Abstract
Objectives
Previous studies have shown that adipose mesenchymal stem cells (AMSCs) share the potency of typical bone marrow mesenchymal stem cells (MSCs); however, there is little information concerning characteristics of canine AMSCs (CAMSCs); it has not previously been made clear whether CAMSCs would be able to differentiate into other cell types.
Materials and methods
In this study, typical AMSC lines were established, and their characteristics including morphology, typical markers and differentiation potentiality were tested.
Results
The cells exhibited typical MSC morphology and were positive for CD90, CD44 and CD166, considered to be MSCs surface markers. They were negative for CD34 and CD45. The CAMSCs also exhibited embryonic stem cell (ESC) markers, including Oct4 and Sox2, at passage 2. In an appropriate microenvironment, CAMSCs differentiated into EBs and were able to produce cells of the three germ layers. These results indicate that established cells were putative adipocyte‐derived MSCs, which also displayed properties of ESCs. Moreover, when the CAMSCs were induced by bone morphogenetic protein 4 (BMP4), they differentiated into PGC‐like cells (PGCLCs) and male germ‐like cells, which were positive for PR domain‐containing 1 (Prdm1), PR domain‐containing 14 (Prdm14), doublesex and mab‐3 related transcription factor (Dmrt1), as well as for promyelocytic leukaemia zinc finger (Plzf). Quantitative real‐time PCR (qRT‐PCR) and western blotting analysis verified higher expression levels of these markers.
Conclusion
This study provides an efficient approach to study germ cell development using CAMSCs.
1. Introduction
Canines are an important type of pet and partners of our families; however, some canines suffer from significant injuries and metabolic diseases.1, 2, 3 Recently, it has been shown that mesenchymal stem cells (MSCs) exist in a variety of tissues, including bone marrow, fat, brain and other adult tissues.4 MSCs possess characteristics of self‐renewal and multipotent differentiation. Under appropriate conditions, MSCs could differentiate into many cell types both in vivo and in vitro. It has been well displayed that MSCs can differentiate into adipocytes, bone, muscle, skin and even neural cells,5 which bring hope to cure multiple diseases of pet. MSCs could be separated from variety adult tissues, including bone marrow, fat, brain and other tissues.6 The most common source of MSCs is bone marrow, from which they were first isolated.7, 8 However, there are other studies that verified the presence of MSCs in canine adipose tissue abundantly.9 Some studies demonstrated that the MSCs found in canine adipose tissue share characteristics with bone marrow MSCs.10 Adipose mesenchymal stem cells (AMSCs) seem preferable to the bone marrow mesenchymal stem cells (BM‐MSCs) because they are more readily available and fat tissue is abundant. On one hand, collection of bone marrow is toilsome, and the procedure is painful for small puppies. The amount of bone marrow that can be harvested is restricted as well. On the other hand, adipose‐derived stem cells have been employed in veterinary therapy, mainly for bone, tendon, ligament injuries and joint diseases.11, 12 Canine MSCs isolated from the adipose tissue have tested well and have shown therapeutic potential in canine models.13
Many studies have found that bone marrow–derived MSCs from mice and humans could differentiate into germ cells under appropriate conditions in vitro.14 However, the procedures for gathering bone marrow from the donors are immoral and cruel and also have high demands for laboratory conditions and operators. Furthermore, the differentiating potential and the amount of bone marrow were decreased with the increasing age of the donors.15 Conversely, compared with BM‐MSCs, AMSCs obtain higher differentiation potential. Therefore, AMSCs are considered one of the best options for tissue engineering and cellular therapy and possess the ability to differentiate into adipocytes,16 osteoblasts,11 chondrocytes12 and even germ‐like cells.14
During the murine embryonic formation period, ectodermal cells begin to secrete bone morphogenetic protein 4 (BMP4) and bone morphogenetic protein 8‐β (BMP8b) at the embryonic stage 5.5, accompanied by cells expressing Prdm1, which is an important marker of primordial germ cells (PGCs). At the embryonic stage 6, secretion of BMP4 and BMP8b is at its highest, and some PRDM1 positive ectoderm cells differentiate into PGCs. PGCs then continue to differentiate into germ cells when they arrive at the genital ridge.17 This illustrates that BMP4 takes an essential part in PGC formation and migration.18 It has been well demonstrated that embryonic stem cells (ESCs) could differentiate into primordial germ cell–like cells (PGCLCs) successfully after induction with BMP4.18 Some studies also found that BM‐MSCs could express early germ cell‐specific markers after BMP4 induction.19 From this perspective, BMP4 is the primary factor to obtain PGCLCs derived from ESCs and BM‐MSCs.19, 20, 21, 22 Our previous studies have illustrated that human umbilical cord mesenchymal stem cells (hUC‐MSCs) possess the ability to differentiate into germ‐like cells with RA, BMP4 or follicle fluid treatment. However, the differentiation mechanism was still not fully understood, and the induction efficiency was low and unstable.23, 24
In this study, we isolated canine adipose mesenchymal stem cells (CAMSCs) and investigated the characteristics of CAMSCs; flow cytometry has been taken to identify the surface markers of these cells, which are positive for MSCs markers CD90, CD44 and CD166,25 and negative for vascular endothelial cells markers CD34, CD45, CD73, CD105, CD11725, 26 and hepatic stem cells marker CD326.27 CAMSCs could differentiate into several kinds of cells indicated that these cells have the strong plasticity. The main point of this study has been explored whether BMP4 could efficiently induce the cells to differentiate into PGC‐like cells even germ‐like cells in vitro.
