Abstract
Objectives: One aspect of the effects of isoflavones against fat deposition might be at least associated with the mechanism by which Wnt/β‐catenin signalling inhibits adipocyte differentiation. However, it remains completely unknown as to whether isoflavones might influence Wnt signalling during commitment of pluripotent mesenchymal stem cells (MSCs) to adipose lineages. In the present study, we have investigated the mechanisms underlying effects of genistein and daidzein, the major soy isoflavones, on anti‐adipogenic Wnt/β‐catenin signalling.
Materials and methods: Adipose tissue‐derived (AD) MSCs were exposed continuously to genistein and daidzein (0.01–100 μm) during adipogenic differentiation (21 days). An oestrogen antagonist, ICI 182,780, was used to determine whether or not the isoflavones activated Wnt signalling via oestrogen receptors (ERs).
Results: Genistein and daidzein suppressed adipogenic differentiation of AD‐MSCs in a dose‐dependent manner and inhibited expression of adipogenic markers, PPARγ, SREBP‐1c and Glut 4, from mid‐phase differentiation. Microarrays showed that anti‐adipogenic effects of genistein were principally attributable to activation of Wnt signalling via ERs‐dependent pathway, such as Erk/JNK signalling and LEF/TCF4 co‐activators. These findings were supported by evidence that the effects of genistein were offset by ICI182,780. Unlike genistein, daidzein inhibited adipogenesis through stimulation of lipolysis, with for example, PKA‐mediated hormone sensitive lipase. This is consistent with the increase in glycerol released from AD‐MSCs. In conclusion, understanding that different sets of mechanisms of the two isoflavones on adipogenesis will help the design of novel strategies to prevent observed current epidemic levels of obesity, using isoflavones.
Introduction
Adipose tissue can be considered as a major organ that maintains energy homeostasis through fat storage/release of surplus energy. However, excessive body fat (obesity) is a principal contributor to chronic diseases, including lipodystrophic conditions and insulin resistance (1). Adipose tissue results from adipogenic differentiation of precursor cells or hypertrophy of pre‐existing adipocytes (2), and these two processes can be prevented by regulation of several pathways relating to adipogenesis (3, 4, 5, 6).
Recently, Wingless‐type MMTV integration site family member (Wnt) signalling has been reported to cause preadipocytes to remain in an undifferentiated state, which is partly associated with the evidence that Wnt target genes, c‐myc, cyclin D1 and PPAR (peroxisome proliferator‐activated receptor)δ, inhibit adipogenic transcription factor PPARγ activity (3).
In many studies, Wnt signalling has been demonstrated to converge with the oestrogen receptor (ER)‐dependent pathway. Non‐genomic ER effectors, such as Jun oncogene (JUN), extracellular signal‐regulated kinase (Erk) and c‐Jun N‐terminal kinase (JNK), inhibit glycogen synthase kinase (GSK)3β‐mediated β‐catenin degradation and enhanced T‐cell factor (TCF)4‐dependent transcriptional activity (7, 8). Moreover, genomic ERs have been reported to interact directly with co‐activators of β‐catenin/TCF4, including p300/CBP (CREB binding protein) or chromatin remodelling complexes, that compel local chromatin structures to become modified for β‐catenin transactivation (9, 10).
A natural compound isoflavone, which mimics action of oestrogen and has only minimal side effects [unlike oestrogen itself (6)], has been reported to reduce fat deposition in vivo and in vitro (11, 12). One aspect of this anti‐adipogenic effect might be at least associated with the mechanism by which Wnt/β‐catenin signalling inhibits adipocyte differentiation. However, it remains completely unknown whether isoflavones might repress adipogenesis via Wnt/β‐catenin signalling, during commitment of pluripotent mesenchymal stem cells (MSCs) to adipose lineages.
Thus, we continuously exposed adipose tissue‐derived (AD)‐MSCs to genistein (0.01–100 μm), daidzein (0.01–100 μm), or oestrogen as positive control (0.1 μm), during adipogenic differentiation. To acquire comprehensive understanding of the effects of genistein and daidzein on Wnt/β‐catenin signalling, we conducted global gene comparisons using Beadchip microarray systems. In addition, we used ICI 182, 780, an oestrogen antagonist, to investigate whether anti‐adipogenic effects of isoflavones might be exerted via ER‐dependent or independent Wnt signalling.
Materials and methods
Isolation and expansion of MSCs from human adipose tissue
AD‐MSCs were obtained from fresh human lipoaspirates and propagated in modified MCDB 153 medium (Keratocyte‐SFM; Gibco‐Invitrogen, Carlsbad, CA, USA), containing 5% foetal bovine serum (FBS), N‐acetyl‐l‐cysteine (NAC, 2 mm; Sigma, St Louis, MO, USA) and L‐ascorbic acid 2‐phosphate (Asc 2P, 0.2 mm; Sigma), as described previously (13). Antioxidants NAC and Asc 2P, have been reported to promote proliferation and lifespan of stem and progenitor cells in culture (14, 15).
Adipogenic differentiation and treatment
Human AD‐MSCs (5 × 104 cells/24 well) were cultured in Dulbecco's modified Eagle's minimum essential medium (DMEM) containing 10% FBS and 1% (v/v) penicillin–streptomycin (10 000 units/ml of penicillin, 10 000 μg/ml of streptomycin in 0.85% saline) at 37 °C, in a humidified atmosphere of 5% CO2.
Next day, post‐confluent AD‐MSCs were exposed to genistein (0.01–100 μm; Sigma), daidzein (0.01–100 μm; Sigma) and positive control oestrogen (0.1 μm; Sigma), or in combination with ICI 182, 780 (10 μm; purity >99%; Tocris, Bristol, UK) in IDI + I‐medium (for 2 days), then in I‐medium (for 1 day), which was repeated seven times. IDI + I‐medium contained 3‐isobutyl‐1‐methylxanthine (0.5 mm; Sigma), dexamethasone (1 μm; Sigma), indomethasone (1 μm; Sigma) and insulin (10 mm; Sigma) in DMEM plus 10% charcoal‐stripped FBS and 1% penicillin–streptomycin. I‐medium contained insulin (10 mm; Sigma) in DMEM plus 10% charcoal‐stripped FBS and 1% penicillin–streptomycin.
Assessment of lipid droplets, apoptosis and cytotoxicity
Adipocyte‐differentiated AD‐MSCs were stained with 0.35% oil red O dye (Sigma) as detailed previously (12). Red‐stained lipid droplets were examined using an optical microscope and quantified using a spectrophotometer (Beckman Coulter, Brea, CA, USA) at 500 nm wavelength.
Apoptosis was observed by chromatin staining with Hoechst 33258 (0.5 mm in PBS; Sigma) as described previously (12). Stained cells were examined under ultraviolet illumination using a fluorescence microscope (Olympus Optical, Tokyo, Japan).
Cytotoxicity of treated compounds was assayed using 50 μl of 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyl tetrazolium bromide (MTT) solution (0.5 mg/ml in PBS; Sigma) as detailed previously (12), which is a means of measuring activity of living cells via mitochondrial dehydrogenase, as it cleaves tetrazolium rings of MTT and yields purple formazan crystals. Crystals were dissolved in isopropanol and the resulting purple solution was measured spectrophotometrically at 540 nm wavelength (Beckman Coulter).
