Abstract
While acute neuroprotection in acute stroke has proven difficult and ended in many failures, there is increasing interest in restorative therapies that target brain remodelling. Cell therapy (transplantation of cells) shows promise, with a growing body of pre‐clinical evidence demonstrating improved functional outcomes in animal models; however, questions still remain concerning mechanisms of action. Clinical trials are already underway and will increase in the next few years; their appropriate design and execution along with continued pre‐clinical work are necessary for the field to advance and satisfy a large unmet clinical need.
Acute neuroprotection in human stroke has been largely unsuccessful with many failed clinical trials; the traditional big pharma ‘small molecule’ approach has yielded no positive results. To date, the only FDA‐approved pharmacological agent for acute stroke is the biological one, tissue plasminogen activator (tPA). While initially the time window for tPA administration has been restricted to 3 h, the ECASS clinical trial has now shown that this time window can be extended to 4.5 h (1). As cascade of injuries in acute ischaemic stroke is mostly complete within 24–48 h, neuroprotection to be effective, must be started within a small number of hours of injury. One of the reasons for failure of neuroprotective treatments for stroke has been the need to start treatments so soon, within 3–6 h of the onset of ischaemia. This has proven to be difficult in clinical practice. On the other hand, for restorative treatment, the target of therapy is to promote repair processes such as angiogenesis, neurogenesis and synaptogenesis. The window for restorative treatment is not precisely known but it may extend to as much as 1 week or even as far as 1 month or perhaps longer. Neurorestorative treatment can be initiated later than neuroprotective treatment and therefore many more stroke patients could potentially benefit (Fig. 1).
Figure 1.
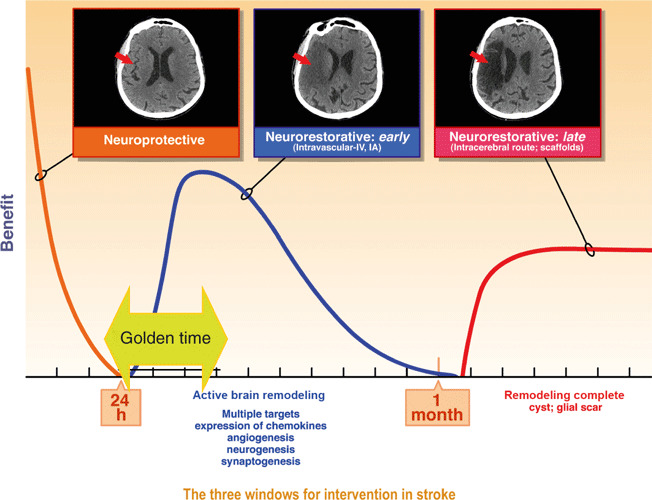
Depiction of the three time windows for therapeutic intervention after stroke. This so‐called golden time is the period of most active brain remodelling.
Ischaemic stroke (cerebral ischaemia) involves destruction of multiple cell types including neurons, astrocytes, oligodendrocytes, endothelial cells and pericytes. Thus, regenerative strategy needs to target not only neural elements but also supportive structures such as blood vessels. After cerebral ischaemia, the brain undergoes active remodelling and Nestin is upregulated in astrocytes after cerebral ischaemia (2, 3). In rodents, subventricular progenitor cells proliferate after middle cerebral artery occlusion and cerebral ischaemia and migrate to the striatum where they contribute to formation of striatal medium‐sized spiny neurons and glial cells (4). However, most of these neuroblasts undergo apoptosis and die. Subventricular progenitor cells continue to migrate to the striatum for at least 4 months and this migration is directed by a gradient of stromal derived factor 1 stromal cell‐derived factor 1 (SDF‐1), upregulated in ischaemic tissue, and CXCR4 expressed on the migrating neuroblasts (5). SDF‐1 is upregulated in astrocytes and endothelial cells for at least 1 month after cerebral ischaemia and serves to direct migration of bone marrow‐derived cells involved in tissue repair (6). There is a close association between angiogenesis and neurogenesis. Neuroblasts in the subventicular zone migrate along blood vessels to the peri‐infarct cortex, mediated by SDF‐1 and angiopoietin (Ang‐1) (7). In squirrel monkeys, after injury to the primary motor cortex, axons sprout and establish new connections to distant sites, providing evidence of ‘rewiring’ the injured brain (8). In this way there is evidence of an endogenous repair mechanism operating after cerebral ischaemia; however, it is insufficient for recovery of function.
