Abstract
Objectives
Previous studies have shown that promyelocytic leukaemia zinc finger (PLZF) is a spermatogonia‐specific transcription factor in the testis, required to regulate self‐renewal and maintenance of the spermatogonia stem cell. Up to now, expression and function of PLZF in the goat testis has not been known. The objectives of this study were to investigate PLZF expression pattern in the dairy goat and its effect on male goat germline stem cell (mGSC) self‐renewal and differentiation.
Materials and methods
Testis development and expression patterns of PLZF in the dairy goat were analysed by haematoxylin and eosin staining, immunohistochemistry and reverse transcription‐polymerase chain reaction (RT‐PCR). Furthermore, effects of PLZF overexpression on mGSC self‐renewal and differentiation were evaluated by quantitative RT‐PCR (QRT‐PCR), immunofluorescence and BrdU incorporation assay.
Results
Promyelocytic leukaemia zinc finger was essential for dairy goat testis development and expression of several proliferation and pluripotency‐associated proteins including OCT4, C‐MYC were upregulated by PLZF overexpression. The study demonstrated that PLZF played a key role in maintaining self‐renewal of mGSCs and its overexpression enhanced expression of proliferation‐associated genes.
Conclusions
Promyelocytic leukaemia zinc finger could function in the dairy goat as well as in other species in maintaining self‐renewal of germline stem cells and this study provides a model to study the mechanism on self‐renewal and differentiation of mGSCs in livestock.
Introduction
Mammalian spermatogenesis derives from spermatogonial stem cells (SSCs), also known as male germline stem cells (mGSCs). Maintaining self‐renewal of SSCs is the basis for continued mammalian spermatogenesis, helping male animal production of large amounts of sperm. Self‐renewal and differentiation of SSCs are affected and regulated by a variety of internal and external environment factors 1 and previous studies have shown that many transcription factors and cytokines are necessary for self‐renewal and differentiation of SSCs 2, 3. TAF‐4b has been shown to be critical for promotion of SSC proliferation and maintenance of normal spermatogenesis 4. Oct4 has been found to be expressed in embryonic primordial germ cells and in juvenile mouse spermatogonia 5. Glial cell line‐derived neurotrophic factor (GDNF) promotes cell proliferation and self‐renewal of SSCs 3 although Akt knockout is detrimental to spermatogenesis in mouse 6. ERM is a key factor for self‐renewal and maintenance of spermatogenesis of SSCs in adult rat 7 and Foxo1 is required for SSC self‐renewal and differentiation in mouse 8.
Promyelocytic leukaemia zinc finger (PLZF) was originally identified in acute promyelocytic leukaemia (APL) as one partner of proteins fused by a reciprocal chromosomal translocation to the retinoic acid receptor‐alpha 9, 10. PLZF is a transcription factor containing nine carboxyterminal zinc fingers and an amino terminal POZ domain, which suppresses transcription by binding to specific DNA sequences with the help of recruiting co‐repressors 11; nevertheless, PLZF can activate transcription 12. It is a key regulator of myeloid development 13, and has a role in the immune response 13. In addition, PLZF is essential for maintaining self‐renewal of stem cells, including SSCs 14. Numerous genes are regulated by PLZF, such as MYC and cyclin A2 15. The goat (Capra hircus) is a convenient domestic species for current biological investigation and application as it has diversified products of commercial value and a relatively short gestation period 16. SSCs could be one of the most effective ways to create transgenic animals and a model could be of use for infertility treatment. Understanding of SSC self‐renewal will be helpful for application of SSCs. However, information on PLZF regulation of goat SSCs has been limited and whether PLZF contributes to dairy goat mGSC self‐renewal has not been known. Recently, Bahadorani et al. initially demonstrated that expression of PLZF, Vasa in goat was highly conserved 17. However, these authors did not provide further study on PLZF function in goat. Here, for the first time, we have compared PLZF expression in dairy goats of different ages and analysed any possible relationship between PLZF and self‐renewal‐associated proteins. Then, dairy goat PLZF mRNA full‐length sequence was obtained by using in silico cloning. Subsequently, PLZF overexpression in mGSC was observed successfully through recombinant vector transfection. Furthermore, results show that PLZF overexpression upregulated proliferative and pluripotent gene expression in dairy goat mGSCs. This study will be valuable for comprehensive evaluation of the role played by PZLF, in mammalian SSCs.
Materials and methods
Collection of dairy goat testes
Guanzhong dairy goat testes were supplied by Yaoan abattoir, Yangling Hi‐tech district, collected from goats of different ages. Animals were killed specifically for our experiment and all procedures were approved by Northwest A&F University and Shaanxi Centre of Stem Cell Engineering & Technology, Northwest A&F University.
Testicular tissue immunohistochemistry
Testicular tissues were derived from goats 30 days postnatal (dpp), 6 M (month) and 10 M; they were dissected and fixed in 4% paraformaldehyde for 24 h embedded in paraffin wax, sections cut, deparaffinized and rehydrated following standard methods 18. Slides were soaked in boiling citrate buffer for 15–25 min, followed by three washes in cold phosphate buffered saline (PBS), each for 5 min. Samples were then incubated in 3% H2O2 for 10 min to inhibit endogenous peroxidise activity and rinsed in PBS. Blocking was performed with 4% goat serum at room temperature for 20 min. Slides were then exposed to primary antibody (anti‐PLZF, 1:100; Santa Cruz, CA, USA) overnight at 4 °C or for 1–2 h at room temperature. Appropriate HRP‐conjugated secondary antibodies and chromogen solution 3, 3‐diaminobenzidine (DAB) were used according to the manufacturer's manual (Beijing Zhongshan Golden Bridge Biochemical Factory, Beijing, China). Concurrently, negative controls were stained with conjugated secondary antibodies alone: goat anti‐rabbit IgG and goat anti‐mouse IgG 19. Testes from the three age groups (30 dpp, 6 M and 10 M, 3 samples from each group) were fixed, sectioned and stained for PLZF expression. Testis seminiferous tubule cross‐sections were used to determine percentage of PLZF‐positive cells (number of PLZF‐positive cells per seminiferous tubule/Total cells in each seminiferous tubule cross‐section). Average percentages of PLZF‐positive seminiferous tubules cross‐section were plotted.