Immunofluorescence
Adipocyte‐differentiated AD‐MSCs were fixed in 4% paraformaldehyde for 20 min, blocked for 1 h in 10% normal goat serum (Zymed Laboratories, San Franciso, CA, USA), incubated with primary antibody Oct‐4 (1:200; Chemicon, Temecula, CA, USA) followed by incubation with anti‐rabbit IgG antibody labelled with FITC (1:1000; Invitrogen, Carlsbad, CA, USA). Nuclei were stained with Hoechest 33258 (0.5 mm in PBS; Sigma). Images were captured using a confocal microscope (Eclipse TE 200; Nikon, Tokyo, Japan).
Triglyceride and free glycerol analysis
Cellular triglyceride and medium glycerol were determined by quantitative enzymatic and colorimetric methods using commercially available kits; for triglyceride, A410 (Sigma), and for glycerol, A540 (Sigma). For triglyceride analysis, cells were washed and scraped in 0.9% saline. Cell suspensions were homogenized by sonication. For glycerol analysis, adipogenic medium at 21 days was collected.
Protein extract and western blotting
Cells (2 × 106 cells/100π dish) were lysed in buffer (20 mm Tris–HCl, 150 mm NaCl, 1 mm EDTA, 1% Triton X‐100) containing proteinase inhibitors (1 mm aprotonin, 1 mm leupeptin, 1 mm PMSF) and protease inhibitors (1 mm NaOV3, 1 mm NaF) at 4 °C. Lysates were cleared by centrifugation at 16 000 g for 15 min, and supernatant protein content was quantified.
Twenty micrograms of protein was separated by SDS–PAGE and transferred to nitrocellulose membranes as described previously (12). Proteins were visualized using p‐ERα/β‐catenin/Erk1/2/p‐Erk1/2/Bcl‐2 (1:1000; Cell Signaling, Danvers, MA, USA), ERα/p‐ERβ/PPARγ (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), Bax/JNK/p‐JNK (1:1000; Upstate Biotechnology, Lake Placid, NY, USA) and β‐actin (1:10 000; Sigma), followed by horseradish peroxidase‐labelled anti‐goat, rabbit, mouse IgG antibody (1:5000, Santa Cruz Biotechnology).
Total RNA extraction and real‐time PCR
Total RNA was isolated and reverse‐transcribed using TRIzol reagent and reverse transcriptase kits (Invitrogen) according to manufacturer’s instructions. For relative quantification of gene expression, real‐time PCR was conducted using a Biosystem 7500 real‐time PCR system (ABI, Carlsbad, CA, USA). cDNA (500 ng) was amplified using a 25 μl SYBR Green PCR master mix (ABI) and specific primer pairs. Primers are listed in Table S1. Relative expression levels of target genes were calculated as: 2−ΔΔCt, where
Microarray analysis
Gene expression profiles were assessed using Sentrix Human Ref 6 Expression Beadchip system (Illumina Inc., USA), which contains 50‐mer oligonucleotide probes representing more than 46 000 individual human genes per piece. Each gene was derived from the National Center for Biotechnology Information (NCBI) Human Reference Sequence (RefSeq) database.
For microarray analysis, isolated RNA was reverse transcribed to cDNA using reverse transcriptase, then biotinylated cRNA was synthesized from cDNA by in vitro transcription (Ambion Inc., USA). Biotinylated cRNA was hybridized to an array treated with Streptavidin‐Cy3. Chemiluminescent signals were scanned, quantified and corrected using the BeadArray system and software (Illumina Beadstation 500, San Diego, CA, USA).
Target sequence signals with detection P‐values of <0.05 were normalized using quantile normalization (16). Differentially expressed probe sets were selected using local‐pooled‐error (LPE) tests (P < 0.05) (17) and fold‐change values (≥|1.5|). Biological processes were analysed using Protein Analysis Through Evolutionary Relationships (PANTHER; http://www.pantherdb.org). Each RNA sample was extracted from AD‐MSCs exposed to vehicle (DMSO), genistein (20 μm), or daidzein (20 μm).
Statistical analysis
Data were expressed as mean ± SEM, and analysed using one‐way analysis of variance (ANOVA) followed by Dunnett’s multiple range test, or t‐test using sas Proprietary Software Release 8.2 (SAS Institute, USA). In micorarray analysis, genes with fold‐change values (≥|1.5|) were analysed by LPE test (17) using Avadis Prophetic version.3.3 (Strand Genomics, Bangalore, India) and R (version 2.5) program. Data with P‐values of <0.05 were considered statistically significant.
Results
Genistein and daidzein inhibited adipogenic differentiation of AD‐MSC in a dose‐dependent manner
As shown in Fig. 1A,B and Fig. S1, genistein and daidzein reduced lipid droplets formation in a dose‐dependent manner compared to vehicle controls.
Figure 1.

Genistein and daidzein inhibited adipogenic differentiation of AD‐MSCs in a dose‐dependent manner. AD‐MSCs were cultured in adipogenic differentiation medium plus genistein or daidzein for 21 days. (A) oil red O staining (original magnification; 200×, scale bar; 100 μm); (B) Quantification of oil red O; (C) Hoechst staining (original magnification; 100×); (D) MTT assay at 1, 2 and 3 days; (E) Protein level of apoptotic Bax and anti‐apoptotic Bcl‐2; (F) mRNA level of adipogenic PPARγ and anti‐adipogenic β‐catenin. Data are expressed as mean ± SEM (n = 3–5 independent experiments). *Mean values were significantly different from those of control groups (Dunnett’s test; P < 0.05). AD‐MSCs, adipose tissue‐derived mesenchymal stem cells; PPARγ, peroxisome proliferator‐activated receptor γ.
Specially above concentration of 20 μm, genistein and daidzein maintained fibroblast‐like appearance of AD‐MSCs during adipogenic differentiation, which might be attributable to downregulation of adipogenic transcription factor PPARγ expression and upregulation of anti‐adipogenic transcription factor β‐catenin expression, as well as expression of stem cell marker, Oct‐4 (1, 2). At concentrations of 50–100 μm, genistein and daidzein maintained more fibroblast‐like appearance of AD‐MSCs than that shown at 20 μm concentration, but also increased expression of apoptotic protein Bax and reduced cell growth by approximately 20% (Fig. 1D,E), even without chromatin condensation and DNA cleavage (Fig. 1C). Indeed, high concentrations (≥50 μm) of genistein have previously been demonstrated to induce developmental toxicity in zebrafish embryos (18), and hydroxylated analogues of daidzein – 3′‐OH‐daidzein and 6′‐OH‐daidzein – and equol have been reported to increase genotoxicity, that is, 10 μm 3′‐OH‐daidzein and 100 μm equol formed micronuclei in Ishikawa cells (19) and in mouse lymphoma cells (20) respectively. Taking all the evidence together, we chose the non‐cytotoxic concentration of 20 μm for the following study.
Figure 2.

