Abstract
Objectives
Protein arginine methyltransferase 5 (PRMT5), is thought to play a role in epigenetic reprogramming of mouse germ cells. However, up to now there has been little information concerning its expression profile and effects on generation of induced pluripotent stem cells (iPSCs) from somatic cells, in livestock. Here, we have explored PRMT5 expression profiles in dairy goats and its consequences to derivation of iPSCs from dairy goat embryonic fibroblasts (GEFs).
Materials and methods
We investigated effects of PRMT5 on iPS‐like cells production in vitro. alkaline phosphatase (AP) staining, QRT‐PCR and western blotting analysis of expression of related markers were used to evaluate efficiency of generation of iPSCs derived from GEFs.
Results
These showed PRMT5 to be a conservative gene widely expressed in various tissues and different‐aged testes. PRMT5 overexpression in combination with OCT3/4, SOX2, KLF4 and C‐MYC (POSKM) significantly increased number of AP positive iPS‐like colony‐derived GEFs compared to OSKM alone, in our dairy goats. Moreover, our results demonstrated that PRMT5 overexpression stimulated GEF proliferation and down‐regulated p53, p21 (a target gene of p53) and the apoptotic marker caspase 3, to enhance somatic cell reprogramming.
Conclusion
This study provides an efficient model for future studies on mechanisms underlying goat somatic cell reprogramming and differentiation.
Introduction
Embryonic stem (ES) cells, derived from the inner cell mass of mammalian blastocysts, have the ability to proliferate infinitely while maintaining pluripotency, and the ability to differentiate into cells of all three germ layers 1. Goat ES cells have a very important use to produce transgenic animals of agricultural significance and can aid as research models for cell proliferation and cell differentiation in vitro; however, there is a lack of stable goat ES cell lines. One way to circumvent this issue is to generate pluripotent cells directly from somatic ones. Mouse induced pluripotent stem cells (iPSCs) have been generated from somatic cells upon forced expression of transcription factors OCT4, KLF4, SOX2 and C‐MYC (OSKM), and it has been definitively shown that iPSCs share similar characteristics and morphology with ES cells 2, 3. Apart from potential clinical applications, iPS cell generation also provides an excellent model system for investigating mechanisms underlying cell reprogramming in vitro via identification of alternative or additional factors involved in the processes 4. Different factor combinations, including OCT4, SOX2, KLF4, C‐MYC, LIN28, NANOG and NR5A2, have been used to reprogram cells of many species, including humans, rat, pig, sheep, horse, rabbit, monkey, goat and cattle 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16. However, low efficiency of iPSC generation is a handicap for mechanistic studies and high‐throughput screening, and bona fide colony isolation is time consuming and costly 17. Efficiency of alkaline phosphatase positive (AP+) colony formation with the four Yamanaka factors (OSKM) in mouse fibroblasts is approximately only 1% of the starting population, but even only 1 in 10 of these colonies is sufficiently reprogrammed to be chimaera competent 17, 18.
Due to this limitation, most studies have focused on the immediate response of somatic cells to factor expression. Protein arginine methyltransferases (PRMT5) is thought to play a role in epigenetic reprogramming in germ cells 19, 20 and PRMTs play important roles in numbers of cellular processes, including signalling, gene regulation and transport of proteins and nucleic acids, to affect growth, differentiation, proliferation and development 21. PRMT5 is a particularly interesting target because it is highly expressed in blood, breast, colon and stomach cancers and promotes cell survival in the presence of DNA‐damaging agents 22. Recently, it has been shown that PRMT5 combined with KLF4 and OCT3/4 reprogrammed mouse somatic cells to a state indistinguishable from ES cells, in terms of their gene expression profile, DNA methylation status, capacity to differentiate into the three germ layers and germline transmission 4. However, there has been lack of information concerning PRMT5 expression profile and its effects on somatic cell reprogramming in livestock 23, 24. Dairy goats have a relatively short period of gestation and provide milk, meat, fur and other valuable products; also they can be used as animal models for biomedical research in production of peptides, using transgenic animals 25, 26.
In this study, first we explored the expression profile of PRMT5 in the dairy goat and found that overexpression of PRMT5, in combination with OSKM, significantly increased numbers of alkaline phosphatase positive (AP+) cells and iPS‐like colony formation, derived from dairy goat fibroblasts via down‐regulation of p53.
Materials and methods
Animals and cell lines
ICR mice were used and maintained in a controlled environment at 20–25 °C, 12/12 h light/dark cycle and 50–70% humidity. All experiments requiring use of animals were approved by the Shaanxi Centre of Stem Cell Engineering & Technology, Northwest A&F University. HEK293T cells were maintained in our laboratory. MEF feeder cells were isolated and prepared according to previous reports 27, 28.
