Abstract
Evidence for a functional role for extracellular matrix (ECM) proteins in adipose tissue is demonstrated in dynamic changes in expression of ECM genes during adipocyte differentiation and in obesity. Components of the ECM may regulate adipose cell number expansion by restricting pre‐adipocyte proliferation, regulating apoptosis and inhibiting adipogenesis. Although pre‐adipocytes express multiple proteoglycans, their role in pre‐adipocyte proliferation up to now has remained unknown. The study described here was conducted to characterize roles of small leucine‐rich proteoglycans (SLRPs) in adipocyte proliferation. Pre‐adipocytes were seeded on plates coated with biglycan and decorin and were allowed to differentiate. In addition, pre‐adipocytes were incubated on plates coated with biglycan, decorin, or fibronectin and measurements were made of cell proliferation and apoptosis. We are able to report that SLRPs decorin and biglycan did not affect differentiation of our 3T3‐L1 cells; however, biglycan and decorin did reduce proliferation of pre‐adipocytes, partly by induction of apoptosis. Furthermore, anti‐proliferative capabilities of decorin and biglycan were nullified with removal of GAG side‐chains suggesting that the chains played key roles in anti‐proliferative effects of the SLRPs. We also found that co‐treatment of decorin or biglycan with the proteoglycan fibronectin restored normal proliferation, an indication that multiple ECM proteins may act in concert to regulate overall proliferation rates of pre‐adipocytes. These studies indicate that SLRPs may compose a regulatory factor in adipose tissue expansion, through hyperplasia.
Introduction
During periods of adipose tissue expansion, the tissue undergoes remodelling to accommodate physiological changes, such as angiogenesis and macrophage infiltration (1, 2); these changes require adaptation of the extracellular matrix (ECM). Microarray studies show that expression of ECM genes in adipose tissue is modulated by changes in energy balance, such as occurs in obesity and weight loss (3, 4). ECM proteins are mainly recognized for their structural roles, however, their components might also regulate function of adipose tissue. Evidence for functional roles of ECM proteins in adipose tissue has been demonstrated in collagen 6 null ob/ob mice, which have lower numbers of inflammatory markers and improved glucose clearance (5). These studies highlight an emerging role of ECM in adipose tissue in obesity.
One hypothesis associated with adipose derived ECM is that improper ECM remodelling may inhibit adipose tissue expansion, leading to increased inflammation and inhibition of lipid storage (6). While ECM rigidity may inhibit adipose expansion by restricting adipocyte lipid storage, another possibility is that ECM components may affect other parameters of adipose tissue expansion, such as pre‐adipocyte proliferation, apoptosis and adipogenesis. In the current study, we have examined effects of biglycan and decorin on pre‐adipocyte proliferation and differentiation. Biglycan and decorin are members of the small leucine‐rich proteoglycan (SLRP) family, which are a group of ECM proteins characterized by leucine‐rich repeats and glycosaminoglycan (GAG) side‐chains (7); biglycan and decorin are known to impact cell proliferation in multiple cell types. Decorin influences cell proliferation in human airway smooth muscle cells and induces apoptosis in squamous carcinoma cells and renal proximal tubular epithelial cells (8, 9, 10). Biglycan influences proliferation of renal mesangial cells (11). Levels of adipose tissue biglycan and decorin are altered in obesity and elevated expression levels of biglycan are found in obese mice compared to lean ones (12). Decorin is highly expressed in adipose tissue of Psammomys obesus, with stromal vascular cells contributing more to decorin secretion (13). Although expression of SLRPs is elevated in obesity, the impact of these SLRPs on expansion of the pre‐adipocyte pool in adipose tissue has remained unknown. In addition, it has remained unknown whether biglycan or decorin impact adipocyte differentiation. Thus, we have investigated roles of SLRPs in regulation of proliferation and apoptosis of pre‐adipocytes, as well as effects of SLRPs on adipocyte differentiation. We report herein that SLRPs reduce proliferation of the pre‐adipocyte cell pool, partly through induction of apoptosis.
Materials and methods
Adipose tissue samples
Human adipose tissue biopsy samples were collected from omental adipose tissue depots of lean (BMI <25, n = 5) and obese (BMI >25, n = 5) patients undergoing elective bariatric surgery. All samples were collected after informed consent of the individuals and were approved by the institutional review board on human subject research. Samples were processed for RNA extraction.
Biglycan knockout mice
Biglycan knockout mice on a C3H background were provided by Marian Young at the National Institute of Dental and Craniofacial Research, NIH. Wild‐type (n = 4) and knockout mice (n = 4) were fed high‐fat diet (60% fat; Research Diets, New Brunswick, NJ, USA) ad libitum for 9 weeks. They were then were killed and mesenteric adipose tissue was harvested for RNA extraction.