Stimulating and enhancing this endogenous repair response is an attractive therapeutic target. The so‐called golden time – the period of active brain remodelling, rich with targets, is restricted to days to weeks after ischaemic stroke. Most growth factor and cell therapies are best delivered in this golden time (Fig. 1). The period of 24 h after onset of ischaemia is the tail end of the neuroprotective period, at which time a therapy may still salvage some penumbral tissue and also act to stimulate brain repair.
Neurorestorative approach using cytokines and growth factors
An early attempt to use fibroblast growth factor in the first 6 h after acute stroke failed as the treatment showed no effect in a late phase randomized trial (9). However, there was some evidence that the drug used might be effective if given late, after 5 h (9). Haematopoietic growth factors, granulocyte colony‐stimulating factor (G‐CSF) and erythropoietin (EPO), combine both neuroprotective and neurorestorative effects in the brain, and are hence ideal therapeutic agents. G‐CSF as a drug, is FDA‐approved, and can be used to mobilize CD34 positive cells for bone marrow transplantation and to treat neutropenia after cancer chemotherapy. G‐CSF is effective in rodent stroke models with a therapeutic window extending for up to 24 h (10). The G‐CSF receptor is dramatically upregulated on neurons during cerebral ischaemia (11) and G‐CSF has direct effects on neurons, reducing neuronal apoptosis and stimulating endogenous neural progenitors (11). A further mechanism of action of G‐CSF in stroke is mobilizing bone marrow‐derived stem cells to participate in neurogenesis and angiogenesis (12). Similarly, erythropoietin is neuroprotective in rodent models for cerebral ischaemia (13) and erythropoietin has a restorative effect when administered 24 h after the stroke, improving functional outcome and stimulating angiogenesis and neurogenesis (14). However, in a clinical trial using erythropoietin within 6 h of symptom onset, it had no effect on functional outcome and increased mortality, suggesting safety issues when used in combination with tPA (15, 16). An early phase trial combining erythropoietin and beta human chorionic gonadotrophin delivered between 24 and 48 h after stroke appeared to be safe and well tolerated in 15 patients (17) and development of carbamylated erythropoietin, although devoid of erythropoietic benefit has maintained neuroprotection and may also be of potential use (18).
Cell therapy approaches
Cell therapy may offer advantages over direct delivery of individual trophic or growth factors. First, cells provide regulated release of trophic factors rather than bolus‐type delivery. Second, cells release a variety of growth factors simultaneously, rather than a solitary one. Third, cells have the ability to migrate to areas of damage. Fourth, many cell types, such as mesenchymal stem cells and neural stem cells, display an immunomodulatory effect. Bone marrow and umbilical cord/placental‐derived stem and progenitor cell types show therapeutic promise and represent a notion of cellular therapy that is likely to have the earliest impact on neurological diseases. As cell therapy products they generate no ethical concerns, they are easily obtainable from bone marrow or placental tissue, are highly expandable in culture and are scalable. Blood banking is already a well‐established technique and cell therapy with blood and bone marrow‐derived stem cells is a logical commercial and technical extension.
Mesenchymal stem cells or bone marrow stromal cells (MSC) are a particularly promising source for cell therapy for stroke. MSC can be isolated from the bone marrow (and other tissues) and differentiated into cartilage, bone, and adipose tissues, amongst others. They can be induced to express a neuronal phenotype in vitro and engraft and migrate in the brain in vivo (19); importantly also, MSC can secrete cytokines and trophic factors that support other cell types (20). MSC have shown efficacy in pre‐clinical animal models of stroke, head trauma and multiple sclerosis (21, 22). The therapeutic effect of human MSC in rodent models of cerebral ischaemia is enhanced when they are modified with growth factor genes such as that for brain‐derived neurotrophic factor (BDNF) and placental growth factor (PlGF) (23, 24). MSC are also anti‐inflammatory, immunomodulatory and immune privileged, allowing them to be potentially used in allogeneic transplantation (25, 26). This is a major advantage for cell therapy as it may permit their isolation from healthy donors (versus autologous) and provide an off‐the‐shelf product (Table 1).
