Abstract
Objectives
Stem cell factor (SCF) is essential in the haematopoietic stem cells (HSCs) niche, and is therefore used extensively in haematopoietic stem and progenitor cells (HSPCs) ex vivo expansion. However, in the literature, dose and schedule of SCF feeding varies widely. We previously proposed a novel SCF feeding regimen with proven effectiveness for HSPCs expansion; however, physiological function of expanded cells with this SCF feeding regimen required further research.
Materials and methods
CD34+ cells were cultured with or without SCF supplementation in serum‐free medium for 10 days. Expanded cells were transplanted into sublethally irradiated non‐obese diabetic/severe combined immune‐deficient (NOD/SCID) mice. Engraftment and multilineage reconstitution of transplanted cells were determined. Also, clonogenic potential of engrafted cells was analysed.
Results
Cells, both cultured with and without SCF supplementation, successfully engrafted and reconstituted blood cell lineages in NOD/SCID mice. However, level of engraftment and multilineage reconstitution reduced when cells were expanded without SCF supplementation. Meanwhile, frequencies of colony‐forming cells (CFCs) amongst bone marrow cells were higher in mice transplanted with CD34+ cells expanded with SCF supplementation.
Conclusions
Reconstitution capacity reduced when CD34+ cells were expanded without SCF supplementation, though this feeding regimen did not have any effect on cell expansion. This finding suggested that SCF was essential for preserving NOD/SCID reconstitution capacity of ex vivo expanded CD34+ cells.
Introduction
Haematopoietic stem cells (HSCs) are defined to be the population of cells being able to renew and give rise to haematopoietic progenitor cells (HPCs), which subsequently generate all blood cell lineages, including lymphoid, myeloid and erythroid cells 1. Consequently, transplantation of haematopoietic stem and progenitor cells (HSPCs) has provided a very promising therapeutical strategy for treating various diseases, such as genetic metabolic disorders, malignant and haematological diseases and more 2, 3. Importantly, HSPCs can be harvested from a variety of sources, including bone marrow, mobilized peripheral blood and cord blood (CB). Notably, CB is superior to the other two, as HSPCs derived from CB (CB‐HSPCs) have high proliferative capacity, low incidence and reduced severity of graft‐versus‐host disease (GVHD), and can be conveniently isolated from a potentially large donor pool 4, 5, 6. Thus, transplantation of CB‐HSPCs holds great promise for clinical translation. Since the first human transplantation of CB was performed in 1989, there have been more than 600 000 units of CB reserved in cord blood banks worldwide, and more than 20 000 transplants of CB‐HSPCs have been performed clinically 7, 8. It should be noted that most transplantations of CB‐HSPCs were carried out for treating child patients. In adults, engraftment delay and transplantation failure often occur, which has mainly been attributed to insufficient numbers of HSPCs in each CB unit, for each adult patient. Although double‐unit CB transplantation has been reported to overcome this problem, the success rate has not improved and increase in incidence of GVHD has been observed 2, 9, 10. Thus, limited numbers of HSPCs in each CB unit remains a bottleneck for clinical application of CB‐HSPCs.
Ex vivo expansion provides methodology for increasing numbers of HSPCs and thus may facilitate their better clinical transplantation 11, 12. However, optimal conditions for ex vivo expansion of HSPCs have not yet been achieved. As HSPCs reside in a specialized niche in vivo, investigation of HSPC ex vivo expansion has been focused on simulating the niche microenvironment 13. In particular, Shestopalov et al. have demonstrated that stem cell factor (SCF) is the dominating feature in the HSPC niche and is indispensable in fate decision of HSPCs, including regulation of proliferation, differentiation, cell cycle and apoptosis 14, 15. Accordingly, SCF has been widely employed in combination with thrombopoietin (TPO) and flt3‐ligand (FL) in ex vivo expansion of HSPCs. 16, 17, 18, 19, 20. Previously, it has been reported that the response of primitive cells in the HSPC population was more sensitive to SCF than in the mature population, which was possibly related to activation of c‐kit, the surface receptor of SCF 21, 22, 23. Consistent with this, our group has also found that expression of c‐kit in HSPC declines sharply after 4 days in ex vivo expansion culture 24. Hence, it was hypothesized that the need of SCF in ex vivo expansion of HSPCs varies during maturation of HSPCs.
