Abstract
Objectives: We have investigated foetal mesenchymal stem cells (MSCs) obtained from first‐trimester chorionic villi (CV) and second‐trimester amniotic fluid (AF), comparing them to adult bone marrow‐derived MSCs.
Materials and methods: We report on cell population growth in human allogeneic serum (HS) and platelet lysate (PL), immunophenotype, cytokine expression profile and immunoregulatory activity, of these foetal MSCs on stimulated peripheral blood mononuclear and lymphocyte subpopulations.
Results: Chorionic villi cells grow rapidly in HS, with 20 populations doublings (PDs) after 59 days (six passages), and also in animal serum, with 27 PDs after 65 days (seven passages). PL allowed for expansion in 60% of the samples tested, although it was lower than in HS. HS supported an average of 40 PDs of expansion in 20% of AF cells after 90 days, whereas animal serum supported 28.5 PDs in 66 days. CV and AF cells inhibited proliferation of stimulated T lymphocytes, suppressing population growth of both CD4+ and CD8+ T subpopulations and sometimes also, CD19+ cells.
Conclusions: Our results indicate that CV would be an optimal source of MSCs with high expansion potential in a HS propagation system and immunoregulatory capacity of T and B lymphocytes. More than 90% of CV samples achieved large‐scale expansion in HS, which is encouraging for potential clinical applications of these cells.
Introduction
Mesenchymal stem cells (MSCs) are multipotential non‐haematopoietic progenitor cells whose population expands rapidly in culture, as adherent stromal colonies. MSC are the subject of investigation in many fields, including tissue engineering, haematopoietic stem‐cell transplantation and gene therapy. The most utilized source of MSCs has been adult bone marrow (BM) (1, 2); however, cells with MSC‐like characteristics have also been isolated from adipose tissue (3), cord blood (4), placenta (5), amniotic fluid (AF) (6), chorionic villi (CV) (7) and other tissues. MSCs typically produce several types of cytokine, growth factors and cell adhesion molecules, important factors that influence the haematopoietic microenvironment and immunomodulatory capacity (8). Several reports have suggested that MSCs could exert a potent immunosuppressive effect in vitro and thus may have therapeutic potential for T‐cell‐dependent pathologies. These properties may be used in clinical therapy in the context of allogeneic stem‐cell transplantation, in particular, to modulate Graft versus Host Disease (GvHD) and graft rejection (9). MSCs are currently a focus of development towards a wide array of cell therapies and their use in clinical applications is documented by a growing number of clinical trials. Limited frequency of MSCs in tissues tested to date makes their in vitro expansion necessary to reach sufficient numbers of cells for therapeutic applications. Animal serum represents the predominant source of growth factors they require, in traditional cell‐culture systems. The main disadvantages of animal serum use are unknown pathogens and possibility of xenoimmunization.
Thus, the aim of this study was to test the ability of a human alternative to animal serum (HS, PL), to generate and expand in vitro, MSCs from foetal sources, as well as to characterize the cells for their immunomodulatory functions. We isolated MSCs from first‐trimester CV and AF used for prenatal investigation and diagnosis. We expanded these cells in pooled allogeneic human serum and in pooled allogeneic human platelet lysate, and tested expression of embryonic stem‐cell‐like genes (10) and multipotent differentiation specific markers (11). In particular, we focused on effects of foetal MSCs on various subtypes of T lymphocytes derived from allogenic peripheral blood. We characterized foetal stem cells for their immunophenotype and for expression of tolerogenic molecules, potentially important for their immunological features. In addition, we have described the cytokine secretion profile of foetal MSCs expanded in a human AB serum and in platelet lysate. Moreover, bringing new information concerning cytokines included in humanized propagation systems, we analysed the same cytokine secretion profile also in human and animal medium supplements.
Materials and methods
Cell cultures
Chorionic villus samples were obtained from 20 pregnant women at 11–13 weeks gestation after written informed consent. Permission for studies on human‐derived tissue was provided by all participants. CV cells were isolated using a villocentesis technique, and we used cells taken after the first passage of back‐up cultures prepared for prenatal diagnosis (generally after 20–30 days of culture) at completion of cytogenetic analysis.
Amniotic fluid samples were collected from 20 pregnant women at 15–18 weeks gestation after written informed consent. Permission for studies on human‐derived tissue was provided by all participants. Cells were harvested from discarded supernatants of AF cell cultures prepared for prenatal diagnosis. A cell culture was established after centrifugation for 10 min at 690 g.
Bone marrow was harvested,as described previously (12), from 9 healthy donors (5 ml, median age 17 years, range 16–45 years) after obtaining written informed consent from all participants, and from parents or guardians for those under eighteen, and seeded at the first passage at 0.5 × 106 cells/ml, then at 2000 cells/cm2.
Foetal cells were seeded at 2000 cells/cm2 in DMEM (Biological Industries) supplemented with 10% human allogeneic serum, 5% human allogeneic platelet lysate and 20% foetal bovine serum (Stem Cell Technologies, Vancouver, Canada) and incubated at 37 °C and 5% CO2 in a fully humidified atmosphere, as described previously (12). To avoid gel formation in medium supplemented with platelet lysate, we added 10 U/ml of heparin.
The proliferative potential in population doublings (PDs) of cultured cells was calculated at every passage, according to the equation: log2 (number of harvested cells/number of seeded cells). Finite PDs were determined by cumulative addition of total numbers generated from each passage until the cells ceased dividing.
