Abstract
Objectives
The balance between survival and death is a key point for regulation of physiology of stem cells. Recently, applications of natural products to enhance efficiencies in culturing and differentiation of stem cells are increasing. Korean mistletoe lectin (Viscum album L. var. coloratum agglutinin, VCA) has been known to be toxic to some cancer cells, but it is still unclear whether VCA has a cytotoxic or indeed a proliferative effect on mesenchymal stem cells (MSCs). Here, we have compared effects of VCA in naïve placenta‐derived stem cells (PDSCs), immortalized PDSCs and cancer cells (HepG2), and analysed their mechanisms.
Materials and methods
MTT assay was performed to analyse effects of VCA on naïve PDSCs, immortalized PDSCs and HepG2. FACS, ROS, caspase‐3 assay, western blotting and immunofluorescence were performed to detect signalling events involved in self‐renewal of the above cell types.
Results
VCA had cancer cell‐specific toxicity to HepG2 cells even with low concentrations of VCA (1–5 pg/ml), toxicity was observed to immortalized PDSCs and HepG2s, while proliferation of naïve PDSCs was significantly increased (P < 0.05). ROS production by VCA treatment in naïve PDSCs was significantly lower compared to controls (P < 0.05). Furthermore, autophagy was activated in naïve PDSCs treated with VCA through increase in type II LC3 and decrease in phosphorylated mTOR.
Conclusions
VCA can promote MSC proliferation through an activated autophagic mechanism.
Introduction
Mesenchymal stem cells (MSCs) derived from a variety of adult tissues have potential for self‐renewal and differentiation into multiple lineages, including mesodermal‐type cells (such as chondrocytes, adipocytes and osteoblasts) 1, 2, 3. Also, MSCs have been highlighted as powerful cells for use in cell therapy for degenerative diseases, as they also have an immunomodulating effect, an important factor in cell therapy 4, 5, 6. Furthermore, MSCs are sensitive to several types of reagent during proliferation and differentiation as they express the ATP‐binding cassette (ABC) multi‐drug transporter; such systems having been commonly observed during cancer chemotherapy. Among several ABC transporters, ABCG2 is known to be widely distributed in both cancer cells and those of normal tissues. It is also highly expressed in stem cells, as first described in bone marrow MSCs, by their ability to efflux Hoechst 33342 dye 7. Since that discovery, ABCG2 has been recognized to be a universal marker for stem cells. Several results have shown that ABCG2 plays a crucial role in protecting stem cells as well as in promoting proliferation of haematopoietic progenitors and stem cells 8, 9.
Bone marrow‐derived mesenchymal stem cells (BM‐MSCs), which are a representative MSCs, have been studied to be able to analyse the unique characterization of MSCs and to evaluate their efficacies in a number of degenerative diseases 10; they have also been used as a source for cell therapy in clinical trials around the world 11, 12, 13. However, only a limited number of cells can be collected from a donor, and self‐renewal activity seems to depend on age of the donor 14. Also, aggressive procedures followed to isolate BM‐MSCs are a drawback.
Recently, placenta‐derived stem cells (PDSCs) have been reported to be an alternative source of MSCs due to the following advantages: (i) there are no ethical problems involved, as PDSCs are isolated from placentas after full‐term birth; (ii) large number of cells are easily accessible; (iii) they have the potential to differentiate into multiple lineages; (iv) they are immunosuppressive; and (v) they have display good self‐renewal activity without dependency on age of the donor 15, 16. However, there are still obstacles to overcome any limits in extent of self‐renewal required to obtain the huge numbers of MSCs needed to be used in clinical trials.
Self‐renewal of MSCs is regulated by their balance between cell survival and cell death 17. A major cause of their DNA damage, followed by apoptosis, or senescence, is by reactive oxygen species (ROS). Several studies have shown that level of ROS generated has an important role in maintaining physiological function in various cell types 18. Alterations in ROS can regulate self‐renewal ability of embryonic stem cells (ESCs) and can control self‐renewal ability by expressing high activity of multidrug transporters 9.
Autophagy and apoptosis play an essential role in regulation of self‐renewal in cancer cells 19, 20. Autophagy, leading to such type II cell death is characterized by autophagic vesicles produced by the cellular lysosomal system 21, 22 and it is well known that autophagosomes (typical markers of autophagy), are produced by the regulatory mechanism of autophagy‐related (Atg) proteins, Beclin‐1 and LC3 23. Also, autophagy can prevent cell death when cells are exposed to various detrimental environments, for instance, absence of growth factors, hypoxic conditions, or presence of a chemotherapeutic agent, through decreased expression of ROS 24.