CD34 is the key surface marker of HSPCs and CD34+ cells are generally considered to be HSPCs 25. In our previous study, a novel SCF feeding regimen was developed and proven to be effective in expanding CD34+ cells, wherein SCF was added only at the time of cell seeding, and no SCF was supplemented during the following culture process 24. This novel SCF feeding regimen did not reduce secondary expansion ability of CD34+ cells, suggesting that initial supplementation of SCF at seeding was sufficient to support expansion of HSPCs 24. However, whether the in vivo physiological function of expanded CD34+ cells was affected by this SCF feeding process warranted further study. The capacities of engraftment and multilineage reconstitution in vivo are critical to assess HSPC ex vivo expansion processes. The non‐obese diabetic/severe combined immune‐deficient (NOD/SCID) mouse is an ideal model to test physiological functions in vivo 26, 27. Thus, in the present study, CB‐derived CD34+ cells were cultured using the novel SCF feeding regimen, then transplanted into NOD/SCID mice, to evaluate engraftment and multilineage reconstitution capacities. Hopefully, results will lead to insight and understanding of effects of SCF on physiological functions of HSPCs, and provide further guidelines in optimizing the ex vivo expansion process for HSPCs.
Materials and methods
Cell preparation
CB units were collected from full‐term deliveries after informed consent of the donors. Mononuclear cells (MNCs) were isolated from CB units, on Ficoll‐Histopaque (density 1.077 g/ml; GE, New York, NY, USA) by density gradient centrifugation. Then, CD34+ cells were purified from the MNCs by magnetic cell sorting, according to the manufacturer's instructions (Miltenyi Biotech, Bergisch Gladbach, Germany). Purity of the CD34+ cells obtained was greater than 95% as confirmed by flow cytometric analysis (FACS Calibur; BD, San Jose, CA, USA).
Cell culture
1 × 105 enriched CD34+ cells were seeded in 2 mL StemSpan serum‐free medium (STEMCELL Technologies, Vancouver, BC, Canada) in each well of 24‐well plates, supplemented with a combination of recombinant human cytokines (Peprotech, Rocky Hill, CT, USA) including SCF at 50 ng/ml, TFO at 20 ng/ml and FL at 50 ng/ml. Twice weekly, cultures were demi‐depopulated and fed fresh medium supplemented with different cytokine combinations as described below. Cell cultures were maintained at 37 °C in a humidified incubator with 5% CO2 and 21% O2 in nitrogen. After 10 days culture, cells were counted and analysed for proportion of CD34+ cells, cell cycle distribution and composition of expanded cells.
Cytokine feeding regimens were set in two groups, as follows:
A: SCF, TPO and FL added to medium at seeding and every feeding.
B: SCF, TPO and FL added to medium at seeding, then TPO and FL only added at every feeding.
Immunophenotype analysis
Total of 1 × 106 cells was collected, washed in phosphate‐buffered saline (PBS) and re‐suspended in 50 μl PBS. Then, cells were incubated in 20 μl respective monoclonal antibodies (BD) at 4 °C for 30 min in the dark. Samples were analysed by flow cytometry.
Cell cycle analysis
Cells were collected, washed in PBS and fixed in 70% iced alcohol for 4 h at −20 °C. Then, they were exposed to 500 μl propidium iodide (PI) solution containing 50 μg/ml RNase, 50 μg/ml PI, 5% Triton‐100 and 0.02% EDTA in PBS for 30 min at 4 °C. Fluorescence intensity of stained cells was measured by flow cytometry.