Quantitative real‐time – polymerase chain reaction
Quantitative real‐time – polymerase chain reaction (QRT‐PCR) was used for detection of expression levels of genes in this experiment. Total RNA from dairy tissues and cells was extracted using Trizol reagent (TaKaRa, Biotech. Co., Ltd, Dalian, China). Single‐strand cDNAs were prepared from 1 μg RNA using a reverse transcription kit (TaKaRa, Biotech. Co. Ltd), and PRMT5 gene expression was analysed. QRT‐PCR primers used are listed in Table 1.
Table 1.
The primer sequences for QRT‐PCR
| Gene | Forward | Reverse |
|---|---|---|
| β‐actin | 5′‐ACGGCATCACCAACT‐3′ | 5′‐AGGAAGGAAGGCTGGAAGAG‐3′ |
| Prmt5 | 5′‐GCTTCTGGGCTCATTTGCTG‐3′ | 5′‐TGCAGCCGTACCACATAAGG‐3′ |
| P53 | 5′‐GGGAATCTTCTGGGACGG‐3′ | 5′‐CTTCTTGGTCTTCGGGTAGC ‐3′ |
| P21 | 5′‐AGGGCAVGTCTCAGGAGGA‐3′ | 5′‐CAGTCTGCGTTTGGAGTGGTAG‐3′ |
Quantitative real‐time – polymerase chain reactions were set up in 25 μl reaction mixtures containing 12.5 μl SYBR@ Premix (BORI, Hangzhou, China), 0.5 μl sense primer, 0.5 μl antisense primer, 11 μl distilled water and 0.5 μl template. QRT‐PCR conditions were initial denaturation at 95 °C for 5 min, followed by 30 cycles of 95 °C for 10 s, annealing temperature of 58 °C for 30 s and 72 °C for 30 s, with final extension at 72 °C for 10 min.
Generation of pCDH‐PRMT5‐EGFP vector
PRMT5 gene CDS region was cloned by PCR from 3‐month‐old Guanzhong dairy goat testicular tissue, and amplified using primers incorporating restriction sites for Xba1 (forward primer: 5′‐TCTCGAGCGCCGCTACCGCCAGCCACCA‐3′) and BamH1 (reverse primer: 5′‐AGGATCCCTGCACCTTCTGTGCTACAGG‐3′). Resulting products of the polymerase chain reaction were purified using TIANGEN gel purification kit (TIANGEN Biotech (Beijing) Co., Ltd.) according to the manufacturer's instructions, cloned into pMD‐18T vector (TaKaRa, Biotech. Co. Ltd) and confirmed by PCR using the same primer sets as described above. Next, purified PRMT5 fragment was constructed into the pCDH‐EGFP vector. Constructed vector pCDH‐PRMT5‐EGFP plasmid was identified by digestion with Xba1/BamH1 restriction enzymes and sequencing.
Cell culture and viral transduction
Dairy goat embryonic fibroblast cells (GEFs) were isolated from a 60‐day embryonic foetus. Muscles and skin were digested with 1 mg/ml collagenase I at 37 °C for 15–30 min. After centrifugation at 350 g for 5 min, specimens were incubated in dissociation solution consisting of a mixture of 0.2% (v/w) trypsin (Invitrogen, Carlsbad, CA, USA) and 1.4 mg/ml DNase (Invitrogen), for 10 min at 37 °C. Samples were again centrifuged at 350 g for 5 min, and the cell suspension in culture medium was transferred to a 100 mm diameter culture plate. GEF cells used in this experiment were cultured in DMEM/F12 (Invitrogen), supplemented with 10% foetal bovine serum (FBS; HyClone, Logan, UT, USA), 2 mm l‐glutamine (Invitrogen), 1% non‐essential amino acids (NEAA; Invitrogen), 0.1 mm β‐mercaptoethanol (Sigma, St. Louis, MO, USA) and 100 mg/ml penicillin/streptomycin.
To produce pseudotyped lentivirus particles, a monolayer of HEK293T cells in a 100 mm diameter dish was cotransfected with three plasmids, pXPAX2 (7 μg), pMD2.G (7 μg) and pCDH‐PRMT5‐EGFP, or the four human reprogramming factors, OCT4, SOX2, KLF4 and C‐MYC, along with individual additional candidate factors, which were amplified by RT‐PCR and cloned into pCDH‐EGFP using the calcium‐phosphate method (ProFection Mammalian Transfection System; Promega, Madison, WI, USA). After overnight incubation, cells were washed and incubated in fresh H‐DMEM (Invitrogen). Supernatant containing lentivirus of the four factors – OSKM in combination with pCDH‐PRMT5‐EGFP, or OSKM, alone were harvested after 48 h and 72 h post‐transfection. Using the supernatant containing lentivirus, transduction of PRMT5 was performed in GEF cells, and medium was changed as normal medium after 6–8 h 26.