RT‐PCR and quantitative RT‐PCR analysis
Total RNAs for RT‐PCR analysis were extracted from all tissue categories and cultured using TRIzol (Tiangen Biotech Co. Ltd, Beijing, China). PCR primers and lengths of amplicons were listed (Table S1). Protocol details for RT‐PCR and gel electrophoresis can be referred from previous studies 20, 21. Quantitative reverse transcriptase‐polymerase chain reaction (QRT‐PCR) was set up in 15 μl reaction mixtures as described by Hu et al. 22. Fluorescence signal was collected every 0.5 °C for 10 s.
In silico cloning and evolutionary relationship of PLZF coding domain sequence (CDS)
Transcriptome Shotgun Assembly (TSA) sequences Database in NCBI (National Center for Biotechnology Information) were used for in silico cloning, splicing and obtaining dairy goat PLZF, which contains the whole CDS. Briefly, bovine PLZF cDNA sequence (GenBank accession number: NM_001037476) was put as the seed sequence, and BLAST alignment in goat TSA database was conducted to obtain goat homology TSA sequences (Fig. 1c); then several overlapping sequences from the result of BLAST alignment were selected, spliced and finally, the nucleotide sequence (we named it putative PLZF cDNA sequence), then the sequence was analysed by homology and evolution.
Figure 1.
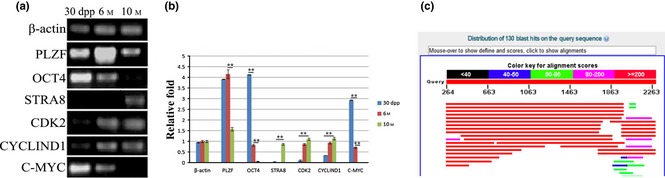
RT ‐ PCR analysis of expression of PLZF and associated markers in dairy goat testicular tissues of 30 dpp, 6 M and 10 M samples. (a) Expression of PLZF, OCT4, STRA8, CDk2, CYCIND1 and C‐MYC in 30 dpp, 6 M and the 10 M testicular tissues, β‐actin as internal control. (b) Relative value of (a) (measured by ImageJ). **P < 0.01. (c) Series of goat homology TSA sequences gained by the Bos taurus seed sequence BLAST alignment in the goat TSA database.
Primers were designed according to the putative PLZF cDNA sequence achieved from the in silico cloning; primers were synthesized by Peking SanBoYuanZhi Company (Beijing, China) (Table S1). Guanzhong dairy goat PLZF CDS was obtained using RT‐PCR, and sequenced by Peking SanBoYuanZhi Company. The sequence was then aligned with known sequences in GenBank by DNAman software and the phylogenetic tree was depicted with MEGA4.1 23.
Construction of recombinant plasmid
Full length coding sequence for PLZF was obtained using RT‐PCR and was cloned into pMD18‐T cloning vector (TaKaRa, Dalian, China). Briefly, DH5α competent cells were used; recombinant plasmid with DNA resistant to ampicillin was introduced to the competent cells and streaked on lysogeny broth agar plates with antibiotic. After blue‐white screening, positive clones were identified by PCR, restriction enzyme digestion and sequencing. Fragments were then cloned into pIRES2‐EGFP eukaryotic expression vector to obtain pPLZF‐IRES2‐EGFP recombinant plasmid 23.
Cell culture and transfection
Goat foetal testes (age of 2–5 months terminated specifically for these experiments) were collected and washed 5–10 times in PBS supplemented with 100 U/ml penicillin and 100 mg/ml streptomycin. Seminiferous tubules from each testis were cut into small pieces using forceps and scissors. Seminiferous epithelial cells were dissociated by modified enzymatic digestion and plated by two‐step successive differential plating methods (Fig. 2a) 20. mGSCs were cultured under optimum culture conditions 19 for 9 days, then cultured in non‐optimum culture conditions in 2–3 serial passages within 8 days, before being passaged into 48‐well plate for transfection with plasmid. Non‐optimum culture conditions contain DMEM/F12 (Invitrogen, Carlsbad, CA, USA), supplemented with 10% serum (Hyclone, Logan, UT, USA), 4 mm l‐glutamine (Invitrogen), 1% non‐essential amino acids (Invitrogen).
Figure 2.