2. Materials and methods
2.1. Animals
Two male beagle canines aged approximately 1 year old with the weight of 5.23 and 6.62 kg were used for the experiment. The canines were housed in an indoor facility lies in Experimental Animal Center of Northwest A&F University. The canines were used according to Chinese Laboratory Animal Guidelines. The experiment was approved by the committee of Shaanxi Centre of Stem Cells Engineering & Technology, Northwest A&F University.
2.2. Adipose tissue collection and CAMSCs isolation
The canines were given general zoletil (Virbac group, Carros, France) injection anaesthesia, and then, adipose tissue was aseptically harvested from abdominal subcutaneous fat.12 Canine adipose tissue was minced using a sterile scalpel and surgical scissors, placed in a 50‐mL tube with an equal volume of pre‐heated PBS and agitated for 30 seconds to wash.
The tissues were allowed to separate into phases for 3 minutes, and then the infranatant solution was removed. The tissue was rinsed with PBS to remove the erythrocytes and white blood cells. The tissue was shaken for 45 seconds and then left float to the top. The sample was rinsed until the infranatant was clear. Collagenase type I solution (Roche Diagnostics, Mannheim, Germany) was added per volume of adipose tissue, and then, it was placed in a 37°C water bath and vortexed every 15 minutes. It was then vortexed for 15 seconds to thoroughly mix cells and then centrifuged at 252xg for 5 minutes. The supernatant was then removed. Two to three microlitres of liquid above the cell pellet was left behind so that the stromal vascular fraction was not disturbed. The cells were resuspended in 1% BSA solution and centrifuged at 252xg for 5 minutes then removed the supernatant. The pellet was then resuspended in a known volume of cell culture medium and centrifuged at 1500 rpm for 5 minutes. The supernatant was then discarded, and the pellet was resuspended in an equal volume of red cell lysis buffer and incubated for 5 minutes. The cells were counted using a haemocytometer. The cells were plated at an appropriate density in complete cell medium.12
2.3. CAMSCs culture
The adipose mesenchymal stem cells were plated in cell culture dishes with low‐glucose DMEM (l‐DMEM), which contains 10% FBS, 0.1 mmol/L β‐mercaptoethanol (BME), 2 mmol/L l‐glutamine, 1% non‐essential amino acids, 10 ng/mL basic fibroblast growth factor (bFGF) and 10 ng/mL epidermal growth factor (EGF) (all Reprotech, Grand Island, NY, USA) at 37°C in a humid atmosphere containing 5% CO2.15 After 2 days, non‐adherent cells were discarded, and adherent cells were cultured. The medium was changed every 2 days. Subconfluent cell monolayers were dissociated every 2 days with trypsin‐EDTA treatment. For all experimental set‐ups, cultures were used between passage 2 and passage 4.
2.4. Cell growth curve, flow cytometry and cell cycle analysis
2.4.1. Cell growth curve
The CAMSCs were cultured in 24‐well plates with a density of 5000 cells per well. The cell growth curve was used to investigate the proliferation ability at a 24‐hour interval. The CAMSCs were trypsinized every day, and the total number of cells was determined on eight consecutive days.
2.4.2. Flow cytometry
Flow cytometry was used to determine the phenotypes of the cells using surface antigens. The cells at passage 3 were dissociated into single cells, stained with fluorescein‐conjugated antibodies and analysed by flow cytometry. Briefly, the adherent cells were detached from the culture dish using a cell scraper, washed twice with phosphate buffer saline (PBS), and then resuspended and centrifuged. Immunofluorescent staining was performed using mouse monoclonal antibodies against the leucocyte antigens, CD45 (BD), CD105 (BD), CD34 (BD), CD44 (BD), CD90 (BD), CD166 (BD), CD73 (BD), CD326 (BD) and CD117 (BD) and conjugated with FITC (fluorescein isothiocyanate) or PE. The results were analysed based on the percentage of positive cells and standard deviation from multiple experiments.
2.4.3. Cell cycle assay
The CAMSCs were suspended into single cells, mixed with cold 70% ethanol for 30 minutes and then incubated with propidium iodide (PI) solution and RNase H for 30 minutes. Then, the cells were harvested to analyse cell cycle using flow cytometry.
2.5. Differentiation of CAMSCs in vitro
Canine adipose mesenchymal stem cells at passage 3 were suspension cultured to form cell colonies; 3 × 105 cells were seeded into 35 mm plates with 1.5 mL normal culture medium. Embryoid bodies (EBs) were obtained after 3 days of suspension culture. Then, the EBs were cultured in normal cell culture dishes with low‐glucose DMEM (l‐DMEM), containing 10% FBS, 0.1 mmol/L β‐mercaptoethanol (BME), 2 mmol/L l‐glutamine, 1% non‐essential amino acids, 10 ng/mL bFGF and 10 ng/mL EGF (all Reprotech) for 7 days to investigate the ability of CAMSCs to spontaneously differentiate. After 7 days of spontaneous differentiation, the EBs were subjected to immunofluorescence staining with endodermal antibodies: PDX1, NGN3, markers of mesoblasts, DESMIN, PAX7, markers of ectoderm: β‐III‐tubulin, NSE.
Markers of osteogenic, adipogenic and chondrogenic differentiation of CAMSCs were also investigated. The cultures were maintained in 24‐well dishes and stimulated with the appropriate differentiation media as described below.
2.5.1. Osteogenic differentiation
To induce osteogenic differentiation, 2 × 104 cells were seeded into 12‐well plates induced by αMEM consisting of 10% FBS, 100 nmol/L dexamethasone, 30 μg/mL ascorbic acid and 10 mmol/L β‐glycerophosphate (Sigma‐Aldrich, St. Louis, MO, USA) for 7 days.
