Abstract
Abstract. Objective: Some normal somatic cells in culture divide a limited number of times before entering a non‐dividing state called replicative senescence and fusion of normal cells with immortal cells claimed to produce hybrid cells of limited proliferation. We reinvestigated the proliferative capacity of hybrid cells between normal cell and immortal cell. Materials and Methods: Normal pig fibroblast cells and cells of immortal mouse fibroblast cell line F7, a derivative of GM05267, were fused by polyethylene glycol treatment and subsequently the fused cells were cultured in a selective medium containing hypoxanthine‐aminopterin‐thymidine in order to enrich the hybrid cells. The hybrid cells were then monitored for chromosome content and proliferation. Results: Cytogenetic analysis revealed that the hybrid cells contained polyploidy chromosomes derived from normal pig fibroblasts. These hybrid cells exhibit no sign of replicative senescence after more than 190 population doublings in vitro. Instead, these hybrid cells have an accelerated growth and proliferate even in the complete absence of glutamine. In addition, these hybrids produce biologically active factors in the conditioned media, which not only can accelerate their own proliferation but also can reinitiate mitotic activity in the senescent–like normal fibroblast cells. Conclusions: Our results question the validity of cellular senescence as a dominant trait. Additionally, the generation of hybrid cells using the specific mouse cell line can be applied to the generation of hybrids with other normal cell types and can be used to produce tissue‐specific growth‐factor(s) to extend the lifespan and/or improve the proliferation of various normal cells, including adult stem cells.
INTRODUCTION
Some normal mammalian somatic cells divide for a limited number of times before entering a non‐dividing state called replicative senescence (Hayflick 1965). Senescent cells have flattened morphology and are unable to synthesize DNA in response to mitogenic stimuli (Goldstein 1990). Several types of senescence have been described in the literature (Ben‐Porath & Weinberg 2005). These include replicative senescence (assumed to be caused by shortening of telomeres with successive cell divisions), premature or induced senescence (associated with oncogenic activation or introduction of activated oncogenes into normal cells) and stress‐induced senescence, resulting from suboptimal culture conditions.
Fusion of normal cells with immortal cells generally produces hybrid cells of limited proliferation status (Pereira‐Smith & Smith 1983). However, the fusion of immortal stem cells with normal cells generates immortal hybrid cells (Tada et al. 1997; Takagi 1997; Pells et al. 2002; Flasza et al. 2003; Ambrosi & Rasmussen 2005). It is suggested that senescence‐escape in hybrid cells is the result of rapid loss of large numbers of normal cell chromosomes (Ran & Pereira‐Smith 2000).
We have previously reported the generation of pig–mouse hybrids containing segregated pig chromosomes against the background of polyploid chromosomes of the mouse cell line, GM05267. By functional studies of the hybrids, we have mapped a suppressor gene on pig chromosome 14 for cellular anchorage independence (Gao & Islam 2001). Unexpectedly, fusion of the F7 cell line, a derivative of GM05267, with normal pig fibroblasts produced hybrids with an excessive number of pig chromosomes compared to mouse chromosomes. Following this serendipitous observation, we have extensively examined the outcome of fusions involving cells of the mouse F7 cell line with normal pig fibroblasts of a variety of strains as well as spontaneously immortalized pig fibroblast lines and monitored cell proliferation and chromosome content of the resulting hybrids. In this communication, we present the results of these investigations.
MATERIALS AND METHODS
Cell strains/lines and culture conditions
The original GM05267 cell line was of SV40‐transformed immortal mouse fibroblasts deficient of the enzyme hypoxanthine‐phosphoribosyl‐transferase (HPRT) (Migeon et al. 1981; Jabs et al. 1984), obtained from the National Institutes of General Medical Sciences (NIGMS), Camden, NJ. Normal primary pig fibroblast strains AG08114 and AG12077 (both at passage 3) were obtained from NIA, Ageing Cell Culture Repository, Coriell Institute for Medical Research, Camden, NJ. All cell lines/strains were maintained in Dulbecco's modified eagle's medium (DMEM, high glucose, Invitrogen Corporation, Paisley, UK), supplemented with 15% foetal bovine serum (FBS, Euroclone Life Sciences, Pero, Italy), 1% DMEM non‐essential amino acids (Invitrogen), and 1% penicillin‐streptomycin (Invitrogen).
Introduction of a neomycin‐resistant gene
In order to selectively isolate the hybrid cells only, and to eliminate both parental cell types, we introduced a G418‐resistant gene to the HPRT‐deficient mouse cell line GM05267 using a modified calcium‐phosphate co‐precipitation method (Islam & Islam 2000). Briefly, exponentially growing cells were trypsinized, seeded into 60‐mm Petri dishes, and incubated at 37 °C under 5% CO2. The following day, 20 µg of plasmid DNA (pSV2neo) was mixed with 0.5 ml of 0.25 m CaCl2, 0.5 ml of double‐strength N,N‐bis(2‐hydroxyethyl)‐2‐aminoethanesulfonic acid‐buffered saline (Fluka‐Riedel‐de Haen, Basel, Switzerland), and the mixture was kept for 20 min at room temperature. Calcium phosphate‐DNA solution (1 ml) was added drop‐wise onto the plated cells, was mixed gently, kept for 30 min at room temperature and incubated overnight. The medium was removed, and selective medium containing DMEM plus 1% G418 (Invitrogen) was added. The cells were fed with fresh selective medium twice a week. After 3 weeks, a colony of G418‐resistant cells was transferred to a culture flask for further increasing cell population and the resulting cell line was designated as F7.
Introduction of the hTERT gene into the immortal AG12077‐IM cell line
The hTERT gene transfer was carried out as described previously (Davis et al. 2003). pBABE‐hTERT retroviral vector was used to generate retroviral supernatant, which was filtered through a 0.45‐µm‐pore‐size filter. Cells of the immortal porcine cell line AG12077‐IM (which had been spontaneously developed at our laboratory from primary AG012077 cells after passage 38), were plated 1 day earlier in a T75 cell culture flask in DMEM containing tunicamycin (0.03 µg/ml, Sigma). Medium of the near‐confluent culture was removed and freshly collected; filtered retroviral supernatant containing polybrane (4 µg/ml, Sigma, Aldrich, Sweden) was added and allowed to settle for 5 h. The following day, drug selection was applied to eliminate non‐transduced cells by adding complete DMEM containing puromycin (2 µg/ml, Sigma). After continuous selection for 2 weeks, a mass population of puromycin‐resistant cells was established as a single cell line, and this was used for cell fusion.
