Abstract
Abstract. Objective: Recently, our team has demonstrated that voltage‐gated delayed rectifier K+ current (IKDR) and Ca2+‐activated K+ current (IKCa) are present in rat bone marrow‐derived mesenchymal stem cells; however, little is known of their physiological roles. The present study was designed to investigate whether functional expression of IKDR and IKCa would change with cell cycle progression, and whether they could regulate proliferation in undifferentiated rat mesenchymal stem cells (MSCs). Materials and Methods: Membrane potentials and ionic currents were recorded using whole‐cell patch clamp technique, cell cycling was analysed by flow cytometry, cell proliferation was assayed with DNA incorporation method and the related genes were down‐regulated by RNA interference (RNAi) and examined using RT‐PCR. Results: It was found that membrane potential hyperpolarized, and cell size increased during the cell cycle. In addition, IKDR decreased, while IKCa increased during progress from G1 to S phase. RT‐PCR revealed that the mRNA levels of Kv1.2 and Kv2.1 (likely responsible for IKDR) reduced, whereas the mRNA level of KCa3.1 (responsible for intermediate‐conductance IKCa) increased with the cell cycle progression. Down‐regulation of Kv1.2, Kv2.1 or KCa3.1 with the specific RNAi, targeted to corresponding gene inhibited proliferation of rat MSCs. Conclusion: These results demonstrate that membrane potential, IKDR and IKCa channels change with cell cycle progression and corresponding alteration of gene expression. IKDR and intermediate‐conductance IKCa play an important role in maintaining membrane potential and they participate in modulation of proliferation in rat MSCs.
INTRODUCTION
Ion channels play important roles in maintaining physiological homeostasis. In proliferative cells, ion channels have been shown to participate in cell proliferation (see review, Wonderlin & Strobl 1996). Recent studies have demonstrated that ion channels modulate the progression of the cells through the cell cycle, and that K+ channel expression changes with different stages in spinal cord astrocyte cycling (MacFarlane & Sontheimer 2000). Blockade of K+ channels has been shown to be antiproliferative for numerous types of cells including T‐lymphocytes (DeCoursey et al. 1984), vascular smooth muscle cells (Grgic et al. 2005) and cancer cells (Ouadid‐Ahidouch et al. 2004b), etc.
Mesenchymal stem cells (MSCs) from bone marrow of various species (e.g. mice, rats and humans) exhibit multilineage potential to incorporate into a variety of tissues (Caplan & Bruder 2001; Jiang et al. 2002; Reyes et al. 2002; Pittenger & Martin 2004), including bone, cartilage, muscle and lung by in vivo transplantation (Pittenger et al. 1999; Deans & Moseley 2000; Jiang et al. 2002; Zhao et al. 2002), and to form a variety of cell types in vitro, for example, hepatocytes, cardiomyocytes and neuronal cells (Caplan & Bruder 2001; Reyes et al. 2001; Jiang et al. 2002). In addition, transplantation of MSCs into infarcted myocardium has been found to improve heart function significantly in experimental studies (Tomita et al. 1999; Orlic et al. 2001; Sussman 2001). Thus, it is believed that MSCs are an ideal cell source for regeneration of the myocardium (Caplan & Bruder 2001; Reyes et al. 2001; Cahill et al. 2003; Pittenger & Martin 2004). Recent studies from ours and other groups have demonstrated that multifunctional ion channels were heterogeneously expressed in undifferentiated human (Kawano et al. 2003; Heubach et al. 2004; Li et al. 2005), rat (Li et al. 2006) and rabbit (Deng et al. 2006) MSCs. We found that the delayed rectifier K+ current (IKDR) (likely to be encoded by Kv1.2 and Kv2.1) and Ca2+‐activated K+ current (IKCa) (likely to be encoded by KCa3.1 and KCa1.1) were major ion channel currents in rat MSCs. IKDR was present in almost all rat MSCs, while IKCa were observed in one‐third of rat MSCs (Li et al. 2006). However, little is known regarding the mechanism underlying heterogeneous expression of ion channels and the biological and physiological roles of these ion channels in rat MSCs. The present study was therefore designed to investigate whether IKDR and IKCa would change during cell cycle progression, and whether they could regulate cell proliferation in undifferentiated rat MSCs.
MATERIALS AND METHODS
Isolation and culture of rat MSCs
Rat MSCs were isolated from the bone marrow of Sprague–Dawley rats (150–200 g, either sex) using a modified procedure described previously (Li et al. 2006). Guidelines for animal care and use from the Committee on the Use of Animals in Teaching and Research, University of Hong Kong, were followed. Briefly, rat MSCs that adhered to the flask bottom gradually proliferated to form colonies in Iscove's modified Dulbecco's medium (IMDM; Sigma‐Aldrich Chemicals, St Louis, MO, USA), 10% foetal bovine serum (FBS, Invitrogen, Hong Kong, China), antibiotics (100 U/mL penicillin G, 100 µg/mL streptomycin sulphate, 0.25 µg/mL amphotericin B; Invitrogen) and 10 ng/mL leukaemia inhibition factor (Invitrogen) until they reached 80–90% confluence. The cells were then detached from the flasks by trypsinization, were centrifuged at 170 g for 8 min and were suspended in the medium for continuous culture or ionic current recording. For ion current studies, detached cells were transferred to a cell chamber for 15–20 min, and were allowed to attach to the bottom of the cell chamber. Subsequently, these cells were superfused with normal Tyrode solution (1.5 mL/min).
Synchronization of rat MSCs and flow cytometric analysis
Rat MSCs (passages from 2 to 5) were synchronized in the cell cycle using a procedure described previously by Ouadid‐Ahidouch et al. (2004b). Briefly, cells were plated initially in 25 cm2 flasks in IMDM containing 10% FBS for 24 h, and then were synchronized to early G1 (i.e. to G0/G1 phase) by starving them for 24 h using IMDM medium containing 0.5% FBS. By returning to 10% FBS in the medium for 8–10 h, cells were progressed to G1 phase. To synchronize the cells at the end of G1 phase, 2 mm thymidine (Sigma‐Aldrich Chemicals) added to the culture medium containing 10% FBS for 24 h and finally the cells were allowed to progress to S phase by removing the thymidine for 8–10 h.