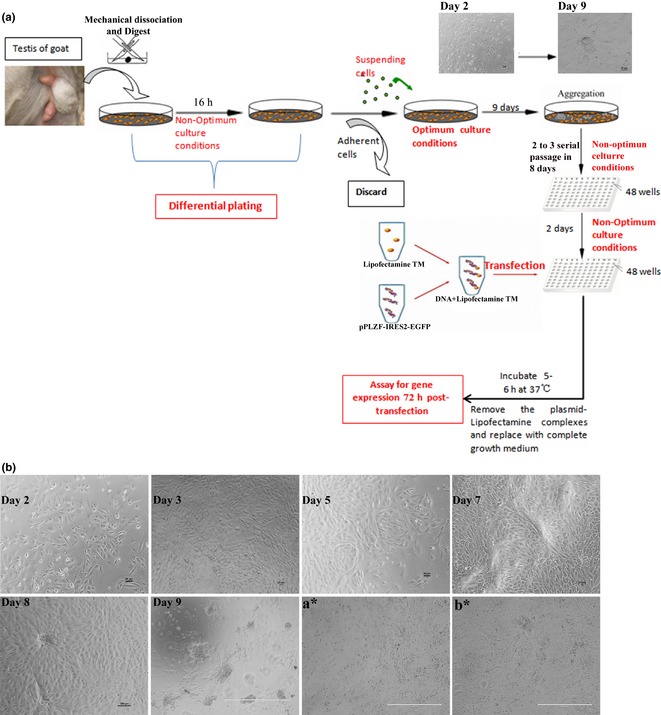
Schematic diagrams of the study. (a) Goat testes were mechanically dissociated and digested into single cells which were then treated with differential plating in 6‐well plates for 16 h. Suspended cells (we named them mGSCs) were isolated and inserted into other wells pre‐treated with matrigel. mGSCs were then cultured in optimum media for 9 days. After aggregation, mGSCs were digested once more and cultured in non‐optimal culture conditions for 2–3 serial passages over 8 days. mGSCs were then plated into 48 wells before being transfected with plasmid, 2 days later.(b) Morphological changes in mGSCs (before, concurrent with, or after medium change), days 2, 3, 5, 7, 8 and 9. Morphological changes in mGSCs under optimum culture conditions at different days. (a*) Morphology of mGSCs before transfection; (b*) Morphology of mGSCs 72 h after transfection.
pPLZF‐IRES2‐EGFP recombinant plasmid and pIRES2‐EGFP were transfected into the mGSCs in 48‐well plates. The specific transfection protocol was followed from Li et al. 18. Briefly, 500 ng plasmid and 1 μl Lipofectamine™ LTX Reagent (Invitrogen) were diluted in 25 μl Opti‐MEM (Invitrogen) reduced serum medium respectively; mixed gently and incubated for 5 min at room temperature. Then diluted Lipofectamine™ LTX Reagent (Invitrogen) was added directly to diluted plasmid and gently rocked and incubated for 20 min at room temperature. Fifty microlitres of plasmid‐Lipofectamine™ LTX complexes was added, and transfection medium was replaced 5 h later by fresh medium; cells were observed after 48 h using inverted phase contrast microscopy. GFP‐positive cells were examined after 72 h, and total RNAs of the two groups were extracted. Expression of Vasa, PLZF, GFRa1, C‐MYC and Oct4 was analysed by QRT‐PCR 22.
Optimization of culture conditions for dairy goat mGSC
Purified cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% knockout serum replacement (KSR; Invitrogen), 4 mm l‐glutamine (Invitrogen), 1% non‐essential amino acids (Invitrogen), 100 IU/ml penicillin and 100 mg/ml streptomycin (Invitrogen), supplemented with 5 ng/ml recombinant human basic fibroblast growth factor (bFGF; Millipore, Billenca, MA, CA, USA) and 2.5 μm 6‐bromoindirubin‐3′‐oxime (BIO; Merck, Munchen, Germany) 19.
Immunofluorescence
Specific markers of pluripotent stem cells and mGSC were analysed by immunofluorescence. Briefly, second to fourth passage mGSCs before, and 72 h after transfection, were fixed in 4% paraformaldehyde (PFA) for 10 min at room temperature. Cells were permeabilized with 0.1% Triton X‐100 for 10 min, blocked in 10% goat serum in PBS at room temperature for 1 h, and incubated with primary antibodies overnight at 4 °C. Primary antibodies included anti‐: PLZF (1:100; Santa Cruz), OCT4 (1:20; Millipore), VASA (1:200; Abcam Cambridge, MA, USA), C‐MYC (1:200; Chemicon Temecula, CA, USA), GFRa1 (1:100; Santa Cruz), CD49F (1:100; Santa Cruz), LIN28 (1:100; Santa Cruz), NANOS2 (1:100; Santa Cruz), NANOG (1:100; Santa Cruz). After three washes in PBS, cells were incubated in secondary FITC‐ or TRITC‐conjugated antibodies (1:500; Chemicon) at room temperature for 30 min followed by three washes in PBS. Concurrently, negative controls were stained with conjugated secondary antibodies alone: goat anti‐rabbit IgG and goat anti‐mouse IgG. Nuclei of cells were stained using Hoechst 33342 (Sigma, St Louis, MO, USA). Images were captured using a Leica fluorescence microscope (Hicksville, NY, USA) 24.
BrdU incorporation
After PLZF overexpression, mGSCs proliferation was determined by BrdU incorporation assay as described previously 25. BrdU‐positive cells were detected by incubating them in TRITC‐conjugated secondary antibody (1:500; Millipore) for 1 h at room temperature. After three washes in PBS, cells were visualized by fluorescence microscopy and analysed for BrdU incorporation.
Statistical analysis
Data are presented as mean ± SEM. Statistical comparisons were assessed by Student's t‐test; P < 0.05 was considered statistically significant and P < 0.01 was considered a significant difference.