Induction of cell fusion
The immortal F7 mouse cells (hypoxanthine‐aminopterin‐thymidine, HAT sensitive and G418 resistant) and the porcine fibroblasts (HAT resistant and G418 sensitive), at passages 7 and 16, were detached from the monolayer by trypsin‐ethylenediaminetetraacetic acid treatment. Approximately, equal numbers of cells (1 × 106) from each parent culture were mixed in a centrifuge tube and plated on 60‐mm Petri dishes, in sufficient numbers to achieve confluence after 3–4 h. The medium was aspirated and fresh serum‐free media containing phytohaemagglutinin‐P (100 µg/ml, Sigma) was added (in order to increase cell–cell contact), and cells were incubated at 37 °C with 5% CO2 for 30 min. After this, the medium was removed and polyethylene glycol (PEG‐Sigma, MW 1500, prepared in serum‐free DMEM, 45% w/v) was added at room temperature and mixed thoroughly with the cell layer. After 1 min, the fluid was aspirated and the cell layer was washed four times with serum‐free medium. Serum‐containing fresh medium was added to the dish after the final wash and cells were incubated at 37 °C with 5% CO2 for 30 min. The medium was aspirated again and fresh serum‐containing medium was added, and incubated at 37 °C with 5% CO2. After 24 h, the fused cell layer was dissociated by trypsin treatment and cells were mixed in a centrifuge tube containing complete growth medium supplemented with 2% HAT (Invitrogen) and 1% G418 (Invitrogen) and were plated onto larger dishes. The HAT medium killed mouse F7 cells and G418 killed the porcine fibroblasts. After 7 days, hybrid cell colonies were visible to the naked eye and these were individually trypsinized in cloning rings and expanded to establish cell lines. In a selection of experiments, mass populations of hybrid cells derived from each fusion were transferred into a single culture flask for further cell population increase.
Chromosome analysis
Chromosome preparation and the chromosome‐banding procedure have been described previously (Islam & Levan 1987). Twenty metaphase cells were captured from each cell strain/line by a CCD camera using the cytovision software program (Applied Imaging, Santa Clara, CA, USA). Chromosomes of the parental cell lines/strains were identified and were counted from printed images of metaphase cells. In the hybrids, chromosomes belonging to the same species were counted together and then the total number of chromosomes determined by adding the chromosomes of two species. In some cases, chromosomes were analysed by using manual karyotyping and the cytovision program.
Acquisition and compilation of images
Cell morphology was recorded by using an inverted microscope (10× objective lens) (Axiovert, Zeiss) equipped with a Canon digital camera (Power Shot A95) setting the zoom at 4.9×. The images obtained were then transferred to a personal computer. Images of the metaphases and karyotypes were transferred from cytovision as bmp files to the same computer. Some images were processed with the Photoshop element program before finally composing them into figures using the Adobe Illustrator 10 program.
RESULTS
Karyotypic analyses of parental cell strains/lines
Parental cell lines were karyotyped by G‐banding. The karyotype of the mouse F7 cell line contained approximately 36 autosomes and one X chromosome without any Ys. Most of the karyotypes contained several single‐copy chromosomes including 6, 7, 8, 9, 12, 17, 18, four copies of chromosome 15 and one small marker (Fig. 1b, Table 1). Similar to the F7 cell line, the majority of the karyotypes of the original GM05267 cell line contained hypo‐diploid chromosomes, but with an additional copy of chromosome 6, instead of the single copy in F7 cells (Fig. 1b, Table 1). Porcine fibroblast strains (AG08114 and AG12077) displayed normal female karyotype with 36 autosomes and two X chromosomes (Fig. 1d, Table 1).
Figure 1.
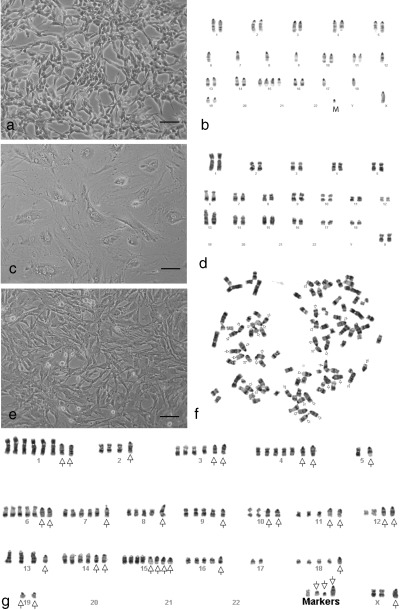
Cell morphology and chromosome composition of parental cell strain/lines and their derived hybrid cells. (a) Morphology of highly proliferative F7 mouse cells. (b) A representative karyotype of F7 cells containing hypo‐diploid chromosomes. (c) Morphology of the senescence‐like AG12077 pig fibroblasts at passage 20. (d) A representative karyotype of the AG12077 cell strain containing diploid chromosomes. (e) Morphology of 2 : 1 pig–mouse hybrid cells with high mitotic activity at confluency. (f) A representative metaphase cell of the 2 : 1 PMH cells containing polyploid pig chromosomes (arrow indicates mouse chromosomes). (g) Karyotype of the same metaphase cell shown in F (arrow indicates mouse chromosomes). In this particular karyotype, no normal copy of mouse chromosome 17 could be found. Bar = 100 µm.
Table 1.
Summary of results of a variety of combinatorial cell fusion experiments involving mouse and pig fibroblasts
| Mouse parental cell lines | Pig fibroblast strains/lines | Selective system used for hybrid isolation | Genomic composition of most metaphase cells | Growth activation in selected cells |
|---|---|---|---|---|
| F7 a | AG08114 c | HAT + G418 | 2 : 1 pig–mouse hybrid | Yes |
| F7 a | AG12077 c | HAT + G418 | 2 : 1 pig–mouse hybrid | Yes |
| F7 a | AG08114 c | HAT only | 2 : 1 pig–mouse hybrid | Yes |
| F7 a | AG12077 c | HAT only | 2 : 1 pig–mouse hybrid | Yes |
| GM05267 b | AG08114 c | HAT only | 2 : 1 pig–mouse hybrid | Yes |
| GM05267 b | AG12077 c | HAT only | 2 : 1 pig–mouse hybrid | Yes |
| F7 a | AG12077‐IM d | HAT only | Pig fibroblast | No |
| F7 a | AG12077‐IM d | HAT + G418 | 2 : 1 and 1 : 1 hybrids | No |
| F7 a | AG12077‐IM‐Aged d | HAT only | Pig fibroblast | No |
| F7 a | AG12077‐IM‐Aged d | HAT + G418 | 2 : 1 and 1 : 1 hybrids | No |
| F7 a | AG12077‐IM + hTERT d | HAT + G418 | 2 : 1 and 1 : 1 hybrids | No |
cells of this mouse cell line had monosomy of chromosomes 6, 7, 8, 9, 12, 17 and 18.
cells of this mouse cell line had monosomy of chromosomes 7, 8, 9, 12, 17 and 18.
most of the karyotypes of the porcine cell strain had normal female pig karyotype (38, XX).
average karyotype of cells of this porcine cell line was 35–36, XX, −4, −5, −9, −10, −11, −12, −12, +3–4mar.