Genistein and daidzein inhibited adipogenic differentiation of AD‐MSCs from mid‐phase differentiation. AD‐MSCs were cultured in adipogenic differentiation medium plus genistein or daidzein for 3 (early), 12 (middle) and 21 (terminal) days. (A) oil red O (original magnification; 200×, scale bar; 100 μm) and Hoechst staining (original magnification; 100×); (B) Quantification of oil red O; (C) Immunofluorescence of Oct‐4 (original magnification; 400×, scale bar; 100 μm). Data are expressed as mean ± SEM (n = 3–5 independent experiments). *Mean values were significantly different from those of control groups (Dunnett’s test; P < 0.05). AD‐MSCs, adipose tissue‐derived mesenchymal stem cells.
Genistein and daidzein inhibited adipogenic differentiation of AD‐MSCs from mid‐phase differentiation
Once committed to adipose lineage, AD‐MSCs underwent a complex adipogenic programme, which requires coordination of several pathways ranging from downregulation of preadipocyte factor (Pref)‐1 (21) and Wnt proteins (3) to induction of CCAT/enhancer‐binding proteins (C/EBPs) (22) and PPARγ (23). However, it is quite complex already at the time when these several pathways participate in normal adipocyte differentiation. Thus, actions of genistein and daidzein involved in adipogenic processes might change according to the differentiation stage cells are in.
As shown in 2, 3, genistein reduced lipid droplets and mRNA levels of adipogenic transcription factors, such as fatty acid‐binding protein 4 (FABP4; aP2), sterol regulatory element‐binding protein (SREBP)‐1c, PPARγ and glucose transporter (Glut) 4, principally in mid‐phase (12 days) differentiation, compared to vehicle control (P < 0.05). In contrast, genistein increased expression of anti‐adipogenic transcription factors, Pref‐1 and β‐catenin, from mid‐phase differentiation.
Figure 3.
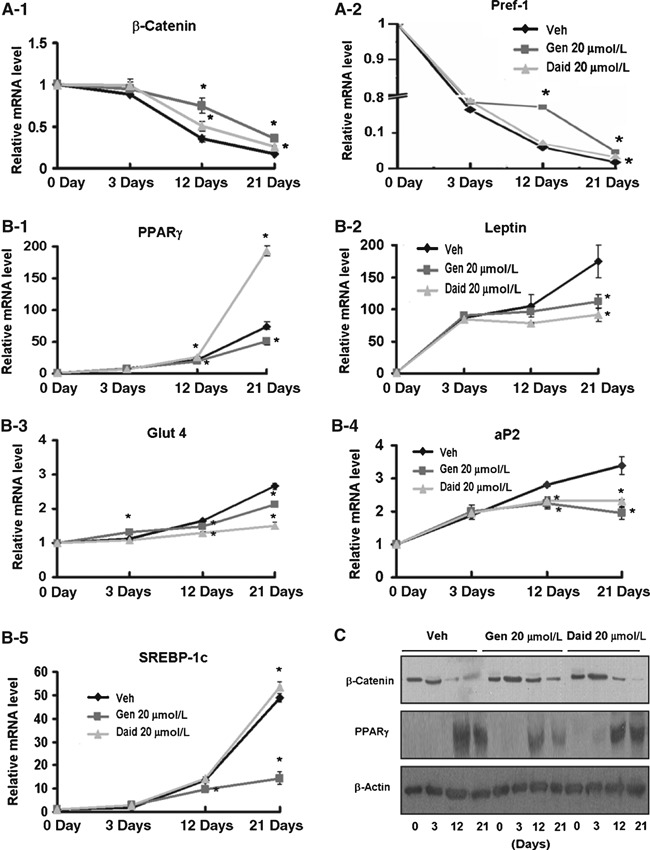
Genistein and daidzein altered expression of adipogenic or anti‐adipogenic markers principally from mid‐phase. AD‐MSCs were cultured in adipogenic differentiation medium plus genistein or daidzein for 3 (early), 12 (middle) and 21 (terminal) days. (A) mRNA levels of anti‐adipogenic genes; (B) mRNA level of adipogenic genes; (C) Protein levels of β‐catenin and PPARγ. Data are presented as mean ± SEM (n = 3–5 independent experiments). *Mean values were significantly different from those of control groups at each differentiation phase (3, 12 and 21 days) (Dunnett’s test; P < 0.05). AD‐MSCs, adipose tissue‐derived mesenchymal stem cells; aP2, fatty acid‐binding protein 4; Glut 4, glucose transporter 4; PPARγ, peroxisome proliferator‐activated receptor γ; SREBP‐1c, sterol regulatory element‐binding protein‐1c.
Daidzein showed a similar pattern with reference to lipid droplets and expressions of aP2, Pref‐1, and Glut4 with genistein. However, daidzein increased expression of PPARγ and SREBP‐1c unlike genistein (P < 0.05).
Collectively, these findings indicate that anti‐adipogenic effects of genistein and daidzien are likely to be exerted mainly from mid‐stage differentiation via regulation of genes involved in adipogenic processes.
Genistein and daidzein regulated adipogenesis by different set of mechanisms
To obtain comprehensive understanding regarding effects of genistein and daidzein on Wnt/β‐catenin signalling, we conducted a global gene analysis using the Beadchip system.
A total of 4363 genes were expressed differentially in genistein‐ or daidzein‐treated cells, compared to vehicle‐treated cells. Among them, we selected the genes associated with Wnt signalling, inflammation, fatty acid β‐oxidation, lipogenesis, lipolysis and insulin signalling (Table 1).
Table 1.