In previous reports, natural products such as buzhong yipi have been shown to regulate self‐renewal of MSCs by controlling the cell cycle through the retinoic acid receptor (RAR) pathway 25. Also, Potu et al. reported that the extract of cissus quadrangularis promotes proliferation of MSCs 26. In addition, the extract of Echinacea purpurea has been shown to increase proliferation and to inhibit apoptosis, in mouse embryonic palatal shelf mesenchymal stem cells 27, although exact mechanisms of this are still unknown.
A galactose‐ and N‐acetyl‐d‐galactosamine‐specific lectin (Viscum album L. var. coloratum agglutinin, VCA) isolated from Korean mistletoe (Viscum album L. var. coloratum), a natural product isolated from semi‐parasitic plants, has been used therapeutically to treat some cancers, and it has been reported that European mistletoe lectin (Viscum album L. agglutinin, VAA) shows anti‐cancer activity through cancer cell‐specific cytotoxicity 28, 29, 30. Also, it has reported that VAA has different effects depending on its different concentrations 31, 32. In a previous report, we demonstrated that VCA can reduce cell proliferation at high concentrations, but promotes cell proliferation at lower concentrations 33. In addition, VCA controlled proliferation of cancer cells by upregulating Bcl2 and downregulating Bak via the Akt pathway 29. Also, VCA has been known to induce population growth of haematopoietic progenitor CD34+ cells 34. There are no reports, however, on effects of VCA regarding proliferation or self‐renewal of MSCs, and their underlying mechanisms for regulating their self‐renewal. Furthermore, the role of autophagy has not previously been studied on self‐renewal of stem cells including of MSCs.
Thus, the objectives of this study were to analyse the effect of VCA on PDSCs and to demonstrate the underlying mechanisms of VCA on self‐renewal regulation on them, by autophagy.
Materials and methods
Isolation and purification of Korean mistletoe lectin
VCA (Viscum album L. var. coloratum agglutinin, VCA) was purified from Korean mistletoe (Viscum album L. var. coloratum) grown on oak trees, by affinity chromatography on asialofetuin‐Sepharose 4B as described previously 35.
Cell culture
PDSCs were isolated as described previously 36 and immortalized PDSCs were produced by overexpression of hTERT genes by Amaxa‐nuceo factor kits (Lonza, Gampel Switzerland). Cells were cultured in DMEM/F12 (Gibco‐BRL, Grand Island, NY, USA), supplemented with 10% foetal bovine serum (FBS; Gibco‐Invitrogen, Grand Island, NY, USA), 100 U/ml penicillin (Gibco‐Invitrogen), 100 μg/ml streptomycin (Gibco‐BRL), 25 ng/ml FGF4 (fibroblast growth factor 4) (Peprotech Inc., Rocky Hill, NJ, USA) and 1 μg/ml heparin (Sigma, St Louis, MO, USA), at 37 °C, in humidified 5% CO2, 95% air. Hepatocellular carcinoma cells (HepG2) cell line provided by Dr. Ja‐Jun Jang (Seoul National University, Korea) were cultured in DMEM (Gibco‐Invitrogen)/high glucose medium supplemented with 10% FBS (Gibco‐Invitrogen), 100 U/ml penicillin (Gibco‐Invitrogen), 100 μg/ml streptomycin (Gibco‐BRL) and 2 mm l‐glutamine (Gibco‐Invitrogen) at 37 °C, in humidified 5% CO2, 95% air.
Cell proliferation assay
Naïve PDSCs (3 × 103 cells/well), immortalized PDSCs (2.5 × 103 cells/well) and HepG2 (3 × 103 cells/well) cells were cultured in 96‐well plates (BD Falcon; BD Bioscience Discovery Labware, Bedford, MA, USA) for 24 h at 37 °C, in humidified 5% CO2, 95% air. Cells were then treated with 0, 1, 5, 10, 50, 100, 500, 1,000, 5,000 and 10,000 pg of VCA diluted in culture medium, and incubated for a further 24 h. In vitro cell viability was measured using 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazollum bromide (MTT) analysis. Culture medium was replaced with MTT (Sigma)‐contained medium (0.5 mg/ml) and incubated for 2 h. Absorbance was measured at 562 nm after being dissolved in 200 μl of dimethylsulphoxide. All experiments were performed in triplicate.
