Abstract
Objectives
Dendritic cells (DCs) are antigen‐presenting cells that participate in the immune response; recently, it has been reported that growth hormone (GH) promotes their maturation. The aim of this study was to investigate mechanisms by which GH acts on DC maturation and activation.
Materials and methods
Human peripheral blood monocytes (HPBMs) were induced to become immature DCs and treated with GH to obtain mature DCs. An osteosarcoma mouse model was established by injection of LM8 cells to investigate anti‐tumour effect of GH‐induced DCs in vivo.
Results
After administration of GH, DCs reduced miR‐200a expression and nuclear Nrf2 accumulation; miR‐200a down‐regulation inhibited DC maturation. Nrf2 ubiquitination level was increased by Keap1 overexpression in murine bone marrow derived dendritic cells (BMDCs), which was cancelled by miR‐200a in GH exposed cells. In vivo, tumour volume was significantly reduced by GH‐treated DCs and the effect was reversed by overexpression of miR‐200a.
Conclusions
GH promoted maturation and activation of DCs, and regulation of miR‐200a played a part in this process by modulation of the Keap1/Nrf2 pathway.
Introduction
Dendritic cells (DCs), as antigen‐presenting cells, are central to the immune response 1. Their function is to process antigenic material and present it on their cell surfaces, to T cells, which are involved in activation or suppression of a specific immune response in the body. Immunization therapy has been shown to be a valuable way of combatting many cancers 2. Cancer‐associated DCs can induce cytotoxic T lymphocytes (CTLs), which harbour specific anti‐tumour antigens 3. Investigating the function of DCs provides a new approach to improvement in cancer therapy.
Immature DCs reside in non‐lymphoid tissues where they can capture and process antigens; their antigen‐presenting function is carried out when they mature and migrate to T‐cell‐containing areas of lymphoid organs 1. Thus, DC maturation is a critical component of the immune response. It is well known that various stimuli, such as evoked by cytokines, bacterial products and sensitizers, can induce DC maturation 4, 5, 6. One recent study has shown that growth hormone (GH) can promote induction and maturation of DCs and facilitate autologous lymphocyte proliferation 7. GH is a peptide hormone that stimulates cell reproduction and regeneration in mammals. However, mechanisms by which GH acts to regulate DC maturation are still unknown.
MicroRNAs (miRNAs) are small non‐coding RNAs that function in post‐transcriptional regulation of gene expression 8, and have been reported to be involved in various physiological and pathological processes 9, 10. More than 100 miRNAs are expressed in immune cells and play pivotal roles in development and differentiation of immune cell subsets 11, 12, 13. Regulation of DC maturation and its function in cytokine production have been shown in previous studies 12, 14, 15. The miR‐200 family has been reported to play an important role in innate responses 16.
The Keap1/Nrf2 signalling pathway is critical in protecting cells from electrophilic and oxidative stress. However, activation of Nrf2 in many tumours contributes to survival and proliferation of cancer cells, as well as to resistance to anti‐cancer therapy, one aspect of carcinogenesis 17. miR‐200a regulates the Keap1/Nrf2 pathway in mammary epithelium 18. We formed the hypothesis that the Keap1/Nrf2 pathway, via miR‐200a regulation, could be involved in maturation and activation of DCs by GH stimulation. This study has aimed to confirm the role of GH in DC maturation, and to investigate underlying mechanisms, according to our hypothesis.
Materials and methods
DC induction and autologous T cell preparation
In the order of 2–5 ml peripheral blood was obtained from healthy children after written informed consent was obtained from their parents/guardians. All human experiments were approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. Mononuclear cells were collected from blood supernatants. After washing in RPMI 1640, mononuclear cells were diluted to 4 × 106 cells/ml with RPMI 1640 containing 10% autoserum, incubated in 24‐well plates (1 ml/well), then cultured at 37°C for 2 h in an atmosphere of 5% CO2. RhIL‐4 (5 ng/ml) and rhGM‐CSF (10 ng/ml) were used to induce differentiation of adherent mononuclear cells into immature DCs. Recombinant GH (10 mg/ml) was supplemented to induce their maturation. Medium containing GH and cytokines was replaced every two days. DCs obtained were identified 5–9 days after incubation.