Gene expression profiles altered in cells exposed to genistein or daidzein
| Gene name | Fold change and LPE <0.051 | ||
|---|---|---|---|
| GeneBank ID | Gene symbol and description | Gen | Daid |
| Wnt/β‐catenin signalling | |||
| NM_030753.3 | WNT 3; wingless‐type MMTV integration site family, member 3 | 1.6 | 1.1ns2 |
| NM_032642.2 | WNT 5B: wingless‐type MMTV integration site family, member 5B | −2.1 | −1.6 |
| NM_001904.2 | CTNNB1(β‐Catenin);catenin (cadherin‐associated protein), beta 1, 88kDa | 1.6 | ↔3 |
| NM_001892.4 | Casein kinase 1, alpha 1(CSNK1A1) | −2.4 | −1.1ns |
| NM_003012.3 | SFRP1; secreted frizzled‐related protein 1 | −1.2 | −1.7 |
| NM_014421.2 | DKK2; dickkopf homologue 2 (Xenopus laevis) | −3.8 | −1.7ns |
| NM_004275.3 | TRFP; Trf (TATA‐binding protein‐related factor)‐proximal homologue | 1.5 | ↔ |
| Converge between Wnt/β‐catenin and ER signalling | |||
| NM_003884.3 | PCAF; p300/CBP‐associated factor | 1.8 | 1.4ns |
| NM_003076.3 | SMARCD1; SWI/SNF‐related, matrix‐associated, actin‐dependent regulator of chromatin, subfamily d, member 1 | 2.1 | ↔ |
| NM_005078.1 | TLE3; transducin‐like enhancer of split 3 | −2.6 | ↔ |
| NM_002745.4 | MAPK1(ERK2); mitogen‐activated protein kinase 1 | 1.6 | ↔ |
| NM_138980.1 | MAPK10(JNK3); mitogen‐activated protein kinase 10 | 1.4 | 1.5 |
| NM_004586.2 | RPS6KA3; ribosomal protein S6 kinase, 90 kDa, polypeptide 3 | 1.5 | ↔ |
| NM_002228.3 | JUN; v‐jun sarcoma virus 17 oncogene homologue (avian) | 1.8 | 1.3ns |
| Inflammation | |||
| NM_000584.2 | IL8; interleukin 8 | −3.8 | −1.5ns |
| NM_002503.3 | NFKBIB; nuclear factor of kappa light polypeptide gene enhancer in B‐cell inhibitor, beta | 2.4 | ↔ |
| NM_201397.1 | GPX1; glutathione peroxidase 1 | −1.5 | ↔ |
| NM_001752.2 | CAT; catalase | −1.5 | ↔ |
| NM_003102.1 | SOD3; superoxide dismutase 3 | −1.6 | −1.5ns |
| Lipolysis | |||
| NM_005357.2 | LIPE; lipase, hormone‐sensitive (LIPE) | −2.1 | 1.9 |
| NM_002736.2 | PRKAR2B(PKA); protein kinase, cAMP‐dependent, regulatory, type II, beta | 2.5 | 1.5 |
| Fatty acid β‐oxidation and lipogenesis | |||
| NM_002861.1 | PCYT2; phosphate cytidylyltransferase 2, ethanolamine | 2.4 | 2.1 |
| NM_198336.1 | INSIG1; insulin‐induced gene 1 | 2.3 | 2.6 |
| NM_015869.3 | PPARG; peroxisome proliferative activated receptor, gamma | −1.5 | 2.6 |
| NM_001033583.1 | ACOT9; acyl‐CoA thioesterase 9 | −3.3 | −1.4ns |
| NM_173467.3 | MT; malonyl‐CoA:acyl carrier protein transacylase (mitochondria) | −3.5 | ↔ |
| NM_203379.1 | ACSL5; acyl‐CoA synthetase long‐chain family member 5 | 2.5 | 1.8ns |
| NM_032169.3 | ACAD11; acyl‐Coenzyme A dehydrogenase family, member 11 | 1.9 | 1.5 |
| NM_003355.2 | UCP2; uncoupling protein 2 (mitochondria, proton carrier) | 1.7 | 1.6ns |
| Insulin signalling | |||
| NM_004383.1 | CSK; c‐src tyrosine kinase | −1.7 | ↔ |
| NM_203349.2 | SHC4; SHC family, member 4 | 1.58 | −1.1ns |
| NM_005544.1 | IRS1; insulin receptor substrate 1 | −1.7 | 1.0ns |
| NM_001014431.1 | AKT1; v‐akt murine thymoma viral oncogene homologue 1 | −1.8 | ↔ |
| NM_030777.3 | SLC2A10(Glut 4); solute carrier family 2, member 10 | −3.1 | ↔ |
1The fold changes are represented with the mean gene expression ratios from each of two independently repeated microarray experiments comparing the genisten‐ and daidzein‐treated cells with the vehicle‐treated cells. The LPE test is used to verify a statistical significance regarding the fold changes of genes that expressed differentially (≥1.5‐fold).
2The fold changes are not statistically significant at P < 0.05 using LPE test.
3The fold changes are below 1.5‐fold.
Genes involved in Wnt/β‐catenin signalling. As shown in Table 1, genes including those belonging to classes of non‐canonical or canonical Wnt antagonists, such as Wnt 5b, secreted frizzled‐related protein (sFRP)1, dickkopf homologue (Dkk)2, casein kinase (CSNK)α1 and transducin‐like enhancer of split (TLE)4, were downregulated by up to 1.5‐ to 2.6‐fold expression in cells exposed to genistein, compared to those exposed to vehicle. Among them, Wnt 5b and sFRP1 were also downregulated by up to 1.6–1.7‐fold expression levels in cells treated with daidzein, compared to those treated with vehicle.
Canonical Wnt 3 and β‐catenin (here, referred to as CTNNB1) had 1.5‐fold higher levels of expression in cells treated with genistein, but not with daidzein, than those treated with vehicle. However, daidzein increased expression of β‐catenin at the protein level (Fig. 4C). TATA‐binding protein‐related factor‐proximal (TRFP), which links β‐catenin to the basal transcriptional machinery or RNA polymerase complex, displayed the same pattern as Wnt 3 and β‐catenin. These results suggest that genistein is a more potent activator of Wnt signalling than daidzein.
Figure 4.
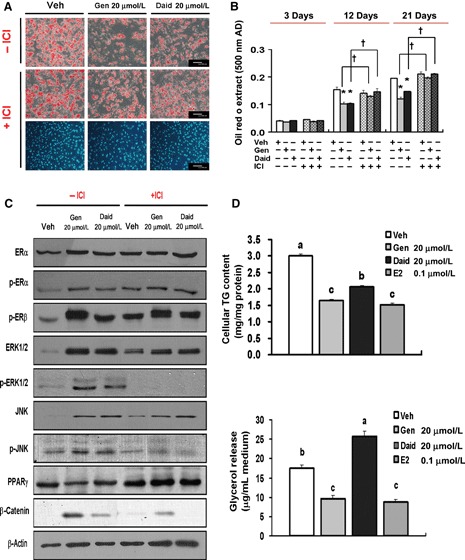
Inhibitory effects of genistein and daidzein on adipogenesis were exerted via ER‐dependent mechanism. AD‐MSCs were cultured in adipogenic differentiation medium plus genistein or daidzein or combination with ICI for 3 (early), 12 (middle) and 21 (terminal) days. (A) oil red O (original magnification; 200×, scale bar; 100 μm) and Hoechst staining (original magnification; 100×); (B) Quantification of oil red O; (C) Protein level of ER signalling‐related intermediates, β‐catenin and PPARγ; (D) Cellular triglyceride and glycerol concentration. Data are expressed as mean ± SEM (n = 3–5 independent experiments). *Mean values were significantly different from those of control groups at each differentiation phase (3, 12 and 21 days) (Dunett’s test; P < 0.05), †mean values were significantly different between groups with ICI and without ICI addition (t‐test; P < 0.05). a,b,cMean values with dissimilar superscript letters were significantly different between each groups (Duncan’s test; P < 0.05).
Genes converged between ER signalling and Wnt signalling. Recent studies have reported cross‐talk between ER signalling and Wnt signalling (24). Thus, we postulated that oestrogenic genistein and daidzein might affect Wnt signalling via ERs. Transcript levels of ERα and ERβ were omitted in our transcript profiles because of data filtering. However, expressions of phosphorylated ERα and ERβ were augmented in cells exposed to genistein or daidzein, compared to those exposed to vehicle (Fig. 4C).
In addition, non‐genomic ER signallers as well as suppressors of GSK3β or PPARγ, Shc (Src homology 2 domain containing family)4‐Erk 2 (here, referred to MAPK 1)‐p90RS6KA3 or Shc4‐JNK3 (here, referred to MAPK 10)‐JUN, were augmented by 1.5‐fold levels of expression in cells treated with genistein, compared to those treated with vehicle.
Other genomic ER signallers as well as β‐catenin/TCF4 co‐activators, p300/CBP‐associated factor (PCAF) and SWI/SNF‐related, matrix‐associated, actin‐dependent regulator of chromatin, subfamily d (SMARCD)1 were upregulated above 1.6‐fold levels of expression in cells exposed to genistein, compared to those exposed to vehicle.