Mice and transplantation of CD34+ cells
Female 6‐ to 8‐week‐old NOD/SCID mice (SLAC Lab Animal, Shanghai, China) were maintained under specific pathogen‐free conditions, and fed sterilized food and filtered water.
Mice were sublethally irradiated with doses of 270 cGy from a 137Cs source, before transplantation. Mice were then divided into four groups: (1) controls, six mice, transplanted with 300 μl medium, (2) fresh cell group, three mice, transplanted with freshly isolated cells, (3) group A, six mice, transplanted with cells cultured with SCF feeding regimen A, (4) group B, six mice, transplanted with cells cultured with SCF feeding regimen B. Exactly, 1 × 106 CD34+ cells and 5 × 106 CD34− cells suspended in 300 μl medium were transplanted into every mouse via the tail vein within 4–6 h after irradiation. For analysis of engraftment and multilineage reconstitution, experimental mice were sacrificed 6 weeks post‐transplantation.
Analysis of human cell engraftment and multilineage reconstitution in NOD/SCID mice
Mouse bone marrow and spleen cells were harvested to analyse human cell engraftment and multilineage reconstitution. Briefly, bone marrow and spleen cells of mice were treated with lysing solution to eliminate red blood cells, while preserving leukocytes. Then, cells were incubated with CD45‐FITC antibody (BD) for analysis of engraftment of human cells. Presence of at least 0.1% human CD45+ cells in mice was considered to be successful engraftment 28. Further, mouse bone marrow and spleen cells were stained with CD3‐PE, CD19‐PE, CD33‐PE, CD34‐PE and CD71‐PE antibodies (BD) in combination with CD45‐FITC antibody, for quantifying multilineage reconstitution. Isotype controls were performed by labelling cells with mouse IgG monoclonal antibodies.
Colony‐forming cell assay
Mouse cells from bone marrow and spleen were seeded at 5 × 104 cells/ml in 24‐well plates containing IMDM, 20% FBS, 0.9% methylcellulose and the cytokines (50 ng/ml SCF, 20 ng/ml G‐CSF,14 ng/ml GM‐CSF, 20 ng/ml IL‐3, 20 ng/ml IL‐6 and 2 U/ml EPO). Plates were incubated at 37 °C for 14 days in a humidified atmosphere containing 5% CO2. Colonies containing more than 50 cells were identified as from colony‐forming cells (CFCs).
Statistical analysis
Values are presented as mean ± standard error. One‐way ANOVA analysis was applied to evaluate significance of differences. P < 0.05 was considered as significant.
Results
Ex vivo expansion of CD34+ cells with different SCF feeding regimens
With SCF feeding regimens A and B, total cells expanded 136.38 ± 22.98‐ and 113.95 ± 15.11‐fold respectively (Fig. 1a), while percentages of CD34+ cells amongst the expanded ones were 22.47 ± 8.03% and 24.28 ± 6.03%, respectively, indicating that CD34+ cells expanded 29.79 ± 8.24‐ and 24.28 ± 6.03‐fold with SCF feeding regimens A and B respectively (Fig. 1b, c). No significant differences were observed for expansion results between these two SCF feeding regimens. Moreover, distribution of cell cycle phases of those cultured with these two SCF feeding regimens showed no significant differences (Fig. 1d).
Figure 1.
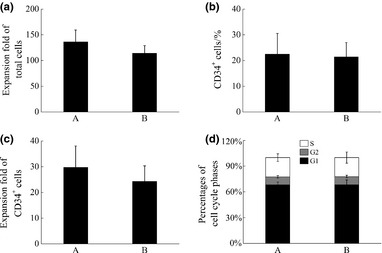
The expansion of HSPCs cultured with SCF feeding regimens A and B on day 10. (a) Expansion folds of total cells, (b) Percentages of CD34+ cells, (c) Expansion folds of CD34+ cells and (d) Distribution of cell cycle phases.