The flow cytometric analysis was performed on the MSCs from different cycling phases. Cells were harvested by trypsinization at the end of each treatment, washed with PBS and were fixed in ice‐cold 70% ethanol at 4 °C for 4 h, followed by centrifuging cell pelleting at 200 g for 5 min and washing with PBS to remove the fixative. Cells were then suspended in 1 mL propidium iodide/Triton X‐100 staining solution with RNase A (final concentration 20 µg/mL propidium iodide) and were incubated for 30 min at room temperature. Stained cells were then analysed using a flow cytometer (Cytomics FC 500, Beckman, Fullerton, CA, USA) as described previously (Collecchi et al. 2000; Tadi et al. 2005) to measure cellular DNA content. The data were stored on a compatible IBM PC computer, and were analysed using ModFit software for cell cycle distribution patterns (G0/G1, S and G2/M phases).
Electrophysiology
Mesenchymal stem cells from passages 2–5 or from different cell cycle phases were used for ion current studies with the whole‐cell patch‐clamp technique, as previously described (Li et al. 2005, 2006). Tyrode solution contained: 136 mm NaCl, 5.4 mm KCl, 1.0 mm MgCl2, 1.0 mm CaCl2, 0.33 mm NaH2PO4, 10 mm glucose and 10 mm 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid; pH adjusted to 7.4 with NaOH. The pipette solution contained (mm): 20 mm KCl, 110 mm K‐aspartate, 1.0 mm MgCl2, 10 mm 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid, 0.05 mm ethyleneglycoltetraacetic acid, 0.1 mm GTP, 5.0 mm Na2‐phosphocreatine, 5.0 mm Mg2‐ATP; pH adjusted to 7.2 with KOH. The experiments were conducted at room temperature (21–22 °C).
Reverse transcriptase‐polymerase chain reaction
Reverse transcriptase‐polymerase chain reaction (RT‐PCR) was performed by the procedure as described previously (Li et al. 2005, 2006). Briefly, total RNA was isolated using the TRIzol method (Invitrogen) from cell cycle phases of the MSCs or from RNAi‐treated MSCs. Reverse transcription was performed using the RT system (Promega, Madison, WI, USA) protocol in a 20‐µL reaction mixture. Then, the polymerase chain reaction was conducted with primers of Kv1.2 (accession no. NM_012970, sense: GAGATGTTTCGGGAGGATGA; antisense: CTCTGTCCCCAGGGTGATAA), Kv2.1 (accession no. NM_013186, sense: GCTGCAGAGCCTAGACGAGT; antisense: TGCTTTTGAACTTGGTGTCG), KCa1.1 (accession no. AF135265, sense: TGTGGGCTCCATCGAGTA; antisense: GCTTAGCGAGTTCCGTGA) and KCa3.1 (accession no. NM_023021, sense: CACGCTGAGATGTTGTGGTT; antisense: CGATGCTGCGGTAAGACG) to amplify corresponding cDNA. PCR was performed using the Promega PCR system with Taq polymerase and accompanying buffers. The cDNA at 3 µL aliquots was amplified by a DNA thermal cycler (Mycycler; Bio‐Rad, Hercules, CA, USA) in a 25‐µL reaction mixture. PCR products were electrophoresed through a 1.5% agarose gel, and amplified cDNA bands were visualized by ethidium bromide staining. The bands, imaged by Chemi‐Genius Bio Imaging System (Syngene, Cambridge, UK), were analysed using GeneTools software (Syngene). Amplified cDNA levels of the genes were expressed as relative values to house keeping glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) gene (accession no. NM_017008, sense: GTGCTGAGTATGTCGTGGAG; antisense: GTCTTCTGAGTGGCAGTGAT). When the cDNA was replaced by the RNA sample, no significant bands were detected (Li et al. 2006).
RNA interference
StealthTM RNAi molecules targeted to specific K+ channels were purchased from Invitrogen Life Technology (Invitrogen) (Wu et al. 2006). Sense RNA sequences of StealthTM RNAi molecules were as follows: 5′‐AAAUAGACAGCACUAGAGAAGAGGA‐3′ for Kv1.2; 5′‐UGCUAGUGCUGUGUGUUUCUCAGGG‐3′ for Kv2.1; 5′‐UCCCUCCCGUGUUUGUGUCUGUAUA‐3′ for KCa1.1 and GCCACUGGUUCGUGGCCAAACUAUA for KCa3.1. In addition, SilencerR GAPDH siRNA (Ambion, Austin, TX, USA) was used as the positive control. StealthTM RNAi molecules at 100 nm were transfected into the MSCs at 60% confluence, using LipofectamineTM 2000 reagent (Invitrogen) for 48 h according to the manufacturer's instructions. StealthTM RNAi of medium GC content (Invitrogen), which had no known target in mammalian genomes, was used as the control. Transfected cells were used for cell proliferation assay and/or RNA extraction. Transfection efficiency was monitored using fluorescent RNA duplex (Invitrogen) according to the manufacturer's instructions.
Cell proliferation assay
The cell proliferation assay was performed on the MSCs by determining incorporation level of [3H]‐thymidine into DNA with a modified procedure as described previously (Wu et al. 2006). Briefly, the MSCs were seeded in 96‐well plates at a density of 2 × 104 cells/well in antibiotic‐free IMDM containing 10% FBS for 24 h, and was incubated in IMDM containing 10% FBS and ion channel blockers or specific StealthTM RNAi ion channels for 24 h, and then was exposed to [3H]‐thymidine (0.5 µCi/well) for an additional 24 h. The level of [3H]‐thymidine incorporation was finally assayed with TopCount·NTXTM microplate scintillation and luminescence counter (PerkinElmer Life and Analytical Sciences, Boston, MA, USA).
Statistical analysis
Results are presented as means ± SEM. Paired and/or unpaired Student's t‐tests were used as appropriate to evaluate the statistical significance of differences between two group means, and analysis of variance (anova) was performed for multiple groups. Values of P < 0.05 were considered to indicate statistical significance.