3T3‐L1 pre‐adipocyte differentiation
3T3‐L1 pre‐adipocytes (ATCC, Manassas, VA, USA) were differentiated as previously described by Ajuwon et al. (14). Briefly, cells were grown in 5% CO2 at 37 °C in DMEM with 10% bovine calf serum supplemented with 1% penicillin‐streptomycin mixture. At 2 days post‐confluence (day 0), cells were differentiated in DMEM with 10% foetal bovine serum containing 1.7 μm insulin, 1 μm dexamethasone and 0.5 mm isobutylmethylxanthine for 48 h. Cells were then treated with 10% foetal bovine serum in DMEM containing insulin, for of 48 h more, only. From this point onwards, cells were treated with DMEM containing 10% foetal bovine serum alone. To study expression pattern of SLRPs, 3T3‐L1 cells were collected at 0, 2, 4 and 8 days post‐differentiation, for RNA isolation and western blotting. To study effects of SLRPs on adipocyte differentiation, 3T3‐L1 cells were seeded at confluence, on to plates previously coated with biglycan and decorin at 0.007 μg/mm2 (2.5 μg/ml) density, and were harvested at 8 days post‐differentiation.
Proliferation assay
First, 96‐well plates were coated with biglycan, decorin and fibronectin dissolved in PBS at the following concentrations: 1, 2.5 and 5 μg/ml (corresponding plating densities: 1 μg/ml = 0.003 μg/mm2, 2.5 μg/ml = 0.007 μg/mm2 and 5 μg/ml = 0.014 μg/mm2). Biglycan, decorin and fibronectin were of bovine origin and were purchased from Sigma‐Aldrich (St. Louis, MO, USA). For those used for interaction assays, 96‐well plates were coated with biglycan + decorin, decorin + fibronectin, biglycan + fibronectin and biglycan + decorin + fibronectin in equal concentrations of 2.5 μg/ml. Samples were incubated for 8 h at 37 °C in 5% CO2 and wells were then incubated in 0.1% BSA in PBS for 30 min. 3T3‐L1 mouse pre‐adipocytes were plated at 13 888 cells/cm2 (5000 cells/well) density and incubated at 37 °C in 5% CO2 for 24 or 48 h. Cell proliferation was determined using CellTiter 96 Aqueous One Solution (Promega, Madison, WI, USA) according to manufacturer’s protocols and as published (15). We validated this assay in our laboratory and found strong positive correlation (R 2 = 0.983, P < 0.05) between cell count data obtained using the CellTiter 96 Aqueous One and direct cell counts obtained using a haemocytometer.
Apoptosis assay
Levels of apoptosis were measured using the ApoStrandTM apoptosis detection kit by Enzo Life Sciences, Inc. (Farmingdale, NY, USA) and ELISAs were performed according to the manufacturer’s instructions. Apoptosis data from pre‐adipocytes plated on biglycan, decorin, fibronectin and BSA were normalized to positive control of single‐stranded DNA (ELISAs detect single‐stranded DNA as a marker of apoptosis) and viable cell number.
Chondroitinase reaction
We used chondroitinase (Sigma‐Aldrich) to remove GAG side‐chains from SLRPs. 96‐well plates were coated as described above. Chondroitinase treatment was performed as previously described (8). Briefly, chondroitinase‐treated wells were incubated in treatment buffer (50 mm Tris, 60 mm sodium acetate, 0.02% BSA, pH 8) with 50 mU of chondroitinase ABC per well, at 37 °C in 5% CO2 for 4 h. Chondroitinase reaction buffer was aspirated and wells were washed in PBS. Samples were blocked with 0.1% BSA in PBS for 30 min, as described above for plate coating.
Immunoblotting
Sample proteins were separated through SDS–PAGE on 10% resolving gel and transferred to nitrocellulose membranes. Proteins were detected by incubating membranes in primary antibody according to manufacturers’ instructions at 4 °C overnight with gentle shaking. Following primary antibodies were used: anti‐biglycan (Abcam, Cambridge, MA, USA), anti‐decorin (LF‐113, National Institutes of Health, Bethesda, MD, USA), anti‐ran, anti‐β‐actin and anti‐PPARγ (Cell Signalling Technology, Danvers, MA, USA). Following primary antibody incubation, membranes were incubated with secondary antibodies linked to horseradish peroxidase at 1:30 000 dilution. Visualization was captured through chemiluminescent HRP substrate (Millipore, Billerica, MA, USA) on autoradiographic film (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Gene expression analysis
Total RNA was extracted from adipocytes using Trizol (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. RNA samples were dissolved in Tris/EDTA pH 8 and concentrations were determined using a Nanodrop reader (Thermo Scientific, Waltham, MA, USA). RNA samples were subjected to gel electrophoresis on 0.8% agarose gel to check for degradation and genomic DNA contamination. We assessed expression of selected genes through RT‐PCR. RNA samples were reverse transcribed using Reverse Transcription system of Promega. PCR was performed on the Bio‐Rad iCycler. PCR reaction mix consisted of 0.5 μg of cDNA, 0.075 nmol of each of the forward and reverse primers, and Faststart SYBR Green Master (Roche, Basel, Switzerland); DEPC‐treated water was added to reach a total reaction volume of 20 μl. Reactions were incubated at 95 °C for 5 min. Afterwards, reactions were cycled 40 times using the following protocol: 10 s at 95 °C, 20 s at 55 °C, 72 °C. Primers used for real time PCR are listed in Table 1.