Preparation of human allogeneic serum
Whole blood from healthy donors was obtained, after written informed consent from all participants, from the Transfusion Medicine Center of our hospital. Each blood sample was centrifuged at 690 g for 10 min at 4 °C. Serum samples obtained were collected and pooled. Aliquots of such sterile allogeneic human serum were stored at −20 °C until use.
Preparation of allogeneic platelet lysate
Platelet‐rich plasma was collected by two aphereses, after written informed consent, at the Transfusion Medicine Unit of our hospital, and samples that contained 1 × 1010 platelets (PLTs)/ml were pooled. Platelets were frozen at −80 °C and subsequently thawed at 37 °C to cause release of growth factors. The product was centrifuged three times at 900 g for 30 min to eliminate platelet bodies. Platelet lysate preparations (PL) obtained were collected, and aliquots were stored at −20 °C until use.
Immunophenotype analysis
CV, AF and BM were characterized by flow cytometry at all passages with antibodies to CD45 (BD Biosciences, Franklin Lakes, NJ, USA), CD34 (BD Biosciences), CD14 (BD Biosciences), CD31 (Dako, Glostrup, Denmark), CD133 (Miltenyi Biotech, Cologne, Germany), HLA‐DR (BD Biosciences), CD117 (Miltenyi), CD271 (Miltenyi), CD90 (BD Pharmingen, San Diego, California), CD105 (Immunostep), CD73 (BD Biosciences), CD44 (BD Biosciences), CD13 (BD Biosciences) and CD29 (BD Biosciences), as described previously (12).
Osteogenic, adipogenic and chondrogenic differentiation
CV, AF and BM cells were were cultured in adipogenic (NH AdipoDiff; Miltenyi Biotech), osteogenic (NH OsteoDiff; Miltenyi Biotech, Cologne, Germany) and chondrogenic media (NH ChondroDiff; Miltenyi Biotech), as described previously (12). Intracellular lipid droplets indicated adipogenic lineage differentiation. Differentiation potential of osteogenic lineage was evaluated by calcium accumulation as assessed by alizarin red S (Sigma‐Aldrich, St Louis, MO, USA) and chondrogenic differentiation was evaluated using the appropriate aggrecan kit (DakoCytomation LSAB System‐AP; Dako).
Reverse‐transcription polymerase chain reaction (RT‐PCR)
Total RNA was extracted using the Invisorb RNA Kit II (Invitek, Berlin, Germany) according to the manufacturer’s instructions. Purity of RNA was confirmed by determining 260 nm/280 nm absorbance ratio (>1.8). PCR was performed as described previously(12). Primers for Oct4 (NM_002701.4; 245 bp), Nanog (NM_024865.2; 213 bp), Sox17 (NM_022454.3; 154 bp), Gata4 (NM_002052.3; 194 bp), Tbx1 (NM_005992.1; 213 bp), Sox1 (NM_005986.2; 199 bp), Pax6 (NM_000280.3; 162 bp), PPARγ (NM_138712.3: 225 bp), LEP (NM_000230.2; 150 bp), LPL (NM_000237.2; 221 bp), Adipoq (NM_004797.2; 173 bp), OST (NM_000582.2; 162 bp), OCN (NM_199173; 355 bp), Sox‐9 (NM_000346; 169 bp), COL2A1 (NM_001844; 213 bp, HLA‐A (NM_002116.6; 237 bp), HLA‐DR (NM_002124.2; 231 bp), HLA‐G (NM_002127.5; 210 bp), CD80 (NM_005191.3; 181 bp), CD86 (NM_175862.3; 197 bp) and CD28 (NM_006139.2; 182 bp) were designed by Primer3 software (http://frodo.wi.mit.edu/primer3/).
PCR conditions consisted of initial denaturation at 94 °C for 4 min followed by 35 cycles of denaturation at 94 °C for 30 s, annealing for 30 s, extension at 72 °C for 30 s and final extension step at 72 °C for 10 min. Annealing temperatures were dependent on primers used [60 °C for all genes except Oct4 (65 °C)].
Analysis of cytokines
Mesenchymal stem cells were expanded in HS, PL and Fetal Bovine Serum (FBS). When cells reached confluence, culture supernatants were collected and frozen at −20 °C. Multiplex human cytokine, chemokine and growth factor detection (BioPlex; BioRad, Segrate, Italy) were utilized to measure production of interleukins (IL)‐1β, IL‐1ra, IL‐2, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8, IL‐9, IL‐10, IL‐12 (p70), IL‐13, IL‐15, IL‐17, basic fibroblast growth factor (FGF‐β), eotaxin, granulocyte colony‐stimulating factor (G‐CSF), granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), interferon‐γ (IFN‐γ), interferon‐γ‐induced protein (IP‐10), macrophage chemotactic protein (MCP‐1), macrophage inflammatory protein (MIP)‐1α, MIP‐1β, platelet‐derived growth factor‐bb (PDGF‐BB), CCL5 (RANTES), tumour necrosis factor‐α (TNF‐α) and vascular endothelium growth factor (VEGF), in culture supernatants.