Generation of dairy goat iPSCs using lentiviral vector
To generate dairy goat iPSCs, GEF cells were cultured in DMEM/F12 (Invitrogen). After 2–3 days culture, cells were remained in excellent condition. Lentiviral transduction was performed for 5–6 h, and fresh DMEM/F12 was added. Two to three days after transduction, cells were gradually replaced with iPSC medium. The dairy goat iPSCs in this study were cultured in knockout DMEM (Invitrogen), supplemented with 17% KSR (Invitrogen), 3% FBS (HyClone), 0.1 mm non‐essential amino acids (Invitrogen), 1 mm l‐glutamine (Invitrogen), 1/100 N2 (Invitrogen), 1/50 B27 (Invitrogen), 50 μg/ml ascorbic acid (Sigma), 2.5 μm PD99023 (Sigma), 2.5 μm CHIR‐99021 (Stemgent, Bergisch Gladbach, Germany), 10 ng/ml recombinant human leukaemia inhibitor factor (LIF; Invitrogen), 10 ng/ml bFGF (Millipore, Billerica, MA, USA), 0.1 mm β‐mercaptoethanol (Sigma), and 50 units/50 mg/ml penicillin/streptomycin, at 37 °C and 5% CO2 in a humidified atmosphere. Culture medium was initially changed every 2 days, subsequently being changed every day. After formation of cell colonies, colonies were picked and seeded with inactivated mouse embryonic fibroblast (MEF) feeder cells 22, 29.
Alkaline phosphatase staining
To determine numbers of iPSC‐like cell colonies, specimens on culture plates were fixed in 4% paraformaldehyde (PFA) in PBS for 15 min at room temperature, and washed three times in PBS. Fixed cells were washed three times in PBS and stained with naphthol AS‐MX phosphate (200 μg/ml; Sigma) and fast red TR salt (1 mg/ml; Sigma) in 100 mm Tris buffer, pH 8.2–8.4, for 10–30 min at room temperature, and washed in PBS to terminate staining 28, 30.
Immunofluorescence analysis
Cell samples were fixed in 4% PFA in PBS for 15 min and washed three times in PBS, treated with 0.2% Triton X‐100 in PBS for 10 min at room temperature, and then washed three times in PBS. After blocking with 1% BSA in PBS for 30 min, cells were incubated with primary antibodies against PRMT5 (1:100; Millipore), OCT4 (1:500; Chemicon, Temecula, CA, USA), SSEA1 (1:200; Chemicon), SSEA4 (1:200; Chemicon), C‐MYC (1:200; Chemicon), NSE (1:200; Chemicon), VASA (1:200; Sino Biological Inc., Beijing, China) and cardiac α‐ACTIN (1:500; Sigma) overnight at 4 °C. Cells were then washed three times in PBS, and appropriate secondary antibodies were applied for incubation for 1 h at room temperature, in the dark. After washing in PBS, cells were finally exposed to Hoechest33342 in PBS to counterstain nuclei, for 2 min.
Western blotting
Total cell extracts were prepared from the PRMT5‐overexpressing group compared to the control group, and proteins were extracted in 1X SDS–‐PAGE sample loading buffer. Total cell proteins were resolved by SDS‐PAGE, transferred to 0.22 mm PVDF membrane for around 55 min in 80 V, and probed with β‐actin (1:1000; Bioss, Beijing, China), p53 (1:1000; Bioss, Beijing, China), CCDN1 (1:1000; Sino Biological Inc., Beijing, China) and caspase 3 (1:1000; Bioworld, Shanghai, China). Horseradish peroxidase‐conjugated anti‐rabbit antibody was used as a secondary antibody (1:1000; Bioworld). Detection was performed using Thermo Scientific Pierce ECL Western blot substrate (Thermo Scientific) 31, 32.
Statistical analysis
Data are presented as mean and SEM. Differences in expression of specific markers were evaluated using Student's t‐test (Excel, Microsoft Corporation). Results of different treatments were considered significantly different when P < 0.05 was obtained 32.