Western blot analysis
VCA‐treated (0, 1, 5 and 10 pg) PDSCs, immortalized PDSCs and HepG2 cells were cultured in 100‐mm dishes for 24 h (6 × 105/well). Cells were gathered by scraping the plates and were lysed in ice‐cold RIPA buffer (50 mmol/l Tris–HCl, pH 7.6, 150 mol/l NaCl, 1% NP40, 0.25% deoxycholic acid, 0.1% SDS, 1 mmol/l EGTA, 1 mmol/l EDTA, 10 mmol/l NaF and 1 mmol/l Na3VO4) in a protease inhibitor cocktail (Sigma). After heating at 95 °C for 10 min, total protein extract (40 μg) was separated on SDS–PAGE (8–12%) and subsequently transferred to PVDF membranes (Bio‐Rad, Berkeley, CA, USA). Samples were incubated in blocking buffer [0.05% Tween 20, 8% bovine serum albumin (BSA) in tris‐buffered saline (TBS)] for 40 min at room temperature. Membranes were incubated in primary antibody in 5% blocking buffer overnight at 4 °C, before being washed once in TBST (0.05% Tween in TBS). Primary antibodies were used at the following dilutions: mouse anti‐phosphorylated ERK, rabbit anti‐phosphorylated Akt, rabbit anti‐PTTG1 and mouse anti‐p53 at 1:1000 (Cell Signaling Technology, Danvers, MA, USA), mouse anti‐nanog at 1:400 (Cell Signaling Technology), rabbit anti c‐myc, rabbit anti‐ cyclin D1, rabbit anti‐ cyclin E1, mouse anti‐ABCG2, mouse anti‐PI3KIII, rabbit anti‐Beclin‐1, mouse anti‐atg5‐12, rabbit anti‐LC3, rabbit anti‐Bcl2, rabbit anti‐Bax, and mouse anti‐HIF1α at 1:1000 (Cell Signaling Technology), rabbit anti‐phosphorylated mTOR at 1:300 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and rabbit anti‐GAPDH at 1:3000 (Cell Signaling Technology). Membranes were then incubated with the secondary antibody, horseradish peroxidase conjugated anti‐rabbit IgG (1:25 000; Cell Signaling Technology) or anti‐mouse IgG (1:25 000; Cell Signaling Technology) for 1 h at room temperature using an orbital shaker. After four washings, bands were detected using an enhanced chemiluminescence reagent (Amersham Biosciences, Arlington Heights, IL, USA).
Detection of autophagy
VCA‐treated PDSCs, immortalized PDSCs and HepG2 cells (4 × 104/well) were grown on glass coverslips in 24‐well plates for 24 h and washed in phosphate‐buffered saline (PBS). Coverslips were blocked with protein block serum‐free buffer at 37 °C for 30 min and cells were incubated in mouse anti‐human LC3 (1:200; Novus Biologicals, Littleton, CO USA) in blocking buffer overnight at 4 °C. Coverslips were washed in PBS and incubated in secondary antibody conjugated to Alexa586 (1:400) for 40 min at room temperature. Nuclear morphology was revealed by 4′, 6‐diaminino‐2‐phenlindole staining (final concentration: 100 ng/ml). Specimens were treated with fluorescence‐mounted medium (Dako North America, Camarillo, CA, USA) and fluorescence was observed using a Nikon Eclipse E600 (Nikon Corp, Tokyo, Japan) microscope; images were obtained using Nikon digital camera DXM‐1200F (Nikon). We also used the immunofluorescence method to detect expression of monodansylcadaverine (MDC) staining. VCA‐treated PDSCs, immortalized PDSCs and HepG2 cells were cultured on glass coverslips in 24‐well plates for 24 h. Afterwards, cells were incubated in 0.05 mm of MDC (Sigma) for 30 min at 37 °C and washed in PBS. Cells were then fixed in 4% (v/v) paraformaldehyde for 30 min and coverslips were exposed to fluorescence‐mounted medium (Dako North America). Fluorescence was observed using the Nikon Eclipse E600 (Nikon) microscope and images were obtained using the Nikon Digital camera DXM‐1200F (Nikon).
Flow cytometric analysis
To analyse the cell cycle, VCA‐treated cells (1 × 106) were harvested, washed and fixed in ice‐cold 70% ethanol for 10 min at room temperature. Subsequently, fixed cells were stained in a solution containing 50 μg/ml propidium iodide and 10 μg/ml RNase, for 30 min at room temperature. Percentages of cells in the different phases of the cell cycle were determined using a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA) and quantified with CellQuest software (BD Biosciences).