Detection of DC phenotypes
9 days after incubation, DCs were harvested and washed. Cells were labelled with fluorescent antibodies anti‐HLA‐DR‐PC7, anti‐CD1α, anti‐CD80‐FITC and anti‐CD83‐APC (BD Pharmingen, USA), according to the manufacturer's instructions. DC phenotypes were detected by FACS using a Canto flow cytometer (Becton Dickinson, San Jose, CA, USA). Data were analysed using BD FACS Diva 6.0 software.
Detection of IL‐12
IL‐12 concentration in DC supernatant was detected using an ELISA kit (GeneStar, Nanjing, China), according to the manufacturer's instructions.
Lymphocyte viability
At days 5, 7 and 9 of DC culture, 1 × 105 cells per ml were harvested and treated with mitomycin (50 μg/mg) for 30 min, to serve as stimulator cells. 1 × 106 cells per ml autogenic lymphocytes, to serve as responding cells, were harvested and co‐incubated with DCs in 96‐well plates for 72 h, in complete RPMI 1640 medium containing 10% FBS and supplemented with 20 U/mL IL‐2. DCs and autogeneic lymphocytes were co‐incubated in 96‐well plates for 72 h. MTT assay was then performed to detect lymphocyte viability, as described in previous studies 19. Viability index of lymphocytes was calculated as follows: value (index of lymphocytes) = (OD570 in experimental group)/(OD570 responding cells in control group + OD570 stimulator cells in control group). In this experiment, DCs treated with GH or DCs from HPBMCs transfected with miR‐200a mimic/inhibitor were considered to be experimental groups.
Cytotoxicity of CTLs
DCs were obtained 9 days after incubation. 1 × 105 per ml DCs and 1 × 106 per ml lymphocytes were co‐incubated in 24‐well plates for 72 h, then CTLs were collected. 1 × 104 per well CTLs and MG‐63 or U2OS osteosarcoma cells were incubated in 96‐well plates at 37°C for 4 h in an atmosphere of 5% CO2. CTL cytotoxicity was detected by MTT assay. Cytotoxicity of CTLs was calculated using the following formula: value (cytotoxicity of CTLs) = [1 − OD570 in the experimental group/(OD570 in responding cells group + OD570 in the target cell group)]. In this experiment, DCs treated with GH were considered to be the experimental group.
Quantitative PCR
Total RNA of DCs or BMDCs was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) and complementary DNA was reverse‐transcribed using a TaqMan miRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. Relative expression of miR‐200a was quantified by Power SYBR® Green PCR Master Mix (Applied Biosystems). U6 served as reference gene. Relative expression of miR‐200a was calculated by the ΔΔCt method. Sequences of miR‐200a were as follows: forward primer, 5′‐GGCTAACACTGTCTGGTAACGATG‐3′ and reverse primer, 5′‐GTGCAGGGTCCGAGGT‐3′. Sequences of U6 were as follows: forward primer, 5′‐GCTTCGGCAGCACATATACTAAAAT‐3′ and reverse primer, 5′‐CGCTTCACGAATTTGCGTGTCAT‐3′.
Western blotting
Cytoplasmic and nuclear proteins were prepared using the Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Shanghai, China) following the manufacturer's instructions and protein was quantified with the Bradford method. After denaturation for 5 min, protein extracts were separated on 10% SDS‐PAGE and transferred to PVDF membranes (Bio‐Rad, Hercules, CA, USA), blocked with 5% non‐fat dry milk in Tris‐HCl buffered saline and incubated with appropriate primary antibodies (Cell Signaling, Beverly, MA, USA). Secondary antibodies were then added and incubated at room temperature for 2 h. All antibodies were diluted with 1:1000 in TBS buffer. After washing membranes, protein activity was detected and imaged with the aid of a GS800 Densitometer Scanner (Bio‐Rad). Data were processed using PDQuest 7.2.0 software (Bio‐Rad); α‐tubulin served as control protein.
Transfection
To overexpress or down‐regulate miR‐200a expression, mimic or inhibitor of miR‐200a was used, by transfection into various DCs. Transfection of the negative control (NC) served as a control, as it has no homology to any known mammalian gene. Lipofectamine 2000 reagent (Invitrogen) was used to perform cell transfection. Mimics and inhibitors of miR‐200a, as well as NC, were synthesized by Ribobio Co., Ltd. (RIBOBIO, Guangzhou, China).