Taken together, our findings indicate that genistein, but not dadizein, appeared to activate Wnt signalling via non‐genomic or genomic ER signalling.
Genes involved in inflammation that functions as regulators of Wnt signalling. Recently, pro‐inflammatory cytokines such as tumour necrosis factor (TNF)α and interleukin (IL)‐6, have been reported to promote lipolysis through activation of Wnt signalling (25).
In our transcription profile, expressions of IL‐8 and IL‐7 were reduced up to 2‐fold in cells exposed to genistein compared to those exposed to vehicle. This reduction results from upregulation of inhibitor of nuclear factor kappa B (NFκB) that controls genes involved in the immune response. Reducing the inflammatory status consequently led to inactivation of antioxidative system. Antioxidant enzymes, glutathione peroxidase (GPX) 1, catalase (CAT) and superoxide dismutase (SOD)3 were downregulated up to 1.5‐fold in cells exposed to genistein, compared to genes in those exposed to vehicle. These findings suggest that genistein did not promote Wnt signalling through a pro‐inflammatory cytokine‐dependent mechanism.
Genes involved in lipolysis and fatty acid β‐oxidation. Although daidzein did not have any substantial effect on Wnt signalling, it reduced numbers of lipid droplets found, similar to genistein (Fig. 1). Thus, there might be yet a different set of pathways in anti‐adipogenesis activity of dadizein.
As shown in Table 1, genes encoding enzymes of lipolysis, such as hormone‐sensitive lipase (LIPE) and its upstream protein kinase A (here, referred to as PPKAR2β), were upregulated up to 1.5‐fold in cells exposed to daidzein, compared to those exposed to vehicle. These results were consistent with the amount of glycerol (a 44% increase, P < 0.05) used as lipolytic index (Fig. 4D).
Also, genistein and daidzein regulated genes involved in lipogenesis or fatty acid β‐oxidation. Ethanolaminephosphate cytidylyltransferase (PCYT)2, acyl‐coenzyme A dehydrogenase (ACAD)11 and insulin‐induced gene (INSIG)1, responsible for inhibition of lipogenesis and catalysis of fatty acid β‐oxidation, were augmented 50–100% in cells exposed to genistein and daidzein, compared to those in cells exposed to vehicle.
Although this overlap is evident between genistein and daidzein, there were also numerous genes regulated by genistein, but not by daidzein. For instance, long‐chain fatty acyl‐coenzyme A synthetase (ACSL)5, involved in a rate‐limiting step in mitochondrial fatty acid β‐oxidation, was observed with 1.5‐fold higher level of expression in cells exposed to genistein than in those exposed to vehicle. Moreover, expression of genes coding for proteins involved in fatty acid importation or synthesis, such as fatty acid‐binding protein (FABP)7, acyl‐CoA thioesterase (ACOT)9 and malonyl‐CoA:acyl carrier protein transacylase (MT), were reduced up to 3‐fold by genistein. Uncoupling protein (UCP)3, which stimulates mitochondrial thermogenesis and energy expenditure, also had 1.5‐fold higher level of expression in cells exposed to genistein than those exposed to vehicle.
Taken together, genistein and daidzein seem likely to inhibit adipogenesis via activation of fatty acid β‐oxidation and lipolysis respectively. These results are in accordance with lower cellular triglyceride concentration (P < 0.05) (Fig. 4D).
Genes involved in insulin signalling. Genistein, but not daidzein, has long been identified as a tyrosine kinase inhibitor (26). This characteristic seems to contribute to inducing differential results between genistein and daidzein on insulin signalling. As shown in Table 1, intermediates of insulin signalling including insulin receptor substrate (IRS)1, c‐Src tyrosine kinase (CSK), v‐akt murine thymoma viral oncogene homologue (AKT)1 and Glut 4 (here, referred to SLC2A10) were reduced up to 1.6‐ to 8‐fold levels of expression in cells exposed to genistein, compared to those in cells exposed to vehicle.
These findings suggest that the anti‐adipogenic effect of genistein might perhaps result from impaired insulin signalling, as genistein can function as a tyrosine kinase inhibitor (26).
Confirmation of microarray data
To verify the results of microarray analysis, we compared gene expression profiles obtained on the microarrays and real‐time RT‐PCR. Both patterns were quite similar in terms of direction (up‐ or downregulation) and degree of changes in expression (Table 2). Moreover, oestrogen, the positive control, produced expression patterns of IRS‐1, AKT 1, Glut 4, PPARγ and β‐catenin more similar to genistein rather than daidzein.
Table 2.
Confirmation of gene expression profiles and comparison with oestrogen, a positive control, using real‐time RT‐PCR
| Gene name | Gen (20 μm) | Daid (20 μm) | E2 (0.1 μm) | ||
|---|---|---|---|---|---|
| Microarray | RT‐PCR | Microarray | RT‐PCR | RT‐PCR | |
| HSL | −2.1 | 0.45 ± 0.01* | 1.9 | 2.26 ± 0.09* | 0.56 ± 0.02* |
| TLE3 | −2.6 | 0.29 ± 0.04* | 1.2 | 1.47 ± 0.16* | 0.70 ± 0.05 |
| Glut 4 | −8.4 | 0.14 ± 0.004* | −2.3 | 0.45 ± 0.01* | 0.30 ± 0.02* |
| c‐JUN | 1.8 | 2.04 ± 0.13* | 1.3 | 1.39 ± 0.16 | 1.44 ± 0.26 |
| Erk2 | 1.6 | 1.59 ± 0.13* | 1.1 | 1.21 ± 0.06 | 0.92 ± 0.05 |
| DKK2 | −3.8 | 0.25 ± 0.004* | −1.7 | 0.66 ± 0.02* | 1.12 ± 0.09 |
| AKT1 | −1.9 | 0.58 ± 0.01* | −1.1 | 0.83 ± 0.01* | 0.91 ± 0.02* |
| IRS1 | −1.7 | 0.51 ± 0.03* | 1 | 1.57 ± 0.20* | 0.53 ± 0.07* |
| β‐Catenin | 1.6 | 2.06 ± 0.06* | −1.2 | 0.89 ± 0.05 | 3.42 ± 0.20* |
| PPARγ | −1.3 | 0.68 ± 0.02* | 2.6 | 2.60 ± 0.01* | 0.49 ± 0.01* |
| WNT 5B | −2.1 | 0.47 ± 0.02* | ‐1.6 | 0.55 ± 0.02 | 1.34 ± 0.22 |
The data are expressed as the relative mRNA levels (mean ± SEM., n = 3–5 independent experiments) using real‐time PCR.
*Significant differences between each treatment group and vehicle control group at P < 0.05 using Dunnett’s multiple range test.
Effects of genistein and daidzein against adipogenesis were offset by ICI 182,780
To confirm whether genistein and daidzein truly regulated Wnt/β‐catenin signalling by an ER‐dependent mechanism, AD‐MSCs had been treated with oestrogen antagonist ICI 182, 780.
Treatment combinations with ICI 182, 780 (10 μm) offset the effects of genistein or daidzein in lipid droplet production from mid‐phase differentiation (Fig. 4A,B). In addition, combinations with ICI 182, 780 suppressed non‐genomic ER cascades (ERα, p‐ERα, p‐ERβ, Erk1/2, p‐Erk1/2, JNK, p‐JNK) and β‐catenin expression, but enhanced PPARγ expression (P < 0.05) (Fig. 4C). These results support the notion that genistein and daidzein are likely to regulate Wnt signalling via an ER‐dependent mechanism.