Reactive oxygen species assay
VCA‐treated PDSCs, immortalized PDSCs and HepG2 cells were cultured in 100 mm dishes for 24 h and were harvested (1 × 106 cells) in 5‐ml FACS tubes. Cells were centrifuged at 280 g for 5 min and the supernatant was discarded. Cells were resuspended in 20 μm DCF‐DA (dichlorofluorescein diacetate) dye in PBS (Invitrogen Corp, Carlsbad, CA, USA) and incubated for 30 min at 37 °C and were washed in PBS. Again, cells were centrifuged at 2500 rpm for 5 min. Then, supernatant was discarded and ROS production was measured using CellQuest software (BD Biosciences) using a FITC emission filter.
Caspase‐3 ELISA assay
Caspase‐3 activity was measured using a Caspase‐Glo 3 assay kit (Promega, Madison, WI, USA) according to the instructions provided by the manufacturer. In brief, 100 μl of Caspase‐Glo reagent was added to each well, and mixed using a 96‐well plate shaker at 40 g for 30 s. The plate was then incubated at room temperature for 1 h. Absorbance was measured at 470 nm. All experiments were performed in duplicate.
Statistical analyses
Statistical analyses were performed using Student's t‐test, or ANOVA using sas (SAS Institute Inc., Cary, NC, USA). P‐value <0.05 was considered statistically significant.
Results
Effect of VCA on proliferation of naïve PDSCs, immortalized PDSCs and HepG2 cells
To confirm effects of VCA on cell proliferation, MTT assay was performed on naïve PDSCs, immortalized PDSCs and HepG2 cells treated with a variety of concentrations of VCA. Consistent with previous reports on VCA cytotoxicity, viabilities of HepG2 cells and immortalized PDSCs were reduced, regardless of concentration of VCA, compared to controls. However, low concentrations including 1, 5 and 10 pg/ml of VCA increased proliferation of naïve PDSCs. Specifically, proliferation of naïve PDSCs was elevated up to 1.42‐fold of control value (P < 0.05) when treated with 10 pg/ml of VCA (Fig. 1). In contrast, there were no changes in viability of naïve PDSCs when treated with concentrations over 50 pg/ml of VCA. These findings indicate that low concentrations of VCA can enhance proliferation of naïve PDSCs without causing any cytotoxic effect.
Figure 1.
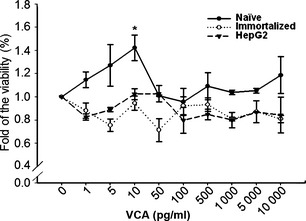
Effects of VCA on viability of naïve PDSC s, immortalized PDSC s and HepG2 cells. MTT analysis of proliferation assay (●) naïve PDSCs, (○) immortalized PDSC and (▼) HepG2. Data are presented as mean ± SD; *P < 0.05, compared to respective controls.
VCA increased proliferation of naïve PDSCs by regulating G 1/S phase of the cell cycle
To further elucidate mechanisms involved in proliferation of naïve PDSCs by VCA, FACS analysis was performed using low concentrations of VCA. Naïve PDSCs in S phase increased in a dose‐dependent manner of VCA, reaching 10.59% when treated with 10 pg/ml of VCA (Fig. 2a). On the other hand, in S phase, immortalized PDSCs and HepG2 composed 12.81% and 8.9% respectively (Fig. 2b,c). In addition, VCA was found to elevate sub G1 phase of immortalized PDSCs compared with others. These results indicate that VCA increases proliferation of naïve PDSCs through induction of S phase of the cell cycle.
Figure 2.
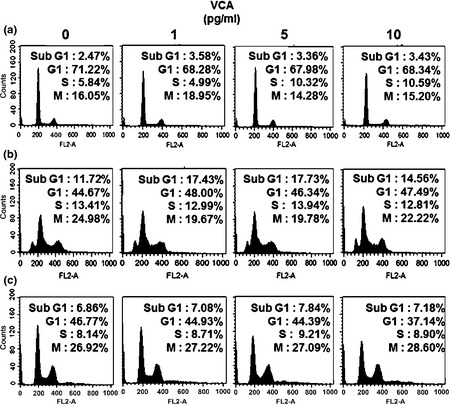
Cell cycle analysis of each group depend on VCA concentration. Cell cycle analysis of (a) naïve PDSCs, (b) immortalized PDSCs and (c) HepG2 when treated with various concentrations of VCA, using FACS analysis. Numbers of cells in each phase of the cell cycle are indicated as percentages (%).