Results
Reproductive status of dairy goat testis
Figure 3c,f,i and Fig. S1 indicate that numbers of germ cells per seminiferous cord/tubule cross‐section initially were low, increasing from birth to 6 months of age. Most cells in seminiferous cords were Sertoli cells. No spermatids could be seen in seminiferous cord/tubule cross‐sections of young animals, thus, reproductive status of 30 dpp and 6 M ones could be defined as pre‐puberty. A very dramatic increase in germ‐cell population per cross‐section of seminiferous tubules occurred by 10 months of age, and some of seminiferous cord/tubule cross‐sections contained spermatids (Fig. S1ΙΙ,ΙΙΙ). Thus, the goats appeared to have begun spermatogenesis by 10 M of age (Fig. S1I,IV).
Figure 3.
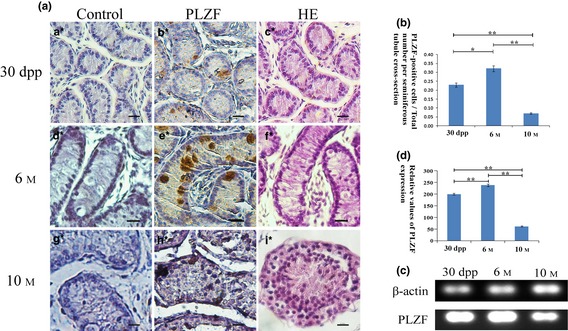
Testis histological phenotype and PLZF expression pattern in the dairy goat. (a) Control groups for immunohistochemical staining of three age groups (a*, d*, g*); HE staining of the three different age groups (c*, f*, i*); PLZF immunohistochemical staining of the testicular tissues of the three different age groups (b*, e*, h*). Bar = 65.34 μm (a*–i*). (b) Percentage of PLZF‐positive cells per seminiferous tubule cross‐section. *P < 0.05, **P < 0.01. (c) PLZF expression at three different ages of testicular tissue was analysed by RT‐PCR. (d) Relative expression values of PLZF at the three different ages (measured by ImageJ). **P < 0.01.
Expression pattern of PLZF
Results of immunohistochemistry manifested that highest expression level of PLZF in our dairy goat testicular tissue was at 6 M (Fig. 3a,b) then declined by 10 M, which was consistent with results of our RT‐PCR analysis (each group contains 3 samples) (Fig. 3c,d), and variation in the trend of PLZF level was similar to real‐time PCR and western blotting results in mice 26. PLZF was located in both cytoplasm and nuclei of spermatogonia (Fig. 3b,e,h). RT‐PCR analysis showed that PLZF expression levels varied testicular tissue of goats at different ages, at 6 M, PLZF expression reaching its highest level. Compared to pubertal (6 M) testis, relative expression level of PLZF was much lower in adult (10 M) and kid (1 M) testicular tissue (Fig. 3c,d).
It has been reported that numerous genes, such as MYC, are specifically regulated by PLZF, 15. Expression patterns above raise the possibility that the C‐MYC and cyclin gene families, and other associated genes, were also expressed variously in the testes of goats of different age groups. Thus, RT‐PCR analysis of C‐MYC, PLZF, STRA8, CYCLIN D1, CDK2 and OCT4 expression levels among 30 dpp, 6 M and 10 M goats were compared. Similar to PLZF, expression levels of CYCLIN D1, CDK2 were much lower in 30 dpp and 10 M goat testes compared to those of 6 M. However, C‐MYC and OCT4 reached their peaks in the 30 dpp group and STRA8 reached its highest level in 10 M adults. (Fig 1a,b).
Identification of the dairy goat PLZF gene
Promyelocytic leukaemia zinc finger nucleotide sequence of Guanzhong dairy goat was obtained by in silico cloning (Fig. 1c) before the whole‐genome of the domestic goat (Capra hircus) was sequenced by the Kunming Institute of Zoology, China 27. NCBI ORF finder was used to analyse the open reading frame (ORF) of the splicing sequence. A 2022 bp ORF was cloned by RT‐PCR and identified by RT‐PCR (Fig. 4a), restriction enzyme digestion (Fig. 4b) and sequencing (data not shown). Furthermore, we aligned the cloned sequence with PLZF CDS of Bos taurus, Homo sapiens and other species. Homology of the nucleotide sequence was analysed by NCBI GEO (Gene Expression Omnibus, GEO) database. Results showed the PLZF nucleotide sequence we cloned, of the Guanzhong dairy goat, had high homology with that of human, rat and mouse, and other species (Fig. 4c), as high as 99% to Bos taurus (Table 1). Phylogenetic trees (Fig. 4c) showed that Bos taurus was the most closely related species to Guanzhong dairy goat's PLZF, followed by human beings, the sequence of PLZF gene having been identified by GenBank (accession number: JX047313, published).
Figure 4.
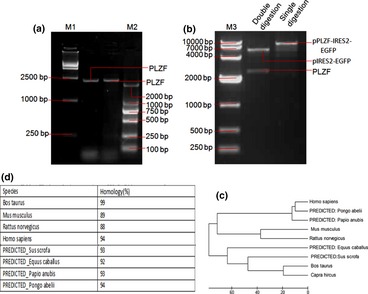
Identification of dairy goat PLZF gene. (a) Agarose gel electrophoresis analysis of PLZF PCR product. (b) pPLZF‐IRES2‐EGFP was digested by SalI and BamHI. M1: 15 000 bp ladder; M3: 10 000 bp ladder; M2: 2000 bp ladder. pIRES2‐EGFP eukaryotic expression vector was in the order of 5300 bp and PLZF fragment was around 2300 bp. (c) Construction of phylogenetic trees with PLZF cDNA sequence of dairy goat and other species. (d) The homologic comparison of Plzf cDNA sequence of dairy goat compare with sequences published in NCBI GenBank.