Immunoprecipitation and immunoblotting
BMDCs were pre‐cultured in 100 mm dishes for 24 h. Cells were supplemented with different treatments for a further 12 h, then lysed in RIPA buffer containing proteinase inhibitor. Lysates were homogenized twice by ultrasonication for 10 s, and incubated on ice for 30 min. Homogenates were then centrifuged at 15 000 g for 15 min. Concentration of protein extracts was detected using the Bradford method with the aid of a protein assay kit (Beyotime).
Immunoprecipitation and immunoblotting were conducted by conventional methods. In brief, whole cell lysates with 0.5 mg proteins were pretreated with protein A‐Sepharose beads for 1 h, cultured with 1 μg anti‐Keap1 or anti‐Nrf2 antibody for 4 h to make Nrf2/Keap1 immunoprecipatitate. Immunoprecipitated complexes were then washed five times in RIPA buffer and boiled in SDS sample buffer for 5 min. Immunoprecipitation products were run on 8% SDS‐PAGE and electrophoretically transferred to PVDF membranes (Bio‐Rad). These were then incubated with primary antibodies (Cell Signaling) according to the manufacturer's protocol, before being incubated with horseradish peroxidase conjugated secondary antibody for 2 h. Protein was finally detected and imaged using a GS800 Densitometer Scanner (Bio‐Rad).
Luciferase activity assay
Luciferase activity assay was conducted as previously described 20. In brief, pGL3‐Nrf2 3′‐UTR reporter plasmid contains wild type Nrf2 3′‐UTR cloned into a pGL3 vector. 3′‐UTR mutant pGL3‐Nrf2 reporter plasmid was generated with point mutations within potential miR‐200a binding sites. Control plasmids or test plasmids were transfected into BMDCs using Lipofectamine 2000 (Invitrogen) in strict accordance with the manufacturer's instructions. Relative luciferase activities were detected using Dual‐Luciferase Reporter Assay (Promega, Madison, WI, USA).
Establishment of osteosarcoma mice
Animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals and approved by the Animal Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University. An osteosarcoma mouse model was obtained by subcutaneous injection of 1 × 106 LM8 cells (a murine osteosarcoma cell line) into female C3H mice. After tumour establishment, mice were treated with the appropriate injections twice weekly: tumour cell lysis (Group A, n = 6), tumour cell lysis + DCs transfected with NC (Group B, n = 6), and tumour cell lysis + DCs transfected with miR‐200a mimic (Group C, n = 6). Tumour volume of lung metastases were detected using Micro‐CT (ZKKS, China) to obtain high‐resolution CT images, in all directions, of the living osteosarcoma mice. Tumour volumes of lung metastases were calculated using the formula: (π × long axis × short axis × short axis)/6.
Statistical analysis
SPSS 17.0 software was used to conduct data analysis. All bars are presented as means ± SD. Differences between two groups were determined by independent‐sample t‐tests for in vitro experiments. Differences between three groups were determined by analysis of variance (ANOVA) and Tukey tests. P < 0.05 was considered statistically significant.
Results
Growth hormone promotes maturation and activation of dendritic cells
Immature DCs were co‐incubated with GH for 9 days to investigate effects of GH on DC maturation. As shown in Fig. 1, immunophenotypes HLA‐DR, CD‐1α, CD80 and CD83 in the GH‐treated group were 49.5%, 36.1%, 32.6% and 34.5%, respectively, which was significantly higher than controls (Fig. 1a). IL‐12 concentration in DC supernatant was also elevated in the GH group at days 5, 7 and 9 (Fig. 1b,c). Results also showed that GH promoted T lymphocyte viability (Fig. 1c) and elevated CTL cytotoxicity (Fig. 1d).
Figure 1.
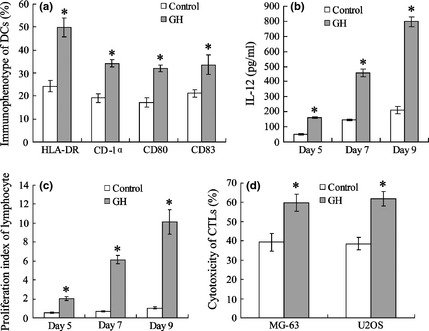
Growth hormone promotes maturation and activation of dendritic cells. FACS was used to detect immunophenotype of DCs (a). IL‐12 in DC supernatant was measured using ELISA (b). Co‐incubated DCs (with or without GH) and allogeneic T lymphocytes, T lymphocyte viability was detected using MTT assay (a). CTL was activated by DC co‐incubated with MG‐63 or U2OS cells; cytotoxicity of CTLs was detected by MTT assay (d). *versus control, P < 0.05.