Discussion
In the present study, phyto‐oestrogens, genistein and daidzein, prevented AD‐MSCs from differentiating into mature adipocytes in a dose‐ or time‐dependent manner (1, 2). One aspect of this effect is at least likely to be associated with the mechanism by which Wnt/β‐catenin signalling potently inhibits adipocyte differentiation (3, 27).
Wnt ligands bind to Frizzled (Fz) receptors and low‐density lipoprotein receptor‐related protein (LRP)5/6 co‐receptors, whereby Dishevelled (Dsh/Dvl) is phosphorylated and subsequently prevents GSK‐3β phosphorylation of β‐catenin at Ser 33, 37, 45, and Thr 41. This allows β‐catenin to escape degradation by the ubiqutin/proteasome pathway, and translocate into the nucleus to activate lymphoid enhancer factor (LEF)/TCF4 responsive genes. Recently, c‐myc, cyclin D1 and PPARδ, target genes of LEF/TCF4, have been reported to inhibit expression and activity of adipogenic transcription factors PPARγ and C/EBPα (3, 4, 5).
Interestingly, in this study, genistein directly promoted Wnt signalling via regulation of Wnt ligands, Wnt antagonists and Wnt intermediates. Until now, 19 different Wnt genes have been identified in mouse and human genomes (28). Among their proteins, genistein increased expression of Wnt 3, which inhibits adipogenesis by regulation of C/EBPα and PPARγ expression in 3T3‐L1 cells (27), and reduces expression of Wnt 5b that otherwise stimulates adipogenesis; this has been identified with type 2 diabetes in the population of Japan (29, 30). Moreover, genistein inhibited Wnt signalling antagonists such as sFRP1, DKK2, CK1 and Axin2 (Table 1). sFRP1 contains a cysteine‐rich domain homologous with the putative Wnt‐binding site, so that it competes with Fz receptor of Wnt ligands (31). DKK2 interacts with LRP5/6, thereby interfering with Wnt‐Fz‐LRP5/6 formation (32). CK1 and Axin 2 create a priming site for GSK‐3β on β‐catenin (33). Genistein and daidzein also augmented mRNA and protein levels of β‐catenin, a final effector of Wnt signalling (Table 1 and Fig. 3C).
These stimulatory effects of genistein on Wnt signalling are likely to be exerted though both non‐genomic and genomic ER‐dependent pathways. Genistein‐activated ER signalling, such as Shc 4‐Erk2‐p90RS6KA3 and Shc4‐JNK3‐JUN (Table 1), has been reported to inactivate GSK‐3β, transactivate LEF/TCF4 and degrade PPARγ (7, 8, 34, 35). In addition, genistein‐augmented p300/CBP and SWI/SNF, binders of nuclear ERs as well as transcriptional co‐activators of LEF/TCF4 (9), have been demonstrated to accelerate access of β‐catenin to the LEF/TCF4 promoter and link β‐catenin to basal transcriptional machinery or to the RNA polymerase complex (10).
These findings were confirmed by evidence that the stimulatory effects of genistein on Wnt signalling were offset by ICI 182,780, an oestrogen antagonist (Fig. 4C).
Daidzein also prevented AD‐MSCs from differentiating into mature adipocytes (1, 2), although it did not function as a potent activator of Wnt signalling and even augmented expression of PPARγ, an inhibitor of β‐catenin (Fig. 3B1 and Table 1). Presumably, there is a different set of pathways in anti‐adipogenic effects of daidzein, for example, PKA‐HSL‐dependent lipolysis (Table 1). PKA activates HSL by phosphorylation of Ser 563, 659, 660 of HSL and subsequently mobilizes free fatty acid and glycerol from triglyceride stored in adipocytes (36). Indeed, glycerol release from the differentiated AD‐MSCs was significantly higher in cells exposed to daidzein, but not genistein, over those exposed to vehicle only (Fig. 4D).
First, this difference between genistein and daidzein might be directly related to oestrogenic activity differences according to ER subtype. Ranking of oestrogenic potency (% activity relative to E2) is E2 (100) >genistein (0.56) >daidzein (0.11) to ERα subtype but E2 (100) >daidzein (5.1) >genistein (2.2) to ERβ subtype. The results are associated with decrease in preference for ERβ by a hydroxyl group at aromatic ring position 5 of genistein (37). Incidentally, responsiveness of Wnt signalling has been reported to be increased in ERα‐positive cells (38, 39). Considering all the evidence mentioned above, genistein seems to inhibit adipogenesis via ERα‐dependent Wnt signalling.
Second, a metabolite of daidzein, O‐desmethylangolensin, has been demonstrated to inhibit transcriptional activity, that is, suppression of PPARγ (40) and SREBP‐1c (41), of 5α‐dihydrotestosterone (DHT), and alpha2‐adrenoceptor antilipolytic signalling (42) by interaction with androgen receptors (43). Indeed, daidzein upregulated expressions of PPARγ and SREBP‐1c and stimulated lipolysis in our study, even though transcriptional activity or expression of 5α‐DHT was not determined. These findings are in accordance with the evidence that daidzein activates PPARγ‐driven reporter‐gene activity in 3T3‐L1 cells (44).
Third, the characteristic of genistein being a tyrosine kinase inhibitor might induce a different set of pathways used between genistein and daidzein. Glucose is a primary energy source for adipogenesis and its transport is controlled by the insulin‐dependent pathway. In our transcription profiles (Table 1), genistein inhibited expression of genes encoding insulin signalling, including those for IRS‐1, AKT and Glut 4, which might be associated with genistein being a tyrosine kinase inhibitor (26).
The anti‐adipogenic effects of genistein and daidzein appeared mainly from mid‐stage differentiation of AD‐MSCs, which might result from augmentation of β‐catenin from mid‐phase. β‐catenin is known as a potent inhibitor of PPARγ that stimulates differentiation of preadipocytes into mature adipocytes from mid to terminal stages of differentiation (3, 4, 5).
Collectively, genistein and daidzein suppressed adipogenic differentiation of AD‐MSCs by different sets of pathways, such as Wnt/β‐catenin signalling and lipolysis, from mid‐phase adipogenic differentiation, although there was some overlap in fatty acid β‐oxidation. In addition, genistein was a more powerful anti‐adipogenic compound than daidzein, which might be understandable considering that ERα is expressed predominantly and identically in most human mature adipocytes regardless of anatomical origin (intra‐abdominal and subcutaneous), and gender (45).
In conclusion, our study describes novel mechanisms by which genistein and daidzein, major components of soy isoflavones, inhibit adipogenesis through prompting of Wnt/β‐catenin signalling or lipolysis. Moreover, considering that radioactive tracer studies have determined that turnover rate (from 6 to 15 months) of MSCs (46, 47) is similar to that (280–420 days) of mature adipocytes (48), an MSC population within adipose tissue is presumably used to replace death or removal of mature adipocytes. Thus, our study using AD‐MSCs will be a useful guide for prevention of adipose tissue‐associated diseases including obesity, diabetes and non‐alcoholic fatty liver disease.
