternatives to animal studies. Human skin cells are potentially more predictive of human skin responses than other in vitro systems; such cultures can be used to determine mechanisms of primary irritant dermatitis, reducing or eliminating the need for animal testing (Osborne & Perkins 1991). The present widespread use of essential oils in pharmacy and industry (antiseptics, soaps, deodorants, flavours and dentistry products) would seem to necessitate research on their cytotoxicity, but such studies are few and by no means comprehensive.
The most commonly used in vitro model of human skin is that of epidermal keratinocytes as these cells are believed to have an important role in the initiation of an inflammatory response. However, such models lack circulatory vascular elements as well as other cell types, such as fibroblasts, that also play an important role in such reactions. On this basis, fibroblasts and endothelial cells were selected here as target cells for assessing the cytotoxicity of lavender oil and its components.
Cytotoxic assays on cultured human skin cells have been reported to provide a useful prediction of skin damage when terpene cytotoxicity was compared with the injury evoked by its topical application to rats in vivo (Kitahara et al. 1993). Relatively good agreement has also been shown between in vitro cytotoxicity end‐points [such as those obtained from the neutral red (NR) assay] and the human skin patch test score (Lee et al. 2000).
MATERIALS AND METHODS
Materials
All chemicals (reagents, buffers, dyes) used in this investigation were purchased from Sigma‐Aldrich Chemical Co. Ltd (Poole, UK) and VWR International (Poole, UK) and, unless specified otherwise, were of analytical grade or higher. Cell culture media components were purchased from Invitrogen Ltd (Paisley, UK) and fetal bovine serum (FBS) was obtained from Biowest Ltd (Ringmer, UK). Lavender oil was obtained from Neal's Yard Remedies Ltd, Battersea, London, and the components linalool and linalyl acetate were obtained from Fisher Scientific UK (Loughborough, UK).
Cell culture
All media, buffers, trypsin and dyes were filter‐sterilized prior to use and warmed to 37 °C. Endothelial cell growth medium was composed of MCDB‐131 supplemented with 10% (v/v) FBS, 1% (v/v) penicillin/streptomycin (10 000 U/10 000 µg/l), 1% (v/v) glutamine (200 mm) and 0.2% (v/v) gentamicin (10 mg/ml). Fibroblast growth medium was composed of minimum essential medium supplemented with 20% (v/v) FBS, 1% (v/v) penicillin/streptomycin, 1% (v/v) glutamine, 0.2% (v/v) gentamicin and 1% (v/v) non‐essential amino acids.
Cell cultures were maintained at 37 °C, 5% CO2 and passaged on confluence by trypsin‐ethylenediaminetetraacetic acid treatment. Following cell detachment, fresh medium was added to the cell suspension which was then centrifuged at 550 g for 5 min. The resulting cell pellet was resuspended in fresh medium and transferred to 75‐cm2 tissue culture flasks. All experiments were conducted on cells between passage 3 and passage 10.
Cytotoxicity studies
The cytotoxicity of lavender oil and its major components linalyl acetate and linalool were evaluated against three cell types 153BR, HNDF and HMEC‐1. The 153BR, human fibroblasts supplied by the European Collection of Cell Cultures (ECACC, Porton Down, Salisbury, UK), were used at 105 cells/ml for all experiments. The human normal dermal fibroblasts (HNDF), a primary culture obtained from biopsy, were similarly used at 105 cells/ml for all experiments. HMEC‐1 cells, an simian virus‐40‐transformed human dermal microvascular endothelial cell line were used at 4 × 105 cells/ml for all experiments.
The major components of lavender oil were identified as 51% linalyl acetate and 35% linalool by gas chromatography and gas chromatography‐linked Fourier Transform Infra Red analysis (data not shown). The concentrations of lavender oil, linalyl acetate and linalool used in this study are summarized in Table 1. The final concentration of the component paralleled that actually present in the oil. As an example, for comparing the cytotoxicity of lavender oil and linalyl acetate, the concentration of the oil used would be 2% (v/v) and the concentration of linalyl acetate used would be 1.02% (v/v), as linalyl acetate constitutes 51% of lavender oil.
Table 1.