Effect of VCA on expression of factors regulating cell cycle and proliferation
Next, we performed western blots on proteins characteristic of cell cycle phases and proliferation. Expressions of cell cycle‐related determinants, including cyclin D1, E1, p53, PTTG1, c‐Myc and SCF rose in naïve PDSCs, depending on VCA dosage. Interestingly, expressions of cyclin D1 and E1 reduced in immortalized PDSCs, and expression of p53 was high. In contrast, no changes in expression of cyclin D1, cyclin E1 and p53 were found in HepG2 cells. In addition, there were no differences in expression of PTTG1, c‐Myc and SCF in immortalized PDSCs and HepG2 cells (Fig. 3a). Subsequently, we analysed expression of proliferation associated proteins nanog, ABCG2, p‐ERK, p‐Akt and p‐STAT3. In naïve PDSCs, expression of nanog and ABCG2, p‐ERK and p‐STAT3 were elevated at 5 and 10 pg/ml of VCA respectively compared to control. Expression of p‐Akt was modified in naïve PDSCs when treated with 10 pg/ml VCA, but there were no changes in expression at other concentrations of VCA. Interestingly, expression level of p‐Akt in immortalized PDSCs decreased depending on concentration of VCA, but expressions of nanog and ABCG2 were constant regardless of VCA treatment. Also, expressions of p‐ERK and p‐STAT3 increased in dose‐dependent manner of VCA. Expressions though of nanog, ABCG2, p‐ERK, p‐Akt and p‐STAT3 reduced in HepG2 cells when treated with VCA in a dose‐dependent manner (Fig. 3b). These results indicate that VCA may enhance proliferation of stem cells including naïve PDSCs and immortalized PDSCs, especially by regulating factors of the cell cycle and proliferation.
Figure 3.
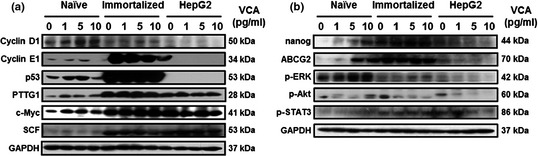
Analysis of the cell cycle and proliferation in naïve PDSC s, immortalized PDSC s and HepG2 cells, depends on VCA . (a) Expression of cell cycle‐related factors and (b) expression of proliferation markers in naïve PDSCs, immortalized PDSCs and HepG2 cells. GAPDH was used as a loading control.
Effect of VCA on cell death by alteration in intracellular ROS
As we found that treatment of VCA could modulate cell proliferation and viability through regulating cell cycle‐ and proliferation‐related factors in naïve PDSCs and immortalized PDSCs, we hypothesized that VCA may be capable of regulating self‐renewal in naïve PDSCs only. To analyse correlation between stress level and programmed cell death (apoptosis) in the cells by VCA, intracellular ROS assay was performed. VCA consistently elevated the level of intracellular ROS in HepG2 cells, depending on concentration of VCA. In case of immortalized PDSCs, intracellular ROS was increased at 10 pg/ml of VCA, but VCA‐induced ROS production in naïve PDSCs significantly reduced in a dose‐dependent manner (P < 0.05, Fig. 4a). In addition, we carried out western blot analysis to detect markers of apoptosis, and caspase 3 assay to determine caspase‐3 activity. Expression of the anti‐apoptotic factor Bcl‐2 was increased in naïve PDSCs in a dose‐dependent manner, but the expression of Bax was reduced. On the other hand, expression patterns of Bcl‐2 and Bax were reduced in immortalized PDSCs according to concentration of VCA used. However, there was no change in the expression patterns in HepG2 cells, although weak expression of Bcl‐2 at 1 pg/ml of VCA was observed (Fig. 4b). In addition, VCA downregulated caspase‐3 activity in naïve PDSCs in a dose‐dependent manner. Also, caspase‐3 activities in HepG2 cells were reduced except at 1 pg/ml of VCA, where caspase‐3 expression was elevated. These findings imply that VCA can induce necrotic cell death in HepG2 cells without them undergoing apoptosis. Interestingly, casapase‐3 activities of immortalized PDSCs were elevated by 1.4‐fold between 1 and 5 pg/ml of VCA, but reduced at 10 pg/ml of VCA (Fig. 4c). These results indicate that VCA may regulate stem‐cell apoptosis including of naïve and immortalized PDSCs by differently inducing intracellular ROS, depending on cell type.
Figure 4.