Role of growth hormone in regulation of miR‐200a and Keap1/Nrf2 pathway
Real‐time PCR results indicated that GH significantly down‐regulated miR‐200a expression in DCs (Fig. 2a). Keap1/Nrf2 was measured by western blotting and GH was found to elevate Keap1 expression (Fig. 2b). Cytoplasmic Nrf2 level increased while its expression in the nucleus was reduced by GH by day 9 (Fig. 2c). As shown in Fig. 2d, expression of target genes NQO1 and HO‐1 in DCs was down‐regulated by GH.
Figure 2.
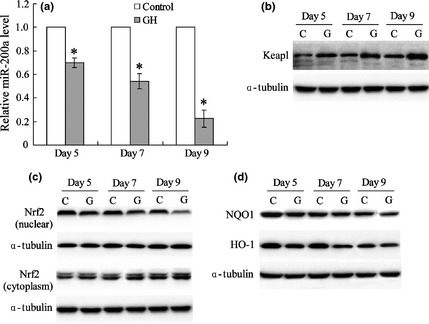
Growth hormone is involved in regulation of miR‐200a and the Keap1/Nrf2 pathway. miR‐200a expression of DCs was detected by real‐time PCR (a). Protein expression of Keap1 (b), Nrf2 (c), and Nrf2 targets (d) in DCs treated with or without GH were measured by western blotting with α‐tubulin as internal reference protein. *versus control, P < 0.05.
MiR‐200a inhibited maturation and activation of dendritic cells
Human peripheral blood mononuclear cells (HPBMCs) were transfected with a miR‐200a mimic to up‐regulate levels of miR‐200a. Immunophenotypes of DCs were detected by FACS, and results showed HLA‐DR, CD‐1α, CD80 and CD83 were significantly reduced by the miR‐200a mimic (Fig. 3a). IL‐12 concentration in DC supernatant and T lymphocyte proliferation also decreased (Fig. 3b,c). HPBMCs were transfected with miR‐200a inhibitor to down‐regulate its expression. Positive expression ratio of DC phenotypes, IL‐12 level and proliferation index of T lymphocytes were markedly elevated compared to groups transfected with a negative control (NC).
Figure 3.
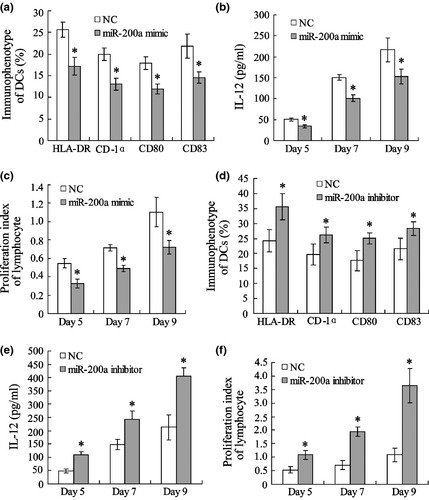
MiR‐200a inhibits maturation and activation of dendritic cells. Human peripheral blood mononuclear cells (PBMC) were transfected with a miR‐200a mimic or inhibitor. Immunophenotype of DCs was detected by FACS (a, d). IL‐12 of DC supernatant was measured using ELISA (b, e). DCs were co‐incubated with allogeneic T lymphocytes and T lymphocyte viability was detected by MTT assay (c, f). *versus negative control (NC), P < 0.05.
MiR‐200a was involved in regulation of growth hormone in the Keap1/Nrf2 pathway
Murine bone marrow derived dendritic cells (BMDCs) were treated with GH for 24 h, after which relative miR‐200a level was significantly down‐regulated (Fig. 4a). GH promoted Keap1 expression and inhibited nuclear Nrf2 level of BMDCs (Fig. 4b,c). Level of Nrf2 in target genes NQO1 and HO‐1 was also reduced in cells (Fig. 4d). However, effects of GH on regulation of Keap1/Nrf2 molecules was reversed by up‐regulation of miR‐200a.
Figure 4.