Results
PRMT5 was expressed in different tissues of dairy goat and co‐localized with OCT4/p53
QRT‐PCR analysis confirmed PRMT5 expression in different tissues; expression levels in heart, liver, kidney and testis were relative higher than in spleen, ovary and lung (Fig. 1a). Levels of PRMT5 varied between different aged testes (Fig. 1b). PRMT5 protein was also detected in the testis and ovary of dairy goats at 90 dpp (Fig. 1c). In addition, PRMT5 protein co‐localized with pluripotentiality marker, OCT4, as analysed using immunofluorescence (Fig. 1d).
Figure 1.
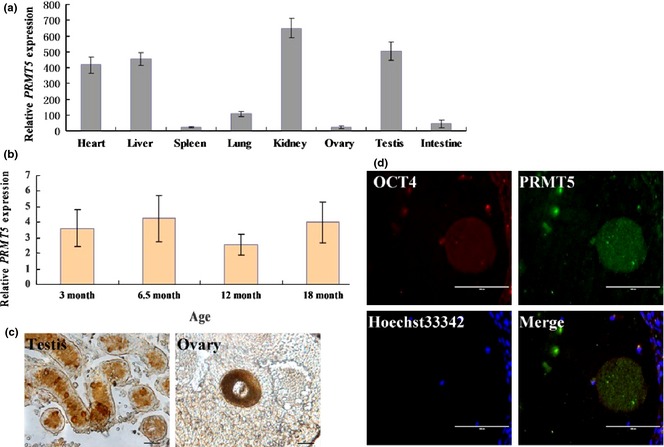
Expression and subcellular location of dairy goat PRMT5. (a) Quantitative PCR detection of PRMT5 expression in different tissues; (b) quantitative PCR detection of PRMT5 expression among different aged testes; (c) immunohistochemical detection of localisation of PRMT5 in testis and ovary; (d) immunofluorescence analysis of the localisation of OCT4 and PRMT5 in the ovary (scale bar = 100 μm).
Construction of pCDH‐CMV‐PRMT5‐EF1‐GreenPuro lentiviral vector
The PRMT5 CDS region (1920 bp) was cloned from Guanzhong dairy goat testicular tissues (Fig. 2a–I) then recombined into the pMD18‐T vector for sequencing. Correctly sequenced PCR product was excised from pMD18‐T (Fig. 2a–II) then recombined with pCDH‐CMV‐EF1‐GreenPuro vector and identified by double digestion, using Xba1/BamHl restriction enzymes (Fig. 2a–III). A schematic of the successful construction vector is shown in Fig. 2b. Furthermore, structure of the dairy goat PRMT5 protein was predicted using Swiss Institute of Bioinformatics online software analysis. These results showed that goat and Boss PRMT5 protein had the same structure (Fig. 2c). We found that dairy goat PRMT5 had high sequence homology compared to corresponding regions in Bos taurus, Callithrix jacchus, Mus musculus, Canis familians and Pan troglodytes. Next, we constructed the phylogenetic tree using MEGA4.1 software, which indicated that PRMT5 of goat and cattle is homologous and conserved (Fig. 2d).
Figure 2.
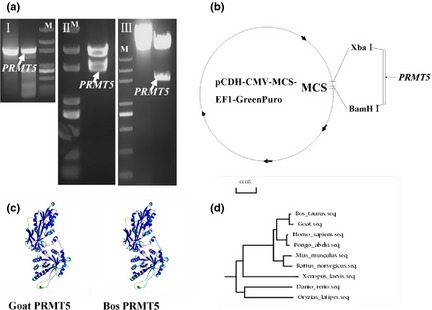
Construction and analysis of pCDH‐CMV‐Prmt5‐EF1‐GreenPuro vector. (a) (I) PRMT5 sequence was amplified using PCR, (II) restriction fragment of PRMT5 from positive T vector, (III), digestion confirms the pCDH‐CMV‐Prmt5‐EF1‐GreenPuro vector; (b) schematic of the recombinant vector; (c) comparison of the PRMT5 protein structure between dairy goat and Bos; (d) evolutionary analysis of PRMT5 gene.
Generation of dairy goat induced pluripotent stem‐like cells using lentivirus vector
Schematic diagram of generation of dairy goat iPSCs is summarized in Fig. 3a. Lentiviral vectors containing four human reprogramming factors OCT4/SOX2/KLF4/C‐MYC (OSKM) were used. AP‐positive cells appeared after 7 days and gradually formed larger and compact colonies (Fig. 3a). ES‐like colonies were formed and selected at 30–40 days after transduction and transferred to a fresh plate with MEF feeders (Fig. 3a). Colonies reprogrammed with OSKM were characterized by well‐defined borders; all features were very similar to iPSC colonies and goat ES cells 23. Within 3–4 passages, typical iPS‐like cell colonies were identified, using RT‐PCR and immunofluorescence analysis. RT‐PCR results showed that these cells were positive for SOX2, KLF4, OCT4, C‐MYC and NANOG (Fig. 3b). Immunofluorescence results also confirmed that the cells were positive for the following pluripotentiality markers: SSEA1, SSEA4, C‐MYC and OCT4 (Fig. 3c).