RESULTS
Pharmacological separation of K+ currents in rat MSCs
Our previous studies have shown that IKDR were present in almost all of rat MSCs, and IKCa was seen in one‐third of them (Li et al. 2006). IKDR was sensitive to inhibition by 4‐aminopyridine (4‐AP) or tetraethylammonium, while IKCa was mostly blocked by the intermediate‐conductance KCa channel blocker clotrimazole. In addition, a small portion of iberiotoxin (a blocker of high‐conductance IKCa)‐sensitive high‐conductance IKCa was detected only in a small population of cells (Li et al. 2006). We therefore used 4‐AP and clotrimazole to separate IKDR and/or IKCa to study changes in these two types of currents during cell cycle progression. In addition, iberiotoxin was also employed in further cases.
Figure 1a displays membrane currents recorded in an MSC, with the voltage protocol as shown in the inset. A gradually activating IKDR and noisy oscillation like IKCa were observed, indicating that the two components of outwards currents were copresent in this cell. IKDR was significantly inhibited by 5 mm 4‐AP (Sigma‐Aldrich Chemicals), while the remaining IKCa was suppressed by co‐application of 5 mm 4‐AP and 1 µm clotrimazole (Sigma‐Aldrich Chemicals). Figure 1b shows membrane currents recorded in another cell. Current was markedly suppressed by 5 mm 4‐AP. No additional inhibition was observed with co‐application of 4‐AP and 1 µm clotrimazole, suggesting that IKDR only was present in this cell.
Figure 1.
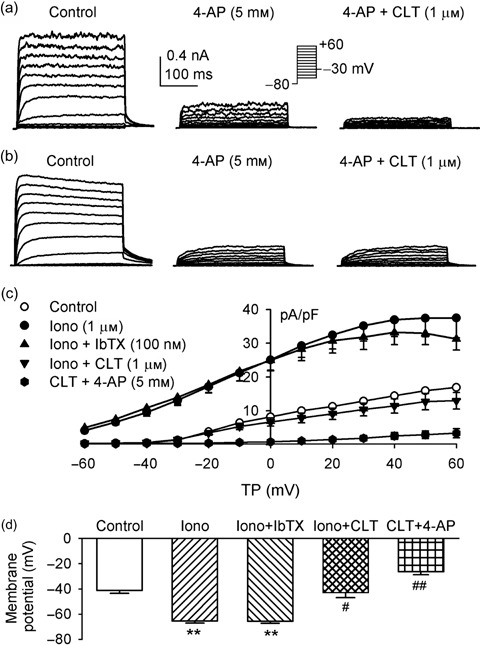
Pharmacological separation of K+ channel currents in rat MSCs. (a) Membrane currents recorded in a cell with 300‐ms voltage steps from –80 to between –50 and +60, and then back to –30 mV as shown in the inset (0.2 Hz). Two components of outwards currents were observed in this cell. One gradually activating current was delayed rectifier K+ current (IKDR), sensitive to inhibition by 5 mm 4‐AP, and another component with noisy oscillation was Ca2+‐activated K+ current (IKCa) sensitive to inhibition by 1 µm clotrimazole (CLT). (b) Membrane current recorded in another cell with the same voltage protocol. Current was inhibited by 5 mm 4‐AP, the remaining current not sensitive to clotrimazole, suggesting that only IKDR is present in this cell. (c) I‐V relationships of membrane currents in rat MSCs (n = 8) expressing both IKDR and IKCa. Application of 1 µm ionomycin (Iono) increased membrane current, iberiotoxin (IbTX, 100 nm) slightly decreased current at +30 to +60 mV, and clotrimazole (1 µm) reversed ionomycin‐induced current and produced a further reduction of membrane current. Remaining current was suppressed by co‐application of clotrimazole and 5 mm 4‐AP. (d) Mean values of membrane potentials determined in current clamp mode in the same rat MSCs as in (c), control, after application of 1 µm ionomycin, co‐application of ionomycin and 100 nm iberiotoxin, combination of ionomycin with 1 µm clotrimazole, and clotrimazole plus 5 mm 4‐AP. **P < 0.01 vs control; #P < 0.05, ##P < 0.01 vs ionomycin plus iberiotoxin.
The MSCs were found to have variable membrane potentials from –15 to –55 mV (Li et al. 2006). To study possible contribution of IKCa and IKDR to membrane potential, the membrane current amplitude and membrane potential were determined, then, the Ca2+ ionophore ionomycin was applied in these cells (n = 8). Ionomycin at 1 µm was found to increase current amplitude, and hyperpolarize membrane potential. Figure 1c illustrates the current‐voltage relationships of membrane current density under control conditions, in the presence of 1 µm ionomycin, ionomycin plus 100 nm iberiotoxin and co‐application of ionomycin and 1 µm clotrimazole. Ionomycin substantially increased current density. The current increased by ionomycin was slightly reduced by 100 nm iberiotoxin from +30 to +60 mV (P > 0.05), while it was substantially suppressed by application of 1 µm clotrimazole. These results indicate that the ionomycin‐activated component is mainly contributed by intermediate‐conductance IKCa. The remaining current, mainly IKDR, was inhibited by co‐application of clotrimazole and 5 mm 4‐AP.
Figure 1d illustrates membrane potential recorded in current clamp mode in the MSCs with different treatments. The membrane potential hyperpolarized to –65.5 ± 1.7 mV from –45.6 ± 2.3 mV of control (n = 8, P < 0.01) by application of 1 µm ionomycin. Increased membrane potential was not affected by 100 nm iberiotoxin (–65.6 ± 1.6 mV), but reduced to –42.8 ± 3.9 mV by 1 µm clotrimazole (P < 0.01 versus ionomycin) treatment. Co‐application of clotrimazole and 5 mm 4‐AP induced additional reduction of membrane potential (to –26.5 ± 2.3 mV, P < 0.01 versus clotrimazole or control). These results suggest that IKDR and intermediate‐conductance IKCa, but not high‐conductance IKCa, play an important role in controlling membrane potential in rat MSCs.
Synchronization of rat MSCs
Mesenchymal stem cells from passages 2–5 were synchronized with the procedure described in the Materials and Methods section. Figure 2 illustrates representative results of flow cytometric analysis of the MSCs from controls and a variety of treatments. In controls, approximately 51% of cells were in G0/G1 stage, 43% were in S phase and only 6% were in G2/M phase. MSCs in low FBS (0.5%) for 24 h (starvation), arrested in early G1 phase, and showed 81% of cells at G0/G1 phase. Cells treated with 2 mm thymidine synchronized to the end of G1 phase, and 88% were at G0/G1 phase, while cells with removal of thymidine for 8 h switched to S phase and showed 92% of cells in there. Average data from four experiments are summarized in Table 1.