Generation of cell hybrids and cytogenetic analysis
A total of 36 independent hybrid cell lines (PMN series) were established from seven fusion experiments. Karyotype analysis of randomly selected 17 cell lines consistently showed excessive numbers of porcine chromosomes, ranging from near triploid to near pentaploid numbers (hereafter called ‘polyploid’), along with hypo‐diploid numbers of mouse chromosomes (Table 2). High chromosome numbers in the hybrids often caused overlapping of some chromosomes in metaphase. Thus, we karyotyped a limited number of metaphase plates with well‐spread chromosomes from each cell line. Identification of individual chromosomes showed that all mouse chromosomes were present in karyotypes with variable frequency among cells, with the exception of the hybrid cell line PMN‐14‐A5, where no normal copy of mouse chromosome 12 was found (Table 3). Because normal pig fibroblasts contained mostly diploid chromosomes, it is likely that many pig–mouse hybrid cells derived from the fusion of three cells; two diploid porcine fibroblasts and one hypo‐diploid mouse F7 cell (hereafter 2 : 1 hybrids). Alternatively, the fusion of one tetraploid or one anaphase pig cell with one mouse F7 cell could also produce similar hybrid cells. None of the hybrid cell lines showed structurally abnormal porcine chromosomes, as judged by G‐banding studies (Fig. 1g).
Table 2.
Chromosome composition of the pig–mouse hybrid cells containing polyploid pig and hypo‐diploid mouse chromosomes
| Cell line | Average number of mouse chromosomes | Range | Average number of pig chromosomes | Range | Average number of total chromosomes | Range | Number of cells analysed |
|---|---|---|---|---|---|---|---|
| Hybrids generated by fusion of F7 with AG08114 | |||||||
| PMN‐14‐A2 | 27 | 19–35 | 67 | 58–75 | 94 | 84–117 | 20 |
| PMN‐14‐A4 | 29 | 22–34 | 71 | 67–80 | 101 | 89–110 | 20 |
| PMN‐14‐A5 | 24 | 18–30 | 72 | 65–79 | 96 | 86–104 | 20 |
| PMN‐14‐C1 | 28 | 21–39 | 71 | 57–78 | 98 | 84–108 | 20 |
| PMN‐14‐C2 | 24 | 18–33 | 71 | 53–76 | 94 | 88–105 | 20 |
| PMN‐14‐C3 | 31 | 28–36 | 72 | 61–77 | 102 | 98–112 | 20 |
| PMN‐14‐C4 | 29 | 19–36 | 72 | 65–77 | 101 | 87–115 | 20 |
| PMN‐14‐D2 | 30 | 16–35 | 69 | 61–79 | 99 | 86–109 | 20 |
| PMN‐14‐D5 | 24 | 16–34 | 103 | 90–120 | 126 | 90–148 | 20 |
| Hybrids generated by fusion of F7 with AG12077 | |||||||
| PMN‐77‐A1 | 29 | 16–34 | 64 | 56–74 | 93 | 80–102 | 20 |
| PMN‐77‐A2 | 28 | 23–35 | 71 | 60–77 | 100 | 91–109 | 20 |
| PMN‐77‐B1 | 28 | 19–33 | 70 | 65–79 | 99 | 87–109 | 20 |
| PMN‐77‐B2 | 26 | 15–33 | 75 | 67–77 | 101 | 94–109 | 20 |
| PMN‐77‐B4 | 26 | 14–34 | 69 | 58–77 | 96 | 88–109 | 20 |
| PMN‐77‐C3 | 31 | 26–35 | 66 | 58–74 | 97 | 93–104 | 20 |
| PMN‐77‐C4 | 30 | 24–36 | 72 | 54–87 | 101 | 81–114 | 20 |
| PMN‐77‐D3 | 26 | 9–33 | 63 | 48–72 | 89 | 82–100 | 20 |
| Mouse parental cell line | |||||||
| F7 | 35 | 33–36 | 45 | ||||
Table 3.
Average number of individual mouse chromosomes per cell of the 2 : 1 hybrids generated by fusion of F7 mouse cells with normal primary pig fibroblasts
| Frequency of mouse chromosomes | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell line | No. cells | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | X | Marker |
| PMN‐14‐A2 | 5 | 1.8 | 1.8 | 1.2 | 1.6 | 1.2 | 1.4 | 0.8 | 1.0 | 0.6 | 1.0 | 1.8 | 0.4 | 1.2 | 2.0 | 3.0 | 1.6 | 0.8 | 0.8 | 1.4 | 0.6 | 0.6 |
| PMN‐14‐A4 | 5 | 1.8 | 1.6 | 1.0 | 1.8 | 1.8 | 1.8 | 1.6 | 1.0 | 0.6 | 1.6 | 2.2 | 1.4 | 1.4 | 1.2 | 3.8 | 1.6 | 1.0 | 1.0 | 1.8 | 1.0 | 1.2 |
| PMN‐14‐A5 | 5 | 1.6 | 1.4 | 1.0 | 1.8 | 0.6 | 1.4 | 0.6 | 0.4 | 0.8 | 0.8 | 1.4 | 0.0 | 1.6 | 1.4 | 1.8 | 1.0 | 1.2 | 1.4 | 1.8 | 0.6 | 1.8 |