Lymphocyte cultures and BrdU incorporation
Peripheral blood mononuclear cells (PB‐MNCs) were obtained from healthy donors, after written informed consent, at the Transfusion Medicine Unit of our hospital and were plated at 105 cells/100 μl concentration. PB‐MNCs were immunoseparated accoding to CD4, CD8 and CD19 (Miltenyi Biotech) expression. PB‐MNCs, CD4+ and CD8+ subpopulations were stimulated with 10 μg/ml phytohemagglutinin‐P (PHA), and the CD19+ subpopulation was stimulated with 2 μg/ml pokeweed mitogen (PWM) (Sigma‐Aldrich, St. Louis, MO). Irradiated MSCs (80 Gy) were resuspended in RPMI 1640 with 10% heat‐inactivated FBS (Stem Cell Technologies) and co‐cultured with lymphocytes at a ratio of 1:10. Cultures were performed in triplicate, incubated at 37 °C in 5% CO2 for 4 days then overnight with BrdU (Roche, Mannheim, Germany). Absorbances were measured using luminometer 1420 Multilabel Counter Victor3 (Perkin Elmer, Massachusetts, USA). Inhibitory effects of MSCs on proliferation of lymphocytes was calculated using the following formula: % inhibition lymphocyte proliferation (IP) = (proliferation of lymphocytes with mitogens in the presence of MSCs/proliferation of lymphocytes with mitogens) × 100.
Transwell experiments
Transwell experiments were performed in 24‐well transwell plates (0.22 μm pore size; Costar, Corning, NY, USA). PHA‐stimulated PB‐MNCs, CD4+ and CD8+ subpopulations and the PWM‐stimulated CD19+ subpopulation were cultured in RPMI 1640 with 10% heat‐inactivated FBS in the lower chamber of the transwell system. Irradiated MSCs (80 Gy) were cultured in HS, PL and FBS in the upper chamber of the system at a ratio of 1:10. Cultures were performed in triplicate, incubated at 37 °C in 5% CO2 for 4 days and then overnight with BrdU (Roche, Germany). Inhibitory effects of MSCs on proliferating lymphocytes were calculated as described above.
Statistical analysis
Data are presented as means ± SD and were analysed using Student’s t‐test. Differences were considered to be statistically significant at P < 0.05.
Results
Cell cultures
We expanded 20 CV samples. In animal serum medium, most samples (15 samples, v3–v17) achieved 8.13 ± 1.39 logues (27 PDs) in an average of 65 days and at passage 7. In allogeneic human serum medium, most samples (14 samples, v2–v3, v5–v6, v9–v18) achieved 5.98 ± 1.39 logues (19.85 PDs) in an average 59 days and after six passages. In allogeneic human platelet lysate medium, we studied seven CV samples (v1–v2, v12, v14–v17). Four of the seven samples tested (v2, v12, v15–v16) achieved 3.26 ± 1.48 logues (10.8 PDs) in an average 45 days and after four passages (Fig. 1).
Figure 1.
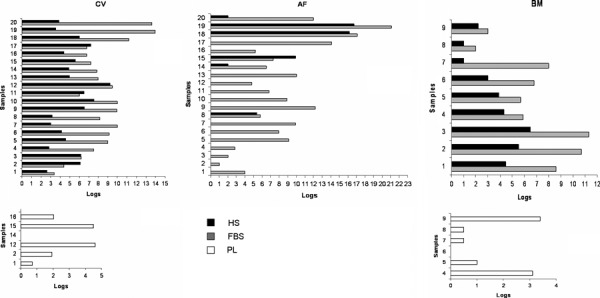
MSCs cultures in vitro. In vitro life span of MSC cultures, defined as logarithms of expansion before observation of senescence, derived from 20 CV, 20 AF and 9 BM samples, cultured in animal serum, allogenic human serum and allogeneic human platelet lysate.
Wide variability of in vitro proliferative capacity and life span was noted between AF samples. This can be explained as AF cells were obtained from discarded supernatants of primary cultures. Thus, presence of viable cells may depend on quantities of cells that did not attach to bottoms of flasks during primary culture and on time between seeding and first change of medium. In human serum medium, only a low percentage of AF samples grew, and no sample expanded in platelet lysate. In animal serum medium, we studied 20 AF samples at each passage. Most samples (A5–A16) achieved average of 7.9 ± 2.2 logues (27.5 PDs) expansion over average of 66 days, and at 4–7 passages. In allogeneic human serum medium, four of the twenty AF samples tested (20% of the samples; A8, A15, A18‐A19) gave rise to proliferation of 16.2, 16.7, 5.4 and 9.9 logues (53.8, 55.4, 17.9, 32.9 PDs, respectively) after average of 90 days, at passages 10, 10, 4 and 7 respectively. Two samples (A14, A20) expanded 2 logues in two passages after 33 days. No proliferation capacity was shown by the other AF samples. In allogeneic human platelet lysate medium, no sample had expansion capacity (Fig. 1).
Proliferation rate of most BM samples (BM1‐BM6) in medium supplemented with allogeneic human serum was on average 4.6 ± 1.2 logues (15.3 PDs) in 54 days and after five passages, while in animal serum medium, 8.1 ± 2.2 logues (26.9 PDs) in 85 days and after seven passages. We analysed six BM samples (BM4–BM9) with allogeneic human platelet lysate medium and, in two samples (BM4, BM9), we observed expansion of 3.25 logues (10.8 PDs) in 73 days and after five passages (Fig. 1).
Immunophenotype analysis
A similar pattern of surface markers was observed throughout the expansions. Foetal cells consistently stained positive for CD90, CD105, CD73, CD44, CD13 and CD29 and negative for CD34, CD133, CD45, CD14, CD117, CD271, CD31 and HLA‐DR (12).
Differentiation capability of MSC
We investigated differentiation potential of CV, AF and BM MSCs expanded in all media tested. As there was no difference between media analysed, we show data of cells expanded in HS. To further investigate differentiation capability, we performed expression analysis of specific adipogenic, osteogenic and chondrogenic markers.