Figure 2.
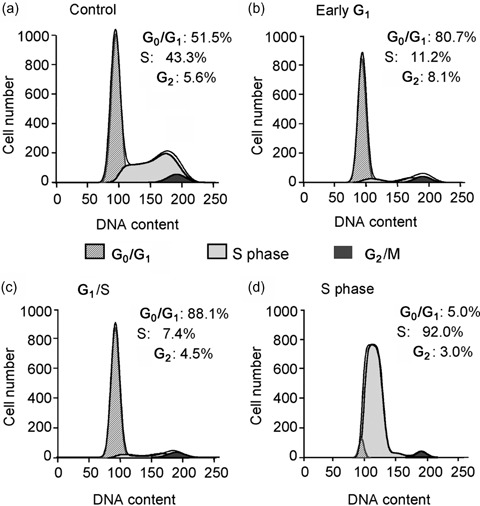
Cell cycle distribution in rat MSCs determined by flow cytometry. (a) Untreated control rat MSCs. (b) Cells from early G1, treated with starvation medium (0.5% FBS) for 24 h. (c) Cells from the end of G1, treated with regular culture medium (10% FBS) containing 2 mm thymidine for 24 h. (d) Cells from S phase, cultured with normal culture medium (10% FBS) for 8–10 h after 24 h of thymidine treatment.
Table 1.
Cell cycle confirmation by flow cytometry (FC)
| FC cell cycle | Control | Early G1 | End G1 | S phase |
|---|---|---|---|---|
| G0/G1 (%) | 52.2 ± 1.6 | 81.0 ± 0.9 | 87.8 ± 0.5 | 7.5 ± 3.6 |
| S (%) | 41.7 ± 2.3 | 11.4 ± 1.4 | 6.5 ± 1.3 | 90.2 ± 2.7 |
| G2/M (%) | 6.1 ± 0.7 | 7.6 ± 2.3 | 5.8 ± 1.8 | 2.3 ± 0.9 |
Cell cycle‐dependent changes in membrane potential, cell size, IKDR and IKCa
Cell cycle‐dependent alterations of membrane potential and cell size (defined by membrane capacitance) were studied in the MSCs from different cycling phases. Figure 3a illustrates membrane potential and membrane capacitance determined in a total of 298 MSCs from the different cycle phases. The membrane potential was –41.3 ± 1.6 mV in early G1 rat MSCs (n = 74), increased to –48.6 ± 1.4 mV (n = 68, P < 0.05) in cells progressing to G1 phase, to –52.2 ± 2.5 mV (n = 62, P < 0.01) in cells at the end of G1 phase and to –52.3 ± 1.3 mV (n = 94, P < 0.01) in cells of S phase. These results suggest that the membrane potential of rat MSCs hyperpolarizes during cycle progression from early G1 to S phase. In addition, cell size significantly increased as the cells developed from early G1 to end of G1, and to S phase (P < 0.01 versus early G1 phase).
Figure 3.
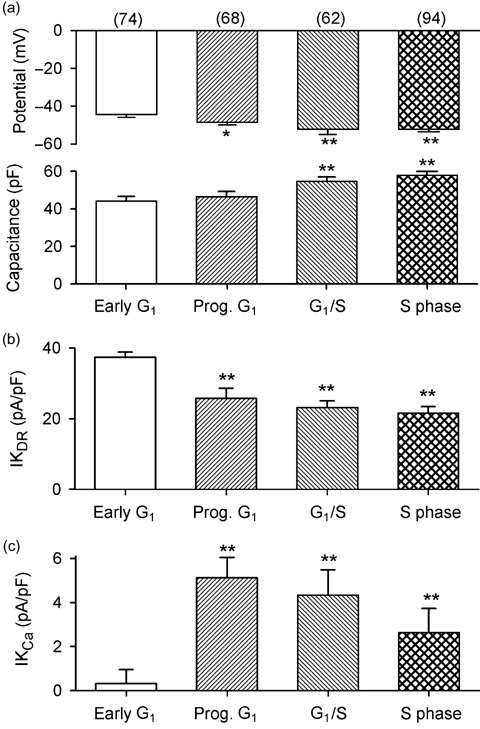
Cell cycle‐dependent changes in membrane potential, cell size, IKDR and IKCa in rat MSCs. (a) Membrane potential (upper panel) hyperpolarized in rat MSCs from progressing (Prog) G1 to S phase, and cell size (lower panel, defined by membrane capacitance, Cm) increased in cells from progressing G1 to S phase. (b) IKDR density reduced in cells from progressing G1 to S phase. (c) IKCa density increased in cells progressing from G1 to S phase.
Cell cycle‐dependent changes of IKCa (defined by 1 µm clotrimazole) and IKDR (defined by 5 mm 4‐AP) were determined in the MSCs (n = 298) from different cell cycle phases. It was found that cell numbers expressing IKCa increased with cell cycle progression. Noise‐like IKCa was copresent with IKDR in only 9% (7 out of 74) of cells from early G1 phase, 63% (43 out of 68) of cells progressing in G1 phase, 76% (47 out of 62), cells from the end of G1 phase and in 82% (77 out of 94) of cells from S phase.
Figure 3(b,c) display the 4‐AP‐sensitive IKDR and clotrimazole‐sensitive IKCa determined in the MSCs from the different cell cycle phases, with a 300‐ms voltage step from –80 to +50 mV. IKDR decreased from 37.3 ± 1.5 pA/pF in cells from early G1 phase to –25.7 ± 1.4, 23.1 ± 2.8, 21.6 ± 1.8 pA/pF in cells from progressing G1, end G1 and in S phase, respectively. However, IKCa density increased from 0.3 ± 0.6 pA/pF in cells from early G1 phase to 5.1 ± 0.9 pA/pF in cells from progressing G1 phase, 4.3 ± 1.2 pA/pF in cells from the end of G1 phase and 2.6 ± 1.1 pA/pF in cells from S phase. These results suggest that membrane hyperpolarization observed in cells from progressing G1 to S phase is most likely related to the increase of IKCa.