Table 1.
Allogeneic versus autologous cell therapy
| Allogeneic | Autologous |
|---|---|
| Scalable | Difficult to scale |
| Universal stem cells obtained | Costly isolation and preparation for everyone |
| From young, healthy patients | Senescent cells from ‘older’ stroke patients |
| ‘Ready to go’ transplantation at early time points | Long preparation period needed – weeks |
| Patentable and commercialized easier | Difficult to patent; little industry interest |
| No bone marrow aspirate or collection needed | Painful bone marrow aspirate |
Much of the early pre‐clinical work on MSC transplantation has been performed by Chopp and colleagues. They improve functional outcome in a dose‐response fashion in rodent middle cerebral artery occlusion models when given intracerebrally, intra‐arterially or intravenously (27, 28, 29) and intravenous transplantation is effective at improving functional outcome even when given as late as 1 month after the insult (30). There was no reduction in infarct size but instead there was a neurorestorative effect with increases in angiogenesis, neurogenesis and synaptogenesis in MSC‐treated groups (31, 32). The mechanism of action here was not direct cell replacement, rather the MSC acted as a kind of trophic factory, elaborating a host of trophic and growth factors (21). A major advantage of MSC and other bone marrow‐derived stem cells is their effectiveness in stroke after intravenous delivery. Combining MSC with a transgene for a trophic factor may enhance their therapeutic effects and MSC expressing a transgene for placental‐derived growth factor or for BDNF have been shown to be more effective than MSC alone in reducing infarct volume and improving functional outcome, in a rodent permanent middle cerebral artery occlusion model. In these studies, MSC were intravenously infused at either 3 or 6 h after onset of ischaemia, used to measure neuroprotective effects more than a restorative benefits (23, 24).
Numbers of investigators have isolated and cultured pluripotential cells from human umbilical cord blood (33, 34). These expressed Oct3/4 and had wide differentiation potential, making them attractive for regenerative therapies. Moreover, Ratajczak et al. (35) isolated very small embryonic stem cells (VSEL) from both bone marrow and umbilical cord blood; they were pluripotential, expressed SSEA‐1(stage specific embryonic antigen) and Oct3/4, and displayed characteristics of embryonic stem cells.
Intravenous and intrastriatal delivery of human umbilical cord blood (HUCB) stem cells has been shown to be effective in animal models of cerebral ischaemia (36, 37, 38, 39); the intravenous route appeared to be more effective than intrastriatal delivery (40) and the intravenous time window extended to at least 48 h post‐stroke (41). Cord blood‐derived CD34 positive cells improved functional outcome and increased angiogenesis and associated neurogenesis when delivered intravenously 48 h after cortical occlusion in mice (39) and entry into the CNS of intravenously delivered HUCB did not appear to be a prerequisite for their beneficial effects (36). However, one issue with umbilical cord stem cells is their scalability (or lack of it); presently, it is difficult to expand HUCB ex vivo making a human clinical product still difficult to achieve.
A subset of MSC that copurify with other MSCs is that of multipotent adult progenitor cells (MAPC) isolated by Verfaillie and colleagues (42, 43, 44). MAPC are multipotent, differentiating into cell types of all three germ layers including neurons. Moreover, they generate all tissues when injected into blastocysts but have never been reported to form teratomas. MAPC can be isolated from human and rodent bone marrow as well as from other tissues such as those of brain and muscle (42); MAPC are highly expandable and represent a promising form of cell therapy. Intracerebral transplantation of MAPC 1 week after cortical stroke has resulted in improved sensorimotor function, but although some of the transplanted cells expressed neuronal markers, their paucity of number suggested that improvement was from a trophic effect of the transplanted cells on the host brain (45). Similarly, intravenous delivery of commercial grade MAPC, known as Multistem, improved long‐term functional outcome in rodents when administered 1 day to 1 week after cerebral ischaemia (46).
Can the effect of intravenously delivered bone marrow stem cells be explained by distant effects from other organs?