Supporting information
Table S1 Sequences of primers used for quantitative real‐time PCR analysis. Sequences of primers sets were designed using Primer Express Software v2.0 (ABI, USA) and verified using BLAST (basic local alignment search tool) provided by NCBI (National Center for Biotechnology Information)
Fig. S1 Genistein and daidzein reduced lipid droplets in a dose‐dependent manner without chromatin condensation and DNA cleavage. AD‐MSCs were exposed to genistein (0.01–100 μm), daidzein (0.01–100 μm) and oestrogen (0.1 μm) for 21 days. Lipid droplets were stained with oil red O (original magnification; 200×, scale bar; 100 μm) and nuclei were stained with Hoechst (original magnification; 100×). AD‐MSCs, adipose tissue‐derived mesenchymal stem cells.
Fig. S2 Pharmacological concentration (20 μm) of genistein and daidzein altered expressions of β‐catenin and PPARγ towards inhibition of adipogenesis, without cytotoxicity. (a) MTT assay in AD‐MSCs exposed to genistein (0.01–100 μm) or daidzein (0.01–100 μm) for 1, 2 and 3 days. (b) mRNA levels of anti‐adipogenic β‐catenin and adipogenic PPARγ in AD‐MSCs exposed to genistein (0.01–100 μm) or daidzein (0.01–100 μm) for 21 days. Data are expressed as mean ± SEM (n = 3–5 independent experiments). *Mean values were significantly different from those of control groups (Dunnett’s test; P < 0.05). AD‐MSCs, adipose tissue‐derived mesenchymal stem cells; PPARγ, peroxisome proliferator‐activated receptor γ.
Supporting info item
Supporting info item
Acknowledgements
This study was supported by a grant from Korea Research Foundation (MEST no. M10841000119‐08N4100‐11910). There are no conflicts of interest.
References
- 1. Perseghin G, Petersen K, Shulman GI (2003) Cellular mechanism of insulin resistance: potential links with inflammation. Int. J. Obes. Relat. Metab. Disord. 27(Suppl. 3), S6–S11. [DOI] [PubMed] [Google Scholar]
- 2. Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE (2000) Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 43, 1498–1506. [DOI] [PubMed] [Google Scholar]
- 3. Freytag SO, Geddes TJ (1992) Reciprocal regulation of adipogenesis by Myc and C/EBP alpha. Science 256, 379–382. [DOI] [PubMed] [Google Scholar]
- 4. Fu M, Rao M, Bouras T, Wang C, Wu K, Zhang X et al. (2005) Cyclin D1 inhibits peroxisome proliferator‐activated receptor gamma‐mediated adipogenesis through histone deacetylase recruitment. J. Biol. Chem. 280, 16934–16941. [DOI] [PubMed] [Google Scholar]
- 5. Shi Y, Hon M, Evans RM (2002) The peroxisome proliferator‐activated receptor delta, an integrator of transcriptional repression and nuclear receptor signaling. Proc. Natl. Acad. Sci. USA 99, 2613–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yasui T, Uemura H, Takikawa M, Irahara M (2003) Hormone replacement therapy in postmenopausal women. J. Med. Invest. 50, 136–145. [PubMed] [Google Scholar]
- 7. Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J et al. (2005) Erk associates with and primes GSK‐3beta for its inactivation resulting in upregulation of beta‐catenin. Mol. Cell 19, 159–170. [DOI] [PubMed] [Google Scholar]
- 8. Nateri AS, Spencer‐Dene B, Behrens A (2005) Interaction of phosphorylated c‐Jun with TCF4 regulates intestinal cancer development. Nature 437, 281–285. [DOI] [PubMed] [Google Scholar]
- 9. Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H (2001) The chromatin remodelling factor Brg‐1 interacts with beta‐catenin to promote target gene activation. EMBO J. 20, 4935–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hecht A, Vleminckx K, Stemmler MP, Van Roy F, Kemler R (2000) The p300/CBP acetyltransferases function as transcriptional coactivators of beta‐catenin in vertebrates. EMBO J. 19, 1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Penza M, Montani C, Romani A, Vignolini P, Pampaloni B, Tanini A et al. (2006) Genistein affects adipose tissue deposition in a dose‐dependent and gender‐specific manner. Endocrinology 147, 5740–5751. [DOI] [PubMed] [Google Scholar]
- 12. Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW et al. (2005) Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP‐activated protein kinase. Biochem. Biophys. Res. Commun. 338, 694–699. [DOI] [PubMed] [Google Scholar]
- 13. Lin TM, Tsai JL, Lin SD, Lai CS, Chang CC (2005) Accelerated growth and prolonged lifespan of adipose tissue‐derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 14, 92–102. [DOI] [PubMed] [Google Scholar]
- 14. Noble M, Smith J, Power J, Mayer‐Proschel M (2003) Redox state as a central modulator of precursor cell function. Ann. N. Y. Acad. Sci. 991, 251–271. [DOI] [PubMed] [Google Scholar]
- 15. Chepda T, Cadau M, Girin P, Frey J, Chamson A (2001) Monitoring of ascorbate at a constant rate in cell culture: effect on cell growth. In Vitro Cell. Dev. Biol. Anim. 37, 26–30. [DOI] [PubMed] [Google Scholar]
- 16. Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193. [DOI] [PubMed] [Google Scholar]
- 17. Jain N, Thatte J, Braciale T, Ley K, O’Connell M, Lee JK (2003) Local‐pooled‐error test for identifying differentially expressed genes with a small number of replicated microarrays. Bioinformatics 19, 1945–1951. [DOI] [PubMed] [Google Scholar]
- 18. Kim DJ, Seok SH, Baek MW, Lee HY, Na YR, Park SH et al. (2009) Developmental toxicity and brain aromatase induction by high genistein concentrations in zebrafish embryos. Toxicol. Mech. Methods 19, 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kulling SE, Lehmann L, Metzler M (2002) Oxidative metabolism and genotoxic potential of major isoflavone phytoestrogens. J. Chromatogr. 777, 211–218. [DOI] [PubMed] [Google Scholar]
- 20. Schmitt E, Metzler M, Jonas R, Dekant W, Stopper H (2003) Genotoxic activity of four metabolites of the soy isoflavone daidzein. Mutat. Res. 542, 43–48. [DOI] [PubMed] [Google Scholar]
- 21. Mei B, Zhao L, Chen L, Sul HS (2002) Only the large soluble form of preadipocyte factor‐1 (Pref‐1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: role of alternative splicing. Biochem. J. 364, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ et al. (2002) C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 16, 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farmer SR (2005) Regulation of PPARgamma activity during adipogenesis. Int. J. Obes. (Lond.) 29(Suppl. 1), S13–S16. [DOI] [PubMed] [Google Scholar]
- 24. Kouzmenko AP, Takeyama K, Ito S, Furutani T, Sawatsubashi S, Maki A et al. (2004) Wnt/beta‐catenin and estrogen signaling converge in vivo. J. Biol. Chem. 279, 40255–40258. [DOI] [PubMed] [Google Scholar]
- 25. Gustafson B, Smith U (2006) Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3‐L1 preadipocytes. J. Biol. Chem. 281, 9507–9516. [DOI] [PubMed] [Google Scholar]