| PMN‐14‐C1 | 5 | 1.4 | 1.8 | 1.2 | 1.0 | 1.8 | 1.4 | 1.2 | 1.0 | 0.6 | 1.2 | 1.6 | 0.8 | 0.6 | 1.4 | 2.8 | 1.6 | 0.8 | 1.4 | 2.0 | 0.6 | 1.2 |
| PMN‐14‐C2 | 8 | 1.2 | 1.7 | 1.1 | 0.9 | 1.4 | 0.7 | 0.7 | 1.1 | 0.6 | 1.2 | 1.4 | 1.1 | 1.4 | 0.4 | 3.0 | 1.6 | 1.0 | 1.4 | 1.6 | 0.6 | 1.1 |
| PMN‐14‐C3 | 5 | 1.8 | 1.8 | 1.2 | 1.6 | 1.4 | 1.2 | 1.0 | 1.0 | 1.0 | 1.8 | 2.0 | 1.6 | 0.6 | 1.6 | 4.2 | 2.0 | 1.0 | 1.4 | 1.8 | 1.0 | 0.8 |
| PMN‐14‐C4 | 5 | 1.6 | 1.6 | 1.6 | 2.0 | 1.2 | 1.6 | 1.0 | 1.0 | 1.0 | 1.2 | 1.6 | 1.2 | 1.4 | 1.8 | 3.4 | 1.0 | 1.0 | 1.0 | 2.0 | 1.0 | 0.8 |
| PMN‐14‐D2 | 5 | 0.6 | 2.0 | 1.4 | 1.6 | 1.4 | 1.6 | 0.8 | 0.8 | 1.0 | 1.6 | 1.8 | 1.2 | 2.0 | 2.0 | 3.2 | 1.8 | 0.8 | 1.0 | 2.2 | 1.0 | 1.0 |
| PMN‐14‐D5 | 5 | 1.6 | 1.4 | 0.8 | 1.4 | 1.0 | 1.2 | 0.8 | 0.8 | 0.8 | 0.6 | 1.8 | 1.2 | 1.4 | 1.0 | 2.6 | 0.4 | 0.6 | 1.0 | 2.0 | 1.0 | 0.4 |
| PMN‐77‐A1 | 5 | 1.2 | 1.6 | 1.2 | 1.4 | 1.2 | 2.2 | 0.8 | 1.0 | 1.0 | 1.2 | 1.4 | 1.0 | 2.0 | 1.8 | 3.0 | 2.0 | 1.0 | 1.0 | 1.8 | 1.0 | 0.8 |
| PMN‐77‐A2 | 5 | 1.4 | 2.2 | 1.2 | 2.0 | 1.4 | 1.8 | 0.8 | 1.0 | 1.2 | 1.4 | 1.8 | 1.0 | 1.6 | 1.4 | 4.0 | 1.0 | 1.0 | 1.0 | 2.0 | 1.0 | 1.4 |
| PMN‐77‐B1 | 5 | 1.4 | 1.4 | 1.0 | 1.2 | 1.4 | 1.0 | 1.0 | 1.0 | 0.8 | 1.8 | 1.8 | 1.0 | 1.8 | 1.4 | 3.6 | 1.6 | 1.0 | 0.8 | 1.8 | 0.8 | 0.4 |
| PMN‐77‐B2 | 5 | 1.0 | 1.8 | 0.8 | 1.6 | 1.6 | 1.0 | 0.8 | 1.0 | 0.6 | 1.2 | 1.8 | 1.2 | 1.2 | 1.8 | 4.4 | 1.6 | 1.0 | 1.0 | 1.8 | 1.0 | 0.2 |
| PMN‐77‐B4 | 5 | 1.0 | 1.6 | 1.0 | 1.4 | 1.2 | 1.2 | 0.6 | 0.8 | 1.2 | 1.8 | 0.8 | 0.6 | 1.0 | 1.6 | 1.8 | 1.6 | 1.0 | 1.0 | 1.8 | 1.0 | 0.2 |
| PMN‐77‐C3 | 5 | 1.8 | 1.6 | 1.4 | 1.2 | 1.8 | 1.6 | 1.0 | 1.0 | 1.2 | 1.8 | 2.0 | 1.2 | 1.4 | 1.6 | 3.6 | 1.8 | 0.8 | 1.2 | 2.0 | 0.8 | 1.2 |
| PMN‐77‐C4 | 5 | 0.4 | 2.0 | 1.4 | 1.8 | 1.2 | 1.6 | 0.6 | 1.0 | 1.0 | 1.6 | 1.6 | 1.0 | 1.4 | 1.4 | 4.0 | 1.6 | 1.0 | 1.0 | 2.0 | 1.2 | 0.4 |
| PMN‐77‐D3 | 5 | 1.2 | 1.6 | 1.2 | 1.0 | 1.8 | 1.0 | 1.0 | 0.6 | 0.6 | 1.6 | 1.8 | 0.8 | 1.8 | 1.8 | 3.2 | 1.6 | 0.8 | 1.0 | 2.0 | 1.0 | 0.4 |
| F7 (parent) | 26 | 1.9 | 2.0 | 2.0 | 2.0 | 2.0 | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 | 2.0 | 1.0 | 2.0 | 1.8 | 4.0 | 2.0 | 1.0 | 1.0 | 2.0 | 1.0 | 1.1 |
Cytogenetic analyses of pig–mouse hybrids with segregated pig chromosomes
Previously, we have generated a mapping panel comprised of pig–mouse hybrid cell lines (PMH‐series), with segregated pig chromosomes by fusion of the mouse cell line GM05267‐HygR, a tetraploid derivative of the hypo‐diploid GM05267 cell line, with primary porcine fibroblasts, in order to map genes on individual pig chromosomes (Gao & Islam 2001). We made a systematic analysis of mouse and pig chromosome contents of 18 PMH hybrid cell lines by G‐banding, and compared the results with the chromosome contents of PMN hybrids. These analyses showed that the PMH hybrids contained near triploid to near pentaploid mouse chromosomes along with extensively segregated pig chromosomes (Table 4). It appears that the mean number of total chromosomes in the two series of hybrids differed by roughly 10 chromosomes (Table 2 and Table 4). However, the composition of pig versus mouse chromosomes in the two series of hybrids was opposite. The loss of porcine chromosomes in PMH hybrids seemed to be random (Table 4), but the loss of mouse chromosomes in the PMN hybrids was not (Table 3). Retention of at least one copy of each mouse chromosome in PMN hybrids may indicate a beneficial role of mouse chromosomes for the continued proliferation of hybrid cells with polyploid pig chromosomes.
Table 4.