Cell cycle‐dependent changes in mRNA levels of IKDR and IKCa
Messenger RNA levels of α‐subunits of K+ channels responsible for IKDR and IKCa were determined in the cells from different cycling phases using RT‐PCR. Our recent study showed that IKDR was likely to be encoded by Kv1.2 and Kv2.1, while IKCa was likely to be encoded by KCa3.1 and KCa1.1 (Li et al. 2006). Thus, mRNA levels of α‐subunits for Kv1.2, Kv2.1, KCa3.1 and KCa1.1 were investigated in cells from different cycling phases. Figure 4(a,b) display the original gels of RT‐PCR. Kv1.2, Kv2.1 and KCa1.1 mRNA levels reduced, while KCa3.1 increased in MSCs from early G1 to progressing G1, to the end of G1, and S phase. No bands were seen (data not shown) when RNA was used directly for PCR (which is without a reverse transcription product), as previously reported (Li et al. 2006). Average cDNA levels relative to the housekeeping gene for GAPDH are summarized in Fig. 4c. Statistically significant changes were observed for KCa1.1 and KCa3.1 from progressing G1 (P < 0.05). These results provide molecular evidence for the cell cycle‐dependent alteration of functional ion channels observed with patch clamp experiments (Fig. 3).
Figure 4.
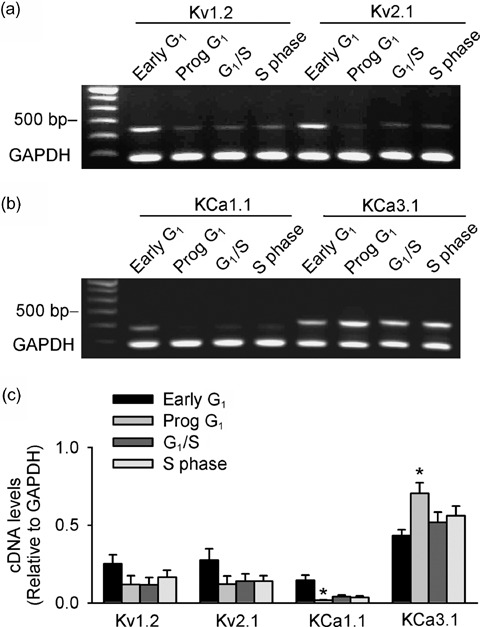
Cell cycle‐dependent changes of mRNA levels of K+ channel α‐subunits in rat MSCs. (a,b) Original gels showing expression of Kv1.2, Kv2.1, KCa1.1 and KCa3.1 mRNA from different cell cycle phases (Prog: progressing). (c) Mean values of cDNA levels (relative to GAPDH) of Kv1.2, Kv2.1, KCa1.1 and KCa3.1 from different cycling phases. *P < 0.05 versus early G1 (n = 4 different treatments).
Effects of IKDR and IKCa on cell proliferation
The cell proliferation assay was initially performed by determining [3H]‐thymidine incorporation level in the MSCs, in the absence or in the presence of varied concentrations of K+ channel blockers, although there were no specific blockers available for IKDR and intermediate‐conductance IKCa. We determined effects of 4‐AP (0.1–3 mm, for inhibiting IKDR), clotrimazole (0.1–1 µm, for inhibiting intermediate‐conductance IKCa) and the specific high‐conductance IKCa inhibitor iberiotoxin (10–100 nm), on cell proliferation, by incubation for 48 h in culture medium. We found that [3H]‐thymidine incorporation levels were reduced by 0.3, 1 and 3 mm 4‐AP (15.6 ± 1.9, 26.4 ± 1.6 and 34.4 ± 1.8%, P < 0.01 versus control), 0.3 and 1 µm clotrimazole (10.5 ± 1.5 and 16.4 ± 2.2%, P < 0.05 or P < 0.01 versus control), but not by 100 nm iberiotoxin (3.5 ± 2.9%, P = NS). These results suggest that IKDR and intermediate‐conductance IKCa, but not high‐conductance IKCa, participate in regulation of cell proliferation.
Results from flow cytometric analysis showed that 54.9 ± 1.9% of the cells were in G0/G1 phase in controls, which increased to 67.1 ± 0.6% of cells treated with 3 mm 4‐AP (n = 4 experiments, P < 0.01), and to 68.9 ± 2.5% of cells treated with 1 µm clotrimazole (n = 4 experiments, P < 0.01), suggesting that blockade of IKDR or intermediate‐conductance IKCa may interfere with cell cycle progression.
Recent studies have shown that specific RNAi was an effective tool to examine the effects of ion channels on cell proliferation (Lan et al. 2005; Weber et al. 2006; Wu et al. 2006). To rule out possible non‐specific effects of ion channel blockers on proliferation, we used specific RNAi targeted to IKDR (i.e. Kv1.2 and Kv2.1) and IKCa (i.e. KCal.1 and KCa3.1). Figure 5 illustrates the effects of the specific RNAi (100 nm) of Kv1.2, Kv2.1, KCa1.1 or KCa3.1 on related gene expression and on cell proliferation. Transfection efficiency of RNAi reached 80–90% (Fig. 5a, right panel). By the use of GAPDH RNAi as positive control, we found that GAPDH RNAi remarkably down‐regulated its mRNA level by 49% (n = 5 experiments). Figure 5b shows that the mRNA level of Kv1.2, Kv2.1, KCa1.1 or KCa3.1 was substantially reduced by specific RNAi targeted to the corresponding gene. In four experiments, cDNA from RT‐PCR was reduced by 51%, 57%, 67% and 64% for Kv1.2, Kv2.1, KCa1.1 and KCa3.1 RNAi, respectively.
Figure 5.