Table 1.
Primers
| Gene | Forward | Reverse |
|---|---|---|
| 18S | 5′‐ATC CCT GAG AAG TTC CAG CA‐3′ | 5′‐CCT CCT GGT GAG GTC GAT GT‐3′ |
| Biglycan | 5′‐GAC AAC CGT ATC CGC AAA GT‐3′ | 5′‐GTG GTC CAG GTG AAG TTC GT‐3′ |
| Decorin | 5′‐TGA GCT TCA ACA GCA TCA CC‐3′ | 5′‐AAG TCA TTT TGC CCA ACT GC‐3′ |
| PREF1 | 5′‐CTA ACC CAT GCG AGA ACG AT‐3′ | 5′‐GCT TGC ACA GAC ACT CGA AG‐3′ |
| PPARγ2 | 5′‐TTG ACC CAG AGC ATG GTG C‐3′ | 5′‐GAA GTT GGT GGG CCA GAA TG‐3′ |
| C/EBPα | 5′‐GCA GTG TGC ACG TCT ATG CT‐3′ | 5′‐AGC CCA CTT CAT TTC ATT GG‐3′ |
| Cyclin D1 | 5′‐GCG TAC CCT GAC ACC AAT CT‐3′ | 5′‐ ATC TCC TTC TGC ACG CAC TT‐3′ |
| p21 | 5′‐CGG TGG AAC TTT GAC TTC GT‐3′ | 5′‐CAG AGA GGA AGT ACT GG‐3′ |
| NFκB | 5′‐GCG TAC ACA TTC TGG GGA GT‐3′ | 5′‐GTT AAT GCT CCT GCG AAA GC‐3′ |
Statistics
Statistical analysis was carried out using analysis of variance (ANOVA) and was followed by means separation by Tukey analysis. All analyses were performed using the general linear model on SAS software (SAS Institute, Cary, NC, USA).
Results
Expression levels of biglycan in the obese
It has been previously established that decorin levels are increased in visceral adipose tissue of type 2 diabetics (13). To substantiate a link between biglycan expression and obesity, we measured transcript levels of biglycan from lean and obese individuals. Biglycan expression was higher (3‐fold) in omental adipose tissue of obese individuals (P < 0.05) when compared to those of lean subjects (Fig. 1a). As obesity is also associated with increased levels of inflammation, which may lead to pathologies such as type 2 diabetes (16), and due to importance of tumour necrosis factor α (TNFα) a major inflammatory cytokine implicated in type 2 diabetes, we examined whether biglycan expression would be correlated to that of TNFα. We found that TNFα levels were higher, by approximately 3 fold, in the obese individuals (Fig. 1b), and biglycan transcript levels correlated positively to those of TNFα (r = 0.37; P < 0.058), suggesting that they may be co‐regulated. For reasons of this possible relationship between biglycan and inflammation in adipose tissue, we examined whether biglycan knockout mice would exhibit lower TNFα expression levels, and we found them to be reduced (57% lower) in mesenteric fat of biglycan knockout mice (Fig. 1c) versus wild type. This may suggest a role for biglycan in regulation of adipose tissue inflammation.
Figure 1.
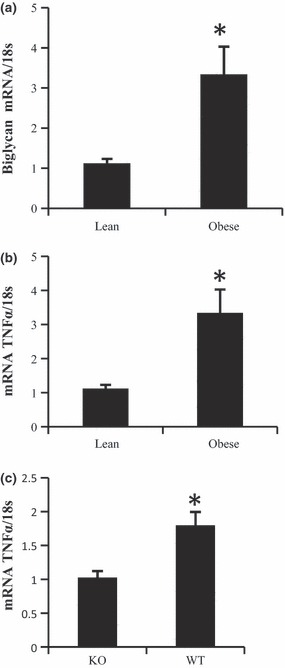
Obesity increases biglycan expression in adipose tissue. Transcript levels of biglycan (a) and TNFα (b) were measured in tissues from lean (BMI <25) and obese (BMI >25) patients. n = 5, *P < 0.05, (b) Expression level of TNFα was measured (c) in biglycan knockout (KO) and wild‐type (WT) mice, n = 4. Bars represent means and SE of treatments. *Significant differences (P < 0.05).
Expression profile of biglycan and decorin during differentiation of 3T3‐L1 cells
Levels of ECM components can fluctuate during 3T3‐L1 differentiation (17). Therefore, we measured biglycan and decorin following 3T3‐L1 differentiation, at mRNA and protein levels. We observed high transcript levels of biglycan and decorin in the pre‐adipocytes, which was followed by sharp drops (approx. 90%) in transcript level post‐differentiation (Fig. 2a,b). Protein levels of biglycan and decorin also dropped after differentiation (Fig. 2c).
Figure 2.