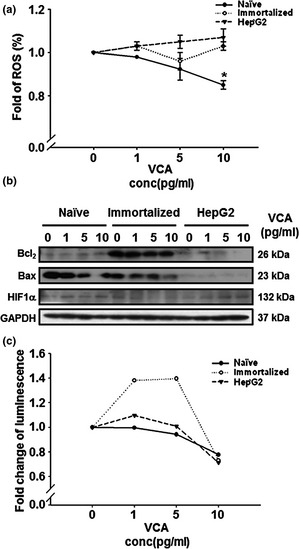
Effect of VCA on cell death in naïve PDSC s, immortalized PDSC s and HepG2 cells. (a) Intracellular ROS production in (●) naïve PDSCs, (○) immortalized PDSCs and (▼) HepG2 by ROS assay. Data are presented as mean ± standard deviation (SD); *mean P < 0.05 when compared with respective controls. (b) Expression of Bcl2 and Bax in cells using western blot analysis. GAPDH was used as a loading control. (c) Expression of active caspase‐3 in (●) naïve PDSCs, (○) immortalized PDSCs and (▼) HepG2 cells by VCA treatment using caspase‐3 assay.
Effects of VCA on autophagic pathway and formation of autophagososomes
Autophagy, type II programmed cell death, is a degradation process involving self‐digestion, by fusion of autophagic vacuoles with the cell's lysosomal machinery. It has a role in cell death as well as in cell survival, through recycling of molecular cell‐building blocks obtained from their own digested organelles. Thus, we performed western blot analysis to detect autophagic markers, and immunostaining of LC3 and MDC to detect autophagosome formation. As shown in Fig. 5a, expression of phosphorylated mTOR (p‐mTOR), a negative regulator of autophagy, was reduced in naïve PDSCs, but was elevated in immortalized PDSCs and HepG2 cells, in a dose‐dependent manner of VCA. Expression patterns of PI3KIII were the opposite of expression of p‐mTOR; VCA‐induced Beclin‐1 increased in all cell types. However, expression of LC3 II (an active form of LC3), was only increased in naïve PDSCs, but expressions of phosphorylated mTOR, PI3KIII and Beclin‐1 in immortalized PDSCs were shown to increase slightly, but not expressions of type II LC3 (active LC3); phosphorylated mTOR and Beclin‐1 in HepG2 were consistently expressed (Fig. 5a).
Figure 5.
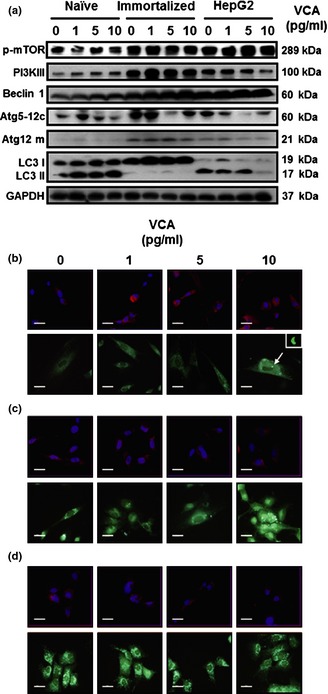
Effects of VCA on autophagic pathways and formation of autophagososomes. (a) Expression of autophagy‐related factors in naïve PDSCs, immortalized PDSCs and HepG2 cells. GAPDH was used as a loading control. (b) Expression of LC3 in cells using immunofluorescence. PI was used for nuclear staining. (c) Monodansylcadaverine (MDC) staining to detect autophagosomes. Scale bar = 80 μm. Arrowhead indicates horseshoe‐shaped structures.
Next, we analysed autophagosome formations at early stages of autophagy, using immunofluorescence/LC3. Immunofluorescence revealed presence of many cup‐shaped and ring‐like structures in both cell types, which represent autophagosomes. As shown in Fig. 5b, expression of LC3, a marker of early stages of autophagosome formation, in naïve PDSCs were revealed as cytoplasmic particles (red) which gradually increased, in a dose‐dependent manner of VCA (Fig. 5b, upper). There were no differences in LC3‐positive vesicles in immortalized PDSCs (Fig. 5b, middle) but, LC3‐positive vesicles in HepG2 cells treated with VCA were low compared to control (Fig. 5b, lower). Additionally, we used the MDC staining method; this is a fluorescent compound that becomes incorporated into autophagosome vacuoles. VCA‐induced MDC signals in naïve PDSCs increased compared to controls and strong horseshoe‐shaped signals were observed at 10 pg/ml of VCA (Fig. 5c, upper). VCA‐induced MDC signals in immortalized PDSCs were similar to controls (Fig. 5c, middle), and there were no changes in MDC signal in HepG2 cells (Fig. 5c, lower). From these results, we confirmed formation of autophagosomes in all cells was induced by VCA treatment, but this progressed faster in naïve PDSCs compared to the other cell types.