Very few intravenously delivered bone marrow stromal cells or other bone marrow‐derived cells, persist in the brain after a few days. It is therefore unlikely, that even a robust paracrine effect from such a small number of engrafted cells in there could explain improved functional outcome observed in many of the animal stroke models. Most intravenously administered stem cells migrate to and are trapped and concentrated in the lung and spleen (47); but such trapping in the lung after intravenous delivery may itself lead to a therapeutic effect. In a rodent model of myocardial infarction, intravenously delivered MSC trapped in lung secreted anti‐inflammatory “tumour necrosis factor‐inducible gene 6 protein” or TSG6, that reduced myocardial damage and improved cardiac performance. MSC transduced with siRNA for TSG6 did not reduce myocardial damage, reduce inflammation nor improve cardiac function.
Similarly, stem cells migrating to the spleen may also exert a therapeutic effect. There is evidence that after cerebral infarction, immune cells in the spleen are activated and splenocytes migrate to the brain where they participate and contribute to tissue injury (48, 49) (Fig. 2). This is associated with shrinking of the spleen and later immunodepression that may be associated with and predispose to infections after stroke. Haematopoietic stem cells delivered intravenously after stroke reduce this inflammatory response in the spleen and associated shrinkage of spleen size, and reduce the size of the cerebral infarct (50). Similarly, HUCB intravenously transplanted into rodents with MCA occlusion, prevent reduction of spleen size and in splenic CD8+ T cells and also increase the anti‐inflammatory cytokine, IL‐10 (51). Intravenous administration of cell therapy may lead to an indirect therapeutic effect on the brain by cells migrating to and acting in peripheral organs such as the lung and spleen – an effect that might be lost if they were administered intracerebrally or intra‐arterially.
Figure 2.
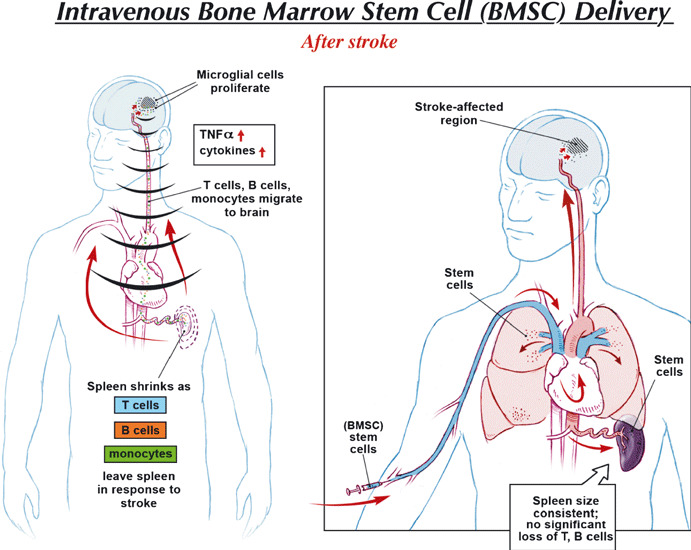
The effect of intravenously delivered stem cells on the peripheral immune response. After ischaemic stroke, there is activation of an inflammatory response in the spleen as T and B cells from the spleen migrate to the brain where they activate local microglial cells and contribute to cerebral injury (49). On the right, an intravenously administered stem cell migrates to the spleen, and reduces this splenic inflammatory response, reduces migration of spleen immune cells to the brain and attenuates splenic atrophy associated with later immunodepression.
Human clinical trials with bone marrow‐derived stem cells
To date, there have been only early phase clinical trials of cell therapy for stroke patients, one of which is a phase I trial of intravenous autologous MSC in ischaemic stroke (52). Thirty patients were randomly assigned to receive MSC; five received MSC and 25 did not, serving as controls. Autologous MSC were delivered intravenously at a dose of 1 × 108 within 1 month of incidence of the ischaemic stroke. The treatment proved to be feasible and safe and there was a trend towards functional improvement. In an extension of this study, an open‐label, blinded endpoint trial of 16 patients treated with MSC appeared to have improved outcomes compared to a control group of 36 patients (53). In a further study, bone marrow mononuclear cells were transplanted using an intra‐arterial approach, in a small number of patients 59–82 days after the stroke (54).