Percentages of CD3+ cells, CD19+ cells, CD33+ cells and CD71+ cells amongst expanded ones are shown in Table 1. It can be observed that percentages of lineage cells did not differ significantly between these two groups.
Table 1.
The percentages of lineage in expanded cells with different SCF feeding regimens (%)
| Groups | CD3+ cells | CD19+ cells | CD33+ cells | CD71+ cells |
|---|---|---|---|---|
| A | 0.17 ± 0.35 | 0.27 ± 0.54 | 73.37 ± 8.55 | 49.12 ± 3.33 |
| B | 1.14 ± 0.88 | 0.32 ± 0.45 | 67.60 ± 17.95 | 45.45 ± 5.95 |
Results above indicate that expansion of CD34+ cells, distribution of cell cycle phases and composition of expanded cells were not affected by SCF supplementation over the 10 day culture process.
CD34+ cell engraftment and multilineage reconstitution in NOD/SCID mice, after culture with different SCF feeding regimens
Physiological function of CD34+ cells ex vivo expanded with different SCF feeding regimens was examined in the NOD/SCID mouse model.
Numbers of mice that survived after transplantation, in different groups, are shown in Table 2. Mice in control groups died within 2 weeks of transplantation, while all mice transplanted with CD34+ cells survived over 6 weeks post‐transplantation, independently of transplanted cells.
Table 2.
The number of survival mice during 6 weeks after cells transplantation (No. of survival mice/No. of experimental mice)
| Group | 1 weeks | 2 weeks | 3 weeks | 4 weeks | 6 weeks |
|---|---|---|---|---|---|
| Control(−) | 6/6 | 6/6 | 0/6 | 0/6 | 0/6 |
| Fresh | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
| A | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| B | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
Percentages of human CD45+ cells in bone marrow and spleen cells of mice were determined, to analyse engraftment of transplanted cells. Results showed that human CD45+ cells were detected in all mice transplanted with CD34+ cells, proving that expanded CD34+ cells with or without SCF supplementation during the ex vivo culture process both engrafted successfully into the NOD/SCID mice (Fig. 2).
Figure 2.
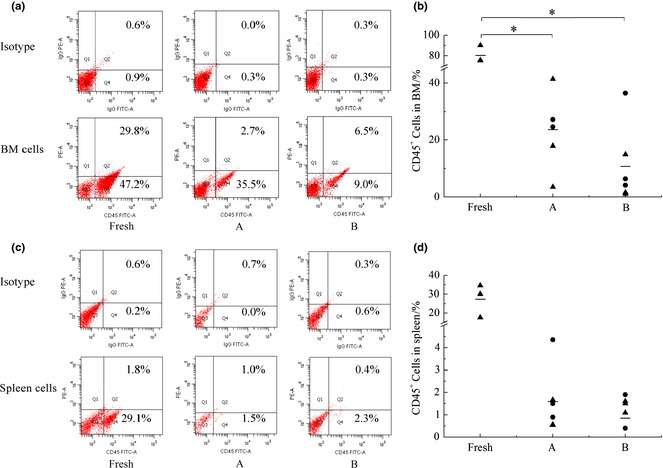
Engraftment levels of expanded cells in bone marrow and spleen cells of recipient mice in different groups. (a) Representative flow cytometric analysis of human CD45+ cells in bone marrow cells of recipient mice, (b) Engraftment levels of human CD45+ cells in bone marrow cells of recipient mice in different groups, (c) Representative flow cytometric analysis of human CD45+ cells in spleen cells of recipient mice and (d) Engraftment levels of human CD45+ cells in spleen cells of recipient mice in different groups. Each black symbol represents one mouse of two independent experiment, the horizontal bars represent the median values. *Represents significant difference (P < 0.05).