Discussion
MSCs as powerful sources for cell therapy have been used in order to treat several degenerative diseases as they have many advantages such as differentiation potential, immunomodulatory effects and paracrine effects. However, applications are restricted by the cells' limited self‐renewal capacity 3, 37, 38. In many previous reports, self‐renewal ability of MSCs has been shown to be regulated by several factors, including markers of stemness (OCT4, NANOG, Sox2), growth factors and environmental factors 39, 40, 41. Jang et al. revealed that heat shock protein 60 (HSP60), a regulator of TERT, c‐myc, p53 and STAT3, contributes to self‐renewal of human adipose‐derived mesenchymal stem cells 42, 43. However, there are few further research studies on this. For this reason, many workers have been further investigating regulation factors of MSCs' self‐renewal activity and their mechanisms, trying to discover new factors capable of enhancing self‐renewal as required by large‐scale and long‐term culture 26, 44. For example, phenytoin enhances proliferation of MSCs by regulating apoptosis and proliferation 27. Also, treatment with Cissus quadrangularis (a perennial plant of the grape family) has been described to enhance BM‐MSC proliferation and facilitate osteoblastogenesis through upregulation of a MAPK‐dependent pathway 45.
It has been reported that VCA can induce apoptosis via protein kinases A or C 46 and the caspase‐3‐dependent pathway, in HL‐60 cells 47 depending on concentration of VCA 31. In this study, PDSC cultured with low concentrations of VCA increased proliferative activity with no cytotoxic effect. To our knowledge, this is the first study to report that VCA, a well‐known anti‐cancer drug, can promote proliferation of PDSCs through autophagic mechanisms, in an MSC‐specific manner, when treated at low concentrations.
Progress through the cell cycle is one of the most important factors seen in regulation of MSC proliferation. G0/G1 phase arrest specially, can reduce their proliferation by reducing cyclin A expression 48. Upregulation of cyclins A1, B1 and E1 are also correlated with proliferative activity of MSCs. Ryu et al. have reported that cyclins D1/E increase proliferation of MSCs via Ca2+/PKC/MAPKs and PI3K pathways 49. Their self‐renewal activity is also regulated by several further factors such as c‐Myc, SCF and Sox2 50, 51. In our results, low concentrations of VCA (5–10 pg/ml) increased proliferative activity of naïve PDSCs; however, we witnessed cytotoxic effects in immortalized PDSCs and HepG2 cells at high concentrations of VCA. These findings indicate that VCA may have a biphasic effect on stem cells, depending on treatment concentrations.
Cell death is also an important event in cell survival via cell cycle regulation in the physiology of cells 52. There are three different types of cell death. Apoptosis, which is a type I programmed cell death, can be induced by high oxidative stress (ROS) 53. Alteration in ROS can regulate cell proliferation or apoptosis of human umbilical cord blood‐derived mesenchymal stem cells (hUCB‐MSCs) via p53/p21 pathway 54. Also, it has been reported that apoptosis by ROS is controlled by Bax, p53 and Bcl2, which can protect against ROS 55, 56, 57. By our results, VCA reduced apoptosis of naïve PDSCs by reducing cytosolic levels of ROS. This indicates that intracellular ROS may control proliferation of naïve PDSCs when treated with VCA.
On the other hand, autophagy (type II programmed cell death), is characterized by appearance of autophagic vesicles engulfing cytoplasmic organelles by fusion with the lysosomal system 58. In contrast to apoptosis, autophagy is an economical way for cells to survive, by recycling their building blocks after degradation 59. Autophagy is regulated by several autophagy key regulators such as the mTOR, PI3KIII, beclin‐1, Atg5‐12 and LC3 series. Interestingly, they also act on MSCs growth factors to increase the cell survival 60. Knockdown of Beclin‐1 inhibits cell proliferation and promotes apoptosis by downregulating cyclin D1, CDK4, Bcl2 and Bcl‐xL expression, as well as caspase‐3 activation 61. We found that VCA increased expression of phosphorylated mTOR, PI3KIII, Beclin‐1, Atg12 and type II LC3 (active LC3 form) in naïve PDSCs. Although expressions of phosphorylated mTOR, PI3KIII and Beclin‐1 in immortalized PDSCs were shown to slightly increase, no expression of type II LC3 (active LC3 form) were expressed. Also, phosphorylated mTOR and Beclin‐1 in HepG2 were consistently expressed. In addition, numbers of autophagosomes were elevated by VCA treatment, in all cell types. These data suggest that VCA can efficiently trigger autophagic mechanism in naïve PDSCs to enhance proliferation, recycling cell building blocks, compared to cancer cells.