Chromosome composition of PMH series of pig–mouse hybrid cells containing polyploid mouse, and extensively segregated pig chromosomes, generated by fusion of the polyploid mouse cell line GM05267‐HygR with primary pig fibroblasts
| Cell line | Average number of mouse chromosomes | Range | Average number of pig chromosomes | Range | Average number of total chromosomes | Range | Number of cells analysed |
|---|---|---|---|---|---|---|---|
| Hybrids generated by fusion of GM05267‐HygR with AG08114 | |||||||
| PMH‐14‐A15 | 67 | 56–74 | 20 | 14–24 | 87 | 82–91 | 20 |
| PMH‐14‐A22 | 71 | 65–79 | 25 | 19–31 | 96 | 88–110 | 25 |
| PMH‐14‐A33 | 71 | 54–135 | 12 | 8–25 | 83 | 64–160 | 20 |
| PMH‐14‐A34 | 63 | 53–73 | 12 | 9–18 | 76 | 62–84 | 22 |
| PMH‐14‐B41 | 64 | 59–71 | 8 | 6–10 | 72 | 65–80 | 20 |
| PMH‐14‐B45 | 56 | 43–60 | 7 | 2–10 | 63 | 45–71 | 20 |
| PMH‐14‐C13 | 74 | 63–82 | 16 | 13–18 | 91 | 76–99 | 10 |
| PMH‐14‐C14 | 65 | 62–71 | 12 | 10–15 | 77 | 74–84 | 10 |
| PMH‐14‐C15 | 65 | 63–72 | 11 | 10–13 | 77 | 73–84 | 11 |
| PMH‐14‐C51 | 65 | 55–74 | 18 | 15–22 | 84 | 72–96 | 14 |
| PMH‐14‐C52 | 80 | 62–131 | 19 | 12–31 | 99 | 79–162 | 20 |
| Hybrids generated by fusion of GM05267‐HygR with AG12077 | |||||||
| PMH‐77‐B12 | 62 | 58–65 | 14 | 11–17 | 76 | 70–81 | 11 |
| PMH‐77‐D11 | 59 | 50–65 | 23 | 20–26 | 83 | 76–89 | 11 |
| PMH‐77‐D13 | 58 | 51–69 | 20 | 16–25 | 78 | 67–84 | 10 |
| PMH‐77‐D24 | 110 | 77–123 | 14 | 10–18 | 124 | 87–139 | 10 |
| Hybrids generated by fusion of GM05267‐HygR with AG08113 | |||||||
| PMH‐13–1D‐21 | 97 | 92–102 | 19 | 17–22 | 116 | 110–122 | 11 |
| PHH‐13–1D‐22 | 100 | 89–118 | 22 | 18–25 | 120 | 108–128 | 10 |
| PMH‐13–2B‐42 | 65 | 54–74 | 15 | 12–19 | 81 | 66–87 | 20 |
| Mouse parental cell line | |||||||
| GM05267‐HygR | 68 | 55–81 | 42 | ||||
Competitive cell proliferation assay
The consistent presence of polyploid pig and hypo‐diploid mouse chromosomes in all clonally derived PMN hybrid cells may indicate a selective growth advantage for this chromosome combination. We reasoned that if this hypothesis was correct, then culture of mass populations of hybrid cells, instead of isolated clones, and subsequent chromosome analyses, would show a 2 : 1 pig–mouse chromosomal content in the majority of metaphase cells. To test this, we developed and performed an assay that we have termed the competitive proliferation assay (CPA). Essentially, CPA is performed on mass populations of cells after fusion of two parental cell types. Selection is applied against mouse (HAT sensitive) immortal cells but not against the porcine (G418 sensitive) fibroblasts (half‐selection) that are allowed to grow along with the hybrid cells. In theory, one would expect a mixture of hybrid cells and fibroblasts in such mass cultures. If the hybrids and the fibroblasts have different growth rates, then the proportion of slow‐growing cells gradually decreases and these eventually disappear from later passages. After fusing mouse F7 cells with pig fibroblasts, we applied HAT selection to eliminate the F7 cells, but porcine fibroblasts were allowed to grow as no G418 was added to the medium. After growing the mass cell populations in the presence of HAT for 2 passages, cytogenetic analyses were performed. The results of these analyses demonstrated that most of the mass cultured cells (95% of the metaphases) contained 2 : 1 PMN cells. Internal controls showed comparable frequencies of hybrid cells (94% of the metaphases) with similar chromosomal combinations (Table 5). These results clearly demonstrate that hybrid cells containing 2 : 1 pig–mouse chromosomes have a selective growth advantage compared with that of the porcine parental fibroblasts or hybrid cells containing other chromosomal combinations. To determine whether the F7 cell line was a clonal variant of the original GM05267 cell line, we fused non‐cloned GM05267 mouse cells with pig fibroblasts. The mixed fused cells were then grown in HAT, but in the absence of G418, and chromosome analyses were performed at passage 2. The results of CPA showed that most of the metaphases were 2 : 1 hybrid cells (Table 1). These results indicate that the original GM05267 cell line also contains cells capable of producing 2 : 1 hybrid cells.
Table 5.
Selective growth advantage of 2 : 1 pig–mouse hybrids over hybrid cells containing other genomic combinations, as well as the pig parental fibroblasts, measured by frequency of metaphases present in the chromosome preparations of the mass cultured cells
| Genomic constitution of the hybrids/parents | F7xAG12077 (%) a , b | F7xAG12077 (%) a , c |
|---|---|---|
| 2 N + 2 N (pig + mouse) | 4 | 5 |
| 4 N + 2 N (pig + mouse) | 94 | 93 |
| 6 N + 2 N (pig + mouse) | 2 | 0 |
| 2 N pig fibroblast | 0 | 2 |
Derived from a common cell fusion experiment but the fused cells were divided into two parts 24 h post‐fusion and were allowed to grow in two different selective media in order to provide an internal control of the fusion experiment.
Hybrid cells were selected in HAT + G418 in order to kill both mouse and pig parental cells but allow the growth of hybrid cells (served as a control). All hybrid cell colonies of the fusion were pooled into a single mass culture, and chromosome preparations were performed at passage 2.
Fused cells were selected in HAT medium in order to kill only the mouse parental cells but not the pig parental fibroblasts and the hybrid cells. All HAT‐resistant cell colonies were pooled and chromosome preparations were made from the presumptive mixture of cells containing hybrids as well as the pig parental fibroblast cells at passage 2.
Morphology and growth properties of 2 : 1 hybrid cells
Mouse F7 cells had fibroblastic morphology with rapid cell proliferation (Fig. 1a), whereas the porcine fibroblasts had flattened morphology with mostly non‐dividing cells (Fig. 1c). The hybrid cells were larger in size and acquired a fibroblastic phenotype with high rates of cell proliferation (Fig. 1e). Similar to immortal cells, the hybrid cells could grow in normal medium at low cell density; they even survived and proliferated in glutamine‐free medium (Fig. 2a,b). Although the 2 : 1 hybrid cells were not kept in continuous culture, they were grown, subsequently cryopreserved, and then recovered from liquid nitrogen on innumerable occasions; according to our evaluation, they have exceeded more than 190 population doublings. Another unusual phenotype of these hybrid cells is that they are not strictly contact inhibited, and continue to proliferate in post‐confluent populations even in acidic media (Fig. 2c). Because mitotic activity of the hybrid cells was visible even at high density, we believed that they may have produced growth factors and released these into the medium. To test this hypothesis, we performed a preliminary study using pooled conditioned media to treat senescent‐like porcine parental fibroblasts. After centrifugation, we diluted the conditioned media with serum containing fresh media and added this to morphologically senescent porcine fibroblast cultures. Treatments by addition of 20% conditioned media with 80% fresh media were sufficient to change the senescent‐like cells (AG012077 at passage 30) into morphologically transformed cells (the culture containing many mitoses) by renewal of medium every 3–4 days for 4 weeks (Fig. 2d). This result indicates that conditioned media, of 2 : 1 PMN cells, contains yet to be identified factors that can stimulate the proliferation of previously senescent‐appearing cells. Although freshly collected, conditioned media was better for stimulating growth of other cells, bioactivity of conditioned media could be preserved for up to 3 months by storing them in the refrigerator, at normal temperature, without freezing (results not shown).