Identification of pPLZF‐IRES‐EGFP recombinant plasmid
The cloned PLZF was cloned into pIRES2‐EGFP eukaryotic expression vector to obtain pPLZF‐IRES2‐EGFP plasmid, which was verified by restriction enzyme (SalI and BamHI) digestion (Fig. 4b).
Overexpression of PLZF promoted mGSC self‐renewal
Our dairy goat mGSCs maintained typical GSC and ES‐like colonies when cultured under optimum culture conditions for 9 days 19; cells expressed PLZF, VASA 28, OCT4 29 (also known as POU5F1), NANOS2 30, NANOG 31, LIN28 32 and CD49F 33 as analysed by immunofluorescence (Fig. 5).
Figure 5.
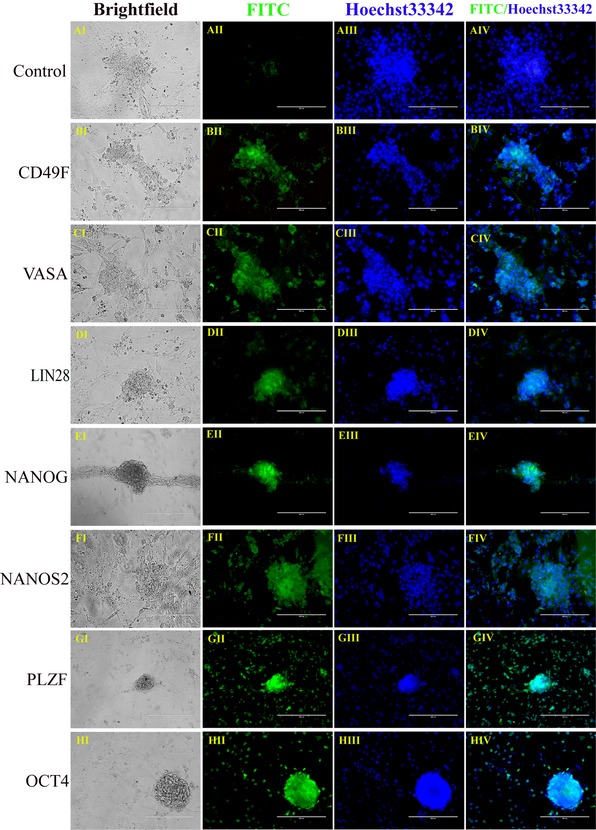
Immunofluorescence staining of mGSC s. Immunofluorescence staining analysis: CONTROL (aI–aIV), CD49F (bI–bIV), VASA (cI–cIV), LIN28 (dI–dIV), NANOG (eI–eIV), NANOS2 (fI–fIV), PLZF (gI–gIV) and OCT4 (hI–hIV). Nucleus stained with Hoechst 33342 (Blue). Bar = 100 μm (a–r). Technical replicate numbers: n = 3.
Culture medium was then changed to DMEM/F12, consisting of 10% FBS, and recombinant vector pPLZF‐IRES2‐EGFP was transfected into the cells. Seventy‐two hours after transfection, cells were analysed by QRT‐PCR, BrdU analysis and immunofluorescence. Expression levels of PLZF, VASA, OCT4, C‐MYC and GFRa1 in PLZF overexpression groups were much higher than in pIRES2‐EGFP group (Fig. 6aa, Table S2); similar results were observed by QRT‐PCR (Fig. 6ab) (n = 3). These results indicate that overexpression of PLZF upregulated expression of VASA‐germ cell marker, GFRa1 (GDNF family receptor alpha‐1) pluripotency marker Oct4 and C‐MYC 20.
Figure 6a.
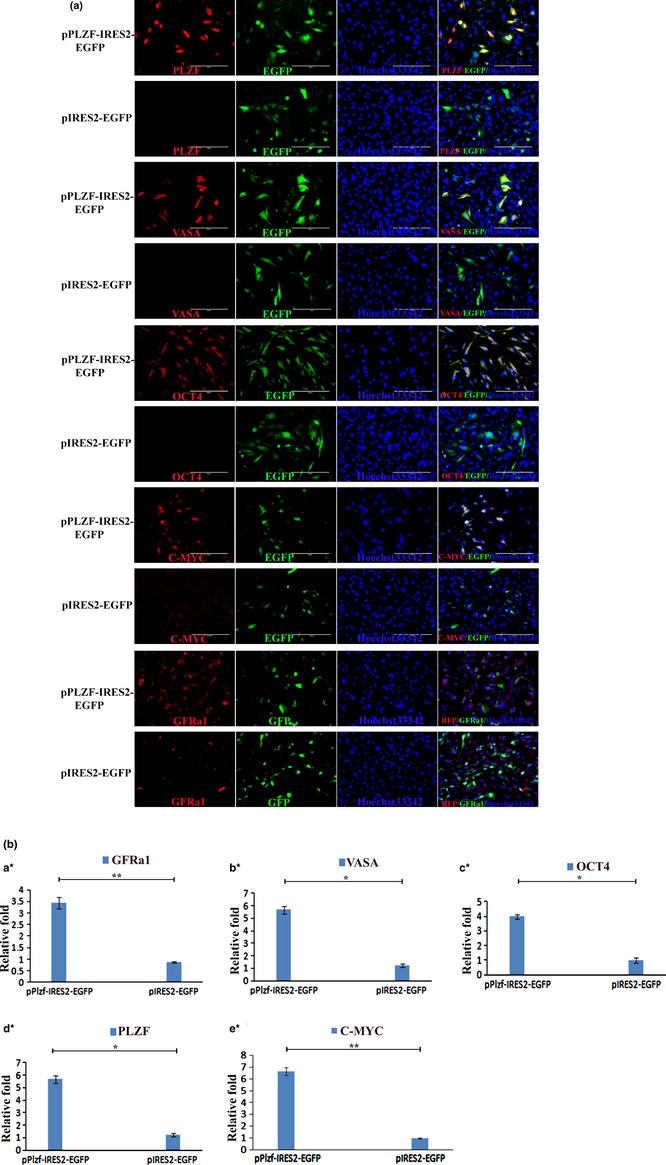
mGSC s detected by immunofluorescence staining and QRT ‐ PCR at 72 h after PLZF transfection. (a) Immunofluorescence staining analysis for PLZF, VASA, OCT4, C‐MYC, GFRa1in both pPLZF‐IRES2‐EGFP (1st and 3rd row) and pIRES2‐EGFP (2nd and 4th row) groups. Bar = 200 μm. (b) QRT‐PCR analysis for GFRa1 (a*), VASA (b*), OCT4 (c*), PLZF (d*) and C‐MYC (e*) in pPLZF‐IRES2‐EGFP groups and pIRES2‐EGFP groups. *P < 0.05; **P < 0.01.