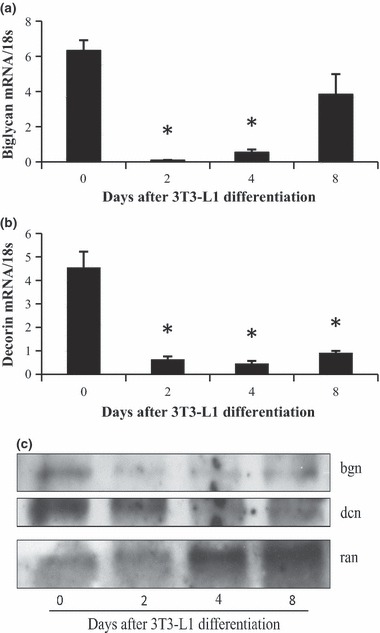
Expression levels of biglycan and decorin during 3T3‐L1 differentiation. Expression levels of biglycan (a) and decorin (b) were measured using RT‐PCR on 3T3‐L1 cells at 0, 2, 4 and 8 days post‐differentiation. Significant difference from day 0 (n = 3, P < 0.05). Representative western blot analyses of biglycan and decorin, normalized, during differentiation (c).
Effects of biglycan and decorin on adipocyte differentiation
To test whether exogenous biglycan or decorin would affect 3T3‐L1 differentiation, cells were plated at confluence on to wells coated with either biglycan or decorin. Levels of markers associated with either pre‐adipocytes or mature adipocytes were also measured. Pre‐adipocyte factor 1 (Pref‐1) is highly expressed in pre‐adipocytes, whereas peroxisome proliferator‐activated receptor γ (PPARγ) and CCAAT‐enhancer‐binding protein α (C/EBPα) are transcription factors associated with mature adipocytes (18, 19). We saw no effect of biglycan or decorin on Pref‐1 (Fig. 3a), C/EBPα (Fig. 3b) or PPARγ, at either transcript (Fig. 3c) or protein levels (Fig. 3d), perhaps suggesting only a limited role, if any, for SLRPs in regulation of adipocyte differentiation.
Figure 3.
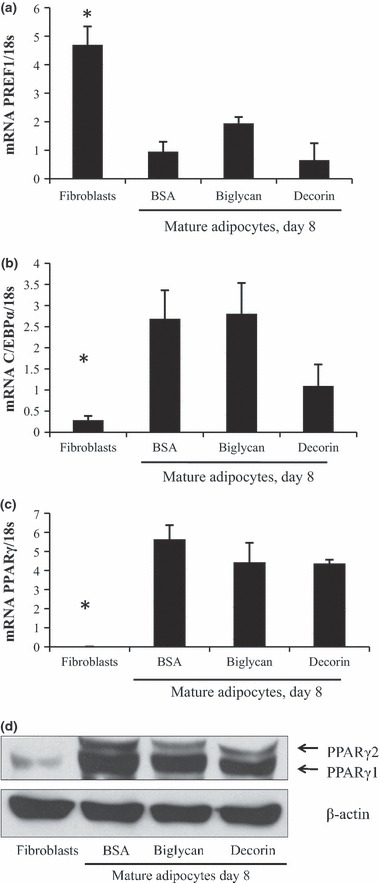
Effects of SLRPs on 3T3‐L1 differentiation. (a) 3T3‐L1 cells were seeded at confluence on to wells that were either coated with biglycan or decorin, or were uncoated. BSA was used to block all wells. Cells were differentiated and harvested at day 8 post‐differentiation. Undifferentiated 3T3‐L1 cells (fibroblasts) served as controls. Transcript levels of Pref‐1 (a), C/EBPα (b) and PPARγ (c) were used to assess differentiation. n = 3, *P < 0.05. Representative western blot analysis of PPARγ (d).
Effects of SLRPs on pre‐adipocyte proliferation
Pre‐adipocytes were seeded into wells coated with decorin, biglycan and ECM glycoprotein fibronectin. Cells plated on plastic coated with BSA only served as controls. Both decorin and biglycan led to lower cell proliferation (approx. 70–90%) at 24 and 48 h timepoints (Fig. 4a,b), whereas fibronectin stimulated pre‐adipocyte proliferation (Fig. 4c).
Figure 4.
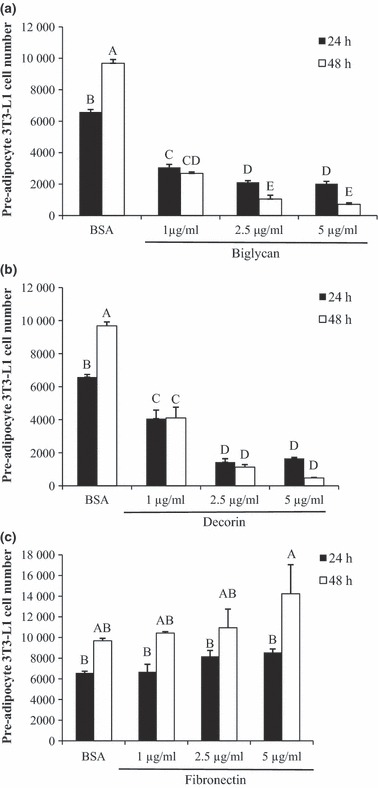
Effect of SLRPs on pre‐adipocyte proliferation. Pre‐adipocytes were seeded on BSA‐coated plastic wells (control) or on biglycan, decorin, or fibronectin coated wells. Both biglycan and decorin reduced proliferation of pre‐adipocytes (a and b). Fibronectin increased proliferation at 5 μg/ml between 24 and 48 h (c). Bars represent means ± SE (n = 5). Superscript letters indicate differences in treatments as determined by ANOVA followed by Tukey means separation (P < 0.05). n = 5 replicates per treatment group.