Transplantation of freshly isolated CD34+ cells generated an average 80.33 ± 8.46% human CD45+ cells in mouse bone marrows, indicating a significantly higher engraftment level than cultured cells (P = 0.0001 < 0.05). Expanded CD34+ cells led to 23.64 ± 12.50% CD45+ cells in mouse bone marrows of group A, while it resulted in lower levels (10.73 ± 13.56%) of CD45+ cells in mouse bone marrows of group B. The same trends were also found in spleen cells of mice. Although there was no significant difference of engraftment level in mouse bone marrows between groups A and B (P = 0.096 > 0.05), it could be found that percentages of CD45+ cells in bone marrow of mice in group A scattered at higher positions. Then, relative engraftment level was calculated to analyse engraftment capacity in groups A and B, of which engraftment levels of expanded CD34+ cells in mouse bone marrows were normalized to that of the fresh group (Table 3). It was found that five group A mice had relative engraftment levels ranging from 22% to 52%, and only one mouse had relative engraftment level of 4%. Although five mice in group B had relative engraftment levels lower than 20% (including four lower than 10%), only one mouse had relative engraftment level of 45%. It was thus visible that relative engraftment level in bone marrow of mice was higher in group A. Thus, feeding SCF during the culture process contributed to maintaining engraftment capacity of CD34+ cells.
Table 3.
The relative engraftment level in bone marrow cells of individual mice (The percentage of CD45+ cells in BM was normalized to that in Fresh group.)
| Group | Relative engraftment level in BM of mice | |||||
|---|---|---|---|---|---|---|
| A | 4% | 22% | 31% | 34% | 34% | 52% |
| B | 1% | 2% | 5% | 8% | 19% | 45% |
To evaluate multilineage reconstitution capacity of transplanted CD34+ cells, bone marrow and spleen cells of recipient mice were characterized for CD45+CD3+ cells, CD45+CD19+ cells, CD45+CD33+ cells, CD45+CD34+ cells and CD45+CD71+ cells (Fig. 3). Results showed that freshly isolated CD34+ cells, cells cultured with SCF feeding regimens A and B all had the capacity of multilineage reconstitution in the NOD/SCID mice. Percentages of CD45+CD19+ cells and CD45+CD34+ cells were significantly higher in the fresh group than groups A and B (P < 0.05), while there were no significant differences in percentages of the other three lineage cells. Comparing percentages of lineage cells in group A and B, no difference was found, indicating that the SCF feeding regimen did not alert multilineage reconstitution capacity of engrafted cells.
Figure 3.
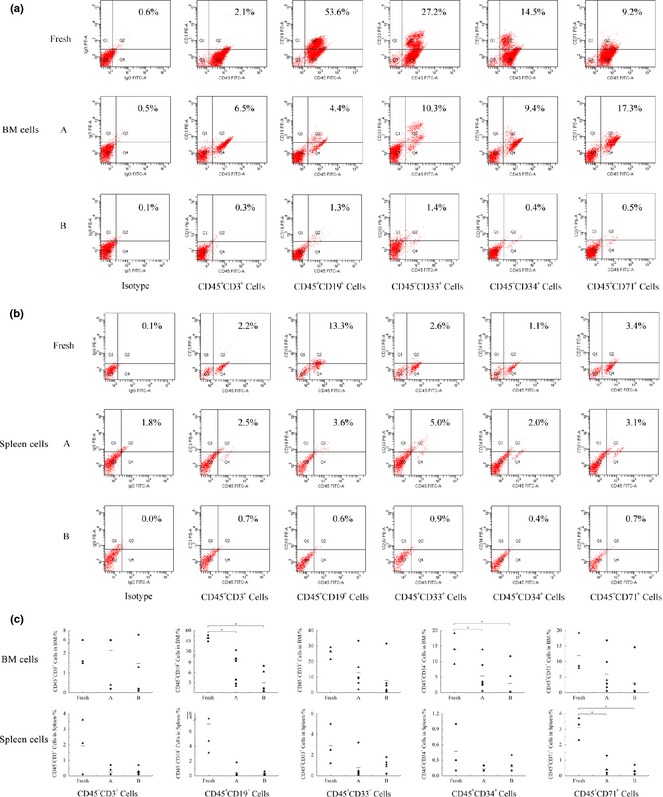
Multilineage reconstitution of expanded cells in bone marrow and spleen cells of recipient mice in different groups. (a) Representative flow cytometric analysis of human lineage cells in bone marrow cells of recipient mice, (b) Representative flow cytometric analysis of human lineage cells in spleen cells of recipient mice and (c) Percentages of lineage cells in bone marrow and spleen cells of recipient mice in different groups. Each black symbol represents one mouse of two independent experiment, the horizontal bars represent the median values. *Represents significant difference (P < 0.05).