BrdU incorporation assay showed that percentage of BrdU‐positive mGSCs in the PLZF overexpression group (48.5%) was significantly higher than in the pIRES2‐EGFP group (20.2%) and blank control group (transfected with no plasmid) (19.8%) (Fig. Figure 7). These results further confirmed that overexpression of PLZF enhanced self‐renewal of the dairy goat mGSCs.
Figure Figure 7.
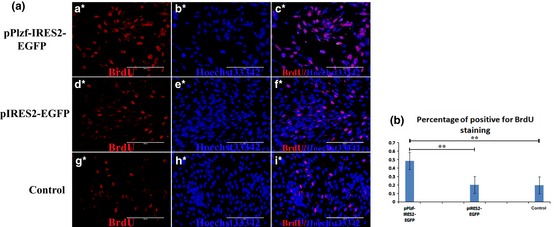
BrdU incorporation assay. (a) Proliferation of mGSCs was determined by BrdU incorporation assay in PLZF overexpression. (a*–c*) Cells treated with pPLZF‐IRES2‐EGFP; (d*–f*) Cells treated with pIRES2‐EGFP; (g*–i*) Blank group, transfected, but with no plasmid. (b) Quantification of (a). **P < 0.01.
Discussion
Maintenance of sperm count in adult germline tissues is dependent on the resident stem‐cell pool with self‐renewal potentiality 34; factors regulating balance between stem cell self‐renewal and differentiation ensure tissue homeostasis. Disruption of these regulatory mechanisms can lead to tissue degeneration 35 and maintenance of high levels of mGSCs is essential for eventual fertility of mammalian spermatogenesis. It has been reported that lack of PLZF expression has resulted in germ cell loss and testicular atrophy in aged male mice, eventually leading to infertility 5, 14. Biallelic PLZF loss of function and gonad hypoplasia in male patients has been reported 36 and studies on both rodent and human have suggested that PLZF plays an important role in self‐renewal of mGSCs.
In our study, mGSCs maintained characteristic stem cell markers under optimization culture conditions, in accordance with results of Zhu et al. 19. However, when mGSCs were treated with DMEM/F12 containing 10% serum, compact, round cell clones scattered and no longer formed aggregates. Interestingly, although expression of pluripotency‐associated markers was lost or reduced in scattered cells of pIRES2‐EGFP groups, in pPLZF‐IRES2‐EGFP groups, they remained in perfect condition. In addition, BrdU incorporation assay also confirmed that proliferation ability of dairy goat mGSCs was higher in the pPLZF‐IRES2‐EGFP group than in pIRES2‐EGFP group.
In vivo expression levels of PLZF, consistent with C‐MYC and OCT4, were higher in 6 months and 30 dpp goat testis than those of 10 M old animals; however, CYCLIN D1, CDK2 and STRA8 were not. This raises the possibility that there exists a relationship between PLZF and pluripotency‐associated genes. In combination with results of PLZF overexpression in mGSCs, our study further suggested that overexpression of PLZF activated expression of proliferation and pluripotent‐associated genes, while lower expression of PLZF lead to downregulation of those genes. Higher levels of PLZF affect differentiation of mGSCs into sperm cells, and maintain self‐renewal of mGSCs. The view that PLZF may inhibit male germ cell differentiation in the goat also could be supported by in vivo evidence. Figure 1a and 1b show younger (30 dpp and 6 M) specimens with higher levels of pluripotency‐associated genes (such as PLZF) and lower levels of differentiation genes (such as STRA8), and lower pluripotency‐associated genes and higher differentiation genes were expressed in older goat testes (10 M). Results are in accordance with the fact that the adult goat needs to activate differentiation genes, and simultaneously, stemness or pluripotency genes are suppressed, to produce enough sperm for reproduction. Also the results have shown that sperm cell numbers increases with age, although number of PLZF‐positive cells declined (Table S3); this raises a hypothesis that proliferation regulation of PLZF is only for mGSCs, but not for somatic cells. In all, these results demonstrated that PLZF plays a key role in maintaining self‐renewal of dairy goat mGSCs, and expression levels of PLZF are essential for this mammals spermatogenesis.