Figure 3.
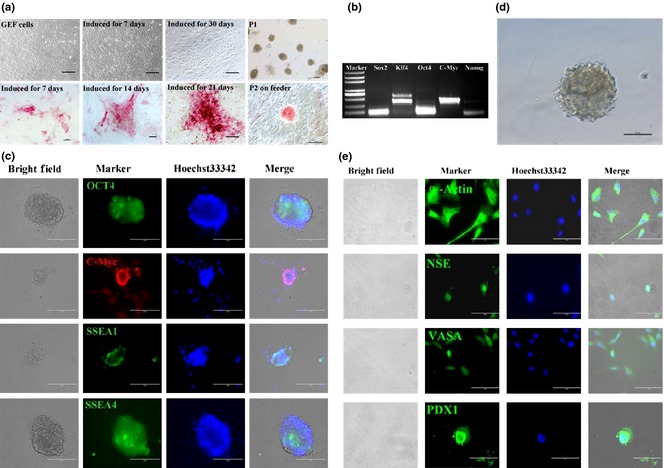
Characterization of dairy goat iPSCs. (a) Induced goat iPSC‐like colonies were positive for AP staining; (b) RT‐PCR analysis showed that goat iPSC‐like cells were positive for SOX2, KLF4, OCT4, C‐MYC and NANOG; (c) immunofluorescence analysis of dairy goat iPSCs at passage 2; pluripotent relative markers (OCT4, C‐MYC, SSEA1, SSEA4) were expressed. Nuclei were counterstained with Hoechst 33342. (d) Goat iPSCs formed embryoid bodies (EBs). (e) Immunofluorescent staining showed that the EBs derived from goat iPSCs differentiated into three germ layer cell types, including the NSE, α‐ACTIN, PDX1 and VASA‐positive cells. Goat iPSCs differentiated into EBs.
To test potential of the iPS‐like cell colonies, cells were cultured in suspension for 2 days. They spontaneously differentiated into typical embryoid bodies (Fig. 3d) and were then transferred into petri dishes containing normal cell culture medium without LIF and bFGF for 7 days. Differentiated cells expressed specific markers of the different germ layers, including α‐ACTIN (marker of mesoderm), NSE (marker of ectoderm), VASA (germ cell marker) and PDX1 (endoderm marker) (Fig. 3e).
PRMT5 enhanced generation of dairy goat iPSCs
We investigated effects of PRMT5 on derivation of iPSCs from dairy goat fibroblasts, and numbers of AP positive colonie, at D7 and D14 in the PRMT5/OCT4/SOX2/KLF4/C‐MYC (POSKM) transduced group and discovered they were significantly higher than in OSKM (Fig. 4a). Moreover, most dairy goat iPS‐like colonies were GFP positive, as analysed using immunofluorescence microscopy. These findings indicated that PRMT5 transduction clearly increased the ability to form iPS‐like colonies derived from GEFs (Fig. 4b). Moreover, the results showed that PRMT5 enhanced generation of goat iPSCs derived from GEFs.
Figure 4.
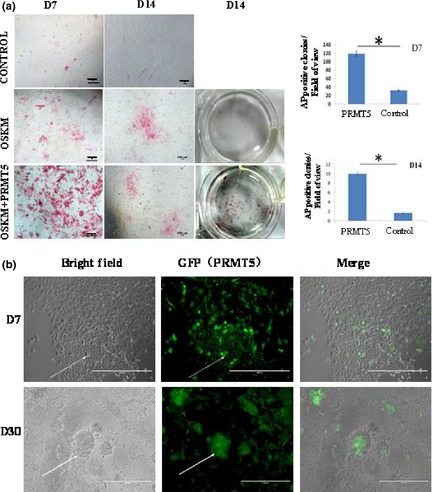
Generation of dairy goat iPSC s using four defined factors (OSKM) and OSKM combined with PRMT5 (OSKMP). (a) Reprogramming efficiencies at 7 days in two groups (OSKM and OSKMP) were determined. Inset shows that overexpression PRMT5 enlarged AP positive cells. AP positive colonies in each 24‐well dish were quantified and plotted in the right panel, and number of AP positive colonies at D7 and D14 in OSKMP (PRMT5) was significantly higher than that in OSKM (Control) group, *P < 0.05. (b) Most iPS‐like clusters were GFP (PRMT5) positive.