2.5.2. Adipogenic differentiation
2 × 104 CAMSCs were seeded into 12‐well plates. Adipogenic differentiation was induced by αMEM supplemented with 10% FBS, 1 μmol/L dexamethasone, 0.5 mmol/L isobutylmethylxanthine, 1 μg/mL insulin and 100 μmol/L indomethacin (all Sigma Aldrich) for 7 days.
2.5.3. Chondrogenic differentiation
2 × 104 CAMSCs were seeded into 12‐well plates. CAMSCs were cultured in chondrogenesis differentiation medium consisting of αMEM, 10% FBS, 40 ng/mL dexamethasone, 50 μg/mL ascorbic acid, 50 μg/mL L‐proline, 1 mmol/L sodium pyruvate (all Sigma Aldrich), insulin‐transferrin‐selenium X (Gibco, Carlsbad, California, USA) and 10 ng/mL transforming growth factor‐β3 (PeproTech, Rocky Hill, NJ, USA) for 7 days. The chondrogenesis differentiation medium was changed every 2 days.
2.5.4. PGCLCs differentiation
For PGCLC differentiation, 2 × 104 CAMSCs were seeded into 12‐well plates. The induction culture medium was made with 12.5 ng/mL BMP4 (PeproTech, Rocky Hill, NJ, USA) in normal culture medium. EBs were added in 48‐well plate cultured with the induction medium, which was replaced every 2 days.
2.6. Quantitative real‐time PCR (qRT‐PCR) analysis
The total RNA of CAMSCs was extracted by Trizol reagent (TakaRa, Dalian,China) according to the manufacturer's instructions. Then, total RNA was reverse transcribed into cDNA by Reverse Transcriptase Reagent Kit (Thermo Scientific, Waltham, MA, USA). The qRT‐PCR was carried out with the CFX96 Real‐Time PCR system (Bio‐Rad Ltd, Berkeley, CA, USA), and each volume contained 0.1 μL Taq DNA polymerase, 0.5 μL cDNA, 0.3 μL forward primer, 0.3 μL reverse primer, 6.3 μL ddH2O and 7.5 μL SYBR. The qRT‐PCR reaction cycles were as follows: 5 minutes at 94°C for predenaturation, followed by repeated amplification including 39 cycles of 30 seconds at 94°C for denaturation, 30 seconds at 58°C for annealing and 30 seconds at 70°C for extending. Glyceraldehyde 3‐phosphate dehydrogenase (Gapdh) was used as the control housekeeping gene. Comparative CT values from qRT‐PCR were used to measure relative gene expression. Primers were designed based on coding sequences from the GenBank of National Center for Biotechnology Information (NCBI) and synthesized by Sangon Biotech Biotechnology (Shanghai, China). Primers are listed in Supplementary table 1.
2.7. Immunofluorescence staining
Canine adipose mesenchymal stem cells were fixed for 15 minutes in 4% paraformaldehyde solution (PFA; pH 7.4; 4% wt/vol PFA, 100 mmol/L NaH2PO4, 0.4 mmol/L CaCl2) at room temperature (RT), washed three times with 1x phosphate‐buffered saline (PBS) and then permeabilized for 10 minutes with PBS containing 0.1% Triton‐X 100 (Sigma Aldrich) at RT. After 30 minutes of blocking with PBS supplemented with 1% bovine serum albumin (BSA), cells were incubated with primary antibodies overnight at 4°C. The antibodies used were anti‐PRDM1 (mouse monoclonal antibody, 1:400; Biolegend, San Diego, CA, USA), anti‐PRDM14 (rabbit monoclonal antibody, 1:400; Sigma‐Aldrich, St. Louis, MO, USA), anti‐SSEA‐1 (mouse monoclonal antibody, 1:100; Chemicon, Billerica, MA, USA), anti‐VASA (rabbit polyclonal antibody, 1:200; Abcam, Cambridge, UK), anti‐PLZF (rabbit polyclonal antibody, 1:200; Santa Cruz, Inc., CA, USA), anti‐DMRT1 (rabbit polyclonal antibody, 1:200; Sigma), anti‐ACROSIN (mouse monoclonal antibody, 1:400; Santa Cruz Biotechnology, Dallas, TX, USA), PDX1 (rabbit polyclonal antibody, 1:200; Chemicon), NGN3 (rabbit polyclonal antibody, 1:200; Abcam), DESMIN (rabbit polyclonal antibody, 1:200; Abcam), PAX7 (mouse monoclonal antibody, 1:200; Active Motif, Carlsbad, CA,USA), β‐III‐tubulin (mouse monoclonal antibody, 1:100; Chemicon) and NSE (rabbit polyclonal antibody, 1:200; Chemicon). Cells were then washed three times with PBS and incubated with secondary antibodies for 1 hour at 37°C in the dark. Following another three washes in PBS, nucleus counterstaining was performed in PBS containing 1 μg/mL Hoechst 33342 (Sigma Aldrich). Finally, after three more PBS washes, fluorescence images were obtained by Evos f1 fluorescence microscope (AMG, America).