According to previous reports, PLZF can regulate self‐renewal of SSCs and suppress their differentiation in rodents and human beings 5, 14, 34. We found that pluripotent gene OCT4 could be activated by PLZF, which is consistent with reports concerning mice 5. Study of APL has revealed that PLZF inhibits expression of C‐MYC directly by binding to the C‐MYC promoter; this could resist progression of the cell cycle by retaining the cells in G0 37, 38. Nevertheless, in contrast to the previous study, we found that expression of C‐MYC was promoted, not inhibited by PLZF. Our results showed that C‐MYC could be upregulated by higher expression of PLZF and downregulated by its lower expression (Fig. 6aa,b). Accordingly, we assumed that the pathway of PLZF regulation of C‐MYC in mGSCs might be different from that in APL 37, 39. Certainly, more work needs to be performed to support this assumption.
In conclusion, our study first explored the expression pattern of PLZF in dairy goats, and cloned its CDS, which further proved that PLZF plays a key role in maintaining self‐renewal of dairy goat mGSCs. Overexpression of PLZF promoted proliferation and upregulated pluripotency‐associated genes of the mGSCs. Our findings will extend further support for the crucial roles of PLZF in mammalian reproduction and also this study will help us better understand self‐renewal of SSCs and therefore be helpful for application of dairy goat SSCs, in for example, breeding.
Supporting information
Fig. S1 HE staining of goat testes in three different age groups.
Table S1 The PCR primers and the length of the amplified products
Table S2 Number of positive cells for PLZF, VASA, OCT4, GFRa1 and C‐MYC for the two groups
Table S3 Number of positive cells for PLZF by immunohistochemistry in the testes of 1 M, 6 M and 10 M goat
Acknowledgements
This work was supported by grants from the Program (31272518, 30972097) of National Natural Science Foundation of China, Doctoral Fund of Ministry of Education of China (RFDP, 20120204110030), Program for New Century Excellent Talents of State Ministry of Education (NCET‐09‐0654), the Scientific Research Program of Shaanxi Province (2011K02‐06) and Fundamental Research Funds for the Central Universities (QN2011012).
References
- 1. Spradling A, Drummond‐Barbosa D, Kai T (2001) Stem cells find their niche. Nature, 414, 98–104. [DOI] [PubMed] [Google Scholar]
- 2. Filipponi D, Hobbs RM, Ottolenghi S, Rossi P, Jannini EA, Pandolfi PP, et al (2007) Repression of kit expression by Plzf in germ cells. Mol. Cell. Biol. 27, 6770–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M (2007) Gdnf upregulates c‐Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells 26, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falender AE, Freiman RN, Geles KG, Lo KC, Hwang KS, Lamb DJ, et al (2005) Maintenance of spermatogenesis requires TAF4b, a gonad‐specific subunit of TFIID. Genes Dev. 19, 794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, et al (2004) Plzf is required in adult male germ cells for stem cell self‐renewal. Nat. Genet. 36, 647–652. [DOI] [PubMed] [Google Scholar]
- 6. Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, et al (2001) Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15, 2203–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, et al (2005) ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature 436, 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH (2011) Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J. Clin. Invest. 121, 3456–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McConnell M, Licht J (2007) The PLZF gene of t (11; 17)‐associated APL. Curr. Top. Microbiol. Immunol. 313, 31–48. [DOI] [PubMed] [Google Scholar]
- 10. Chen S, Zelent A, Tong J, Yu H, Wang Z, Derre J, et al (1993) Rearrangements of the retinoic acid receptor alpha and promyelocytic leukemia zinc finger genes resulting from t (11;17)(q23;q21) in a patient with acute promyelocytic leukemia. J. Clin. Invest. 91, 2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu D, Holko M, Sadler AJ, Scott B, Higashiyama S, Berkofsky‐Fessler W, et al (2009) Promyelocytic leukemia zinc finger protein regulates interferon‐mediated innate immunity. Immunity 30, 802–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Labbaye C, Quaranta MT, Pagliuca A, Militi S, Licht JD, Testa U, et al (2002) PLZF induces megakaryocytic development, activates Tpo receptor expression and interacts with GATA1 protein. Oncogene 21, 6669–6679. [DOI] [PubMed] [Google Scholar]
- 13. Dick JE, Doulatov S (2009) The role of PLZF in human myeloid development. Ann. N. Y. Acad. Sci. 1176, 150–153. [DOI] [PubMed] [Google Scholar]
- 14. Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, et al (2004) Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 36, 653–659. [DOI] [PubMed] [Google Scholar]
- 15. Kolesnichenko M, Vogt PK (2011) Understanding PLZF. Cell Cycle 10, 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pawar S, Malakar D, De A, Akshey Y (2009) Stem cell‐like outgrowths from in vitro fertilized goat blastocysts. Indian J. Exp. Biol. 47, 635–642. [PubMed] [Google Scholar]