Effects of SLRPs on expression of p21, cyclin D1 and NFκB
From our initial proliferation assay, we observed that SLRPs decorin and biglycan, reduced proliferation of pre‐adipocytes. Next, we examined whether decorin and biglycan would affect pre‐adipocyte proliferation by halting the cell cycle. Thus, we measured transcript levels of proteins involved in cell cycle progression. p21 is under transcriptional control of p53 (20). It inhibits cyclin/CDK2 complexes, and overall impacts cell cycle progression negatively (21). Cyclin D promotes cell cycle progression, and knockdown of cyclin D inhibits pre‐adipocyte proliferation (22, 23). We did not observe any statistically significant differences in p21 expression due to our treatments (Fig. 5a). Fibronectin significantly increased expression of cyclin D1 (Fig. 5b), which is in agreement with previous findings (23), and supports its proliferative effects. NFκBp65 is a transcription factor involved in inflammation that can inhibit cell death (24). None of the treatments we tested changed NFκB expression from the BSA control levels (Fig. 5c).
Figure 5.
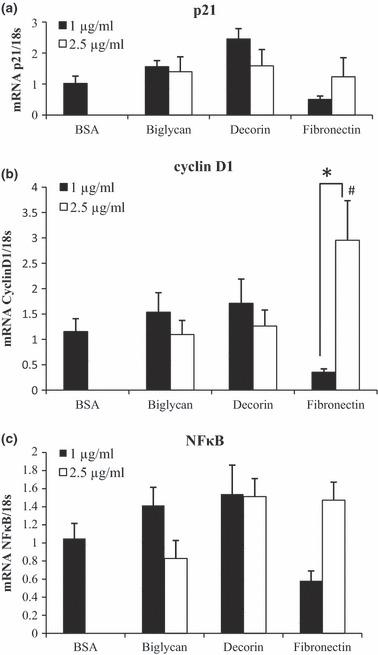
Expression levels of cell cycle mediators p21 and cyclin D1, and transcription factor NFκB. 3T3‐L1 pre‐adipocytes were plated into wells either left uncoated (BSA) or coated with 1 and 2.5 μg/ml of biglycan, decorin, or fibronectin, for 48 h, and cells were incubated for 24 h. RT‐PCR was conducted to determine gene expression levels of p21, cyclin D1, NFκB. Expressions of p21 (a) and cyclin D1 (b) were normalized to 18S. Bars represent means ± SE. *P < 0.05, #Difference from BSA levels.
SLRPs induced apoptosis in pre‐adipocytes
To observe whether anti‐proliferative effects of biglycan and decorin involved induction of apoptosis, a quantitative apoptosis assay was conducted to determine their effects plus effects of fibronectin on apoptosis. Both decorin and biglycan induced (2‐ to 8‐fold) increase in pre‐adipocyte apoptosis compared to fibronectin (Fig. 6). Thus, biglycan and decorin may suppress pre‐adipocyte proliferation through a mechanism that includes induction of apoptosis.
Figure 6.
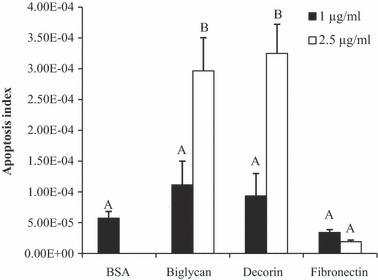
Apoptosis of pre‐adipocytes due to biglycan, decorin and fibronectin. Wells were either uncoated (BSA) or coated with 1 or 2.5 μg/ml of biglycan, decorin, or fibronectin and cells were incubated for 48 h. Amounts of apoptosis are expressed as arbitrary numbers. Superscript letters indicate differences in treatments as determined by ANOVA followed by Tukey means separation (P < 0.05). n = 5 replicates per treatment group.
Effects of glycosaminoglycan side‐chain removal
To determine contribution of GAG side‐chains on suppressive effects of biglycan and decorin (both SLRPs) on pre‐adipocyte proliferation, their GAG chains were removed by chondroitinase enzyme digestion. This treatment does not affect fibronectin, which is not an SLRP. Chondroitinase treatment on decorin restored proliferation to levels comparable to pre‐adipocytes plated on plastic only (Fig. 7). Chondroitinase digestion of biglycan reversed its inhibitory effect on pre‐adipocyte proliferation, but levels of proliferation were still significantly lower compared to controls.
Figure 7.