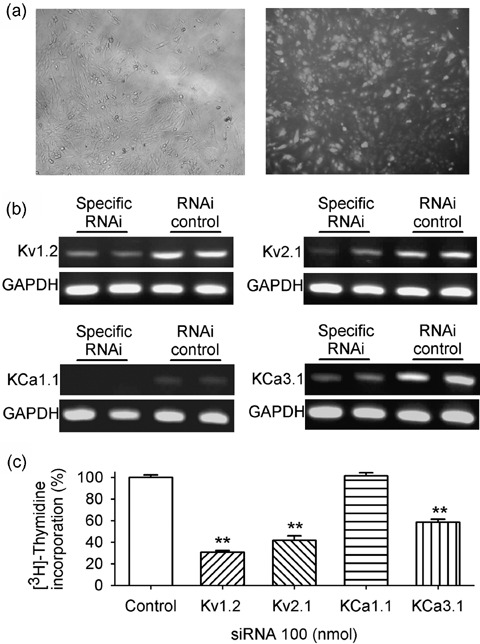
Effects of down‐regulation of K+ channels with specific RNAi on cell proliferation in rat MSCs. (a) Images showing an example of transfecting efficiency with fluorescent RNA duplex, phase contrast (left panel) and fluorescence (right panel). (b) Original gels showing reduced messenger RNA levels of Kv1.2, Kv2.1, KCa1.1 and KCa3.1 with the corresponding specific RNAi, compared to RNAi control. (c) Cell proliferation was reduced by the down‐regulation of the specific RNAi of Kv1.2, Kv2.1 or KCa3.1, but not KCa1.1. ** P < 0.01 vs control
Flow cytometric analysis showed that 58.4 ± 2.6% of the cells were in G0/G1 phase in cells treated with control RNAi, which increased to 91.2 ± 2.1%, 83.6 ± 2.6% and 85.4 ± 3.5% in cells (n = 3, P < 0.01 versus control) treated, respectively, with Kv1.2, Kv2.1 and KCa3.1 siRNAs, while no change (59.3 ± 2.6%, P = NS) was observed in cells treated with KCa1.1 siRNA, suggesting that down‐regulation of IKDR or intermediate‐conductance IKCa interfered with cell cycle progression.
Figure 5c shows [3H]‐thymidine incorporation levels in the MSCs treated by RNAi targeted to different genes. Cell proliferation was reduced by 70%, 58% and 42.0% with the specific RNAi targeted to Kv1.2, Kv2.1 and KCa3.1, respectively. However, cell proliferation was not affected by knockdown of KCa1.1. These results further suggest that IKDR and intermediate‐conductance IKCa, but not high‐conductance IKCa, regulate cell proliferation in these cells.
DISCUSSION
In the present study, we have demonstrated for the first time that membrane potential and cell size increased with cell cycle progression from early G1 to S phase in undifferentiated rat MSCs. IKDR decreased, while IKCa increased with corresponding changes of mRNA levels likely responsible for these two types of K+ channels. Pharmacological blockade of IKDR, with 4‐AP or IKCa with clotrimazole, but not with iberiotoxin, decreased cell proliferation. Moreover, specific RNAi targeted to Kv1.2, Kv2.1 or KCa3.1 down‐regulated the corresponding genes, and inhibited cell proliferation.
Cell proliferation is a crucial function, and is strictly controlled by a number of independent mechanisms, and one of them is ion channel activity. It is generally believed that cells require K+ channels to proliferate (see reviews, Wonderlin & Strobl 1996; Pardo 2004). There has been increasing evidence that K+ channels participate in regulation of cell cycle progression, because the leading study in lymphocytes by DeCoursey et al. (1984). Inhibition of K+ channels causes a decrease of proliferation in a variety of types of cells under both physiological (e.g. lymphocytes) and pathological (e.g. cancer cells) conditions (see reviews, Wonderlin & Strobl 1996; Pardo 2004), and therefore K+ channel functions have been proposed to be involved in cell cycle progression in general (Gollapudi et al. 1988; Wonderlin & Strobl 1996; Bruggemann et al. 1997; Cahalan et al. 2001; Parihar et al. 2003; Grgic et al. 2005).
Although MSCs have been used for a number of years in the investigation of cell therapy and differentiation (Bruder et al. 1997; Deans & Moseley 2000; Caplan & Bruder 2001; Janderova et al. 2003), ion channel expression and roles of ion channels in biological and physiological activity are not well understood. It is generally accepted that K+ channels are key players in controlling membrane potential, and therefore they are important in controlling proliferation processes. Here, we have demonstrated that enhancement of IKCa by ionomycin, hyperpolarized membrane potential in rat MSCs, while inhibition of intermediate IKCa remarkably reduced IKCa amplitude and membrane potential. In addition, blockade of IKDR produced an additional depolarization in these cells (Fig. 1), suggesting that IKDR and intermediate IKCa are important in controlling membrane potential. High‐conductance IKCa was not as significant as that of intermediate conductance IKCa (Li et al. 2006), and iberiotoxin‐sensitive high‐conductance IKCa was low in these cells; therefore membrane potential was not affected by blocking the current in cells treated with ionomycin (Fig. 1).
Cell cycle‐dependent changes in IKDR and IKCa were observed by synchronizing the cells to early G1, progressing through G1, end G1, and S phase, with a modified procedure (Ouadid‐Ahidouch et al. 2004a). Results from flow cytometry demonstrated that 81–88% of cells from early G1 and end G1 were in G0/G1 phase, and only 7.5% of those from S phase were in G0/G1 phase (Table 1). However, flow cytometric analysis could not differentiate the G0 cells from G1 cells, which is a limitation of the present study. Nevertheless, our data from electrophysiology and RT‐PCR have revealed that cell size, membrane potential, IKDR and IKCa densities, and mRNA levels of Kv1.2, Kv2.1, KCa1.1 and KCa3.1 altered with cell cycle progression (3, 4).
Our results have shown that membrane potential relatively depolarized in rat MSCs from early G1, compared to that in cells from progressing G1, end G1 and S phase, suggesting that most cells are most likely arrested in G0 by (24 h) starvation using low serum (0.5%) medium. The cells from early G1 exhibited very low density of IKCa in a small population of cells (9%, 7 out of 74 cells), and a higher density of IKDR, which implies that membrane potential is likely controlled by IKDR at this stage. In addition, cell numbers expressing IKCa increased, and clotrimazole‐sensitive IKCa were augmented 81–159‐fold in cells from early G1 to progressing G1, end G1 and S phase, while density of 4‐AP‐sensitive IKDR decreased by 31–42% (Fig. 3), suggesting that the membrane potential of rat MSCs progressing through G1 to S phase is controlled by IKCa. However, contribution of IKDR to membrane potential could not be excluded, as density of IKDR was always greater than that of IKCa through G1 to S phase (Fig. 3).