2.8. Western blotting
Total cell extracts were prepared from CAMSCs and induced cells, and cell protein concentration was detected using the BCA Protein Quantification Kit (Vazyme, Piscataway, NJ, USA), after heat denaturation in 5× sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) sample loading buffer. Total cell proteins were resolved by SDS‐PAGE and then transferred to polyvinylidene difluoride membrane. They were probed with β‐III‐tUBULIN (1:1000, Beyotime, Haimen, Jiangsu, China), and antibodies against PRDM1 (mouse monoclonal antibody, 1:400; Biolegend), PRDM14 (rabbit monoclonal antibody, 1:400; Sigma) and SSEA‐1 (mouse monoclonal antibody, 1:100; Chemicon); horse‐radish peroxidase‐conjugated anti‐rabbit was used as a secondary antibody (1:1000; Beyotime). Detection was performed using the Thermo Scientific Pierce–enhanced chemiluminescence Western blotting substrate (Thermo Scientific). The results were analysed with a Tanon‐410 automatic gel imaging system (Shanghai Tianneng Corporation, China).
2.9. Statistical analysis
The Newman‐Keuls multiple range test was conducted to analyse variance and post‐test. P<.05 were considered statistically significant. T tests were used when only two sets of data were compared. All data were represented as the mean±SD, and statistical significance was expressed as follows: *P<.05; **P<.01; ***P<.001. All data were collected from three independent experiments and were analysed using Graphpad Prism software (La Jolla, CA, USA).
3. Results
3.1. Characterization of canine adipose mesenchymal stem cells
The canine adipose mesenchymal stem cells (CAMSCs) were adherent cultured cells that presented spindle or irregular morphology (Fig. 1A a,b). These cells still exhibit characteristics of MSCs at passage 10 (Fig. 1A c). To demonstrate the growth characteristics of CAMSCs, cell growth curve for primary CAMSCs has been performed. The quantity of CAMSCs increased 30‐fold after eight passage (Fig. 1B). They expressed putative pluripotent stem cell markers including Sox2 and Oct4, as demonstrated by RT‐PCR (Fig. 1C). In addition, the isolated adipose mesenchymal stem cells were positive for mesenchymal stem cell markers CD44 and CD16611 and negative for CD34 and CD45 as shown by RT‐PCR (Fig. 1C), which was consistent with the flow cytometry assay (Fig. 2A). According to the flow cytometry results, CAMSCs are positive for MSCs markers CD44 (Fig. S1a), CD90 (Fig. S1b) and CD166 and are negative for vascular endothelial cells markers CD34, CD45, CD73, CD105, CD117 and hepatic stem cells marker CD326. The speed of cell proliferation was determined by analysing the DNA synthesis (S) stage. HEK293T cells have strong proliferative capacity, which as control for cell cycle assay. FACS cell cycle detection showed that approximately 23.2% of CAMSCs were at S stage (Fig. 2B a); however, HEK293T cells only got 21.5% (Fig. 2B b), which means the speed of the cell proliferation in CAMSCs was higher compared with HEK293T cells. These results demonstrated that CAMSCs not only possess the characteristics of mesenchymal stem cells but also have a strong proliferation ability.
Figure 1.
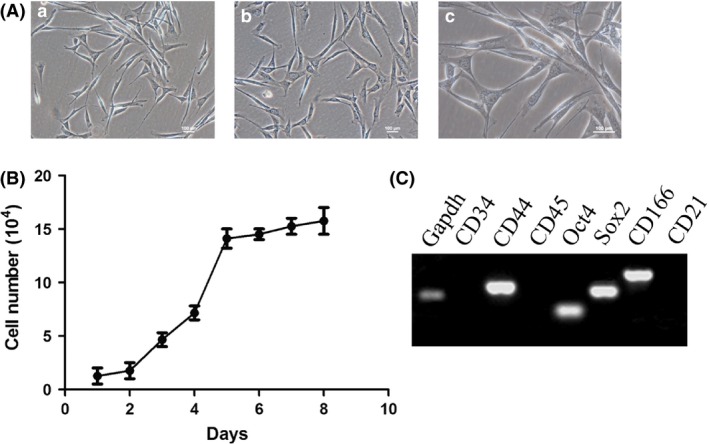
Characterization of canine adipose mesenchymal stem cells. (A) Morphology of adherent CAMSCs, the cells present spindle‐like structures. (a, b) Cells were magnified 100‐fold. (c) Cells were magnified 200‐fold. (B) The growth curve of CAMSCs. After 8 days of culture, the cell number increased by 30‐fold. (C) PCR detection of cell surface and pluripotency markers. CAMSCs are positive for Sox2, Oct4, CD44 and CD166 and negative for CD21, CD34 and CD45, which share the same characteristics with mesenchymal stem cells
Figure 2.
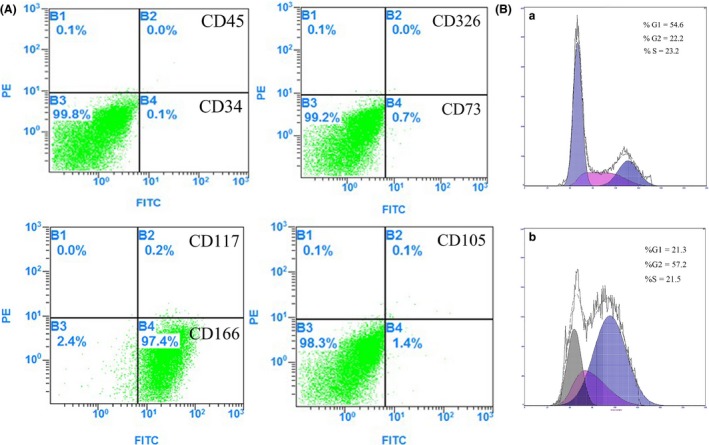
Flow cytometry of CAMSCs. (A) The flow cytometry detection chart of the primary CAMSCs. (B) The FACS cell cycle chart of primary CAMSCs
3.2. CAMSCs could differentiate into variety of cell types
To clarify the differentiation ability of CAMSCs, four induction regimens were designed to investigate the differentiation potential of canine adipose mesenchymal stem cells, which were performed by inducing the cells into adipocytes, osteoblasts, chondrocytes and neural cells. As expected, after 1 week of treatment, the induced CAMSCs were examined and found that corresponding induced groups were positive for adipocytes marker oil red O (Fig. 3a–c), osteoblasts marker Alizarin red (Fig. 3d–f) and chondrocytes marker Alcian blue (Fig. 3g–i). In addition, Giemsa staining showed that some spindle‐shaped CAMSCs turn into protruding which are similar with neural cells (Fig. 3j,k). To further prove these cells had neural‐like cells characteristics, immunofluorescence staining has been taken to illustrate that the induced cells are positive for the neuronal stem cell marker NESTIN28 (Fig. 3l).