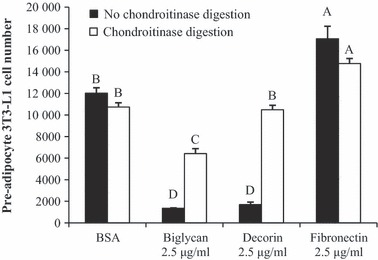
GAG chain removal by chondroitinase digestion and pre‐adipocyte proliferation. Proliferation assay was conducted on uncoated wells (BSA), wells coated with ECM, or wells coated with chondroitinase digested ECM, for 48 h. Removal of GAG chains mitigated anti‐proliferative effects of SLRPs biglycan and decorin, but not fibronectin. Bars represent means ± SE. Superscript letters indicate differences in treatments as determined by ANOVA followed by Tukey means separation (P < 0.05). n = 5 replicates per treatment group.
Effects of SLRP interactions
To determine whether SLRPs, biglycan and decorin could antagonize proliferative effects of fibronectin, pre‐adipocytes were seeded in wells coated with biglycan + decorin, biglycan + fibronectin and decorin + fibronectin. Plating pre‐adipocytes on biglycan + decorin did not reduce proliferation to levels lower than pre‐adipocytes plated on either biglycan or decorin alone (Fig. 8). However, pre‐adipocytes plated on biglycan + fibronectin and decorin + fibronectin restored proliferation to levels similar to pre‐adipocytes plated on plastic alone (Fig. 8a), and addition of both biglycan and decorin to fibronectin did not result in any additive suppression of fibronectin‐induced proliferation beyond that exerted by either decorin or biglycan alone (Fig. 8b).
Figure 8.
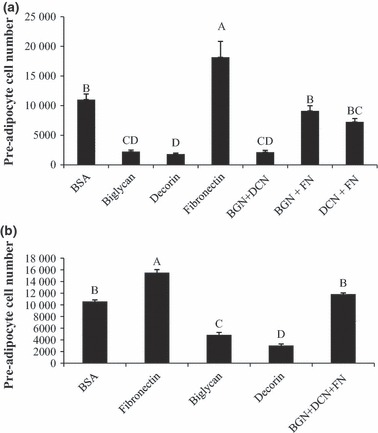
Effect of co‐incubation of SLRPs and fibronectin. (a) Pre‐adipocytes were plated either in uncoated wells (BSA), biglycan, decorin, or fibronectin alone, or a combination of biglycan + decorin (BGN + DCN), biglycan + fibronectin (BGN + FN), or decorin + fibronectin (DCN + FN), for 48 h. (b) Pre‐adipocytes were plated either in uncoated wells (BSA), biglycan, decorin, or fibronectin alone, or a combination of biglycan + decorin + fibronectin (BGN + DCN + FN), for 48 h. Interaction treatments were coated at equal concentration of 2.5 μg/ml. Both biglycan and decorin suppressed fibronectin’s proliferative effect. Bars represent means ± SE (n = 5). Superscript letters indicate differences in treatments as determined by ANOVA followed by Tukey means separation (P < 0.05).
Discussion
In this investigation we have shown that biglycan and decorin negatively impacted pre‐adipocyte proliferation by increasing their apoptosis in the pre‐adipocyte pool. These results indicate that biglycan and decorin may affect adipose tissue expansion, which may be a determining factor in development of insulin resistance. Availability of sufficient adipose tissue is critical for maintenance of energy homoeostasis. In support of this, adipose tissue transplants reduce circulating levels of glucose and insulin in mice lacking adipose tissue, clear evidence of a major role for adipose tissue availability in regulating insulin resistance (25, 26). Therefore, any impairment in proliferation and differentiation of pre‐adipocytes may limit ability of adipose tissue to store lipids, possibly leading to ectopic storage of fat (27). There are varied sources of biglycan and decorin in adipose tissue. Biglycan has been shown to be expressed by adipocytes and stem cells of adipose tissue, while decorin is strongly associated with vascular cells (13, 17, 28), and expression of both proteins is increased in obesity. We also show that biglycan may have a role in inflammation of adipose tissue, as absence of biglycan in knockout mice reduces TNFα in adipose tissue.
Our results show that biglycan and decorin expression levels decreased immediately after induction of differentiation of 3T3‐L1 cells, at both protein and transcript levels. These data indicate that expression of these SLRPs may be biphasic, a pattern which is observed in other ECM components during 3T3‐L1 differentiation (29). As biglycan and decorin expression decrease shortly after differentiation, it is unlikely that these SLRPs play a significant role in adipocyte differentiation. Indeed, we observed no effect of biglycan or decorin on markers of adipocyte differentiation.
Our results indicate that biglycan and decorin may influence pre‐adipocyte number more prominently. Coating of plastic plates with biglycan and decorin, as performed for this report, stimulated ECM rigidity or else a fibrotic state in adipose tissue characterized by overexpression of ECM proteins (3). As a signalling molecule, biglycan may have differing bioactivity depending on whether it is soluble or attached (30). Indeed, addition of SLRPs to cell culture media to simulate free forms of the proteins did not affect cell proliferation (data not shown). Therefore, proliferative and apoptotic effects of biglycan and decorin may be dependent on their available form.