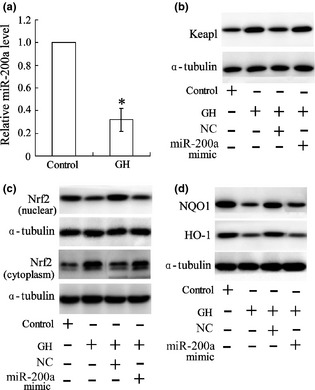
MiR‐200a is involved in regulation of growth hormone in the Keap1/Nrf2 pathway. Murine bone marrow‐derived dendritic cells (BMDCs) were treated with GH for 24 h and miR‐200a expression was then detected by real‐time PCR (a). BMDCs were transfected with an miR‐200a mimic or negative control (NC) after treatment with GH; protein expressions of Keap1 (b), Nrf2 (c), and Nrf2 targets (d) in BMDCs were measured by western blotting with α‐tubulin as internal reference protein; *versus control, P < 0.05.
Involvement of MiR‐200a in regulation of growth hormone and Nrf2 ubiquitination
Nrf2 ubiquitination was detected by immunoprecipitation and immunoblotting. As presented, GH elevated ubiquitination level of Nrf2, and this elevation was reversed by down‐regulation of Keap1 (Fig. 5a) or transfection of a miR‐200a mimic (Fig. 5b). To overexpress BMDC Keap1 by pCMV5‐Keap1, Nrf2 ubiquitination level was increased, which cancelled the effect of miR‐200a on GH exposed cells (Fig. 5c).
Figure 5.
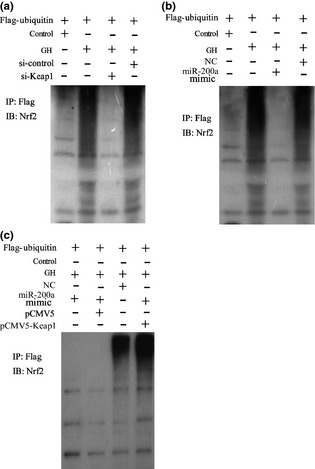
MiR‐200a is involved in regulation of growth hormone, which affects Nrf2 ubiquitination. Murine bone marrow‐derived dendritic cells (BMDCs) were treated with GH, si‐Keap1/si‐control, miR‐200a mimic/miR‐NC and pCMV5‐Keap1/pCMV5; immunoprecipitation (IP) and immunoblotting (IB) were used to detect Nrf2 ubiquitination.
MiR‐200a inhibited Keap‐1 3′UTR activity in murine bone marrow‐derived dendritic cells
BMDCs were co‐transfected with Keap1 3′‐UTR reporter along with inhibitors or mimics of miR‐200a, for 24 h and luciferase activity assay as then performed. Results indicated that miR‐200a inhibitor significantly increased Keap 3′‐UTR 1 activity (Fig. 6a) and miR‐200 mimic markedly reduced it (Fig. 6c), compared to the group transfected with NC. In addition, protein expression of Keap1 was also up‐regulated by miR‐200a inhibitor (Fig. 6b) and down‐regulated by miR‐200a mimic (Fig. 6d).
Figure 6.
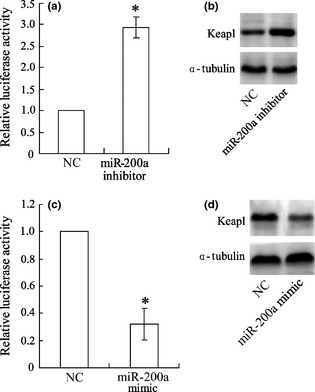
miR‐200a inhibits Keap‐1 3′UTR activity in murine bone marrow‐derived dendritic cells. Murine bone marrow‐derived dendritic cells (BMDCs) were transfected with an miR‐200a inhibitor or mimic; Keap‐1 3′UTR activity was detected by luciferase activity assay (a, c), protein expression of Keap1 was detected by western blotting (b, d); *versus negative control (NC), P < 0.05.
MiR‐200a was involved in tumour metastasis in osteosarcoma mice
An osteosarcoma mouse model was established by administration with subcutaneous injection of LM8 cells. As shown in Fig. 7, osteosarcoma mice were treated with DCs (Group B) and volume of lung metastases was significantly reduced compared to controls (Group A). However, the effect was reversed by treatment with miR‐200a mimic transfected DCs (Group C).