Delayed time window
Most human functional recovery after stroke is complete by 30 days when a plateau is reached (55). At this point, the brain has finished its period of remodelling and there is gliosis and cavitation; there are fewer targets then in days and weeks after stroke. However, there are some advantages to a later time of transplantation. Baseline deficit is well established and hence, changes from the baseline are easier to detect and there are thousands of desperate stroke victims with few other options. As it is doubtful that intravascularly delivered stem cells would naturally home to areas of damage, it is more likely that an intracerebral approach to deliver neural stem cells to the point of injury would be effective.
An early attempt at cell therapy for stroke involved intracerebral transplantation of human hNT cells, neuronal‐like cells derived from a teratocarcinoma line (NT2‐N) exposed to retinoic acid (56, 57). Twelve stroke patients with basal ganglia stroke and stable motor deficits were transplanted between 6 months and 6 years after their stroke. No tumour formation took place and PET scanning indicated increased uptake of metabolites in six out of 11 patients. A subsequent small randomized early phase II study showed no functional benefit from such transplantation but there were no major safety issues (58). In addition, a small trial with foetal porcine cells transplanted into the basal ganglia of patients with chronic stroke was terminated by the FDA after five subjects were enrolled, due to potential safety issues (59). Intracerebral transplantation of a cloned neural stem cell line, into the basal ganglia of chronic stroke patients, with the cells conditionally immortalized using transgene c‐mycERTAM to allow controlled expansion when cultured in the presence of 4‐hydroxytamoxifen, is about to enter a phase I clinical trial in Glasgow, UK (60).
For reasons of cavity formation, late transplantation of stem cells might be best combined with scaffolds of biodegradable matrix (61). Neural stem cells transplanted intracerebrally inside Matrigel led to better cavity filling than neural stem cells transplanted alone, enhanced survival of transplanted cells and improved functional recovery, in a rodent model of stroke (62). In the future, ability to use iPS cells differentiated to neural stem cells would allow both an allogeneic (more scalable) or autologous approach (personalized) (Fig. 3). However, there remains a risk of tumorigenesis if undifferentiated iPS cells are transplanted. (63)
Figure 3.
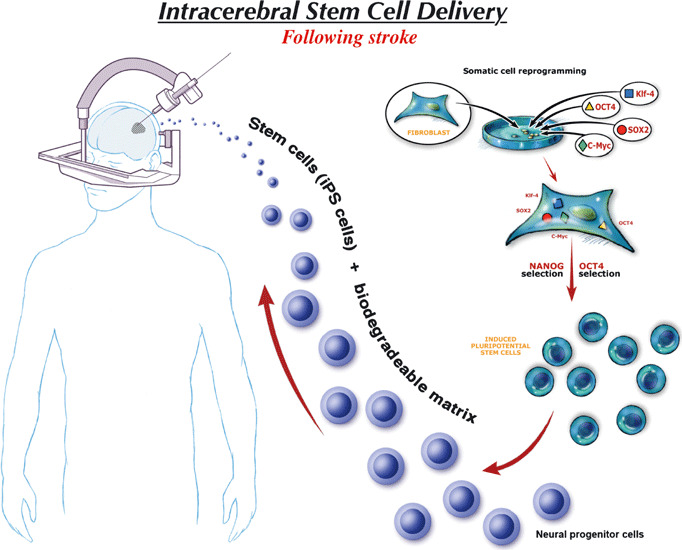
Schematic diagram depicting potential use of transplantation of iPS cells differentiated into neural progenitor cells within a biodegradable matrix in late‐stroke patients, months after injury. This process could allow autologous or allogeneic therapy. Note that this would require production of iPS cells with no viral gene integration and no genomic instability and careful selection of neural progenitor cells to ensure that no residual undifferentiated iPS cells would be transplanted.