Figure 3.
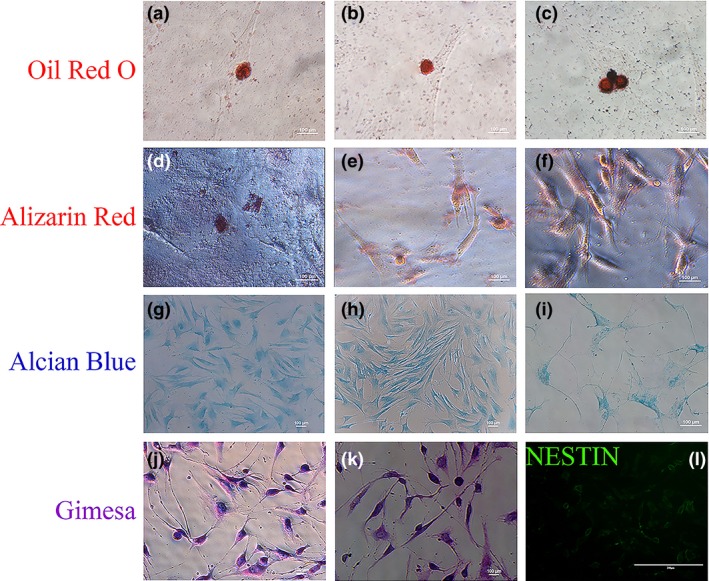
CAMSCs could differentiate into variety of cell types. After induction by appropriate conditions, the cells are positive for oil red (a–c), alizarin red (d–f), alcian blue (g–i) and immunofluorescence staining for NESTIN (l); Giemsa staining (j, k) showed that the induced cells were neural‐like cells
Embryoid bodies (EBs) are similar to the embryo in developmental pattern. Thus, EB has a strong ability to proliferate and differentiate under appropriate induction conditions. After 3 days of suspension culture, we obtained representative CAMSCs‐EBs, which were similar with embryonic cell clusters in morphology (Fig. 4a). It has been considered that whether the EBs could differentiate into embryonic germ layers in vitro. In addition, qRT‐PCR and immunofluorescence staining have been conducted to test the expression of endoblast, mesoblast and ectoblast markers at the mRNA and protein level. The results showed that mRNA levels of Pdx1, Desmin and Nestin increased significantly in CAMSC‐EBs compared with control cells, which were adherent cultured (Fig. 4b). Moreover, the immunofluorescence staining results for endoblast markers PDX1, NGN3,29 mesoblast markers DESMIN, PAX7,30 ectoblast markers β‐III‐tubulin, NSE31 (Fig. 4c–e) were also supporting that the induced cells were positive for three germ layers (endoderm, mesoderm and ectoderm) markers. Therefore, the protein expression level was consistent with the qRT‐PCR results. These data revealed that CAMSC‐EBs are more likely differentiate into embryonic germ layers.
Figure 4.
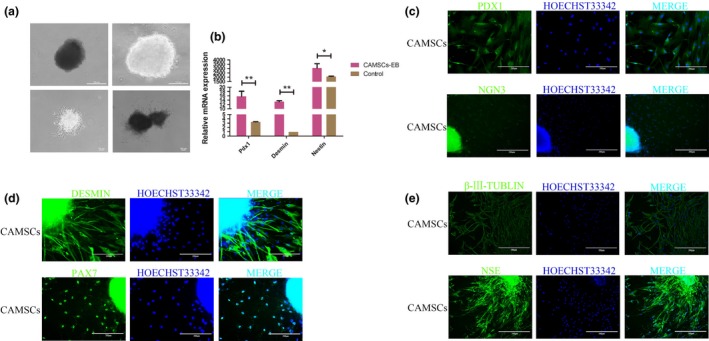
CAMSC‐EBs could differentiate into triploblastica cells. (a) EBs were formed after suspension culture for 3 days. (b) qRT‐PCR analysed relative expression levels of Pdx1, Desmin and Nestin, which were corresponding to markers of endoblast, mesoblast and ectoblast; these markers were expressed at higher levels in the EBs suspension cultured group. (c–e) Immunofluorescence staining for the cells that migrated out from EBs. (c) Immunofluorescence staining for PDX1 and NGN3 (endoblast markers). (d) Immunofluorescence staining for DESMIN and PAX7 (the mesoblast markers). (e) Immunofluorescence staining for β‐III‐Tubulin, NSE (ectoblast markers)
3.3. BMP4‐induced CAMSCs‐EBs to differentiate into PGC‐like cells
Bone morphogenetic protein 4 (12.5 ng/mL) could effectively promote MSCs to differentiate into PGC‐like cells, which has been reported by many studies.32, 33, 34 The spherical PGC‐like cells were migrated from CAMSCs‐EBs that were treated by 12.5 ng/mL BMP4 for 1 week (Fig. 5a). PRDM1, PRDM14 and AP2γ are considered to have essential roles in the formation and specification of PGCs.35 To test whether these cells possessed PGC‐specific features, qRT‐PCR assessment confirmed that mRNA expression levels of Prdm1, Prdm14, AP2γ and CD61 were higher in the BMP4‐induced group compared with the control (Fig. 5c). According to the qRT‐PCR results, immunofluorescence staining was detected furthermore, which showed that the cells either internal or migrated from EBs were positive for PGCs markers including PRDM1 and PRDM14 (Fig. 5b). Western blotting assay was consistent with the mRNA detection results (Fig. 5d). Taken together, it could draw the conclusion that spherical PGC‐like cells could be obtained from EBs after induced culture of BMP4, the PGC‐specific markers at higher level of the induced CAMSC‐EBs according to qRT‐PCR, western blotting and immunofluorescence staining detections.