Adipocyte differentiation involves a series of transcriptional events. PPARγ is one of the key positive transcriptional regulators of adipocyte differentiation and adipogenesis, activating a large number of target genes whose expression determines adipogenic differentiation, such as ADIPOQ, LPL, LEP. CV and AF cells had poor adipogenic potential with few and small fat vacuoles, weak expression (thin and not very intense band in RT‐PCR) of ADIPOQ and no detection of LPL. Otherwise, BM differentiated cells rich in lipid droplets, strongly expressed ADIPOQ and LPL. PPARγ and LEP were expressed by all samples tested.
Foetal MSCs were able to differentiate into osteogenic and chondrogenic cells with differentiation potential analogous to that of BM cells. Mineralized matrix aggregates stained by alizarin red S and positive expression of OSC and OCN in RT‐PCR, showed osteogenic differentiation of all cells studied. Positive staining for aggrecan and positive expression of Sox9 and COL2A1 had chondrogenic differentiation of all cells studied (Fig. 2A,B).
Figure 2.
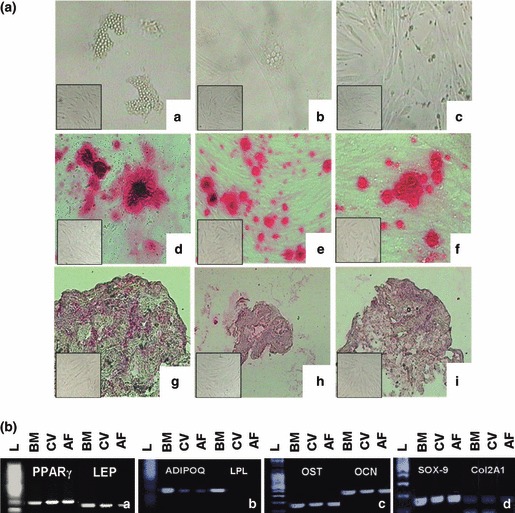
Differentiation capacity of MSCs expanded in HS. [A] Intracytoplasmatic lipid vacuoles (a,b,c); alizarin red S staining (d,e,f); aggrecan staining (g,h,i). Undifferentiated controls for respective time points are in lower left boxes. [B] Expression profile of BM, CV and AF adipogenic‐related genes PPAR γ and LEP (a), ADIPOQ and LPL (b), osteogenic‐related genes OST and OCN (c) and chondrocyte‐related genes Sox9 and Col2A1 (d), after adipogenic, osteogenic and chondrogenic induction, respectively. (L) 100 bp DNA ladder.
Embryonic stem‐cell gene expression profile
Embryonic stem cell genes required for self‐renewal and pluripotency including Nanog, Oct4, Sox17, Gata4, Tbx1 and Pax6 were expressed at high levels during culture at variable levels in both animal and human sera‐supplemented media (Fig. 3).
Figure 3.
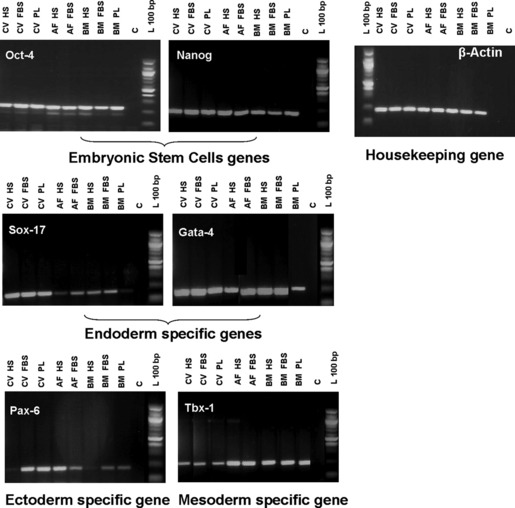
Embryonic stem‐cell gene expression profile. Expression profile of CV, AF and BM cells in HS, FBS and PL for embryonic stem‐cell genes and markers of endoderm, ectoderm and mesoderm. The expression of β‐actin, the housekeeping gene, was used as RT‐PCR internal control. (L) 100 bp DNA and (C) negative control.
MSC immune recognition‐related gene expression profile
We determined that CV and AF MSCs were negative for type II MHC (HLA‐DR), whereas it was expressed in BM samples (maybe, below the level of detection by flow cytometry). HLA‐A and HLA‐G were expressed by CV, AF and BM MSCs in all media tested. (Figure 4a). AF and CV cells did not express CD80 in culture with human and animal sera‐supplemented media. There was CD86 expression in some samples expanded in human and animal serum (data of cells in animal serum, not shown). CD28 was always expressed in all media tested. BM cells expressed CD80, but no CD86, in both human and animal sera. CD28 was expressed in all media tested (Fig. 4b).
Figure 4.
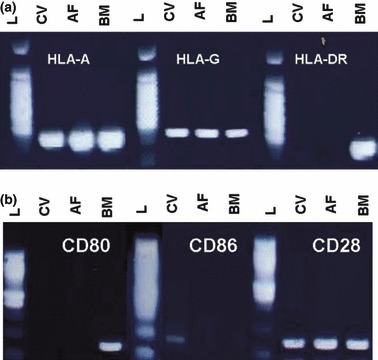
Gene expression profiles of CV, AF and BM cells expanded in HS for immune recognition specific markers. (a) MHC Class I and II; (b) co‐stimulatory molecules for T cell activation. (L) 100 bp DNA ladder. There was no significant difference between media tested.