Figure 7.
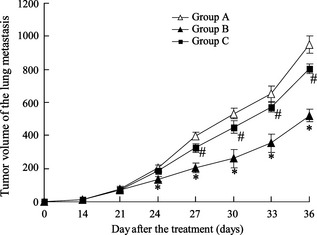
MiR‐200a is involved in tumour metastasis in osteosarcoma mice. 18 female C3H mice were administered with subcutaneous injection of LM8 cells (1 × 106 cells/mouse) to establish an osteosarcoma mouse model. Mice were then treated with appropriate injections: tumour cell lysis (Group A, n = 6), tumour cell lysis + DCs transfected with NC (Group B, n = 6) and tumour cell lysis + DCs transfected with miR‐200a mimic (Group C, n = 6). Volumes of lung metastases were detected. *versus Group A, P < 0.05; #versus Group B, P < 0.05.
Discussion
Growth hormone is a peptide that stimulates cell reproduction and regeneration in mammals. GH and its receptors are expressed in various immune cells such as T lymphocytes, B lymphocytes, monocytes and natural killer cells, in thymus, spleen and bone marrow 21. Biological functions of GH in the immune system include modulating cytokine production 22, 23, regulating thymocyte development 24, enhancing lymphocyte development and antibody production 25, 26, enhancing monocyte migration 27 and neutrophil adhesion 28. However, few previous articles have revealed effects of GH on DC activation and maturation. Liu et al. recently reported that GH promoted induction and maturation of DCs, and facilitated autologous lymphocyte proliferation 7. In this study, effects of GH promoting activation and maturation of DCs was confirmed. In addition, we found that the Keap1/Nrf2 pathway via miR‐200a regulation, played a part in this process.
Dendritic cells (DCs) are potent antigen‐presenting cells participating in the immune response; ability of DCs to regulate immunity is dependent on their maturation. Immature DCs reside in non‐lymphoid tissues where they can capture and process antigens; antigen‐presenting properties arise when mature DCs migrate to T‐cell‐containing areas of lymphoid organs. Immunophenotypes HLA‐DR, CD1α, CD80 and CD83 are expressed in DCs, expression implying the cells maturation. Various factors can induce DC maturation, including cytokines, bacteria or bacteria‐derived products, and ligation of select cell surface receptors. In this study, immunophenotypes HLA‐DR, CD1α, CD80 and CD83 levels were significantly increased in immature DCs after treatment with GH, proving that GH promoted DC maturation. Elevated IL‐12 concentration in DC supernatants, as well as viability index of T lymphocytes and CTL cytotoxicity, confirmed activation of DCs.
It was shown that GH promoted DC maturation and activation, but the mechanisms for this remain unknown. Nuclear erythroid 2 p45‐related factor 2 (Nrf2) is a redox‐sensitive transcription factor that regulates expression of antioxidant and detoxification genes. Williams et al. have found that deletion of Nrf2 affects important constitutive functions of both bone marrow‐derived and highly purified myeloid lung DCs, such as secretion of inflammatory cytokines and their ability to take up exogenous Ag 29. Nrf2 is activated following its detachment from its cytosolic inhibitor, Keap1, then translocates to the nucleus where it binds to antioxidant response elements in the promoter region of target genes, leading to their transcriptional induction. Nrf2 targets to HO‐1 and NQO1, which have antioxidant activities. Chauveau et al. have demonstrated that induction of HO‐1 expression, with cobalt protoporphyrin in human and rat DCs, inhibits lipopolysaccharide‐induced phenotypic maturation and secretion of proinflammatory cytokines, resulting in inhibition of alloreactive T‐cell proliferation 30. We also investigated role of the Keap1/Nrf2 pathway in the process through which GH promotes DC maturation and activation. It was revealed that GH inhibited Nrf2 pathway activation, due to high levels of Nrf2 ubiquitination induced by Keap1. Expression of Nrf2 target genes HO‐1 and NQO1 was also down‐regulated by GH. These data suggest that the Nrf2 pathway maybe involved in the process of GH‐induced DC maturation.