Figure 2.
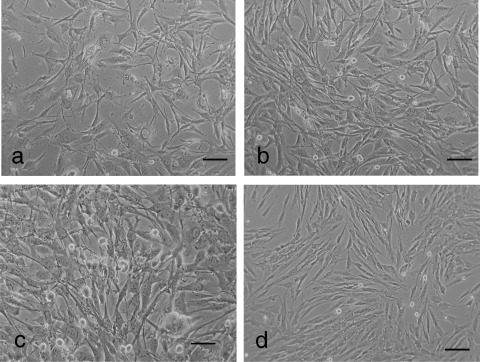
Unusual growth properties of 2 : 1 hybrid cells and effect of conditioned media of hybrid cells on proliferation of senescence‐like pig parental fibroblasts. (a) Hybrid cells grown in DMEM devoid of glutamine supplemented with 15% FCS. (b) Hybrid cells grown in DMEM devoid of glutamine supplemented with 15% FCS and 1% non‐essential MMEM amino acids lacking glutamine. (c) High mitotic activity of 2 : 1 PMN cells in acidic medium and at high density. (d) Addition of 20% conditioned media of 2 : 1 hybrid cells reversed the non‐dividing characteristic of the pig fibroblasts (shown in Fig. 1c) to retain a youthful state – with numerous mitotic cells. Bar = 100 µm.
Immortal porcine fibroblasts did not produce 2 : 1 hybrid cells
To determine whether formation of 2 : 1 pig‐mouse hybrids was limited to fusions between F7 cells and normal fibroblasts, we fused F7 cells with the cells of a spontaneously immortalized pig fibroblast cell line AG12077‐IM. After CPA at passage 2, morphology of cells from a mass population of HAT‐selected fused cells was found to be similar to the immortal pig fibroblasts (Fig. 3a,b). Chromosome analysis revealed that most of the metaphase‐stage cells contained pig chromosomes exclusively (Table 1).
Figure 3.
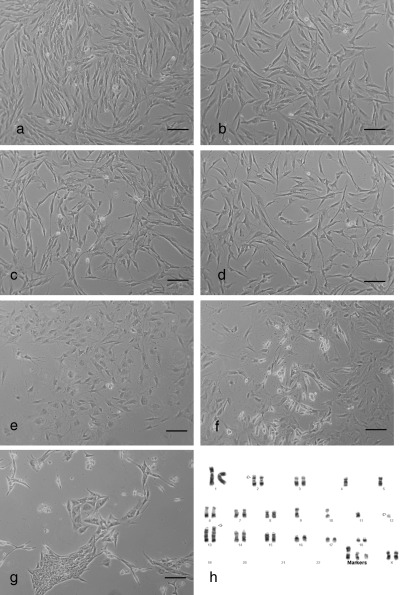
Morphology and cell population growth properties of spontaneously immortalized pig fibroblasts and those of the derived hybrid cells. (a) Morphology of spontaneously immortalized pig fibroblasts AG12077‐IM. (b) Fusion of AG12077‐IM cells with F7 cells to produce a mass culture, with immortal fibroblast‐like morphology when selected in HAT medium (note: no growth activation). (c) Morphology of immortal AG12077‐IM‐hTERT after introduction of hTERT. (d) Fusion of AG12077‐IM‐hTERT cells with F7 cells produced cells similar to immortal pig fibroblasts when fused cells were grown in HAT medium (note: no growth activation). (e) Morphology of the immortal AG12077‐IM cells after artificially induced ageing. (f) Fusion of aged AG12077‐IM cells with F7 cells occasionally produced colonies of slow‐growing hybrids in the background of ageing fibroblasts. (g) Tightly packed, slow‐growing hybrids resulted from the fusion of AG12077‐IM‐hTERT cells with F7 cells, selected in DMEM containing G418 and puromycin. (h) Karyotype of the immortal cell line AG12077‐IM showing monosomy of chromosomes 4, 5, 9, 10, 11 and nullisomy of chromosome 12, in addition to three marker chromosomes (indicated by arrows). Βar = 100 µm.
Introduction of the telomerase‐coding gene into the immortal porcine cell line failed to generate 2 : 1 hybrid cells
It is known that introduction of the human telomerase gene (hTERT) into normal cells can extend their lifespans and in some instances immortalize them (Harley 2002). To test whether hTERT had any effect on improving cell population growth of hybrids, we introduced it into AG12077‐IM cells by retroviral transduction, then fused these transduced cells with F7 cells. The CPA of mass cultured cells at passage 2 showed no difference from the fusion results of the original AG12077‐IM cells (Fig. 3c,d), indicating that the introduction of hTERT‐coding gene had no visible effect on growth characteristics of the hybrid cells. Because the fusion of F7 cells with morphologically ageing and senescent porcine fibroblasts, produced hybrid cells with accelerated population growth, we ‘aged’ the original immortal AG12077‐IM cells by depriving them of fresh growth medium for at least 3 weeks. When we observed morphologically flat cells with retarded growth (Fig. 3e), we fused these with F7 cells. The result of this fusion was not different from the results of previous cell fusions involving the AG12077‐IM and AG12077‐IM‐hTERT cell lines (Fig. 3e–g). Chromosome analysis of the immortal porcine fibroblast cell line AG12077‐IM showed a hypo‐diploid chromosome number represented by single copies of several chromosomes as well as some rearranged chromosomes (Fig. 3h).
DISCUSSION
Here we have demonstrated that fusion of cells of the immortal mouse fibroblast cell line F7, with the normal porcine fibroblasts consistently produces hybrid cells that contain polyploid pig and hypo‐diploid mouse chromosomes. Despite the unusual gain of large numbers of porcine chromosomes derived from the normal fibroblasts, these hybrids exhibit no sign of replicative senescence even after approximately 190 population doublings. Retention of normal cell‐derived polyploid chromosomes by the hybrid cells is exceptional (Vassilopoulos & Russell 2003; Wang et al. 2003; Matveeva et al. 2005). These pig–mouse cell hybrids show accelerated population growth and proliferate in the nature of immortal cells. They can be grown at low cell density and even in glutamine‐free medium.