Analysis of cytokines
MSC culture supernatants were analysed using a multiplex detection assay for 27 different factors. Results were divided into four groups: (−) value <50 pg/ml, (+) 50 pg/ml <value< 500 pg/ml, (++) 500 pg/ml < value< 5000 pg/ml, (+++) 5000 pg/ml < value. Samples were studied at three different passages, analysing their growth curves: at initiation of the exponential phase, at its centre and at the end. As there was no significant difference between the results, we show data at the beginning of the culture time, only.
Three CV samples expanded in human serum, three in animal serum and two in human platelet lysate medium were assayed. While many levels of secreted factors were not significantly different between media tested, MIP‐1β was produced at higher levels in cells in human allogeneic serum over those in human platelet lysate or animal serum. CV samples expanded in platelet lysate had lower levels of IL‐17, FGF‐β, MIP‐1α, GM‐CSF, IP‐10 and VEGF than those human and animal sera.
Three AF samples expanded in human allogeneic serum and three in animal serum medium were analysed. In contrast to CV cells, IL‐10 and IL‐12(p70) were produced from AF cells in human and animal sera, while IP‐10 was expressed at low levels.
Three BM samples expanded in human serum, platelet lysate and animal serum were used as controls for comparison with foetal MSCs. IL‐9, IL‐12(p70), IL‐17, IP‐10, MIP‐1α, MIP‐1β, GM‐CSF and FGF‐β were produced in human and animal sera, but not in platelet lysate. Major cytokines produced by foetal and BM MSCs were IL‐6, IL‐8, MCP‐1 and VEGF in all media tested (Table 1).
Table 1.
Levels of factors secreted from MSCs derived from CV, AF and BM at the beginning of the culture time. Results from the average of three samples are shown for each MSC. (−) value <50 pg/ml, (+) 50 pg/ml < value < 500 pg/ml, (++) 500 pg/ml<value<5000 pg/ml, (+++) value > 5000 pg/ml. There were no significant differences between the time points analysed
| 1 × 106 cells | CV MSCs HS | CV MSCs PL | CV MSCs FBS | AF MSCs HS | AF MSCs FBS | BM MSCs HS | BM MSCs PL | BM MSCs FBS |
|---|---|---|---|---|---|---|---|---|
| IL‐1β | − | − | − | − | − | − | − | − |
| IL‐1ra | + | + | + | − | − | + | + | + |
| IL‐2 | − | − | − | − | − | − | − | − |
| IL‐4 | − | − | − | − | − | − | − | − |
| IL‐5 | − | − | − | − | − | − | − | − |
| IL‐6 | ++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ |
| IL‐7 | − | − | − | − | − | − | − | − |
| IL‐8 | +++ | ++ | +++ | +++ | +++ | +++ | ++ | +++ |
| IL‐9 | + | − | + | − | + | + | − | + |
| IL‐10 | − | − | − | + | + | + | + | + |
| IL‐12p70 | + | − | − | + | + | + | − | + |
| IL‐13 | − | − | − | − | − | − | − | − |
| IL‐15 | + | + | − | − | − | + | + | + |
| IL‐17 | + | − | + | + | + | + | − | + |
| Eotaxin | + | − | − | − | − | − | − | − |
| FGF‐β | + | − | + | + | + | + | − | + |
| G‐CSF | + | − | − | − | − | − | − | − |
| GM‐CSF | + | − | + | + | + | + | − | + |
| IFN‐γ | + | + | + | + | + | + | + | + |
| IP‐10 | ++ | + | ++ | + | + | + | − | + |
| MCP‐1 | ++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ |
| MIP‐1α | + | − | + | + | + | + | − | + |
| MIP‐1 β | ++ | − | + | + | + | + | − | + |
| PDGFbb | − | − | − | − | − | − | − | − |
| RANTES | − | − | − | − | − | − | − | − |
| TNF‐α | + | − | + | + | + | + | + | + |
| VEGF | +++ | − | +++ | +++ | +++ | +++ | ++ | +++ |
AF, amniotic fluid; BM, bone marrow; CV, chorionic villi; HS, human allogeneic serum; MSCs, mesenchymal stem cells; PL, platelet lysate.
In addition, we analysed all growth factors tested in human allogeneic serum, platelet lysate and animal serum. FBS did not express any cytokine tested, HS only IP‐10 and PDGFbb, while LP expressed IL‐1ra, IL‐2, IL‐7, IL‐9, IL‐10, IL‐17, eotaxin, FGF‐ β, G‐CSF, GM‐CSF, IFN‐γ, IP‐10, MCP‐1, MIP‐1 β, TNF‐α and VEGF.
Immunomodulatory capacity of CV, AF and BM MSCs
After immunomagnetic separation, purity percentages of CD4+, CD8+ and CD19+ separated cell fractions were 98%, 98% and 96% respectively (data not shown).
In direct contact experiments, PB‐MNC and CD4+ cell proliferation were inhibited by all cells tested in the three media studied. There was high variability between MSC sources and media analysed. Indeed, CD8+ cells were inhibited only by AF in HS and FBS, and by BM in PL and FBS.
In transwell experiments, as in direct contact experiments, PB‐MNC proliferation was inhibited by all MSC sources tested in all media. CD4+ cell proliferation was inhibited only by CV and AF in FBS, and by BM in PL. Interestingly, MSC sources analysed seemed to be more effective at inhibiting CD8+ cell proliferation in the transwell system, with respect to direct contact experiments. CD19+ cells were inhibited only by CV in HS in direct contact, and in transwell experiments, and by BM in HS (Table 2).