Concentrations of lavender oil and its components tested
| Oil/component | Limits of concentrations used |
|---|---|
| Lavender oil | 0.016–2.00% (v/v) |
| Linalyl acetate | 0.008–1.02% (v/v) |
| Linalool | 0.005–0.7% (v/v) |
Cytotoxicities of whole lavender oil, lynalyl acetate and linalool were determined by the NR assay (Babich et al. 1993) with slight modifications. Briefly, sterile 96‐well tissue culture microtitre plates were inoculated with 100 µl medium containing a defined number of cells (described above). Cells were allowed to grow to 70% confluence (approximately 48 h). Growth medium was then replaced with medium containing various concentrations of lavender oil or its components and incubated for 1 h at 37 °C in 5% CO2. After exposure, the medium containing the test agent was removed and 200 µl of medium containing 40 µg/ml NR was added to each well. The plate was then further incubated for 3 h to allow for uptake of the dye by viable, uninjured cells. The NR‐containing medium was then removed and cells were first quickly washed with 200 µl fixative (1% CaCl2−0.5% formaldehyde). Then 200 µl of a solution of 1% acetic acid−50% ethanol solution was added to each well to extract the dye. The plate was allowed to stand at room temperature for 10 min followed by rapid agitation on a microtitre plate shaker. The absorbance of the extracted dye was read at 540 nm on a microtitre plate reader (Dynex Technologies Ltd, Worthing, UK). All experiments were performed at least three times. The total NR uptake was a measure of the viability (% NR uptake is directly proportional to the number of live, uninjured cells).
Control plates (cells + medium) were run alongside in every experiment. The use of a test blank (oil or component diluted in the medium, no cells) was introduced in all experiments to account for any reaction between the medium and the test agent. The oils and/or their components were diluted first in medium to the maximum concentration used and doubling dilutions were then carried out over the 96‐well plates.
Computation of viability, the NR50 value and statistical analysis
Individual cytotoxicity data points for each concentration of the oil or component, presented as the arithmetic mean ± standard deviation, were used to construct dose‐dependent cytotoxicity graphs. The percentage viability was calculated as follows:
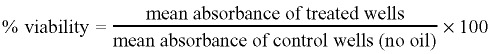 |
The cytotoxicity of the oils or components was expressed in terms of an NR50 value (the concentration that caused 50% cell death). NR50 values were calculated by non‐linear regression analysis. All NR50 values were expressed as a percentage (v/v). The baseline of 100% viability corresponded to the absorbance of untreated cells. Background absorbances due to non‐specific reactions between test materials and the NR dye were deducted from exposed cell values. In general, this background absorbance was only observed at high concentrations of the test materials.
A one‐way analysis of variance (anova) was employed to compare group means. To correlate the activity of the components with the corresponding essential oil, a linear regression was carried out and the r 2 values thus obtained were used to predict such relationships. Where required, a further multiple regression was performed. Statistical analysis was carried out using GraphPad Prism software.
RESULTS
Lavender oil
Across all cell types, mean cell viability of 80–100% was observed at 0.125% (v/v) lavender oil (Fig. 1), while 50% growth inhibition was calculated to be between approximately 0.17 and 0.19% (v/v) lavender oil (NR50 values are shown in Table 2). The cell viability decreased markedly when lavender oil concentration was increased from 0.125% (v/v) to 0.25% (v/v). The oil activity was constant across all the cell types (one‐way anova, P = 0.2906).
Figure 1.
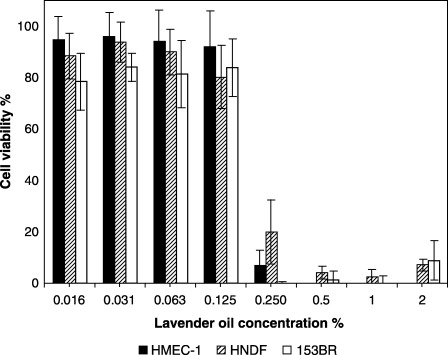
Dose‐dependent cytotoxicity of lavender oil (1‐h exposure) to HMEC‐1 endothelial cells, HNDF fibroblasts and 153BR fibroblasts as determined by the NR assay. Error bars indicate the standard deviation (n = 16–36).
Table 2.
NR50 values of lavender and its major components expressed as percentage of the oil (component) diluted in the medium (v/v)
| Oil/component | NR50 value (%) | ||
|---|---|---|---|
| HMEC‐1 | HNDF | 153BR | |
| Lavender oil | 0.195 | 0.184 | 0.169 |
| Linalyl acetate | 0.360 | 0.028 | 0.031 |
| Linalool | 0.069 | 0.065 | 0.060 |
Linalyl acetate
Linalyl acetate (51% lavender oil) was cytotoxic to both HNDF and 153BR fibroblasts (Fig. 2). The NR50 values obtained for linalyl acetate with fibroblasts (0.028 for HNDF, 0.031 for 153BR) were much lower than those of lavender oil, while an exceptionally high NR50 value (0.36) was obtained with HMEC‐1 endothelial cells (Fig. 2).
Figure 2.