To detect clonogenic potential of engrafted cells, cells from bone marrow of recipient mice were seeded in methylcellulose medium for 14 days. As shown in Fig. 4, all cells derived from transplanted mice generated colonies. Mean numbers of CFCs were 86 (ranging from 14 to 126), 82 (ranging from 22 to 147) and 35 (ranging from 5 to 108) in 1 × 105 mouse bone marrow cells of fresh, A and B groups respectively. It was found that mean CFCs frequency of cells of mice in group A was higher than that of mice in group B. This result demonstrated that SCF supplementation preserved clonogenic potential of the engrafted cells.
Figure 4.
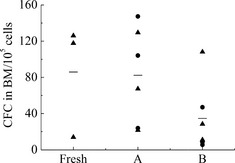
The frequencies of CFCs in bone marrow cells of recipient mice in different groups(/10 5 cells). Each black symbol represents one mouse of two independent experiment, the horizontal bars represent the median values.
Discussion
Ex vivo expansion has been recognized as an attractive way to increase cell number of HSPCs and thus overcome the bottleneck in clinical application of HSPCs. Various attempts have been carried out to improve the ex vivo expansion process. Capacities of engraftment and multilineage reconstitution in vivo are important to assess ex vivo expansion of HSPCs. A well‐conditioned ex vivo expansion process needs to balance both quantity and quality of HSPCs.
Application of cytokines in ex vivo expansion of HSPCs has been developed over a long period. However, few studies have concerned themselves with cytokine feeding regimens, which however, importantly influence ex vivo expansion of HSPCs 18. In our previous study, a novel cytokine feeding regimen was proposed in which SCF was just added to the initial medium, then no further SCF was supplemented during the culture process 24. This SCF feeding regimen was proven to be effective for expanding HSPCs ex vivo. Percentage of CD34+ cells and CD34+ CD38− cells, composition and secondary ex vivo expansion ability of cultured cells also showed similar trends compared to traditional cytokine feeding regimens, in which SCF was supplemented at every feeding. This novel SCF feeding regimen can simplify the operation and reduce costs, without compromising quantity of expanded HSPCs.
To investigate effects of SCF feeding regimen on quality of expanded HSPCs, engraftment and mulitilineage reconstitution capacities of ex vivo expanded CD34+ cells were analysed in NOD/SCID mice, which represent a suitable model for evaluation of engraft and reconstitution capacities of transplanted cells 27. Results showed that CD34+ cells expanded with these two SCF feeding regimens had engraftment and multilineage reconstitution capacities in NOD/SCID mice.