PRMT5 enhanced somatic cell reprogramming by mediating down‐regulation of p53
Expression of PRMT5 was significantly increased in GEFs with overexpressed PRMT5 (Fig. 5a,d). In addition, overexpression of PRMT5 resulted in more rapid cell proliferation compared to the control group (Fig. 5b,c). FACS cell cycle analysis showed that there were more cells in S‐phase in overexpressed PRMT5 group compared to controls (Fig. 5c). Furthermore, we confirmed that PRMT5 enhanced somatic cell reprogramming via regulation of p53. PRMT5 overexpression in GEFs resulted in down‐regulation of expression of p53 and p21 (target gene of p53) (Fig. 5d). In addition, Western blotting detection also demonstrated that p53, CCDN1 and caspase 3 protein expression was reduced by PRMT5 (Fig. 5e).
Figure 5.
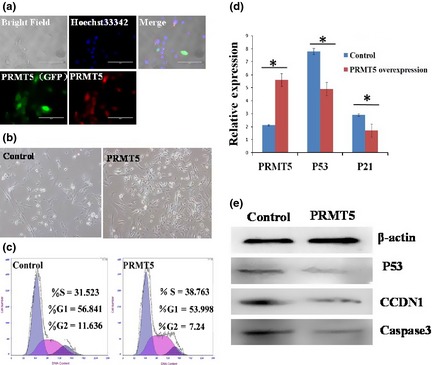
PRMT5 promoted GEF cell proliferation. (a) Overexpression of PRMT5 in GEF cells. Immunostaining of PRMT5 (Red). Nuclei were stained with Hoechst 33342. (b) Overexpression of PRMT5 resulted in more rapid cell proliferation compared to control group. (c) Cell cycle analyses using FACS showed that there were 31.52% and 38.76% S‐phase cells in control and PRMT5‐overexpressing group, respectively. (d) QRT‐PCR showed that expression of p53 and p21 was lower in PRMT5‐overexpressing group compared to the control group, *P < 0.05. (e) Western blotting analyses showed that p53, CCDN1 and caspase 3 were reduced in the PRMT5‐overexpressing group.
Discussion
Previous studies have shown that PRMT5 is important for establishment of ES cell lines derived from blastocysts, and is indispensable for embryonic development 33. In addition, PRMT5 exhibits remarkable effects on reprogramming of mouse embryonic fibroblasts into iPSCs in which PRMT5, KLF4, and OCT3/4 have been introduced in combination 4. However, there has been little information on reprogramming of somatic cells and effects of PRMT5 in livestock. In this study, we first examined effects of PRMT5 gene on goat iPSC generation and found that it is expressed at high levels in iPS‐like cells. Furthermore, addition of PRMT5 in combination with the four typical OSKM pluripotentiality transcription factors clearly increased numbers of AP positive colonies generated, thus sustaining the notion that PRMT5 up‐regulated somatic reprogramming of our dairy goat cells. GEFs overexpressing PRMT5 combined with OSKM exhibited lower levels of p53 and p21 mRNA and protein compared to GEFs lacking PRMT5, thereby promoting reprogramming efficiency.
Reducing signals to p53 by expressing a mutated version of one of its negative regulators, by deletion or knocking down p53 or its target gene, p21 (also known as CDKN1A), or by antagonizing reprogramming‐induced apoptosis, has been shown to increase reprogramming efficiency in mouse fibroblasts 34. This finding indicates that methylation of arginine residues is an underlying mechanism of control during the p53 response 35. p53 knockdown heterozygous MEFs also exhibited three‐factor higher reprogramming efficiency than wild‐type MEFs. These data indicated that the p53 pathway is one determinant of reprogramming efficiency 34. It has been shown that PRMT5 protein (as a co‐factor in a DNA damage responsive co‐activator complex that interacts with p53), was responsible for p53 methylation 35. We first determined whether PRMT5, individually or in combination with the reprogramming factors, OSKM, activated the p53 pathway in dairy goat GEFs. Our results support the hypothesis that PRMT5 promoted dairy goat induced pluripotent stem cell generation via the p53 pathway, at least in part via repression of p53. These results suggest that PRMT5 regulates somatic cell reprogramming by down‐regulation of p53/p21, thereby promoting cell proliferation and expression of KLF4 and C‐MYC, and inhibiting apoptosis (Fig. 6). Previous studies have demonstrated that the p53–p21 pathway serves as a barrier, not only in tumourigenicity, but also in murine and human iPS cell generation 29, 36, 37.
Figure 6.