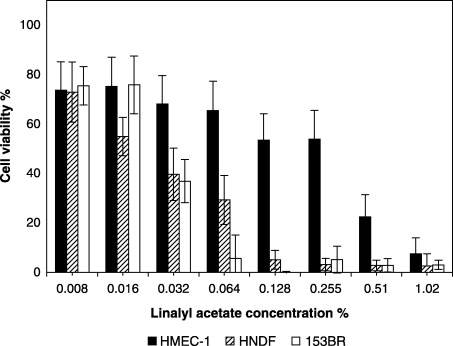
Dose‐dependent cytotoxicity of linalyl acetate (1‐h exposure) to HMEC‐1 endothelial cells, HNDF fibroblasts and 153BR fibroblasts as determined by the NR assay. Error bars indicate the standard deviation (n = 17–24). (The concentrations of linalyl acetate used were proportionate to that actually present in the oil.)
The r 2 values (linear regression) calculated to compute the relationships between the activity of the oil and linalyl acetate were: HMEC‐1: r 2 = 0.69, P = 0.009; HNDF: r 2 = 0.82, P = 0.001; 153BR: r 2 = 0.53, P = 0.031).
Linalool
The NR50 values of linalool across the cell types were analogous to the corresponding lavender oil values (Table 2) since linalool comprises only 35% of the parent lavender oil. The effect of linalool on the viability of endothelial cells and fibroblasts was not significantly different (one‐way anova, P = 0.9785). The cell viability also decreased when linalool concentration was increased from 0.125% (v/v) to 0.25% (v/v) (Fig. 3), in a manner similar to that observed with lavender oil. In the limits of the concentrations used, a linear relationship between lavender oil and linalool was confirmed (HMEC‐1, r 2 = 0.99, P < 0.0001; HNDF, r 2 = 0.97, P < 0.0001; 153BR, r 2 = 0.99, P < 0.0001).
Figure 3.
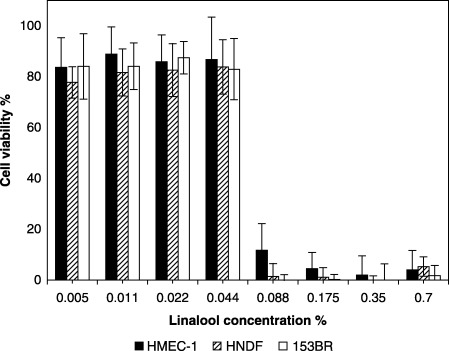
Dose‐dependent cytotoxicity of linalool (1‐h exposure) to HMEC‐1 endothelial cells, HNDF fibroblasts and 153BR fibroblasts as determined by the NR assay. Error bars indicate the standard deviation (n = 16–24). (The concentrations of linalool used were proportionate to that actually present in the oil.)
Single variable regression analysis showed both linalool and linalyl acetate to have high r 2 values. On a multiple regression analysis however, the inclusion of linalyl acetate with linalool to assess the cytotoxicity relationships improved the r 2 only marginally (less than 1%), further indicating that the variability in the cytotoxicity of lavender oil was a function of the variability in the cytotoxicity of linalool.
DISCUSSION
To date, there are very few reports on the cytotoxicity of lavender oil or essential oils in general. Indeed, literature cited in terms of lavender oil cytotoxicity often has lavender oil placed in the category of ‘safe’ oils (Tisserand & Balacs 2000). To our knowledge however, there is no evidence in support of this view, and the data generated in our study indicate a dose‐dependent cytotoxicity for lavender oil and its major components linalyl acetate and linalool. Lavender oil has a history of use in wound healing and is commonly applied to skin without dilution (personal communication, J. Whitehouse, University of Westminster, London) but, similarly, there is no scientific evidence suggesting that lavender accelerates wound healing or reduces scarring. With reports of contact dermatitis associated with lavender oil, concerns are building about the potential for either allergic or skin irritation reactions with its use. One 9‐year study in Japan has already found that up to 13.9% of subjects had contact dermatitis on exposure to lavender oil. The time period of this study (1990–97) corresponded to a time when world‐wide use of essential oils increased and it was suggested that contact dermatitis and other skin reactions may have become more prevalent as product use increased (Sugiura et al. 2000).