In addition, to further investigate mechanisms underlying promotion of GH on DC maturation, miR‐200a was detected. The miR‐200 family has been found to play an important role in innate responses by modulating expression of chemokines 16. Our experiments showed that miR‐200a level in DCs was significantly reduced by GH treatment. In addition, miR‐200a overexpression inhibited DC maturation while its down‐regulation promoted DC maturation, implying that miR‐200a takes part in DC maturation. Eades et al. have demonstrated that miR‐200a regulates the Keap1/Nrf2 pathway in mammary epithelium 18. We also observed that down‐regulation of miR‐200a elevated Keap1 expression and inhibited accumulation of nucleus Nrf2 in GH‐exposed BMDCs. Results of luciferase activity assay showed that miR‐200a reduced Keap1 3′UTR activity in BMDCs, further indicating regulation of miR‐200a in the Keap1/Nrf2 pathway. In vivo, anti‐tumour effects of GH‐treated DCs was reversed by miR‐200a overexpression.
In conclusion, we have shown that GH promoted maturation and activation of DCs, and regulation of miR‐200a took part in this process by modulating the Keap1/Nrf2 pathway. However, Keap1/Nrf2 is not the only regulation pathway of DC maturation and activation. Further studies must be conducted to clarify mechanisms by which GH affects DC function; GH stimulation of DCs can also be applied to aim to combat various cancers, in animal experiments.
Conflict of interest
All authors have no conflict of interest to state.
Acknowledgements
This work was funded by the Henan Provincial Key Science and Technology Project in 2011 (No. 112102310106) and Youth Innovation Fund Project of the First Affiliated Hospital of Zhengzhou University in 2011.
References
- 1. Cella M, Sallusto F, Lanzavecchia A (1997) Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 9, 10–16. [DOI] [PubMed] [Google Scholar]
- 2. Palucka K, Banchereau J (2012) Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 12, 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dhodapkar MV, Dhodapkar KM, Palucka AK (2008) Interactions of tumor cells with dendritic cells: Balancing immunity and tolerance. Cell Death Differ. 15, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aiba S (1998) Maturation of dendritic cells induced by cytokines and haptens. Tohoku J. Exp. Med. 184, 159–172. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y‐C, Hu X‐B, He F, Feng F, Wang L, Li W et al (2009) Lipopolysaccharide‐induced maturation of bone marrow‐derived dendritic cells is regulated by notch signaling through the up‐regulation of CXCR4. J. Biol. Chem. 284, 15993–16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kagatani S, Sasaki Y, Hirota M, Mizuashi M, Suzuki M, Ohtani T et al (2010) Oxidation of cell surface thiol groups by contact sensitizers triggers the maturation of dendritic cells. J Invest Dermatol. 130, 175–183. [DOI] [PubMed] [Google Scholar]
- 7. Liu QL, Wang YS, Xiang WJ (2010) Effect of growth hormone on the immune function of dendritic cells. Chin. Med. J. (Engl). 123, 1078–1083. [PubMed] [Google Scholar]
- 8. Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297. [DOI] [PubMed] [Google Scholar]
- 9. Pizzini S, Bisognin A, Mandruzzato S, Biasiolo M, Facciolli A, Perilli L et al (2013) Impact of microRNAs on regulatory networks and pathways in human colorectal carcinogenesis and development of metastasis. BMC Genom. 14, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strickland ER, Hook MA, Balaraman S, Huie JR, Grau JW, Miranda RC (2011) MicroRNA dysregulation following spinal cord contusion: implications for neural plasticity and repair. Neuroscience 186, 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D (2010) Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122. [DOI] [PubMed] [Google Scholar]
- 12. Turner ML, Schnorfeil FM, Brocker T (2011). MicroRNAs regulate dendritic cell differentiation and function. J. Immunol. (Baltimore, Md.: 1950) 187, 3911–3917. [DOI] [PubMed] [Google Scholar]