Previously, we have found that fusion of cells of the GM05267‐HygR line (a polyploid derivative of the hypo‐diploid GM05267 cell line), with primary diploid pig fibroblasts produced hybrid cells with polyploid mouse chromosomes and extensively segregated pig chromosomes (Gao & Islam 2001 and Table 4). In the present study, fusion of F7 cells, a hypo‐diploid derivative of the GM05267 cell line, with primary diploid fibroblasts produced hybrid cells containing polyploid pig and hypo‐diploid mouse chromosomes (Table 2). These results at first glance may indicate that the direction of chromosome loss is determined by ploidy level of the immortal cell parent. The role of parental cell ploidy in the segregation of chromosomes of somatic cell hybrids has been suggested by other investigators (Graves 1984). However, results of our more recent cell fusion experiments suggest that chromosome segregation in somatic cell hybrids is far more complex than was previously anticipated. Whereas fusion of the F7 mouse cell line (that has monosomy of chromosomes 9 and 17) with normal pig cells consistently produces hybrid cells containing an excessive number of pig chromosomes with high levels of cell population growth, the fusion of two sister cell lines (VJ‐1‐HR and VJ‐4‐HR) of F7 (which are karyotypically similar to F7 but contain disomy of chromosomes 9 and 17) produce hybrid cells containing variable chromosome compositions with no sign of growth activation. On the other hand, fusion of a tetraploid clone of F7 cells with normal pig fibroblasts produces hybrid cells with activated growth characteristics but without significant loss of pig chromosomes (Islam & Islam, unpublished data). It seems that loss or gain of chromosomes in the somatic cell hybrids is controlled by ‘dosages’ of certain genes of the immortal donor cell parent and not by ploidy numbers. Further studies will be needed to identify the specific chromosome(s) that may control the directional loss of chromosomes from the somatic cell hybrids.
We have shown by repeated experiments with high reproducibility that activation of growth in 2 : 1 PMN cells is a general phenomenon rather than a random event. We speculate that interactions of 2 : 1 pig–mouse chromosomes in the hybrid cells constitutively activate a growth‐promoting pathway that requires the involvement of multiple genes for completion. This is probably why the normal cells containing all porcine chromosomes in multiple copies fully activate the pathway, apparently in a single step, through fusion. This fusion between immortal pig and immortal mouse cells, both containing genomic deletion, are unable to produce hybrid cells with activated growth phenotypes probably because of the lack of indispensable genes. The karyotype analysis of the AG12077‐IM cell line revealed that a substantial portion of the genome is no longer available because of complete or partial loss of certain chromosomes. This may indicate that the result of immortal cell growth is caused by loss of genetic information. Lack of growth activation in the hybrid cells, generated by fusion of F7 and AG012077, may indicate that the union of two incomplete genomes of independently derived immortal cell lines has complemented some of the lost functions, producing partial phenotypes of what had been normal cells. It is possible that the characteristic of immortal cell growth and that of activated cell growth in the hybrids are controlled by different mechanisms. It is likely that the former phenotype is a qualitative trait caused by loss of function. On the other hand, the later phenotype may be a quantitative trait caused by gain of function. Gain of function seems to be controlled by the input of normal cell derived chromosomes. It has been suggested that comparatively, it is easier to reprogram morphologically aged normal cells for animal cloning than to reprogram immortal cells for this purpose (Lanza et al. 2000; Kasinathan et al. 2001). Our results show that the fusion of F7 cells with normal porcine cells (containing complete genomic information) produce hybrid cells with activated growth, through reprogramming of normal cell genome. This may indicate that in cellular reprogramming the genetic information of donor cells is more important than their ageing status (Zakhartchenko et al. 1999; Shi et al. 2003).
Although the exact mechanism of growth activation in the 2 : 1 hybrid cell system is currently unknown, the epigenetic basis of this phenomenon cannot be excluded (Efstratiadis 1998; Young et al. 1998; Young et al. 2001). It should be noted that in the mouse parental F7 cell line, cells contain single copies of several chromosomes including 6, 7, 8, 9, 12, 17 and 18. Except for chromosome 8, all these mouse chromosomes carry imprinted genes where only one of the two alleles is functional (Morison & Reeve 1998). Because the F7 cells and the original GM05267 cells have high cell proliferation rates, it is possible that one or more paternally expressed growth‐promoting genes has accumulated and the antagonistically acting maternally expressed growth inhibitory genes have been lost, as predicted by the conflict theory for imprinted genes (Haig & Graham 1991). This might have caused the constitutive activation of a growth‐promoting pathway as a result of overall imbalance in contents between growth‐promoting and growth‐inhibitory genes in the 2 : 1 hybrid cells.
We have demonstrated that the 2 : 1 PMN cells produced unidentified factors in their conditioned media, which not only accelerate their own cell proliferation cycles but also stimulate proliferation of the previously non‐dividing parental fibroblasts. It should be noted that generation of 2 : 1 cell hybrids is not limited to fusion of porcine fibroblasts and mouse F7 cells. Hybrid cells with similar properties have been generated by fusing pig mesenchymal stem cells (MSC) with F7 cells. These hybrids have an accelerated growth pattern and the conditioned media of the hybrid cells also has the ability to improve the proliferation rate of both freshly isolated as well as aged pig MSCs. These hybrid cells largely maintained the mesenchymal stem cell parent phenotype, as evident from their functional studies (Islam et al. 2006). In addition, 2 : 1 hybrid cells have been generated by fusing F7 cells with porcine chondrocytes; human, horse and mouse MSCs; bovine nucleus pulposus cells and human, chimpanzee, dog and mouse fibroblasts, with or without slight modification of the present protocol (Islam & Islam, in preparation), indicating that generation of hybrid cells with higher levels of cell proliferation is not limited to fusion between mouse and pig cells. Hybrid cells generated in the manner described here can be used to produce tissue‐specific growth factor(s) to extend the lifespan and/or improve proliferation rates of various normal cells including adult stem cells.
ACKNOWLEDGEMENTS
We thank R. Weinberg and D. Kipling for providing pBABE‐hTERT retroviral vector and producer cells; P. Magnusson for computer help; C. Sharp and G. Adam for critical reading of the manuscript. This work was partly supported by a grant from the Swedish Cancer Society (M.Q.I.).