While cell therapy and neurorestorative treatment of stroke holds much promise, it will be important to proceed with prudence and design early phase clinical trials with care (Table 2). There are sufficient pre‐clinical data to now embark upon early phase clinical trials in stroke. Early trials in both stroke and the cardiac field have employed autologous cells; however, allogeneic cells show more long‐term potential (Table 1). Intravenous allogeneic MSC, MAPC, placental stem cells and HUCB will likely enter early phase clinical trials and be infused intravenously in the golden time after stroke. It remains unclear which cell type holds the most promise as direct head‐to‐head pre‐clinical data are lacking. While early phase clinical trials will be focused on safety and tolerability, evidence of cell activity should also be sought. Biomarkers to indicate activity, might include MRI measures (diffusion tensor imaging, volumetric measurements, measures of angiogenesis), spleen size by ultrasound, or MRI, and evidence of immunomodulation (serum microarrays, serum lymphocyte subpopulations, IL‐6, IL‐10, and more). Direct implantation of neural stem cells also shows promise but optimal time to transplantation, need for matrices to enhance cell survival and lack of tumorigenicity in any iPS‐derived NSC will need to be fully investigated before clinical trials can proceed.
Table 2.
Issues in early phase clinical trials in cell therapy
| • Adequate pre‐clinical data derived from multiple laboratories in multiple species |
| • Choice of cell type |
| • Route of administration – intravenous, intra‐arterial or intracerebral |
| • Optimal timing, e.g. 24 h, 48 h, 1 week, 6 months |
| • Dose choice – need for dose escalation studies |
| • Single dose versus ‘booster dosing’ |
| • Safety issues – infusional toxicity, engraftment, tumorigenesis |
| • All subjects including controls receive rehabilitation |
| • Endpoint selection include domain‐specific endpoints such as aphasia scales, motor scales |
| • Need for surrogate endpoints such as MRI‐diffusion tensor imaging (DTI), perfusion, volumetric measurements |
Summary
There are promising pre‐clinical data showing that a wide variety of stem and progenitor cells improve nervous system functional outcome after stroke. Using intravenously delivered stem cells, the mechanism of action does not involve cell replacement but instead may be a paracrine effect on neighbouring neurons and blood vessels, an immunomodulatory effect on both brain and peripheral immune system, and perhaps a distant endocrine effect from production of TSG‐6 from cells embolized and established in the lung. The large unmet clinical need has fuelled the industry of stem cell tourism as desperate patients travel internationally in search of a cure. Careful selection of optimal cell types and proper design and execution of clinical trials will be necessary to avoid mistakes and failures of acute neuroprotection. Allogeneic cell therapies, more scalable and patentable, are the ones likely to attract big pharma and move into large clinical trials.
Acknowledgements
The authors would like to acknowledge Michael A. Jensen, M.S., C.M.I., Medical College of Georgia, for the illustrations
References
- 1. Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D et al. (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 359, 1317–1329. [DOI] [PubMed] [Google Scholar]
- 2. Duggal N, Schmidt‐Kastner R, Hakim AM (1997) Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain Res. 768, 1–9. [DOI] [PubMed] [Google Scholar]
- 3. Li Y, Chopp M (1999) Temporal profile of nestin expression after focal cerebral ischemia in adult rat. Brain Res. 838, 1–10. [DOI] [PubMed] [Google Scholar]
- 4. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970. [DOI] [PubMed] [Google Scholar]
- 5. Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V et al. (2006) Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24, 739–747. [DOI] [PubMed] [Google Scholar]
- 6. Hill WD, Hess DC, Martin‐Studdard A, Carothers JJ, Zheng J, Hale D et al. (2004) SDF‐1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 63, 84–96. [DOI] [PubMed] [Google Scholar]
- 7. Ohab JJ, Fleming S, Blesch A, Carmichael ST (2006) A neurovascular niche for neurogenesis after stroke. J. Neurosci. 26, 13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV et al. (2005) Extensive cortical rewiring after brain injury. J. Neurosci. 25, 10167–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bogousslavsky J, Victor SJ, Salinas EO, Pallay A, Donnan GA, Fieschi C et al. (2002) Fiblast (trafermin) in acute stroke: results of the European‐Australian phase II/III safety and efficacy trial. Cerebrovasc. Dis. 14, 239–251. [DOI] [PubMed] [Google Scholar]
- 10. Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS et al. (2004) Functional recovery of stroke rats induced by granulocyte colony‐stimulating factor‐stimulated stem cells. Circulation 110, 1847–1854. [DOI] [PubMed] [Google Scholar]