Different scatter of engraftment level between groups A and B were observed, though there were no statistical differences in percentages of CD45+ cells between these two groups, which may result from the limited sample size and the individual differences between mice. Thus, engraftment level relative to the fresh group was calculated, to analyse engraftment capacity of cells cultured with different SCF feeding regimens; results showed that SCF feeding regimen A achieved higher relative engraftment levels in the mice. In addition, it was found that transplantation of freshly isolated cells resulted in a 3.40‐ and 7.49‐fold engraftment compared to cells expanded with SCF feeding regimens A and B, respectively, when mice were injected with the same number of cells. As CD34+ cells expanded 29.79‐ and 24.28‐fold with SCF feeding regimens A and B, respectively, 1 × 106 CD34+ cells for transplantation came from about 3.4 × 104 and 4.1 × 104 initial CD34+ cells in groups A and B. Accordingly, SCF regimens A and B most likely led to an approximately 8.77‐ and 3.24‐fold increase in engrafting cells, respectively, when cell expansion folds were taken into account. Also, similar CFC densities of engrafted cells from mice in group A and the fresh group representing a 28.40‐fold increase of clonogenic potential, came from SCF feeding regimen A. In addition, SCF feeding regimen B resulted in 11.59‐fold increase of clonogenic potential compared to freshly isolated CD34+ cells. This result further demonstrated that SCF feeding regimen A was more effective to expand engrafting cells than group B.
In conclusion, capacities of engraftment and multilineage reconstitution in NOD/SCID mice, of expanded CD34+ cells, were well preserved when SCF was supplemented into the medium during the culture process. SCF is important in stimulating proliferation of HSPCs and supports their engraftment and reconstitution in vivo.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (20776043), the Key Project of Medicine, Shanghai (074319109) and the Open Project of State Key Laboratory of Bioreactor Engineering.
References
- 1. Kishimoto S, Nakamura S, Hattori H, Oonuma F, Kanatani Y, Tanaka Y et al (2009) Cytokine‐immobilized microparticle‐coated plates for culturing hematopoietic progenitor cells. J. Control. Release 133, 185–190. [DOI] [PubMed] [Google Scholar]
- 2. Sozos JF, Schenker JG (2000) Human umbilical cord blood banking and transplantation: a state of the art. Eur. J. Obstet. Gynecol. Reprod. Biol. 90, 13–25. [DOI] [PubMed] [Google Scholar]
- 3. Felfly H, Haddad GG (2014) Hematopoietic stem cells: potential new applications for translational medicine. J. Stem Cells. 9, 163–197. [PubMed] [Google Scholar]
- 4. Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D et al (1989) Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. PNAS 86, 3828–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gluckman E (2000) Current status of umbilical cord blood hematopoietic stem cell transplantation. Exp. Hematol. 28, 1197–1205. [DOI] [PubMed] [Google Scholar]
- 6. Yvonne JS, Clare MH, Erika AW, Claire AH, James C, Testa NG (2004) AC133+ G0 cells from cord blood show a high incidence of long‐term culture‐initiating cells and a capacity for more than 100 million‐fold amplification of colony‐forming cells in vitro . Stem Cells 22, 704–715. [DOI] [PubMed] [Google Scholar]
- 7. Gluckman E, Ruggeri A, Volt F, Cunha R, Boudjedir K, Rocha V (2011) Milestones in umbilical cord blood transplantation. Br. J. Haematol. 154, 441–447. [DOI] [PubMed] [Google Scholar]