The in vitro testing of lavender oil in this study showed that a viability of 80–100% was observed at an oil concentration of 0.125% (v/v) across all cell types and thereafter an increase in the concentration affected cell viability. The pattern of cytotoxicity of linalool (35% of lavender oil) in this study was found to be equivalent to that of lavender oil, suggesting that the active component of the oil may be linalool. Linalyl acetate (51% of lavender oil) was found to be more cytotoxic to fibroblasts than the whole oil. Since linalool and lavender oil showed a good linear relation (P < 0.0001) for all the three human skin cell types tested, we suggest that linalyl acetate activity may be suppressed in the oil by some other component. Hence, we suggest that, though the proportion of linalyl acetate in the oil is more, linalool is the primary active component of lavender oil. This hypothesis is supported by in vivo studies of other effects of lavender oil and its components where linalool activity reflects that of lavender oil in terms of spasmolytic activity (Lis‐Balchin & Hart 1999) and depressive/narcotic effects on the rat central nervous system (Atanassova‐Shopova et al. 1973). Relating activity with structure, we suggest that the acetate group (linalyl acetate) is responsible for higher cytotoxicity than the alcohol (linalool).
Lavender oil and its components have been reported to possess other possible therapeutic values. Linalyl acetate and linalool have both been shown to reduce carrageenin‐induced oedema in rats at doses ranging from 25 to 75 mg/kg (Peana et al. 2002), suggesting a major role for linalool and its ester in the anti‐inflammatory effect of these oils (Peana et al. 2003). Linalool has been shown to have several different bioactive properties, such as anti‐inflammatory activity, analgesic and spasmolytic effects (Lis‐Balchin & Hart 1999). Further evidence that linalool‐producing plant species are potentially analgesic and anti‐inflammatory has recently been established based on experiments with two murine models of pain (Peana et al. 2003).
Studies investigating the frequencies of occurrence of the most common fragrance ingredients in cosmetics and other scented products have shown that linalool is the most frequently incorporated fragrance (De Groot & Frosch 1997; Rastogi et al. 2001). In one study (Rastogi et al. 1998), 97% of deodorants analysed were shown to contain linalool. Furthermore, linalool, together with d‐limonene, is the most common fragrance in domestic and occupational products (Rastogi et al. 2001). In spite of this, reports of contact allergy to linalool are rare. It might be the case that linalool itself is non‐allergenic and that allergens are formed on handling and storage. This hypothesis is supported by sensitization studies on the guinea‐pig where linalool of high purity provoked no reaction, whilst oxidized linalool caused sensitization in animals (Skold et al. 2002). These workers concluded that auto‐oxidation of linalool was essential for its sensitizing potential. With regard to structure–activity relationships, linalool (a component of several essential oils) would not be considered a contact allergen since the molecular structure of linalool does not contain any protein‐reactive groups (Basketter et al. 2002; Skold et al. 2002). Contact allergy to lavender oil has been reported in Japan where the oil has the highest frequency of use among essential oils (Sugiura et al. 2000). These findings suggest that cell damage plays an important role in the mechanism of dermal inflammation for many irritants and also provides a tool in predicting skin responses to untested agents. The importance is evident in essential oil research as there are hundreds of untested oils and their chemical constituents that possess useful properties, but their cytotoxicity has to be determined prior to use.
Previous studies into the antimicrobial activity of essential oils and their components have ascribed their activity to their action on the cell membrane (Bard et al. 1988; Sikkema et al. 1995; Prashar et al. 2003). It has also been suggested that tissue damage by essential oils may be related to membrane lysis and surface activity (Manabe et al. 1987). Studies with eugenol, menthol and thymol on rat erythrocytes and hepatocytes along with dipalmitoyl phosphatidylcholine‐liposomes indicate that their penetration into tissue may be related to membrane and lipid affinity (Manabe et al. 1987). These studies, coupled with the fact that the NR assay indicates membrane integrity (Cornelis et al. 1992), would suggest that the mechanism of the cytotoxic action of lavender oil, linalyl acetate and linalool may also be one of membrane damage.
The reported antimicrobial activity of essential oils, together with their flavouring/aromatic properties, have made the use of these substances common in the pharmaceutical, cosmetic and food industries at low concentrations (Furia & Bellanca 1975). However, if the use of these compounds is extended to other applications that may require higher doses the increased exposure of humans to them is a matter of concern and therefore issues of safety and toxicity will need to be addressed. The cytotoxicity‐related observations of this study suggest that lavender oil or its components should be used with care and in highly diluted forms especially when directly applied to the skin. It is concluded that essential oils are potent aromatic compounds and evaluation of their toxicity is important before their beneficial antimicrobial activity can be put to safe economic use.