- 11. Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R et al. (2005) The hematopoietic factor G‐CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Invest. 115, 2083–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K et al. (2006) Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow‐derived neuronal cells. Circulation 113, 701–710. [DOI] [PubMed] [Google Scholar]
- 13. Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C et al. (2000) Erythropoietin crosses the blood‐brain barrier to protect against experimental brain injury. Proc. Natl. Acad. Sci. USA 97, 10526–10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, Zhang Z, Wang Y, Zhang R, Chopp M (2004) Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 35, 1732–1737. [DOI] [PubMed] [Google Scholar]
- 15. Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M et al. (2002) Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 8, 495–505. [PMC free article] [PubMed] [Google Scholar]
- 16. Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K et al. (2009) Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 40, e647–e656. [DOI] [PubMed] [Google Scholar]
- 17. Cramer SC, Fitzpatrick C, Warren M, Hill MD, Brown D, Whitaker L et al. (2010) The beta‐hCG+erythropoietin in acute stroke (BETAS) study: a 3‐center, single‐dose, open‐label, noncontrolled, phase IIa safety trial. Stroke 41, 927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doggrell SA (2004) A neuroprotective derivative of erythropoietin that is not erythropoietic. Expert Opin. Investig. Drugs 13, 1517–1519. [DOI] [PubMed] [Google Scholar]
- 19. Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ (1998) Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats – similarities to astrocyte grafts. Proc. Natl. Acad. Sci. USA 95, 3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084. [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Li Y, Chen J, Gautam SC, Zhang Z, Lu M et al. (2002) Ischemic cerebral tissue and MCP‐1 enhance rat bone marrow stromal cell migration in interface culture. Exp. Hematol. 30, 831–836. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB et al. (2005) Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp. Neurol. 195, 16–26. [DOI] [PubMed] [Google Scholar]
- 23. Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H et al. (2006) Neuroprotection by PlGF gene‐modified human mesenchymal stem cells after cerebral ischaemia. Brain 129, 2734–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2005) I.V. infusion of brain‐derived neurotrophic factor gene‐modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 136, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822. [DOI] [PubMed] [Google Scholar]
- 26. Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H et al. (2005) Human mesenchymal stem cells alter antigen‐presenting cell maturation and induce T‐cell unresponsiveness. Blood 105, 2214–2219. [DOI] [PubMed] [Google Scholar]
- 27. Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M (2001) Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J. Neurol. Sci. 189, 49–57. [DOI] [PubMed] [Google Scholar]
- 28. Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M et al. (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32, 1005–1011. [DOI] [PubMed] [Google Scholar]
- 29. Li Y, Chen J, Wang L, Lu M, Chopp M (2001) Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56, 1666–1672. [DOI] [PubMed] [Google Scholar]
- 30. Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A et al. (2006) Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J. Cereb. Blood Flow Metab. 27, 6–13. [DOI] [PubMed] [Google Scholar]
- 31. Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC et al. (2003) Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ. Res. 92, 692–699. [DOI] [PubMed] [Google Scholar]
- 32. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D et al. (2003) Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 73, 778–786. [DOI] [PubMed] [Google Scholar]
- 33. McGuckin CP, Forraz N, Baradez MO, Navran S, Zhao J, Urban R et al. (2005) Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif. 38, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N et al. (2004) A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J. Exp. Med. 200, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kucia M, Reca R, Campbell FR, Zuba‐Surma E, Majka M, Ratajczak J et al. (2006) A population of very small embryonic‐like (VSEL) CXCR4(+)SSEA‐1(+)Oct‐4 + stem cells identified in adult bone marrow. Leukemia 20, 857–869. [DOI] [PubMed] [Google Scholar]
- 36. Borlongan CV, Hadman M, Sanberg CD, Sanberg PR (2004) Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 35, 2385–2389. [DOI] [PubMed] [Google Scholar]
- 37. Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE et al. (2001) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32, 2682–2688. [DOI] [PubMed] [Google Scholar]