- 8. Gluckman E, Rocha V (2009) Cord blood transplantation: state of the art. Haematologica 94, 451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sideri A, Neokleous N, Brunet De La Grange P, Guerton B, Le Bousse Kerdilles MC, Uzan G et al (2011) An overview of the progress on double umbilical cord blood transplantation. Haematologica 96, 1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wagner JE, Eapen M, Carter S, Wang Y, Schultz KR, Wall DA et al (2014) One‐unit versus two‐unit cord‐blood transplantation for hematologic cancers. N. Engl. J. Med. 371, 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ann D, Colleen D, Bernstein ID (2011) Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood 117, 6083–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Csaszar E, Cohen S, Zandstra PW (2013) Blood stem cell products: toward sustainable benchmarks for clinical translation. BioEssays 35, 201–210. [DOI] [PubMed] [Google Scholar]
- 13. Martinez‐Agosto JA, Mikkola HKA, Hartenstein V, Banerjee U (2007) The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 21, 3044–3060. [DOI] [PubMed] [Google Scholar]
- 14. Ding L, Thomas LS, Grigori E, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shestopalov IA, Zon LI (2012) Stem cells: The right neighbour. Nature 481, 453–455. [DOI] [PubMed] [Google Scholar]
- 16. Kishimoto S, Nakamura S, Hattori H, Nakamura S, Oonuma F, Kanatani Y et al (2009) Human stem cell factor (SCF) is a heparin‐binding cytokine. J. Biochem. 145, 275–278. [DOI] [PubMed] [Google Scholar]
- 17. Wohrer S, Knapp DJ, Copley MR, Benz C, Kent DG, Rowe K et al (2014) Distinct stromal cell factor combinations can separately control hematopoietic stem cell survival, proliferation, and self‐renewal. Cell Rep. 7, 1956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andrade PZ, Santos Fd, Cabral JMS, da Silva CL (2013) Stem cell bioengineering strategies to widen the therapeutic applications of haematopoietic stem/progenitor cells from umbilical cord blood. J. Tissue Eng. Regen. Med. doi: 10.1002/term.1741. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19. Delaney C, Heimfeld S, Brashem‐Stein C, Voorhies H, Manger RL, Bernstein ID (2010) Notch‐mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat. Med. 16, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tursky ML, Collier FM, Ward AC, Kirkland MA (2012) Systematic investigation of oxygen and growth factors in clinically valid ex vivo expansion of cord blood CD34(+) hematopoietic progenitor cells. Cytotherapy 14, 679–685. [DOI] [PubMed] [Google Scholar]
- 21. Kent D, Copley M, Benz C, Dykstra B, Bowie M, Eaves C (2008) Regulation of hematopoietic stem cells by the steel factor/kit signaling pathway. Clin. Cancer Res. 14, 1926–1930. [DOI] [PubMed] [Google Scholar]
- 22. Michelle BB, David GK, Michael RC, Eaves CJ (2007) Steel factor responsiveness regulates the high self‐renewal phenotype of fetal hematopoietic stem cells. Blood 109, 5043–5048. [DOI] [PubMed] [Google Scholar]
- 23. Michelle BB, David GK, Brad D, Kristen DM, Lindsay M, Pamela AH et al (2007) Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. PNAS 104, 5878–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Du Z, Cai H, Ye Z, Tan W‐S (2012) Optimization of SCF feeding regimen for ex vivo expansion of cord blood hematopoietic stem cells. J. Biotechnol. 164, 211–219. [DOI] [PubMed] [Google Scholar]
- 25. Kikushige Y, Yoshimoto G (2008) Human flt3 Is expressed at the hematopoietic stem cell and the granulocyte macrophage progenitor stages to maintain cell survival. J. Immunol. 180, 7358–7367. [DOI] [PubMed] [Google Scholar]
- 26. Ewa S, Natalija BV, Staffan L, Jens MN, Karina L, Jacobsen SE. (2003) Human CD34+ hematopoietic stem cells capable of multilineage engrafting NOD/SCID mice express flt3: distinct flt3 and c‐kit expression and response patterns on mouse and candidate human hematopoietic stem cells. Blood 102, 881–886. [DOI] [PubMed] [Google Scholar]
- 27. van der Loo JCM, Hanenberg H, Cooper RJ, Luo F‐Y, Lazaridis EN, Williams DA (1998) Nonobese Diabetic/Severe Combined Immunodeficiency (NOD/SCID) Mouse as a Model System to Study the Engraftment and Mobilization of Human Peripheral Blood Stem Cells. Blood 92, 2556–2570. [PubMed] [Google Scholar]
- 28. Wang J, Kimura T, Asada R, Harada S, Yokota S, Kawamoto Y et al (2003) SCID‐repopulating cell activity of human cord blood–derived CD34− cells assured by intra–bone marrow injection. Blood 101, 2924–2931. [DOI] [PubMed] [Google Scholar]