- 17. Bahadorani M, Hosseini S, Abedi P, Hajian M, Hosseini S, Vahdati A, et al (2012) Short‐term in‐vitro culture of goat enriched spermatogonial stem cells using different serum concentrations. J. Assist. Reprod. Genet. 29, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li M, Yu M, Liu C, Zhu H, He X, Peng S, et al (2013) miR‐34c works downstream of p53 leading to dairy goat male germline stem‐cell (mGSCs) apoptosis. Cell Prolif. 46, 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu H, Liu C, Li M, Sun J, Song W, Hua J (2012) Optimization of the conditions of isolation and culture of dairy goat male germline stem cells (mGSC). Anim. Reprod. Sci. 137, 45–52. [DOI] [PubMed] [Google Scholar]
- 20. Hua J, Zhu H, Pan S, Liu C, Sun J, Ma X, et al (2011) Pluripotent male germline stem cells from goat fetal testis and their survival in mouse testis. Cell. Reprogram. 13, 133–144. [DOI] [PubMed] [Google Scholar]
- 21. Cao H, Chu Y, Zhu H, Sun J, Pu Y, Gao Z, et al (2011) Characterization of immortalized mesenchymal stem cells derived from foetal porcine pancreas. Cell Prolif. 44, 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu Y, Sun J, Wang J, Wang L, Bai Y, Yu M, et al (2012) Characterization of female germ‐like cells derived from mouse embryonic stem cells through expression of GFP under the control of Figla promoter. J. Cell. Biochem. 113, 1111–1121. [DOI] [PubMed] [Google Scholar]
- 23. Li M, Liu C, Zhu H, Sun J, Yu M, Niu Z, et al (2013) Expression pattern of Boule in dairy goat testis and its function in promoting the meiosis in male germline stem cells (mGSCs). J. Cell. Biochem. 114, 294–302. [DOI] [PubMed] [Google Scholar]
- 24. Zhu H, Liu C, Sun J, Li M, Hua J (2012) Effect of GSK‐3 inhibitor on the proliferation of multipotent male germ line stem cells (mGSCs) derived from goat testis. Theriogenology. 77, 1939–1950. [DOI] [PubMed] [Google Scholar]
- 25. Cao H, Chu Y, Lv X, Qiu P, Liu C, Zhang H, et al (2012) GSK3 inhibitor‐BIO regulates proliferation of immortalized pancreatic mesenchymal stem cells (iPMSCs). PLoS One 7, e31502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ketkar AA, Reddy KVR (2012) Expression pattern of OCT‐4 and PLZF transcription factors during the early events of spermatogenesis in mice. J. Cell Sci. Ther. 03, doi: 10.4172/2157-7013.1000120. [DOI] [Google Scholar]
- 27. Dong Y, Xie M, Jiang Y, Xiao N, Du X, Zhang W, et al (2012) Sequencing and automated whole‐genome optical mapping of the genome of a domestic goat (Capra hircus). Nat. Biotechnol. 31, 135–141. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka SS, Toyooka Y, Akasu R, Katoh‐Fukui Y, Nakahara Y, Suzuki R, et al (2000) The mouse homolog of Drosophila vasa is required for the development of male germ cells. Genes Dev. 14, 841–853. [PMC free article] [PubMed] [Google Scholar]
- 29. Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe‐Nebenius D, Chambers I, et al (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95, 379–391. [DOI] [PubMed] [Google Scholar]
- 30. Sada A, Suzuki A, Suzuki H, Saga Y (2009) The RNA‐binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science 325, 1394–1398. [DOI] [PubMed] [Google Scholar]
- 31. Hatano SY, Tada M, Kimura H, Yamaguchi S, Kono T, Nakano T, et al (2005) Pluripotential competence of cells associated with Nanog activity. Mech. Dev. 122, 67–79. [DOI] [PubMed] [Google Scholar]
- 32. Aeckerle N, Eildermann K, Drummer C, Ehmcke J, Schweyer S, Lerchl A, et al (2012) The pluripotency factor LIN28 in monkey and human testes: a marker for spermatogonial stem cells? Mol. Hum. Reprod. 18, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riboldi M, Rubio C, Pellicer A, Gil‐Salom M, Simón C (2012) In vitro production of haploid cells after coculture of CD49f+ with Sertoli cells from testicular sperm extraction in nonobstructive azoospermic patients. Fertil. Steril. 98, 580–590. [DOI] [PubMed] [Google Scholar]
- 34. Hobbs RM, Seandel M, Falciatori I, Rafii S, Pandolfi PP (2010) Plzf regulates germline progenitor self‐renewal by opposing mTORC1. Cell 142, 468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biteau B, Hochmuth CE, Jasper H (2008) JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3, 442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fischer S, Kohlhase J, Böhm D, Schweiger B, Hoffmann D, Heitmann M, et al (2008) Biallelic loss of function of the promyelocytic leukaemia zinc finger (PLZF) gene causes severe skeletal defects and genital hypoplasia. J. Med. Genet. 45, 731–737. [DOI] [PubMed] [Google Scholar]
- 37. McConnell MJ, Chevallier N, Berkofsky‐Fessler W, Giltnane JM, Malani RB, Staudt LM, et al (2003) Growth suppression by acute promyelocytic leukemia‐associated protein PLZF is mediated by repression of c‐myc expression. Mol. Cell. Biol. 23, 9375–9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doulatov S, Notta F, Rice KL, Howell L, Zelent A, Licht JD, et al (2009) PLZF is a regulator of homeostatic and cytokine‐induced myeloid development. Genes Dev. 23, 2076–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rice KL, Hormaeche I, Doulatov S, Flatow JM, Grimwade D, Mills KI, et al (2009) Comprehensive genomic screens identify a role for PLZF‐RARα as a positive regulator of cell proliferation via direct regulation of c‐MYC. Blood 114, 5499–5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 HE staining of goat testes in three different age groups.
Table S1 The PCR primers and the length of the amplified products
Table S2 Number of positive cells for PLZF, VASA, OCT4, GFRa1 and C‐MYC for the two groups
Table S3 Number of positive cells for PLZF by immunohistochemistry in the testes of 1 M, 6 M and 10 M goat