Biglycan and decorin are recognized as proteins that can effect cell proliferation. Biglycan treatment reduces proliferation, but not apoptosis in renal mesangial cells (11). Cells made to express high levels of decorin display increased expression of p21 at both protein and transcript levels, thus halting cell cycle progression (31, 32). We saw no increase in p21 expression levels in decorin‐treated pre‐adipocytes that could show reduced cell number. Our results instead indicate that biglycan and decorin lowered cell number by increasing apoptosis, not by population growth arrest. Precise mechanisms of apoptotic effects of these SLPRs in adipocytes are still unclear, but in squamous carcinoma cells, decorin targets epidermal growth factor receptor to activate caspase‐3 (10). It is possible that our SLRPs induced apoptosis in pre‐adipocytes through a similar mechanism. Additionally, effects of the SLPRs on proliferation or apoptosis may be cell type‐specific. Decorin is reported to have differing effects on apoptosis, depending on cell type. In diabetic mice, decorin inhibits apoptosis in proximal tubular epithelial cells (10). However, decorin treatment prompts apoptosis in mouse tumour xenografts and A431 human squamous carcinoma cells (10). Biglycan, on the other hand, has a protective effect against apoptosis in mesangial cells (11). To the best of our knowledge, we are not aware of any studies that show biglycan promotes apoptosis.
Fibronectin increases proliferation in pre‐adipocytes and prevents adipogenesis (23, 33). Our results show that the proliferative action of fibronectin can be negated by biglycan or decorin. A previous study showed that pre‐adipocytes from obese individuals express more fibronectin (23). The same study showed that culture media from activated macrophages increased biglycan and fibronectin expression in human pre‐adipocytes; however, the net result was increased pre‐adipocyte proliferation. It is possible that in obesity, inflammation increases fibronectin levels in pre‐adipocytes to levels not checked by other ECM components. It is also possible that when SLRPs negate proliferative effects of fibronectin, adipogenesis is favoured.
Anti‐proliferative capability of biglycan and decorin was reduced when their GAG chains were removed through chondroitinase digestion. GAG chain removal leads to increased pre‐adipocyte cell numbers. However, chondroitinase digestion of biglycan was not sufficient to completely reverse its inhibitory effect on pre‐adipocyte proliferation. This raises the possibility that the core protein of biglycan is also able to inhibit cell proliferation. Overall, our results show that GAG side‐chains inhibit pre‐adipocyte proliferation. Chondroitin and dermatan sulphate side‐chains can be further modified by appropriate cell processes (34), and it is possible that anti‐proliferative capacity of biglycan and decorin is modified by side‐chain modifications.
In summary, here we have shown that biglycan and decorin are capable of inhibiting proliferation of 3T3‐L1 pre‐adipocytes by activating apoptosis and GAG side‐chains appear very critical for induction of apoptosis by the SLPS. In addition, strong association between biglycan and inflammation in adipose tissue suggests possible involvement of biglycan in regulation of adipose tissue inflammation, and adipose tissue perturbation observed in obesity. Further studies are needed to elucidate mechanisms of apoptosis induction and to determine whether this phenomenon occurs in vivo.
References
- 1. Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ (2001) The biology of white adipocyte proliferation. Obes. Rev. 2, 239–254. [DOI] [PubMed] [Google Scholar]
- 2. Bourlier V, Zakaroff‐Girard A, Miranville A, De Barros S, Maumus M, Sengenes C et al. (2008) Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 117, 806–815. [DOI] [PubMed] [Google Scholar]
- 3. Henegar C, Tordjman J, Achard V, Lacasa V, Cremer I, Guerre‐Millo M et al. (2008) Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 9, R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kolehmainen M, Salopuro T, Schwab US, Kekäläinen J, Kallio P, Laaksonen DE et al. (2007) Weight reduction modulates expression of genes involved in extracellular matrix and cell death: the GENOBIN study. Int. J. Obes. Relat. Metab. Disord. 32, 292–303. [DOI] [PubMed] [Google Scholar]
- 5. Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N et al. (2008) Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halberg N, Wernstedt I, Scherer PE (2008) The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. North Am. 37, 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McEwan PA, Scott PG, Bishop PN, Bella J (2006) Structural correlations in the family of small leucine‐rich repeat proteins and proteoglycans. J. Struct. Biol. 155, 294–305. [DOI] [PubMed] [Google Scholar]
- 8. D’Antoni ML, Torregiani C, Ferraro P, Michoud M, Mazer B, Martin JG et al. (2008) Effects of decorin and biglycan on human airway smooth muscle cell proliferation and apoptosis, Am . J. Physiol. Lung Cell Mol. Physiol. 294, L764–L771. [DOI] [PubMed] [Google Scholar]