- 38. Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T et al. (2004) Infusion of human umbilical cord blood cells in a rat model of stroke dose‐dependently rescues behavioral deficits and reduces infarct volume. Stroke 35, 2390–2395. [DOI] [PubMed] [Google Scholar]
- 39. Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H et al. (2004) Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest. 114, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S et al. (2003) Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J. Neurosci. Res. 73, 296–307. [DOI] [PubMed] [Google Scholar]
- 41. Newcomb JD, Ajmo CT Jr, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE (2006) Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant. 15, 213–223. [DOI] [PubMed] [Google Scholar]
- 42. Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM (2002) Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp. Hematol. 30, 896–904. [DOI] [PubMed] [Google Scholar]
- 43. Jiang Y, Henderson D, Blackstad M, Chen A, Miller RF, Verfaillie CM (2003) Neuroectodermal differentiation from mouse multipotent adult progenitor cells. Proc. Natl. Acad. Sci. USA 100(Suppl. 1), 11854–11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz‐Gonzalez XR et al. (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49. [DOI] [PubMed] [Google Scholar]
- 45. Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC (2002) Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. 174, 11–20. [DOI] [PubMed] [Google Scholar]
- 46. Mays R, Borlongan CV, Yasuhara T, Hara K, Maki M, Carroll JE et al. (2010) Development of an allogeneic adherent stem cell therapy for treatment of ischemic stroke. J. Exp. Stroke Transl. Med. 3, 34–46. [Google Scholar]
- 47. Fischer UM, Harting MT, Jimenez F, Monzon‐Posadas WO, Xue H, Savitz SI et al. (2009) Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first‐pass effect. Stem Cells Dev. 18, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD (2006) Experimental stroke induces massive, rapid activation of the peripheral immune system. J. Cereb. Blood Flow Metab. 26, 654–665. [DOI] [PubMed] [Google Scholar]
- 49. Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A et al. (2006) Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J. Immunol. 176, 6523–6531. [DOI] [PubMed] [Google Scholar]
- 50. Schwarting S, Litwak S, Hao W, Bahr M, Weise J, Neumann H (2008) Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke 39, 2867–2875. [DOI] [PubMed] [Google Scholar]
- 51. Vendrame M, Gemma C, Pennypacker KR, Bickford PC, Davis Sanberg C, Sanberg PR et al. (2006) Cord blood rescues stroke‐induced changes in splenocyte phenotype and function. Exp. Neurol. 199, 191–200. [DOI] [PubMed] [Google Scholar]
- 52. Bang OY, Lee JS, Lee PH, Lee G (2005) Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 57, 874–882. [DOI] [PubMed] [Google Scholar]
- 53. Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY (2010) A long‐term follow‐up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 6, 1099–1106. [DOI] [PubMed] [Google Scholar]
- 54. Barbosa da Fonseca LM, Gutfilen B, Rosado de Castro PH, Battistella V, Goldenberg RC, Kasai‐Brunswick T et al. (2010) Migration and homing of bone‐marrow mononuclear cells in chronic ischemic stroke after intra‐arterial injection. Exp. Neurol. 221, 122–128. [DOI] [PubMed] [Google Scholar]
- 55. Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J (1992) Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke 23, 1084–1089. [DOI] [PubMed] [Google Scholar]
- 56. Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J et al. (2000) Transplantation of cultured human neuronal cells for patients with stroke. Neurology 55, 565–569. [DOI] [PubMed] [Google Scholar]
- 57. Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR (1998) Transplantation of cryopreserved human embryonal carcinoma‐derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp. Neurol. 149, 310–321. [DOI] [PubMed] [Google Scholar]
- 58. Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J et al. (2005) Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J. Neurosurg. 103, 38–45. [DOI] [PubMed] [Google Scholar]
- 59. Savitz SI, Dinsmore J, Wu J, Henderson GV, Stieg P, Caplan LR (2005) Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: a preliminary safety and feasibility study. Cerebrovasc. Dis. 20, 101–107. [DOI] [PubMed] [Google Scholar]
- 60. Stroemer P, Hope A, Patel S, Pollock K, Sinden J (2008) Development of a human neural stem cell line for use in recovery from disability after stroke. Front. Biosci. 13, 2290–2292. [DOI] [PubMed] [Google Scholar]
- 61. Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST (2010) Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil. Neural Repair 24, 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y et al. (2010) Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J. Cereb. Blood Flow Metab. 30, 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X et al. (2010) Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J. Cereb. Blood Flow Metab. 30, 1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]