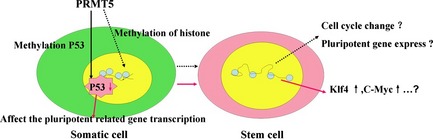
Models for the role of PRMT5 in promoting dairy goat somatic cell reprogramming by down‐regulation of p53/p21, thereby promoting KLF4 and C‐MYC expression, cell proliferation and inhibiting apoptosis.
p53 protein functions mainly as a transcription factor that regulates important cellular processes, such as cell proliferation, cell cycle arrest, DNA repair and apoptosis, in response to stress signals 38. Unlike in the mouse, Gkountela et al. 39 revealed that PRMT5 had no role in hESC pluripotency, and depletion of PRMT5 had no effect on expression of OCT4, NANOG or SOX2, and did not prevent teratoma formation. Instead, PRMT5 seemed to function in hESCs to regulate proliferation in the self‐renewal state by regulating the fraction of cells in G1 phase of the cell cycle, and by increasing expression of G1 cell cycle inhibitor p57. These data indicated a distinct role for PRMT5 in hESCs and identified p57 as a new target 39. Our study showed that PRMT5 promoted GEF reprogramming into iPS‐like cells via regulation of cell proliferation and inhibition of apoptosis via the p53–p21 pathway.
In conclusion, this study showed PRMT5 to be a conserved gene and that its overexpression could stimulate GEF proliferation and down‐regulate p53, p21 and caspase 3, to enhance GEF reprogramming into iPSCs, in combination with OSKM. This investigation provides an efficient method for study of the mechanisms of goat somatic cell reprogramming.
Acknowledgements
This work was supported by grants obtained from the Program of the National Natural Science Foundation of China (31272518), the National Major Project for Production of Transgenic Breeding (2014ZX08007002‐010), the Doctoral Fund of Ministry of Education of P.R. China (RFDP, 20120204110030), National High Technology Research and Development Program of China (SS2014AA021605), the Inner Mongolia Autonomous Region Open Major Basic Research Project (20130903).
References
- 1. Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- 3. Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- 4. Nagamatsu G, Kosaka T, Kawasumi M, Kinoshita T, Takubo K, Akiyama H et al (2011) A germ cell‐specific gene, Prmt5, works in somatic cell reprogramming. J. Biol. Chem. 286, 10641–10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bao L, He L, Chen J, Wu Z, Liao J, Rao L et al (2011) Reprogramming of ovine adult fibroblasts to pluripotency via drug‐inducible expression of defined factors. Cell Res. 21, 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang B, Li T, Alonso‐Gonzalez L, Gorre R, Keatley S, Green A et al (2011) A virus‐free poly‐promoter vector induces pluripotency in quiescent bovine cells under chemically defined conditions of dual kinase inhibition. PLoS One 6, e24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagy K, Sung HK, Zhang P, Laflamme S, Vincent P, Agha‐Mohammadi S et al (2011) Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev. 7, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ren J, Pak Y, He L, Qian L, Gu Y, Li H et al (2011) Generation of hircine‐induced pluripotent stem cells by somatic cell reprogramming. Cell Res. 21, 849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heng JC, Orlov YL, Ng HH (2010) Transcription factors for the modulation of pluripotency and reprogramming. Cold Spring Harb. Symp. Quant. Biol. 75, 237–244. [DOI] [PubMed] [Google Scholar]
- 10. Tomioka I, Maeda T, Shimada H, Kawai K, Okada Y, Igarashi H et al (2010) Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28. Genes Cells 15, 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esteban MA, Xu J, Yang J, Peng M, Qin D, Li W et al (2009) Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J. Biol. Chem. 284, 17634–17640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li W, Zhou H, Abujarour R, Zhu S, Young JJ, Lin T et al (2009) Generation of human‐induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells 27, 2992–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liao J, Cui C, Chen S, Ren J, Chen J, Gao Y et al (2009) Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell 4, 11–15. [DOI] [PubMed] [Google Scholar]
- 14. Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C et al (2009) Generation of pig induced pluripotent stem cells with a drug‐inducible system. J. Mol. Cell Biol. 1, 46–54. [DOI] [PubMed] [Google Scholar]
- 15. Liu H, Zhu F, Yong J, Zhang P, Hou P, Li H et al (2008) Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell 3, 587–590. [DOI] [PubMed] [Google Scholar]
- 16. Yu J, Vodyanik MA, Smuga‐Otto K, Antosiewicz‐Bourget J, Frane JL, Tian S et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920. [DOI] [PubMed] [Google Scholar]