Figure 5.
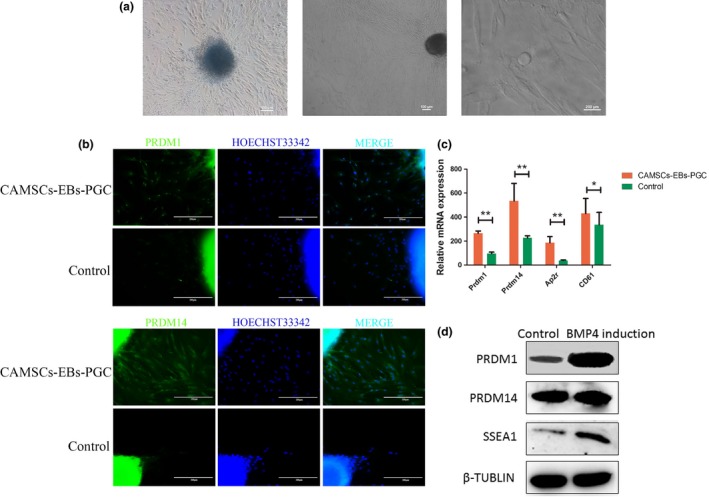
BMP4‐induced CAMSC‐EBs to differentiate into PGC‐like cells. (a) Morphology of adherent CAMSCs‐EB after 7 days with induction of 12.5 ng/mL BMP4, round‐shaped cells obtained as well. (b) The inducted group is more positive for the PGC marker PRDM1 and PRDM14 than the control group by immunofluorescence staining assay. (c) The qRT‐PCR to detect relative mRNA expression of Prdm1 and Prdm14. (d) Western blotting detection to evaluate protein expression level of PRDM1 of PRDM14
3.4. BMP4‐induced CAMSC‐EBs to differentiate into male germ‐like cells
Primordial germ cells developed into germ cells under normal conditions in vivo, so it has been wondered whether canine adipose mesenchymal stem cells could differentiate into male germ cells furthermore in vitro. Some markers of male germ cells were examined to estimate the degrees of differentiation in germ‐like cells derived from CAMSCs‐EBs, including Dmrt1,36 PLZF37 and VASA.37 Immunofluorescence staining confirmed that the CAMSC‐EBs were positive for PLZF, VASA and DMRT1 (Fig. 6a–c). It has been also found that the mRNA expression levels of PLZF and Dmrt1 were increased significantly after CAMSCs‐EBs were induced by BMP4 (Fig. 6d), which demonstrated that these cells have the features of male germ cells. Our work indicates that 12.5 ng/mL BMP4 not only induced CAMSCs differentiation into PGC‐like cells but also further induced them to differentiate into male germ‐like cells.
Figure 6.
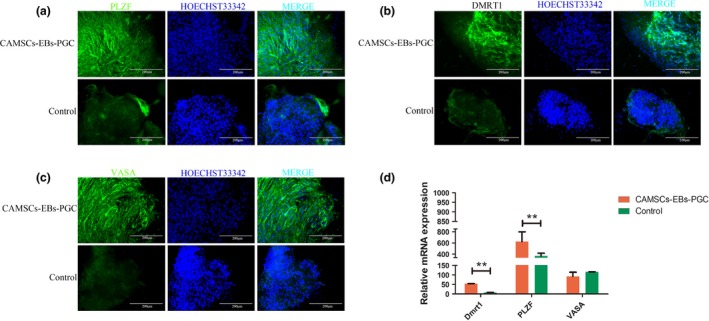
BMP4‐induced CAMSC differentiate into male germ‐like cells. (a–c) Immunofluorescence staining to test germ cell markers. (a) CMSCs‐EB were induced with 12.5 ng/mL BMP4. After 7 days, the expression level of PLZF was distinctly higher than in the control group. (b) Detection of DMRT1 protein expression level by immunofluorescence staining. (c) Detection of VASA protein expression level by immunofluorescence staining. (d) qRT‐PCR analysis of mRNA expression levels of PLZF, Dmrt1 and VASA in the induced group was significantly higher than those of the control group
4. Discussion
AMSCs as a novel putative pet stem cells which were widely used in tissue engineering, cellular therapy and regenerative medicine.38 AMSCs share features of MSCs‐derived bone marrow after long‐term culture in vitro and cryopreservation and also have the capacity to differentiate into adipocytes, chondrocytes, osteoblasts, tendon and ligament, muscle, skin and even neural cells in vitro.11, 12, 14, 16 Moreover, the transplantation assay showed that MSCs can rescue degenerative injuries and damaged organs in animal models.39 In this study, AMSC lines were established, which exhibited typical MSC morphology, and they were positive for CD44, CD90 and CD166, negative for CD34 and CD45, and simultaneously positive for ESC markers including Oct4 and Sox2 at passage 2. In an appropriate microenvironment, the cells can differentiate into EBs, and the typical three germ layer cells were obtained. These results showed that the established cells were putative adipocyte‐derived MSCs and that these cells also shared the properties of ESCs.40 The cultured AMSCs are characterized by their expression of characteristic markers and their capacity to differentiate into three germ layer cell types, including meso‐, ecto‐ and endodermal lineages. ASCs possess a high plasticity and can differentiate into various cell types, including adipocytes, osteoblasts and chondrocytes.40
Primordial germ cells are the precursors of germ cells, and the mechanism on PGC specification and development in mammals is not clear in organisms other than mice and humans. Recent studies have shown that mouse and human pluripotent stem cells including ESCs and iPSCs can be induced to differentiate into PGCs and that offspring can be obtained from PGC derived from pluripotent stem cells.24, 41, 42 Furthermore, bone marrow–derived MSCs and hUC‐MSCs can differentiate into gamete‐(sperm or oocyte) like cells both in vivo and in vitro.8, 9 However, the differentiation potential and number of bone marrow‐derived MSCs decreases gradually as the donor ages. Thus, many scientists want to find alternative sources of MSCs. Recent evidences have demonstrated that AMSCs are a new type of MSCs.10, 11, 12 Canines are the most popular pet in modern life, and canine health is becoming an issue.43 Our experiments have demonstrated that CAMSCs possessed characteristics of PGCs and germ cells after induced with BMP4. From this perspective, PGC‐like cells (PGCLCs) and germ‐like cells could be obtained from the canines which are suffering from reproductive defects.