Importantly, RT‐PCR revealed that cell cycle‐dependent changes in IKDR and IKCa were parallel to alterations in mRNA levels of Kv1.2 and Kv2.1 (responsible for IKDR) and KCa3.1 (responsible for intermediate‐conductance IKCa). Expression of KCa1.1 (responsible for high‐conductance IKCa) was not as great as KCa3.1, and Kv1.2 and Kv2.1. Although KCa1.1 mRNA level reduced in cells from early G1 to S phase, it may not play a significant role in controlling membrane potential during cell cycling of rat MSCs.
It is believed that membrane hyperpolarization is required for cell cycle progression from G0/G1 to progressing G1 and to S phases (see reviews, Wonderlin & Strobl 1996; Pardo 2004). Earlier reports showed that membrane hyperpolarization increased the electrochemical gradient for Ca2+ influx (Wang et al. 2000) and elevation of intracellular Ca2+ levels enhanced activity of Ca2+‐dependent kinases that regulate cyclins and cyclin‐dependent kinases (Santella et al. 1998). For instance, Ca2+‐dependent calmodulin kinases are believed to increase cell cycle progression at several transition checkpoints (Kahl & Means 2003). Blockade of IKDR or IKCa with 4‐AP or clotrimazole resulted in a depolarization of membrane potential in rat MSCs (Fig. 1). This effect is likely related to inhibition of cell proliferation by interference with cell cycle progression. Application of 4‐AP or clotrimazole inhibited proliferation, although the effect was not as strong as the specific RNAi. Specific RNAi, targeted to Kv1.2, Kv2.1 or KCa3.1, but not KCa1.1, decreased the corresponding mRNA levels, substantially inhibiting cell proliferation (Fig. 5), and arrested cells at the G0/G1 phase. The lower effect of 4‐AP and clotrimazole on cell proliferation may be due to incomplete blockade of the potassium channels. These results strongly suggest that IKDR and intermediate‐conductance IKCa regulate proliferation of rat MSCs.
One of the limitations of this study was that it focused mainly on observation whether the dominant K+ currents IKDR and IKCa changed with cell cycle progression and whether they participated in cell proliferation in rat MSCs. However, possible contributions of other ion channel currents (e.g. Ito, INa.TTX, and ICa.L expressed in a small population of cells) (Li et al. 2006) to regulation of cell proliferation could not be excluded (see review, Wonderlin & Strobl 1996). Another limitation was that specific ion channel blockers are still unavailable to separate IKDR from IKCa. We used 4‐AP (5 mm) to define IKDR; however, high concentrations of 4‐AP might affect other K+ currents (see review, Gutman et al. 2005). This might underestimate intermediate‐conductance IKCa. Clotrimazole was used to define intermediate‐conductance IKCa, and this compound was reported to have Kv channel inhibition at high concentrations (see review, Wei et al. 2005). Maximum concentration (1 µm) we used here had no effect on IKur and Ito (Kv1.5 and Kv4.3) on human atrial myocytes (Tian et al. 2006) or IKDR in rat MSCs (Fig. 1b). Thus, possible non‐specific action of clotrimazole on Kv channels may not be involved in the effect on intermediate IKCa observed in the present study. In addition, although a number of ion channel genes (mRNAs) were detected proteins of these have not been demonstrated, which remains to be studied in the future.
In summary, the present study provides novel information that IKDR and intermediate IKCa channels exhibit cell cycle‐dependent expression, and play an important role in controlling membrane potential in rat MSCs. IKDR and intermediate‐conductance IKCa, but not high‐conductance IKCa, participate in regulation of proliferation in rat MSCs.
ACKNOWLEDGEMENTS
This study was supported by a grant (HKU 7347/03M) from Research Grant Council of Hong Kong. The authors would like to thank Ms Hai‐Ying Sun for her excellent technical assistance, Professor Tak‐Ming Wong for his substantial support and Dr Heather J. Ballard for her critical reading of the manuscript.
REFERENCES
- Bruder SP, Jaiswal N, Haynesworth SE (1997) Growth kinetics, self‐renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J. Cell. Biochem. 64, 278–294. [DOI] [PubMed] [Google Scholar]
- Bruggemann A, Stuhmer W, Pardo LA (1997) Mitosis‐promoting factor‐mediated suppression of a cloned delayed rectifier potassium channel expressed in Xenopus oocytes. Proc. Natl Acad. Sci. USA 94, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Wulff H, Chandy KG (2001) Molecular properties and physiological roles of ion channels in the immune system. J. Clin. Immunol. 21, 235–252. [DOI] [PubMed] [Google Scholar]
- Cahill KS, Toma C, Pittenger MF, Kessler PD, Byrne BJ (2003) Cell therapy in the heart: cell production, transplantation, and applications. Methods Mol. Biol. 219, 73–81. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Bruder SP (2001) Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol. Med. 7, 259–264. [DOI] [PubMed] [Google Scholar]
- Collecchi P, Santoni T, Gnesi E, Giuseppe NA, Passoni A, Rocchetta M, Danesi R, Bevilacqua G (2000) Cyclins of phases G1, S and G2/M are overexpressed in aneuploid mammary carcinomas. Cytometry 42, 254–260. [DOI] [PubMed] [Google Scholar]
- Deans RJ, Moseley AB (2000) Mesenchymal stem cells: biology and potential clinical uses. Exp. Hematol. 28, 875–884. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD (1984) Voltage‐gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature 307, 465–468. [DOI] [PubMed] [Google Scholar]
- Deng XL, Sun HY, Lau CP, Li GR (2006) Properties of ion channels in rabbit mesenchymal stem cells from bone marrow. Biochem. Biophys. Res. Commun. 348, 301–309. [DOI] [PubMed] [Google Scholar]
- Gollapudi SV, Vayuvegula BS, Thadepalli H, Gupta S (1988) Effect of K+ channel blockers on anti‐immunoglobulin‐induced murine B cell proliferation. J. Clin. Lab. Immunol. 27, 121–125. [PubMed] [Google Scholar]
- Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J, Kohler R (2005) Selective blockade of the intermediate‐conductance Ca2+‐activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo . Arterioscler. Thromb. Vasc. Biol. 25, 704–709. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X (2005) International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage‐gated potassium channels. Pharmacol. Rev. 57, 473–508. [DOI] [PubMed] [Google Scholar]
- Heubach JF, Graf EM, Leutheuser J, Bock M, Balana B, Zahanich I, Christ T, Boxberger S, Wettwer E, Ravens U (2004) Electrophysiological properties of human mesenchymal stem cells. J. Physiol. 554, 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janderova L, McNeil M, Murrell AN, Mynatt RL, Smith SR (2003) Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes. Res. 11, 65–74. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz‐Gonzalez XR, Reyes M, Lenvik T, Lund T, Du Blackstad MJ, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49. [DOI] [PubMed] [Google Scholar]
- Kahl CR, Means AR (2003) Regulation of cell cycle progression by calcium/calmodulin‐dependent pathways. Endocr. Rev. 24, 719–736. [DOI] [PubMed] [Google Scholar]
- Kawano S, Otsu K, Shoji S, Yamagata K, Hiraoka M (2003) Ca(2+) oscillations regulated by Na(+)‐Ca(2+) exchanger and plasma membrane Ca(2+) pump induce fluctuations of membrane currents and potentials in human mesenchymal stem cells. Cell Calcium 34, 145–156. [DOI] [PubMed] [Google Scholar]
- Lan M, Shi Y, Han Z, Hao Z, Pan Y, Liu N, Guo C, Hong L, Wang J, Qiao T, Fan D (2005) Expression of delayed rectifier potassium channels and their possible roles in proliferation of human gastric cancer cells. Cancer Biol. Ther. 4, 1342–1347. [DOI] [PubMed] [Google Scholar]
- Li GR, Deng XL, Sun H, Chung SS, Tse HF, Lau CP (2006) Ion channels in mesenchymal stem cells from rat bone marrow. Stem Cells 24, 1519–1528. [DOI] [PubMed] [Google Scholar]
- Li GR, Sun H, Deng X, Lau CP (2005) Characterization of ionic currents in human mesenchymal stem cells from bone marrow. Stem Cells 23, 371–382. [DOI] [PubMed] [Google Scholar]
- MacFarlane SN, Sontheimer H (2000) Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia 30, 39–48. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal‐Ginard B, Bodine DM, Leri A, Anversa P (2001) Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705. [DOI] [PubMed] [Google Scholar]
- Ouadid‐Ahidouch H, Roudbaraki M, Ahidouch A, Delcourt P, Prevarskaya N (2004a) Cell‐cycle‐dependent expression of the large Ca2+‐activated K+ channels in breast cancer cells. Biochem. Biophys. Res. Commun. 316, 244–251. [DOI] [PubMed] [Google Scholar]
- Ouadid‐Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N (2004b) Functional and molecular identification of intermediate‐conductance Ca(2+)‐activated K(+) channels in breast cancer cells: association with cell cycle progression. Am. J. Physiol. Cell Physiol. 287, C125–C134. [DOI] [PubMed] [Google Scholar]
- Pardo LA (2004) Voltage‐gated potassium channels in cell proliferation. Physiology (Bethesda) 19, 285–292. [DOI] [PubMed] [Google Scholar]
- Parihar AS, Coghlan MJ, Gopalakrishnan M, Shieh CC (2003) Effects of intermediate‐conductance Ca2+‐activated K+ channel modulators on human prostate cancer cell proliferation. Eur. J. Pharmacol. 471, 157–164. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Martin BJ (2004) Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 95, 9–20. [DOI] [PubMed] [Google Scholar]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM (2002) Origin of endothelial progenitors in human postnatal bone marrow. J. Clin. Invest. 109, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM (2001) Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 98, 2615–2625. [DOI] [PubMed] [Google Scholar]
- Santella L, Kyozuka K, De Riso L, Carafoli E (1998) Calcium, protease action, and the regulation of the cell cycle. Cell Calcium 23, 123–130. [DOI] [PubMed] [Google Scholar]
- Sussman M (2001) Cardiovascular biology. Hearts and bones. Nature 410, 640–641. [DOI] [PubMed] [Google Scholar]
- Tadi K, Chang Y, Ashok BT, Chen Y, Moscatello A, Schaefer SD, Schantz SP, Policastro AJ, Geliebter J, Tiwari RK (2005) 3,3′‐Diindolylmethane, a cruciferous vegetable derived synthetic anti‐proliferative compound in thyroid disease. Biochem. Biophys. Res. Commun. 337, 1019–1025. [DOI] [PubMed] [Google Scholar]
- Tian M, Dong MQ, Chiu SW, Lau CP, Li GR (2006) Effects of the antifungal antibiotic clotrimazole on human cardiac repolarization potassium currents. Br. J. Pharmacol. 147, 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ (1999) Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100, II247–II256. [DOI] [PubMed] [Google Scholar]
- Wang JY, Wang J, Golovina VA, Li L, Platoshyn O, Yuan JX (2000) Role of K(+) channel expression in polyamine‐dependent intestinal epithelial cell migration. Am. J. Physiol. 278, C303–C314. [DOI] [PubMed] [Google Scholar]
- Weber C, Mello DQ, Downie BR, Suckow A, Stuhmer W, Pardo LA (2006) Silencing the activity and proliferative properties of the human EagI Potassium Channel by RNA Interference. J. Biol. Chem. 281, 13030–13037. [DOI] [PubMed] [Google Scholar]
- Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H (2005) International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium‐activated potassium channels. Pharmacol. Rev. 57, 463–472. [DOI] [PubMed] [Google Scholar]
- Wonderlin WF, Strobl JS (1996) Potassium channels, proliferation and G1 progression. J. Membr. Biol. 154, 91–107. [DOI] [PubMed] [Google Scholar]
- Wu WK, Li GR, Wong HP, Hui MK, Tai EK, Lam EK, Shin VY, Ye YN, Li P, Yang YH, Luo JC, Cho CH (2006) Involvement of Kv1.1 and Nav1.5 in proliferation of gastric epithelial cells. J. Cell. Physiol. 207, 437–444. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC (2002) Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. 174, 11–20. [DOI] [PubMed] [Google Scholar]