REFERENCES
- Ambrosi DJ, Rasmussen TP (2005) Reprogramming mediated by stem cell fusion. J. Cell. Mol. Med. 9, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Porath I, Weinberg RA (2005) The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37, 961–976. [DOI] [PubMed] [Google Scholar]
- Davis T, Singhrao SK, Wyllie FS, Haughton MF, Smith PJ, Wiltshire M, Wynford‐Thomas D, Jones CJ, Faragher RG, Kipling D (2003) Telomere‐based proliferative lifespan barriers in Werner syndrome fibroblasts involve both p53‐dependent and p53‐independent mechanisms. J. Cell Sci. 116, 1349–1357. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A (1998) Genetics of mouse growth. Int. J. Dev. Biol. 42, 955–976. [PubMed] [Google Scholar]
- Flasza M, Shering AF, Smith K, Andrews PW, Talley P, Johnson PA (2003) Reprogramming in inter‐species embryonal carcinoma‐somatic cell hybrids induces expression of pluripotency and differentiation markers. Cloning Stem Cells 5, 339–354. [DOI] [PubMed] [Google Scholar]
- Gao X, Islam MQ (2001) A gene on pig chromosome 14 suppresses cellular anchorage independence of the mouse cell line GM05267. Cytogenet. Cell Genet. 94, 62–66. [DOI] [PubMed] [Google Scholar]
- Goldstein S (1990) Replicative senescence: the human fibroblast comes of age. Science 249, 1129–1133. [DOI] [PubMed] [Google Scholar]
- Graves JA (1984) Chromosome segregation from cell hybrids. I. The effect of parent cell ploidy on segregation from mouse‐Chinese hamster hybrids. Can. J. Genet. Cytol. 26, 557–563. [DOI] [PubMed] [Google Scholar]
- Haig D, Graham C (1991) Genomic imprinting and the strange case of the insulin‐like growth factor II receptor. Cell 64, 1045–1046. [DOI] [PubMed] [Google Scholar]
- Harley CB (2002) Telomerase is not an oncogene. Oncogene. 21, 494–502. [DOI] [PubMed] [Google Scholar]
- Hayflick L (1965) The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636. [DOI] [PubMed] [Google Scholar]
- Islam MQ, Islam K (2000) Suppressor genes for malignant and anchorage‐independent phenotypes located on human chromosome 9 have no dosage effects. Cytogenet. Cell Genet. 88, 103–109. [DOI] [PubMed] [Google Scholar]
- Islam MQ, Levan G (1987) A new fixation procedure for quality G‐bands in routine cytogenetic work. Hereditas 107, 127–130. [DOI] [PubMed] [Google Scholar]
- Islam MQ, Ringe J, Reichmann E, Migotti R, Sittinger M, Da Meirelles LS, Nardi NB, Magnusson P, Islam K (2006) Functional characterization of cell hybrids generated by induced fusion of primary porcine mesenchymal stem cells with an immortal murine cell line. Cell Tissue Res. 326, 123–137. [DOI] [PubMed] [Google Scholar]
- Jabs EW, Wolf SF, Migeon BR (1984) Characterization of a cloned DNA sequence that is present at centromeres of all human autosomes and the X chromosome and shows polymorphic variation. Proc. Natl. Acad. Sci. USA 81, 4884–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinathan P, Knott JG, Moreira PN, Burnside AS, Jerry DJ, Robl JM (2001) Effect of fibroblast donor cell age and cell cycle on development of bovine nuclear transfer embryos in vitro. Biol. Reprod. 64, 1487–1493. [DOI] [PubMed] [Google Scholar]
- Lanza RP, Cibelli JB, Blackwell C, Cristofalo VJ, Francis MK, Baerlocher GM, Mak J, Schertzer M, Chavez EA, Sawyer N, Lansdorp PM, West MD (2000) Extension of cell life‐span and telomere length in animals cloned from senescent somatic cells. Science 288, 665–669. [DOI] [PubMed] [Google Scholar]
- Matveeva NM, Pristyazhnyuk IE, Temirova SA, Menzorov AG, Vasilkova A, Shilov AG, Smith A, Serov OL (2005) Unequal segregation of parental chromosomes in embryonic stem cell hybrids. Mol. Reprod. Dev. 71, 305–314. [DOI] [PubMed] [Google Scholar]
- Migeon BR, Brown TR, Axelman J, Migeon CJ (1981) Studies of the locus for androgen receptor: localization on the human X chromosome and evidence for homology with the Tfm locus in the mouse. Proc. Natl. Acad. Sci. USA 78, 6339–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison IM, Reeve AE (1998) A catalogue of imprinted genes and parent‐of‐origin effects in humans and animals. Hum. Mol. Genet. 7, 1599–1609. [DOI] [PubMed] [Google Scholar]
- Pells S, Di Domenico AI, Gallagher EJ, McWhir J (2002) Multipotentiality of neuronal cells after spontaneous fusion with embryonic stem cells and nuclear reprogramming in vitro. Cloning Stem Cells 4, 331–338. [DOI] [PubMed] [Google Scholar]
- Pereira‐Smith OM, Smith JR (1983) Evidence for the recessive nature of cellular immortality. Science 221, 964–966. [DOI] [PubMed] [Google Scholar]
- Ran Q, Pereira‐Smith OM (2000) Genetic approaches to the study of replicative senescence. Exp. Gerontol. 35, 7–13. [DOI] [PubMed] [Google Scholar]
- Shi W, Hoeflich A, Flaswinkel H, Stojkovic M, Wolf E, Zakhartchenko V (2003) Induction of a senescent‐like phenotype does not confer the ability of bovine immortal cells to support the development of nuclear transfer embryos. Biol. Reprod. 69, 301–309. [DOI] [PubMed] [Google Scholar]
- Tada M, Tada T, Lefebvre L, Barton SC, Surani MA (1997) Embryonic germ cells induce epigenetic reprogramming of somatic nucleus in hybrid cells. EMBO J. 16, 6510–6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi N (1997) Mouse embryonal carcinoma cell‐somatic cell hybrids as experimental tools for the study of cell differentiation and X chromosome activity. Cancer Genet. Cytogenet. 93, 48–55. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Russell DW (2003) Cell fusion: an alternative to stem cell plasticity and its therapeutic implications. Curr. Opin. Genet. Dev. 13, 480–485. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al‐Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M (2003) Cell fusion is the principal source of bone‐marrow‐derived hepatocytes. Nature 422, 897–901. [DOI] [PubMed] [Google Scholar]
- Young LE, Fernandes K, McEvoy TG, Butterwith SC, Gutierrez CG, Carolan C, Broadbent PJ, Robinson JJ, Wilmut I, Sinclair KD (2001) Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat. Genet. 27, 153–154. [DOI] [PubMed] [Google Scholar]
- Young LE, Sinclair KD, Wilmut I (1998) Large offspring syndrome in cattle and sheep. Rev. Reprod. 3, 155–163. [DOI] [PubMed] [Google Scholar]
- Zakhartchenko V, Alberio R, Stojkovic M, Prelle K, Schernthaner W, Stojkovic P, Wenigerkind H, Wanke R, Duchler M, Steinborn R, Mueller M, Brem G, Wolf E (1999) Adult cloning in cattle: potential of nuclei from a permanent cell line and from primary cultures. Mol. Reprod. Dev. 54, 264–272. [DOI] [PubMed] [Google Scholar]