Table 2.
The proliferative response of T and B cells to the different MSC sources. Table shows the results of: three BM MSCs in HS, three in LP and six in FBS; six CV in HS, three CV in LP and five CV in FBS; three AF in HS and eight in FBS. (IP) inhibition percentage of lymphocyte proliferation
| CV MSCs HS | CV MSCs PL | CV MSCs FBS | AF MSCs HS | AF MSCs FBS | BM MSCs HS | BM MSCs PL | BM MSCs FBS | |
|---|---|---|---|---|---|---|---|---|
| Direct contact | IP | IP | IP | IP | IP | IP | IP | IP |
| PB‐MNC | 24% | 35% | 74% | 20% | 23% | 47% | 20% | 24% |
| CD4+ cells | 31% | 53% | 65% | 34% | 35% | 57% | 24% | 31% |
| CD8+ cells | – | – | – | 47% | 39% | – | 41% | 52% |
| CD19+ cells | 31% | – | – | – | ‐ | – | – | – |
| Transwell | IP | IP | IP | IP | IP | IP | IP | IP |
| PB‐MNC | 16% | 16% | 58% | 33% | 46% | 28% | 20% | 30% |
| CD4+ cells | – | – | 68% | – | 40% | – | 28% | – |
| CD8+ cells | 64% | – | 35% | – | 44% | 36% | – | 33% |
| CD19+ cells | 40% | – | – | – | – | 30% | – | – |
AF, amniotic fluid; BM, bone marrow; CV, chorionic villi; HS, human allogeneic serum; MSCs, mesenchymal stem cells; PL, platelet lysate.
Discussion
Mesenchymal stem cells are non‐haematopoietic stem cells that grow rapidly in culture as adherent stromal colonies (1). Several reports (3, 4, 5, 6, 7) have indicated that foetal tissues are a potential source for populations of cells phenotypically resembling BM‐MSC. In this study, we reported cell population growth, characterization, immunomodulatory capacity and cytokine expression profiles of MSCs isolated from first trimester CV and second trimester amniotic fluid. We compared these cells to adult BM derived cells grown in a humanized propagation system.
We successfully expanded MSCs isolated from CV, AF and BM. In particular, we focused for the first time on foetal MSCs proliferative capacity in humanized systems (HS and PL) in the search for an animal serum substitute. CV cells achieved 19.85 PDs in six passages and 10.8 PDs (57% of the samples) in four passages in HS and PL, respectively. AF cells (20% of samples) achieved average of 40 PDs in eight passages in HS, and no expansion in PL.
In BM MSCs, we achieved higher expansion capacity in HS than in PL (15.3 versus 10.8 PDs). Thus, our data are in agreement with Bernardo et al. (13) that showed similar proliferation ability (11.6 PDs) of BM MSCs expanded in 5% PL, yet Tarte et al. (14) achieved 17 PDs. These different results could be explained by PL preparation. Theoretical levels of platelet‐derived growth factors in PL might be expected to depend on number of platelets involved. Weibrich et al. (15) however, in agreement with our study, showed no statistically significant correlation between platelet count and growth factor levels. This result might be explained by high individual variability in cell production or storage of cytokines (16). Indeed, increasing numbers of platelet donors in the pool increased concentration of many cytokines. Good manufacturing practice production of PL requires compliance with rules such as using freshly collected platelet‐rich‐plasma containing minimum of 1 × 109/ml platelets, and performing high‐speed centrifugation of PL to avoid residual platelet bodies for allogenic application (17). Kocaoemer et al. (18) have shown that handling of PL is decidedly more difficult than of FBS and HS. It was not possible to purify platelet‐factor rich supernatant of platelet membranes that formed huge aggregates and adhered to MSC surfaces. At the same time, centrifugation at high g to clear the membranes resulted in decreased proliferative effect. However, in our results, foetal and adult MSCs expanded more in HS than PL. HS does not require complicated manufacturing processes and has represented an easily accessible and less expensive resource of growth factors. Thus, there was too much variability in proliferation capacity of PL and its preparation should be standardized to compare studies in the literature and to assess its cell population growth potential.
We analysed CV, AF and BM for their immunophenotype, differentiation capacity and expression of stem‐cell markers. Foetal cells had immunophenotypic characteristics similar to those of MSCs derived from other foetal and adult sources (19, 20, 21) and were able to differentiate into mesenchymal lineages. Moreover, MSCs expressed embryonic stem‐cell genes, confirming the concept that these stem cells indeed are of embryonic origin, as reported by Zipori (22).
We have reported the immunomodulatory properties of the different MSCs sources studied with focussing on their effects on mitogen‐stimulated T‐ (PBMNC, CD4+ and CD8+ cells) and B‐cell proliferation. We have demonstrated that most samples failed to induce any allogeneic T‐cell response (23, 24, 25).
Foetal MSCs were capable of inhibiting PHA‐stimulated PBMNC proliferation not only when co‐cultured with cell‐cell contact but also when separated by a transwell membrane (26, 27). This finding suggests that soluble factors are involved in this phenomenon (28). A strong inhibitory effect was also evident in CD4+ T cell subpopulations when co‐cultured in direct contact with MSCs expanded in all media tested, while only in some cases, was there inhibition in the transwell system. Proliferation of PHA‐stimulated CD8+ T cell subpopulation seemed to be inhibited more strongly in the transwell system than with cell‐cell contact with all MSCs tested, whereas proliferation of PMW‐stimulated CD19+ B cells was inhibited only by low percentage of CV and BM cells. Therefore, there was immunosuppressive activity of CV MSCs expanded in HS as efficiently as BM MSCs, as reported in the literature (29, 30) and this characteristic was evident on total lymphocytes, but variable on T subpopulations and on B lymphocytes.