- 26. Szkudelski T, Nogowski L, Pruszynska‐Oszmalek E, Kaczmarek P, Szkudelska K (2005) Genistein restricts leptin secretion from rat adipocytes. J. Steroid Biochem. Mol. Biol. 96, 301–307. [DOI] [PubMed] [Google Scholar]
- 27. Kennell JA, MacDougald OA (2005) Wnt signaling inhibits adipogenesis through beta‐catenin‐dependent and ‐independent mechanisms. J. Biol. Chem. 280, 24004–24010. [DOI] [PubMed] [Google Scholar]
- 28. Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810. [DOI] [PubMed] [Google Scholar]
- 29. Kanazawa A, Tsukada S, Kamiyama M, Yanagimoto T, Nakajima M, Maeda S (2005) Wnt5b partially inhibits canonical Wnt/beta‐catenin signaling pathway and promotes adipogenesis in 3T3‐L1 preadipocytes. Biochem. Biophys. Res. Commun. 330, 505–510. [DOI] [PubMed] [Google Scholar]
- 30. Kanazawa A, Tsukada S, Sekine A, Tsunoda T, Takahashi A, Kashiwagi A et al. (2004) Association of the gene encoding wingless‐type mammary tumor virus integration‐site family member 5B (WNT5B) with type 2 diabetes. Am. J. Hum. Genet. 75, 832–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhat RA, Stauffer B, Komm BS, Bodine PV (2007) Structure‐function analysis of secreted frizzled‐related protein‐1 for its Wnt antagonist function. J. Cell. Biochem. 102, 1519–1528. [DOI] [PubMed] [Google Scholar]
- 32. Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A et al. (2001) LDL‐receptor‐related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321–325. [DOI] [PubMed] [Google Scholar]
- 33. Amit S, Hatzubai A, Birman Y, Andersen JS, Ben‐Shushan E, Mann M et al. (2002) Axin‐mediated CKI phosphorylation of beta‐catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16, 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Camp HS, Tafuri SR, Leff T (1999) c‐Jun N‐terminal kinase phosphorylates peroxisome proliferator‐activated receptor‐gamma1 and negatively regulates its transcriptional activity. Endocrinology 140, 392–397. [DOI] [PubMed] [Google Scholar]
- 35. Hu E, Kim JB, Sarraf P, Spiegelman BM (1996) Inhibition of adipogenesis through MAP kinase‐mediated phosphorylation of PPARgamma. Science 274, 2100–2103. [DOI] [PubMed] [Google Scholar]
- 36. Krintel C, Osmark P, Larsen MR, Resjo S, Logan DT, Holm C (2008) Ser649 and Ser650 are the major determinants of protein kinase A‐mediated activation of human hormone‐sensitive lipase against lipid substrates. PLoS One 3, e3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takeuchi S, Takahashi T, Sawada Y, Iida M, Matsuda T, Kojima H (2009) Comparative study on the nuclear hormone receptor activity of various phytochemicals and their metabolites by reporter gene assays using Chinese hamster ovary cells. Biol. Pharm. Bull. 32, 195–202. [DOI] [PubMed] [Google Scholar]
- 38. Miyakoshi T, Kajiya H, Miyajima K, Takei M, Tobita M, Takekoshi S et al. (2009) The expression of Wnt4 is regulated by estrogen via an estrogen receptor alpha‐dependent pathway in rat pituitary growth hormone‐producing cells. Acta Histochem. Cytochem. 42, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ray S, Xu F, Wang H, Das SK (2008) Cooperative control via lymphoid enhancer factor 1/T cell factor 3 and estrogen receptor‐alpha for uterine gene regulation by estrogen. Mol. Endocrinol. 22, 1125–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Du J, Zhang L, Wang Z (2009) Testosterone inhibits the activity of peroxisome proliferator‐activated receptor gamma in a transcriptional transaction assay. Pharmazie 64, 692–693. [PubMed] [Google Scholar]
- 41. Aragno M, Tomasinelli CE, Vercellinatto I, Catalano MG, Collino M, Fantozzi R et al. (2009) SREBP‐1c in nonalcoholic fatty liver disease induced by Western‐type high‐fat diet plus fructose in rats. Free Radic. Biol. Med. 47, 1067–1074. [DOI] [PubMed] [Google Scholar]
- 42. Arner P (2005) Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie 87, 39–43. [DOI] [PubMed] [Google Scholar]
- 43. Mentor‐Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A (2001) Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP). Cancer Res. 61, 6777–6782. [PubMed] [Google Scholar]
- 44. Shen P, Liu MH, Ng TY, Chan YH, Yong EL (2006) Differential effects of isoflavones, from Astragalus membranaceus and Pueraria thomsonii, on the activation of PPARalpha, PPARgamma, and adipocyte differentiation in vitro. J. Nutr. 136, 899–905. [DOI] [PubMed] [Google Scholar]
- 45. Dieudonne MN, Leneveu MC, Giudicelli Y, Pecquery R (2004) Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am. J. Physiol. Cell Physiol. 286, C655–C661. [DOI] [PubMed] [Google Scholar]
- 46. Neese RA, Misell LM, Turner S, Chu A, Kim J, Cesar D et al. (2002) Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc. Natl. Acad. Sci. USA 99, 15345–15350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Strawford A, Antelo F, Christiansen M, Hellerstein MK (2004) Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am. J. Physiol. Endocrinol. Metab. 286, E577–E588. [DOI] [PubMed] [Google Scholar]
- 48. Hellerstein MK (2003) Turnover of adipose components and mitochondrial DNA in humans: kinetic biomarkers for human immunodeficiency virus‐associated lipodystrophy and mitochondrial toxicity? Clin. Infect. Dis. 37, S52–S61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Sequences of primers used for quantitative real‐time PCR analysis. Sequences of primers sets were designed using Primer Express Software v2.0 (ABI, USA) and verified using BLAST (basic local alignment search tool) provided by NCBI (National Center for Biotechnology Information)
Fig. S1 Genistein and daidzein reduced lipid droplets in a dose‐dependent manner without chromatin condensation and DNA cleavage. AD‐MSCs were exposed to genistein (0.01–100 μm), daidzein (0.01–100 μm) and oestrogen (0.1 μm) for 21 days. Lipid droplets were stained with oil red O (original magnification; 200×, scale bar; 100 μm) and nuclei were stained with Hoechst (original magnification; 100×). AD‐MSCs, adipose tissue‐derived mesenchymal stem cells.
Fig. S2 Pharmacological concentration (20 μm) of genistein and daidzein altered expressions of β‐catenin and PPARγ towards inhibition of adipogenesis, without cytotoxicity. (a) MTT assay in AD‐MSCs exposed to genistein (0.01–100 μm) or daidzein (0.01–100 μm) for 1, 2 and 3 days. (b) mRNA levels of anti‐adipogenic β‐catenin and adipogenic PPARγ in AD‐MSCs exposed to genistein (0.01–100 μm) or daidzein (0.01–100 μm) for 21 days. Data are expressed as mean ± SEM (n = 3–5 independent experiments). *Mean values were significantly different from those of control groups (Dunnett’s test; P < 0.05). AD‐MSCs, adipose tissue‐derived mesenchymal stem cells; PPARγ, peroxisome proliferator‐activated receptor γ.
Supporting info item
Supporting info item