- 9. Seidler D, Goldoni S, Agnew C, Cardi C, Thakur M, Owens R et al. (2006) Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase‐3 activation. J. Biol. Chem. 281, 26408–26418. [DOI] [PubMed] [Google Scholar]
- 10. Merline R, Lazaroski S, Babelova A, Tsalastra‐Greul W, Pfeilschifter J, Schulter K et al. (2009) Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J. Physiol. Pharmacol. 60, 5–13. [PMC free article] [PubMed] [Google Scholar]
- 11. Schaefer L, Beck KF, Raslik I, Walpen S, Mihalik D, Micegova M et al. (2003) Biglycan, a nitric oxide‐regulated gene, affects adhesion, growth, and survival of mesangial cells. J. Biol. Chem. 278, 26227–26237. [DOI] [PubMed] [Google Scholar]
- 12. Nadler S, Stoehr J, Schueler K, Tanimoto G, Yandell B, Attie A (2000) The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc. Natl. Acad. Sci. USA 97, 11371–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bolton K, Segal D, McMillan J, Jowett J, Heilbronn L, Abberton K et al. (2008) Decorin is a secreted protein associated with obesity and type 2 diabetes. Int. J. Obes. 32, 1113–1121. [DOI] [PubMed] [Google Scholar]
- 14. Ajuwon K, Spurlock M (2005) Palmitate activates the NF‐κB transcription factor and induces IL‐6 and TNFα expression in 3T3‐L1 edipocytes. J. Nutr. 135, 1841–1846. [DOI] [PubMed] [Google Scholar]
- 15. Cho YY, Tang F, Yao K, Lu C, Zhu F, Zheng D et al. (2009) Cyclin‐dependent kinase‐3‐mediated c‐Jun phosphorylation at Ser63 and Ser73 enhances cell transformation. Cancer Res. 69, 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hotamisligil G (2006) Inflammation and metabolic disorders. Nature 444, 860–867. [DOI] [PubMed] [Google Scholar]
- 17. Molina H, Yang Y, Ruch T, Kim J, Mortensen P, Otto T et al. (2009) Temporal profiling of the adipocyte proteome during differentiation using a 5‐plex SILAC based strategy. J. Proteome Res. 8, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smas C, Sul H (1993) Pref‐1, a protein containing EGF‐like repeats, inhibits adipocyte differentiation. Cell 73, 725–734. [DOI] [PubMed] [Google Scholar]
- 19. Rosen E, MacDougald O (2006) Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896. [DOI] [PubMed] [Google Scholar]
- 20. El‐Deiry WS (2003) The role of p53 in chemosensitivity and radiosensitivity. Oncogene 22, 7486–7495. [DOI] [PubMed] [Google Scholar]
- 21. Gartel AL, Radhakrishnan SK (2005) Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 65, 3980–3985. [DOI] [PubMed] [Google Scholar]
- 22. Knudsen K, Diehl J, Haiman C, Knudsen E (2006) Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene 25, 1620–1628. [DOI] [PubMed] [Google Scholar]
- 23. Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D (2009) Macrophage‐secreted factors promote a profibrotic phenotype in human preadipocytes. Mol. Endocrinol. 23, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang C, Mayo M, Baldwin A Jr (1996) TNF‐and cancer therapy‐induced apoptosis: potentiation by inhibition of NF‐κB. Science 274, 784–787. [DOI] [PubMed] [Google Scholar]
- 25. Chao L, Marcus‐Samuels B, Mason MM, Moitra J, Vinson C, Arioglu E et al. (2000) Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J. Clin. Invest. 106, 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rasouli N, Molavi B, Elbein SC, Kern PA (2007) Ectopic fat accumulation and metabolic syndrome. Diabetes Obes. Metab. 9, 1–10. [DOI] [PubMed] [Google Scholar]
- 27. Heilbronn L, Smith SR, Ravussin E (2004) Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int. J. Obes. Relat. Metab. Disord. 28, S12–S21. [DOI] [PubMed] [Google Scholar]
- 28. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al. (2002) Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13, 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mariman E, Wang P (2010) Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 67, 1277–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Babelova A, Moreth K, Tsalastra‐Greul W, Zeng‐Brouwers J, Eickelberg O, Young MF et al. (2009) Biglycan, a danger signal that activates the NLRP3 inflammasome via toll‐like and P2X receptors. J. Biol. Chem. 284, 24035–24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Luca A, Santra M, Baldi A, Giordano A, Iozzo R (1996) Decorin‐induced growth suppression is associated with up‐regulation of p21, an inhibitor of cyclin‐dependent kinases. J. Biol. Chem. 271, 18961–18965. [DOI] [PubMed] [Google Scholar]
- 32. Santra M, Mann D, Mercer E, Skorski T, Calabretta B, Iozzo R (1997) Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin‐dependent kinases. J. Clin. Invest. 100, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spiegelman BM, Ginty CA (1983) Fibronectin modulation of cell shape and lipogenic gene expression in 3T3‐adipocytes. Cell 35, 657–666. [DOI] [PubMed] [Google Scholar]
- 34. Hannes EG, Hobert O (2006) The molecular diversity of glycosaminoglycans shapes animal development. Annu. Rev. Cell Dev. Biol. 22, 375–407. [DOI] [PubMed] [Google Scholar]