Our results have demonstrated that foetal MSCs were not substantially immunogenic towards PBMNCs. They did not induce allogeneic PB lymphocyte proliferation and inhibit stimulated PBMNCs. These results, together with the data of immunogenic molecular markers, in which MSCs displayed MHC I and T cell co‐stimulatory molecules, showed that immunosuppressive action of MSCs mainly targeted T‐cell proliferation rather than T‐cell activation (29, 30, 31). Moreover, CV, AF and BM MSCs expressed HLA‐G, a non‐classical MHC class I antigen, which may prevent immune responses against MSCs (32, 33).
In our results, the major cytokines produced by foetal and BM MSCs were IL‐6, IL‐8, MCP‐1 and VEGF (34). CV and BM expanded in PL did not secrete IL‐9, IL‐17, FGF‐β, GM‐CSF, IP‐10, MIP‐1α or MIP‐1β, but these cytokines were expressed in HS and FBS. PL displayed more pronounced pro‐inflammatory and immunoregulatory cytokines such as IL‐2, IL‐17, IFN‐γ, MCP‐1, MIP‐1β and TNF‐α. These findings could explain why MSCs were not able to expand in long‐term culture in PL. MSCs in human serum spontaneously produced cytokines capable of supporting haematopoiesis, for example GM‐CSF, to stimulate neovasculo/angiogenesis, like VEGF and FGF‐β, and to stimulate neuroregeneration such as FGF‐β and IL‐6. MSCs’ immunoregulatory property is possibly related to high production of IL‐6 and IL‐8, whereas high level of VEGF is probably related to their neovasculo/angiogenesis ability.
Conclusion
In addition to bringing new insight into the immunomodulatory functions of cells isolated from CV, AF and BM, data obtained in this study may be relevant for creating a humanized system to isolate and expand these alternative source‐derived MSCs with immunoregulatory capacity on T lymphocytes.
Our data have shown that CV would be an optimal source of MSCs and that medium supplemented by 10% pooled allogeneic human serum represented optimal growth conditions for generating and expanding MSCs. Indeed, CV cells had higher expansion potential with respect to BM MSCs in HS, and very low variability between samples. Moreover, there was no significant difference in tolerance induction and in cytokines produced with respect to AF and BM cells.
Thereafter, CV cells represented a potential source of stem cells for clinical application, better than AF for their capacity to expand in human serum. More than 90% of CV samples achieved large‐scale expansion in human pooled allogeneic serum medium. Our study indicates that clinical‐scale expansion of 10 × 108 MSC from one back up culture of CV is feasible in 30 days in presence of pooled allogeneic human serum. Use of CV cells could potentially contribute to achieving clinical‐scale expansion in less time compared to BM‐derived MSCs, without xenogenic serum addition. These results are encouraging, for potential clinical applications of these cells.
Acknowledgements and disclosure of interests
This work has been supported by grants from Associazione Italiana Contro le Leucemie, Linfomi e Mieloma (AIL), Sezione di Ancona‐ONLUS.
None of the authors has any conflict of interest to disclose.
References
- 1. Cananzi M, Atala A, De Coppi P (2009) Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod. Biomed. Online 18, 17–27. [DOI] [PubMed] [Google Scholar]
- 2. Poloni A, Maurizi G, Rosini V, Mondini E, Mancini S, Discepoli G et al. (2009) Selection of CD271+ cells and human AB serum allows a large expansion of mesenchymal stromal cells. Cytotherapy 11, 153–162. [DOI] [PubMed] [Google Scholar]
- 3. Rada T, Reis RL, Gomes ME (2011) Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev. 7, 64–76. [DOI] [PubMed] [Google Scholar]
- 4. Chang YJ, Tzu‐Bi Shih D, Tseng CP, Hsiehn TB, Lee DC, Hwang SM (2006) Disparate mesenchyme‐lineage tendences in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells 24, 679–685. [DOI] [PubMed] [Google Scholar]
- 5. Li C, Zhang W, Jiang X, Mao N (2007) Human‐placenta‐derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cells. Cell Tissue Res. 330, 437–446. [DOI] [PubMed] [Google Scholar]
- 6. De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L et al. (2007) Isolation of amniotic stem cells lines with potential for therapy. Nat. Biotechnol. 25, 100–106. [DOI] [PubMed] [Google Scholar]
- 7. Poloni A, Rosini V, Mondini E, Maurizi G, Mancini S, Discepoli G et al. (2008) Characterization and expansion of mesenchymal progenitor cells from first‐trimester chorionic villi of human placenta. Cytotherapy 10, 690–697. [DOI] [PubMed] [Google Scholar]
- 8. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M et al. (2004) Treatment of severe acute graft‐versus‐host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441. [DOI] [PubMed] [Google Scholar]
- 9. Le Blanc K, Samuelsson H, Lonnies L, Sundin M, Rigden O (2007) Generation of immunosuppressive mesenchymal stem cells in allogeneic human serum. Transplantation 84, 1055–1059. [DOI] [PubMed] [Google Scholar]