In our study, we obtained typical MSCs derived from canine adipose tissue and demonstrated that the CAMSCs have the capacity to differentiate into EBs and the three germ layer cells. Moreover, the CAMSCs induced by bone morphogenetic protein 4 (BMP4) differentiated into PGCLCs and male germ‐like cells.
5. Conclusion
Adipose mesenchymal stem cells line were established, and these cells shared the typical properties of MSCs. Moreover, the AMSCs were efficiently induced to differentiate into PGCLs and male germ‐like cells by BMP4.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Acknowledgements
This work was supported by grants from the Program of National Natural Science Foundation of China (31272518, 31572399), the National High Technology Research and Development Program of China (SS2014AA021605) and the Key Project of Chinese Ministry of Science and Technology (2013CB967401).
‘Correction added on 8 July 2016, after first online publication: An author's name was previously incorrect and has been amended in this version’.
References
- 1. Abdulrazzak H, Moschidou D, Jones G, Guillot PV. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface. 2010;7:S689–S706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng F, Hsieh M, Chou C, Hsu W, Lee Y. Detection of indoxyl sulfate levels in dogs and cats suffering from naturally occurring kidney diseases. Vet J. 2015;205:399–403. [DOI] [PubMed] [Google Scholar]
- 3. Sobirey M. Compositions for use in treating or preventing metabolic stress‐induced lung injury and methods for increasing physical capacity of mammalian livestock. U.S. Patent Application. 2015;No. 14/643, 487. [Google Scholar]
- 4. Hipp J, Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008;4:3–11. [DOI] [PubMed] [Google Scholar]
- 5. Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue‐derived stem cells. Keio J Med. 2005;54:132–141. [DOI] [PubMed] [Google Scholar]
- 6. Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. [DOI] [PubMed] [Google Scholar]
- 7. Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. [DOI] [PubMed] [Google Scholar]
- 8. Marcus AJ, Woodbury D. Fetal stem cells from extra‐embryonic tissues: do not discard. J Cell Mol Med. 2008;12:730–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guercio A, Marco P, Casella S, et al. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int. 2012;36:189–194. [DOI] [PubMed] [Google Scholar]
- 10. Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue‐derived canine mesenchymal stem cells. BMC Vet Res. 2012;8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang B‐J, Ryu H‐H, Park SS, et al. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton's jelly for treating bone defects. J Vet Sci. 2012;13:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reich CM, Raabe O, Wenisch S, Bridger PS, Kramer M, Arnhold S. Isolation, culture and chondrogenic differentiation of canine adipose tissue‐ and bone marrow‐derived mesenchymal stem cells – a comparative study. Vet Res Commun. 2012;36:139–148. [DOI] [PubMed] [Google Scholar]
- 13. Yang Q, Peng J, Lu S‐B, et al. Evaluation of an extracellular matrix‐derived acellular biphasic scaffold/cell construct in the repair of a large articular high‐load‐bearing osteochondral defect in a canine model. Chin Med J. 2011;124:3930–3938. [PubMed] [Google Scholar]
- 14. Nayernia K, Lee JH, Drusenheimer N, et al. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006;86:654–663. [DOI] [PubMed] [Google Scholar]
- 15. Zaim M, Karaman S, Cetin G, Isik S. Donor age and long‐term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 2012;91:1175–1186. [DOI] [PubMed] [Google Scholar]
- 16. Marx C, Silveira MD, Selbach I, et al. Acupoint injection of autologous stromal vascular fraction and allogeneic adipose‐derived stem cells to treat hip dysplasia in dogs. Stem Cells Int. 2014;2014:391274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M. A signaling principle for the specification of the germ cell lineage in mice. Cell. 2009;137:571–584. [DOI] [PubMed] [Google Scholar]
- 18. Dudley BM, Runyan C, Takeuchi Y, Schaible K, Molyneaux K. BMP signaling regulates PGC numbers and motility in organ culture. Mech Dev. 2007;124:68–77. [DOI] [PubMed] [Google Scholar]
- 19. Mazaheri Z, Movahedin M, Rahbarizadeh F, Amanpour S. Different doses of bone morphogenetic protein 4 promote the expression of early germ cell‐specific gene in bone marrow mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2011;47:521–525. [DOI] [PubMed] [Google Scholar]
- 20. Erb TM, Schneider C, Mucko SE, et al. Paracrine and epigenetic control of trophectoderm differentiation from human embryonic stem cells: the role of bone morphogenic protein 4 and histone deacetylases. Stem Cells Dev. 2011;20:1601–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiller M, Liu C, Blumenthal PD, Gearhart JD, Kerr CL. Bone morphogenetic protein 4 mediates human embryonic germ cell derivation. Stem Cells Dev. 2010;20:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kee K, Gonsalves JM, Clark AT, Pera RAR. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831–837. [DOI] [PubMed] [Google Scholar]