- 13. Zhang M, Liu F, Jia H, Zhang Q, Yin L, Liu W et al (2011). Inhibition of microRNA let‐7i depresses maturation and functional state of dendritic cells in response to lipopolysaccharide stimulation via targeting suppressor of cytokine signaling 1. J. Immunol. (Baltimore, Md.: 1950) 187, 1674–1683. [DOI] [PubMed] [Google Scholar]
- 14. Martinez‐Nunez RT, Louafi F, Friedmann PS, Sanchez‐Elsner T (2009) MicroRNA‐155 modulates the pathogen binding ability of dendritic cells (DCs) by down‐regulation of DC‐specific intercellular adhesion molecule‐3 grabbing non‐integrin (DC‐SIGN). J. Biol. Chem. 284, 16334–16342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stumpfova Z, Hezova R, Meli AC, Slaby O, Michalek J (2014) MicroRNA profiling of activated and tolerogenic human dendritic cells. Mediators Inflamm. 2014, 259689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wendlandt EB, Graff JW, Gioannini TL, McCaffrey AP, Wilson ME (2012) The role of MicroRNAs miR‐200b and miR‐200c in TLR4 signaling and NF‐ B activation. Innate Immun. 18, 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Namani A, Li Y, Wang XJ, Tang X (2014) Modulation of NRF2 signaling pathway by nuclear receptors: implications for cancer. Biochim. Biophys. Acta 1843, 1875–1885. [DOI] [PubMed] [Google Scholar]
- 18. Eades G, Yang M, Yao Y, Zhang Y, Zhou Q (2011) miR‐200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. J. Biol. Chem. 286, 40725–40733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu Z, Ma B, Zhou Y, Zhang M, Long H, Wang Y et al (2007) Allogeneic tumor vaccine produced by electrofusion between osteosarcoma cell line and dendritic cells in the induction of antitumor immunity. Cancer Invest. 25, 535–541. [DOI] [PubMed] [Google Scholar]
- 20. Yang M, Yao Y, Eades G, Zhang Y, Zhou Q (2011) MiR‐28 regulates Nrf2 expression through a Keap1‐independent mechanism. Breast Cancer Res. Treat. 129, 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hattori N (2009) Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res. 19, 187–197. [DOI] [PubMed] [Google Scholar]
- 22. Uronen‐Hansson H, Allen ML, Lichtarowicz‐Krynska E, Aynsley‐Green A, Cole TJ, Hoiden‐Guthenberg I et al (2003) Growth hormone enhances proinflammatory cytokine production by monocytes in whole blood. Growth Horm IGF Res. 13, 282–286. [DOI] [PubMed] [Google Scholar]
- 23. Sodhi A, Tripathi A (2008) Prolactin and growth hormone induce differential cytokine and chemokine profile in murine peritoneal macrophages in vitro: involvement of p‐38 MAP kinase, STAT3 and NF‐kappaB. Cytokine 41, 162–173. [DOI] [PubMed] [Google Scholar]
- 24. Smaniotto S, de Mello‐Coelho V, Villa‐Verde DMS, Pléau J‐M, Postel‐Vinay M‐C, Dardenne M et al (2005) Growth hormone modulates thymocyte development in vivo through a combined action of laminin and CXC chemokine ligand 12. Endocrinology 146, 3005–3017. [DOI] [PubMed] [Google Scholar]
- 25. Dobashi H, Sato M, Tanaka T, Tokuda M, Ishida T (2001) Growth hormone restores glucocorticoid‐induced T cell suppression. FASEB J. 15, 1861–1863. [DOI] [PubMed] [Google Scholar]
- 26. Sumita K, Hattori N, Inagaki C (2005) Effects of growth hormone on the differentiation of mouse B‐lymphoid precursors. J. Pharmacol. Sci. 97, 408–416. [DOI] [PubMed] [Google Scholar]
- 27. Kähler CM, Pischel AB, Haller T, Meierhofer C, Djanani A, Kaufmann G et al (2001) Signal transduction pathways in directed migration of human monocytes induced by human growth hormone in vitro. Int. Immunopharmacol. 1, 1351–1361. [DOI] [PubMed] [Google Scholar]
- 28. Wiedermann CJ, Niedermühlbichler M, Geissler D, Beimpold H, Braunsteiner H (1991) Priming of normal human neutrophils by recombinant human growth hormone. Br. J. Haematol. 78, 19–22. [DOI] [PubMed] [Google Scholar]
- 29. Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TW et al (2008). Disruption of the transcription factor Nrf2 promotes pro‐oxidative dendritic cells that stimulate Th2‐like immunoresponsiveness upon activation by ambient particulate matter. J. Immunol. (Baltimore, Md.: 1950) 181, 4545–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chauveau C, Rémy S, Royer PJ, Hill M, Tanguy‐Royer S, Hubert F‐X et al (2005) Heme oxygenase‐1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL‐10 expression. Blood 106, 1694–1702. [DOI] [PubMed] [Google Scholar]