- 10. Cai J, Li W, Su H, Qin D, Yang J, Zhu F et al. (2010) Generation of human pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal stem cells. J. Biol. Chem. 285, 11227–11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sensebè L, Krampera M, Schrezenmeier H, Bourin P, Giordano R (2010) Mesenchymal stem cells for clinical application. Vox Sang. 98, 93–107. [DOI] [PubMed] [Google Scholar]
- 12. Poloni A, Maurizi G, Babini L, Serrani F, Berardinelli E, Mancini S et al. (2011) Human mesenchymal stem cells from chorionic villi and amniotic fluid are not susceptible to transformation after extensive in vitro expansion. Cell Transplant. 20, 643–654. [DOI] [PubMed] [Google Scholar]
- 13. Bernardo ME, Avanzini MA, Perotti C, Cometa AM, Moretta A, Lenta E et al. (2007) Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell‐therapy approches: further insights in the search for a fetal calf serum substitute. J. Cell. Physiol. 211, 121–130. [DOI] [PubMed] [Google Scholar]
- 14. Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H et al. (2010) Clinical‐grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 25, 1549–1553. [DOI] [PubMed] [Google Scholar]
- 15. Weibrich G, Kleis WKG, Hafner G, Hitzler WE (2002) Comparison of the platelet concentrate collection system with the plasma‐rich‐in‐growth‐factors kit to produce platelet‐rich plasma: a technical report. J. Craniomaxillofac. Surg. 30, 97–102. [PubMed] [Google Scholar]
- 16. Horn P, Bokermann G, Cholewa D, Bork S, Waleda T, Koch C et al. (2010) Impact of individual platelet lysates on isolation and growth of mesenchymal stromal cells. Cytotherapy 12, 888–898. [DOI] [PubMed] [Google Scholar]
- 17. Doucet C, Ernou I, Zhang Y, Llense JR, Begot L et al. (2005) Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell‐based therapy applications. J. Cell. Physiol. 205, 228–236. [DOI] [PubMed] [Google Scholar]
- 18. Kocaoemer A, Kenr S, Kluter H, Bieback K (2007) Human AB serum and thrombin‐activated platelet‐rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 25, 1270–1278. [DOI] [PubMed] [Google Scholar]
- 19. Bossolasco P, Montemurro T, Cova L, Zangrossi S, Calzarossa C, Buiatiotis S et al. (2006) Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 16, 329–336. [DOI] [PubMed] [Google Scholar]
- 20. Pieternella S, Sherjon SA, Fibbe WE (2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22, 1338–1345. [DOI] [PubMed] [Google Scholar]
- 21. Bernardo ME, Zaffarono N, Novara F, Cometa AM, Avanzino MA, Moretta A et al. (2007) Human bone marrow‐derived mesenchymal stem cells do not undergo transformation after long term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 67, 9142–9149. [DOI] [PubMed] [Google Scholar]
- 22. Zipori D (2004) The nature of stem cells: state rather than entity. Nature 5, 873–878. [DOI] [PubMed] [Google Scholar]
- 23. Barry FP, Murphy JM, English K, Mahon BP (2005) Immunogenecity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 14, 252–265. [DOI] [PubMed] [Google Scholar]
- 24. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E et al. (2005) Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T‐cell anergy. Blood 106, 1755–1761. [DOI] [PubMed] [Google Scholar]
- 25. Sudres M, Norol F, Trenado A, Grégoire S, Charlotte F, Levacher B et al. (2006) Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft‐versus‐host disease in mice. J. Immunol. 176, 7761–7767. [DOI] [PubMed] [Google Scholar]
- 26. Le Blanc K, Ringden O (2006) Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr. Opin. Immunol. 18, 586–591. [DOI] [PubMed] [Google Scholar]
- 27. Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H et al. (2006) Mesenchymal stem cells for treatment of therapy‐resistant graft versus‐host disease. Transplantation 81, 1390–1397. [DOI] [PubMed] [Google Scholar]
- 28. Magatti M, De Munari S, Verta E, Gibelli L, Wengler GS, Parolini O (2008) Human amnion mesenchyme harbors cells with allogeneic T‐cell suppression and stimulation capabilities. Stem Cells 26, 182–192. [DOI] [PubMed] [Google Scholar]
- 29. Ramasamy R, Tong CK, Seow HF, Vidyadaran S, Dazzi F (2008) The immunosuppressive effects of human bone marrow‐derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell. Immunol. 251, 131–136. [DOI] [PubMed] [Google Scholar]
- 30. Krampera M, Cosmi L, Angeli R, Pasini A, Lotta F, Andreini A et al. (2006) Role for interferon‐γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24, 386–398. [DOI] [PubMed] [Google Scholar]
- 31. Lenschow DJ, Walunas TL, Bluestone JA (1996) CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258. [DOI] [PubMed] [Google Scholar]
- 32. Nasef A, Mathieu N, Chapel A, Frick J, François S, Mazurier C et al. (2007) Immunosuppressive effects of mesenchymal stem cells: involvement of HLA‐G. Transplantation 84, 231–237. [DOI] [PubMed] [Google Scholar]
- 33. Najar M, Rouas R, Raicevic G, Boufker HI, Lewalle P, Meuleman N et al. (2009) Mesenchymal stromal cells promote or suppresse the proliferation of T lymphocytes from cord blood and pheripheral blood: the importance of low cell ratio and role of inleukine‐6. Cytotherapy 11, 570–583. [DOI] [PubMed] [Google Scholar]
- 34. Yoo KH, Jang KJ, Lee WM, Kim HE, Yang MS, Eom J et al. (2009) Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell. Immunol. 259, 150–156. [DOI] [PubMed] [Google Scholar]