- 23. Qiu P, Bai Y, Liu C, et al. A dose‐dependent function of follicular fluid on the proliferation and differentiation of umbilical cord mesenchymal stem cells (MSCs) of goat. Histochem Cell Biol. 2012;138:593–603. [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Wang X, Feng X, et al. Generation of male germ cells from mouse induced pluripotent stem cells in vitro. Stem Cell Res. 2014;12:517–530. [DOI] [PubMed] [Google Scholar]
- 25. Mizuno H, Tobita M, Uysal AC. Concise review: adipose‐derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. [DOI] [PubMed] [Google Scholar]
- 26. Sullivan M, Gordon‐Evans WJ, Fredericks LP, Kiefer K, Conzemius M, Griffon DJ. Comparison of mesenchymal stem cell surface markers from bone marrow aspirates and adipose stromal vascular fraction sites. Front Vet Sci. 2015;2:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fomin ME, Tai LK, Bárcena A, Muench MO. Coexpression of CD14 and CD326 discriminate hepatic precursors in the human fetal liver. Stem Cells Dev. 2010;20:1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishiwata T, Teduka K, Yamamoto T, Kawahara K, Matsuda Y, Naito Z. Neuroepithelial stem cell marker nestin regulates the migration, invasion and growth of human gliomas. Oncol Rep. 2011;26:91–99. [DOI] [PubMed] [Google Scholar]
- 29. Þãranu T, Sava A, Pãduraru D, Nedelcu A, Perianu L, Moraru M Clinical, histopathological, immunohistochemical and therapeutical aspects of the heterotopic accessory pancreas in the proximal gastrointestinal tract. Clin Anat. 2012;XI Nr:310–320. [Google Scholar]
- 30. He WA, Berardi E, Cardillo VM, et al. NF‐κB – mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Investig. 2013;123:4821–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu H, Ma J, Du R, et al. Characterization of immortalized dairy goat male germline stem cells (mGSCs). J Cell Biochem. 2014;115:1549–1560. [DOI] [PubMed] [Google Scholar]
- 32. Kassmer SH, Krause DS. Very small embryonic‐like cells: biology and function of these potential endogenous pluripotent stem cells in adult tissues. Mol Reprod Dev. 2013;80:677–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghasemzadeh Hasankolaei M, Sedighi Gilani MA, Eslaminejad MB. Induction of ram bone marrow mesenchymal stem cells into germ cell lineage using transforming growth factor‐β superfamily growth factors. Reprod Domest Anim. 2014;49:588–598. [DOI] [PubMed] [Google Scholar]
- 34. Hu X, Lu H, Cao S, et al. Stem cells derived from human first‐trimester umbilical cord have the potential to differentiate into oocyte‐like cells in vitro. Int J Mol Med. 2015;35:1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magnúsdóttir E, Dietmann S, Murakami K, et al. A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat Cell Biol. 2013;15:905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kobayashi Y, Nozu R, Horiguchi R, Nakamura M. Histological observation of doublesex‐mab 3‐related transcription factor 1 (DMRT1) localization in the adult testis of three‐spot wrasse. Int Aquat Res. 2014;6:1–9. [Google Scholar]
- 37. Bahadorani M, Hosseini SM, Abedi P, et al. Comparative immunohistochemical analysis of VASA, PLZF and THY1 in goats and sheep suggests that these markers are also conserved in these species. J Cytol Histol. 2012;2011:126. [Google Scholar]
- 38. Minteer D, Marra KG, Rubin JP. Adipose‐derived Mesenchymal Stem Cells: Biology and Potential Applications In Mesenchymal Stem Cells‐Basics and Clinical Application I. 2012:59–71. [DOI] [PubMed] [Google Scholar]
- 39. Zhang ZL, Tong J, Lu RN, Scutt AM, Goltzman D, Miao DS. Therapeutic potential of non‐adherent BM‐derived mesenchymal stem cells in tissue regeneration. Bone Marrow Transplant. 2009;43:69–81. [DOI] [PubMed] [Google Scholar]
- 40. Baer PC, Geiger H. Adipose‐derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hayashi K, Saitou M. Perspectives of germ cell development in vitro in mammals. Anim Sci J. 2014;85:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bazley FA, Liu CF, Yuan X, et al. Direct reprogramming of human primordial germ cells into induced pluripotent stem cells: efficient generation of genetically engineered germ cells. Stem Cells Dev. 2015;24:2634–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cortinovis C, Pizzo F, Caloni F. Poisoning of dogs and cats by drugs intended for human use. Vet J. 2015;203:52–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