- 17. Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J et al (2010) Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71–79. [DOI] [PubMed] [Google Scholar]
- 18. Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A (2008) Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 6, e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pal S, Sif S (2007) Interplay between chromatin remodelers and protein arginine methyltransferases. J. Cell. Physiol. 213, 306–315. [DOI] [PubMed] [Google Scholar]
- 20. Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, Kouzarides T et al (2006) Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat. Cell Biol. 8, 623–630. [DOI] [PubMed] [Google Scholar]
- 21. Antonysamy S, Bonday Z, Campbell RM, Doyle B, Druzina Z, Gheyi T et al (2012) Crystal structure of the human PRMT5:MEP50 complex. Proc. Natl. Acad. Sci. USA 109, 17960–17965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang M, Xu RM, Thompson PR (2013) Substrate specificity, processivity, and kinetic mechanism of protein arginine methyltransferase 5. Biochemistry 52, 5430–5440. [DOI] [PubMed] [Google Scholar]
- 23. Song H, Li H, Huang M, Xu D, Gu C, Wang Z et al (2013) Induced pluripotent stem cells from goat fibroblasts. Mol. Reprod. Dev. 80, 1009–1017. [DOI] [PubMed] [Google Scholar]
- 24. Lu Y, Mumaw JL, West FD, Stice SL (2012). Livestock induced pluripotent stem cells. Reprod. Domest. Anim. 47(Suppl 4), 72–76. [DOI] [PubMed] [Google Scholar]
- 25. Wu J, Liao M, Zhu H, Kang K, Mu H, Song W et al (2014). CD49f‐positive testicular cells in Saanen dairy goat were identified as spermatogonia‐like cells by miRNA profiling analysis. J. Cell. Biochem. 115, 1712–1723. [DOI] [PubMed] [Google Scholar]
- 26. Zhu H, Ma J, Du R, Zheng L, Wu J, Song W et al (2014) Characterization of immortalized dairy goat male germline stem cells (mGSCs). J. Cell. Biochem. 115, 1549–1560. [DOI] [PubMed] [Google Scholar]
- 27. Hu Y, Sun J, Wang J, Wang L, Bai Y, Yu M et al (2012) Characterization of female germ‐like cells derived from mouse embryonic stem cells through expression of GFP under the control of Figla promoter. J. Cell. Biochem. 113, 1111–1121. [DOI] [PubMed] [Google Scholar]
- 28. Zhang S, Sun J, Pan S, Zhu H, Wang L, Hu Y et al (2011) Retinol (vitamin A) maintains self‐renewal of pluripotent male germline stem cells (mGSCs) from adult mouse testis. J. Cell. Biochem. 112, 1009–1021. [DOI] [PubMed] [Google Scholar]
- 29. Zhao Y, Yin X, Qin H, Zhu F, Liu H, Yang W et al (2008) Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell 3, 475–479. [DOI] [PubMed] [Google Scholar]
- 30. Hua J, Zhu H, Pan S, Liu C, Sun J, Ma X et al (2011) Pluripotent male germline stem cells from goat fetal testis and their survival in mouse testis. Cell Reprogram. 13, 133–144. [DOI] [PubMed] [Google Scholar]
- 31. Cao H, Chu Y, Lv X, Qiu P, Liu C, Zhang H et al (2012) GSK3 inhibitor‐BIO regulates proliferation of immortalized pancreatic mesenchymal stem cells (iPMSCs). PLoS One 7, e31502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu M, Mu H, Niu Z, Chu Z, Zhu H, Hua J (2014) miR‐34c enhances mouse spermatogonial stem cells differentiation by targeting Nanos2. J. Cell. Biochem. 115, 232–242. [DOI] [PubMed] [Google Scholar]
- 33. Tee WW, Pardo M, Theunissen TW, Yu L, Choudhary JS, Hajkova P et al (2010) Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev. 24, 2772–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A et al (2009) Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460, 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jansson M, Durant ST, Cho EC, Sheahan S, Edelmann M, Kessler B et al (2008) Arginine methylation regulates the p53 response. Nat. Cell Biol. 10, 1431–1439. [DOI] [PubMed] [Google Scholar]
- 36. Ng WL, Chen G, Wang M, Wang H, Story M, Shay JW et al (2014) OCT4 as a target of miR‐34a stimulates p63 but inhibits p53 to promote human cell transformation. Cell Death Dis. 5, e1024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37. Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M et al (2009) Suppression of induced pluripotent stem cell generation by the p53‐p21 pathway. Nature 460, 1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scoumanne A, Zhang J, Chen X (2009) PRMT5 is required for cell‐cycle progression and p53 tumor suppressor function. Nucleic Acids Res. 37, 4965–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gkountela S, Li Z, Chin CJ, Lee SA, Clark AT (2014) PRMT5 is required for human embryonic stem cell proliferation but not pluripotency. Stem Cell Rev. 10, 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]