REFERENCES
- Atanassova‐Shopova S, Roussinov KS, Boycheva I (1973) On certain central neurotropic effects of lavender essential oil. II. Communications: studies on the effects of linalool and of terpinenol. Bull. Inst. Physiol. 15, 149. [Google Scholar]
- Babich H, Stern A, Borenfreund E (1993) Eugenol cytotoxicity evaluated with continuous cell lines. Toxicol. In Vitro 7, 105. [DOI] [PubMed] [Google Scholar]
- Bard M, Albrecht MR, Gupta N, Guynn CJ, Stillwell W (1988) Geraniol interferes with membrane functions in strains of Candida and Saccharomyces . Lipids 23, 534. [DOI] [PubMed] [Google Scholar]
- Basketter DA, Wright ZM, Colson NR, Patlewicz GY, Pease CKS (2002) Investigation of the skin sensitizing activity of linalool. Contact Dermatitis 47, 161. [DOI] [PubMed] [Google Scholar]
- Cornelis M, Dupont C, Wepierre J (1992) Prediction of eye irritancy potential of surfactants by cytotoxicity tests in vitro on cultures of human skin fibroblasts and keratinocytes. Toxicol. In Vitro 6, 119. [DOI] [PubMed] [Google Scholar]
- De Groot AC, Frosch PJ (1997) Adverse reactions to fragrances. A clinical review. Contact Dermatitis 36, 57. [DOI] [PubMed] [Google Scholar]
- Furia TE, Bellanca N (eds) (1975) Fenaroli's Handbook of Flavour Ingredients, 2nd edn, p.321 Boca Raton: CRC Press. [Google Scholar]
- Jager W, Buchbauer G, Jirovetz L, Fritzer M (1992) Percutaneous absorption of lavender oil from a massage oil. J. Soc. Cosmet. Chem. 43, 49. [Google Scholar]
- Kitahara M, Ishiguro F, Takayama K, Isowa K, Nagai T (1993) Evaluation of skin damage of cyclic monoterpenes, percutaneous absorption enhancers, by using cultured human skin cells. Biol. Pharm. Bull. 16, 912. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kim DB, Kim JI, Kim PY (2000) In vitro cytotoxicity tests on cultured human skin fibroblasts to predict skin irritation potential of surfactants. Toxicol. In Vitro 14, 345. [DOI] [PubMed] [Google Scholar]
- Lis‐Balchin M (1995) Aroma Science. The Chemistry and Bioactivity of Essential Oils. Rochester, UK: Amberwood Publishing Ltd. [Google Scholar]
- Lis‐Balchin M, Hart S (1999) Studies on the mode of action of the essential oil of lavender (Lavandula angustifolia P. Miller). Phytother. Res. 13, 540. [DOI] [PubMed] [Google Scholar]
- Manabe A, Nakayama S, Sakamoto K (1987) Effects of essential oils on erythrocytes and hepatocytes from rats and dipalmitoyl phosphatidylcholine‐liposomes. Jpn J. Pharmacol. 44, 77. [DOI] [PubMed] [Google Scholar]
- Osborne R, Perkins MA (1991) In vitro skin irritation testing with human skin cell cultures. Toxicol. In Vitro 5, 563. [DOI] [PubMed] [Google Scholar]
- Peana AT, D’Aquila PS, Panin F, Serra G, Pippia P, Moretti MDL (2002) Anti‐inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 9, 721. [DOI] [PubMed] [Google Scholar]
- Peana AT, D’Aquila PS, Chessa ML, Moretti MDL, Serra G, Pippia P (2003) (‐)‐Linalool produces antinociception in two experimental models of pain. Eur. J. Pharmacol. 460, 37. [DOI] [PubMed] [Google Scholar]
- Prashar A, Hili P, Veness RG, Evans CS (2003) Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae . Phytochemistry 63, 569. [DOI] [PubMed] [Google Scholar]
- Rastogi SC, Johansen JD, Frosch P, Menne T, Bruze M, Lepoittevin JP, Dreier B, Andersen KE, White IR (1998) Deodorants on the European market: quantitative chemical analysis of 21 fragrances. Contact Dermatitis 38, 29. [DOI] [PubMed] [Google Scholar]
- Rastogi SC, Heydorn S, Johansen JD, Basketter DA (2001) Fragrance chemicals in domestic and occupational products. Contact Dermatitis 45, 221. [DOI] [PubMed] [Google Scholar]
- Sikkema J, De Bont JA, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skold M, Borje A, Matura M, Karlberg A (2002) Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis 46, 267. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Hayakawa R, Kato Y, Sugiura K, Hashimoto R (2000) Results of patch testing with lavender oil in Japan. Contact Dermatitis 43, 157. [DOI] [PubMed] [Google Scholar]
- Tisserand R, Balacs T (2000) Essential Oil Safety: A Guide for Health Care Professionals. London: Churchill Livingstone. [Google Scholar]