Previously we have showed that VCA‐induced apoptosis is associated with inhibition of telomerase via mitochondria‐controlled pathways, independent of p53 62. Also, VCA induces apoptosis and telomerase inhibition in human A253 cancer cells by controlling dephosphorylation of Akt 29. These results suggest that VCA may regulate apoptosis by controlling the TERT gene. Similar to our previous results, VCA‐induced apoptosis in immortalized PDSCs was observed by increased sub G1 fractions, using FACS assay. However, autophagosome formation and autophagic mechanisms were not observed in immortalized PDSCs with overexpressed human TERT gene. Further studies are needed to determine correlations between TERT and autophagy.
In conclusion, our results suggest that low concentrations of VCA increase self‐renewal of naïve PDSCs through regulation of passage through the cell cycle by increased cyclin expression. Also, VCA can induce expression of survival factors and can reduce cell death by regulating apoptosis and autophagy. Therefore, VCA may prove to be a useful supplement, capable of enhancing self‐renewal of mesenchymal stem cells, including PDSCs.
Acknowledgements
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MEST) (KRF‐2011‐0019610).
References
- 1. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU (1998) In vitro chondrogenesis of bone marrow‐derived mesenchymal progenitor cells. Exp. Cell Res. 238, 265–272. [DOI] [PubMed] [Google Scholar]
- 2. Aoki S, Toda S, Ando T, Sugihara H (2004) Bone marrow stromal cells, preadipocytes, and dermal fibroblasts promote epidermal regeneration in their distinctive fashions. Mol. Biol. Cell 15, 4647–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. [DOI] [PubMed] [Google Scholar]
- 4. Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK et al (2011) Human chorionic‐plate‐derived mesenchymal stem cells and Wharton's jelly‐derived mesenchymal stem cells: a comparative analysis of their potential as placenta‐derived stem cells. Cell Tissue Res. 346, 53–64. [DOI] [PubMed] [Google Scholar]
- 5. Jiang SS, Chen CH, Tseng KY, Tsai FY, Wang MJ, Chang IS et al (2011) Gene expression profiling suggests a pathological role of human bone marrow‐derived mesenchymal stem cells in aging‐related skeletal diseases. Aging (Albany NY) 3, 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maumus M, Guerit D, Toupet K, Jorgensen C, Noel D (2011) Mesenchymal stem cell‐based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res. Ther. 2, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ et al (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side‐population phenotype. Nat. Med. 7, 1028–1034. [DOI] [PubMed] [Google Scholar]
- 8. Ahmed F, Arseni N, Glimm H, Hiddemann W, Buske C, Feuring‐Buske M (2008) Constitutive expression of the ATP‐binding cassette transporter ABCG2 enhances the growth potential of early human hematopoietic progenitors. Stem Cells 26, 810–818. [DOI] [PubMed] [Google Scholar]
- 9. Susanto J, Lin YH, Chen YN, Shen CR, Yan YT, Tsai ST et al (2008) Porphyrin homeostasis maintained by ABCG2 regulates self‐renewal of embryonic stem cells. PLoS One 3, e4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orlic D (2003) Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Ann. N. Y. Acad. Sci. 996, 152–157. [DOI] [PubMed] [Google Scholar]
- 11. Tayman C, Uckan D, Kilic E, Ulus AT, Tonbul A, Murat Hirfanoglu I et al (2011) Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr. Res. 70, 489–494. [DOI] [PubMed] [Google Scholar]
- 12. Kassem M, Kristiansen M, Abdallah BM (2004) Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin. Pharmacol. Toxicol. 95, 209–214. [DOI] [PubMed] [Google Scholar]
- 13. Dazzi F, Horwood NJ (2007) Potential of mesenchymal stem cell therapy. Curr. Opin. Oncol. 19, 650–655. [DOI] [PubMed] [Google Scholar]
- 14. Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G et al (2008) Donor age and cell passage affects differentiation potential of murine bone marrow‐derived stem cells. BMC Cell Biol. 9, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pilz GA, Ulrich C, Ruh M, Abele H, Schafer R, Kluba T et al (2011) Human term placenta‐derived mesenchymal stromal cells are less prone to osteogenic differentiation than bone marrow‐derived mesenchymal stromal cells. Stem Cells Dev. 20, 635–646. [DOI] [PubMed] [Google Scholar]
- 16. Fariha MM, Chua KH, Tan GC, Tan AE, Hayati AR (2011) Human chorion‐derived stem cells: changes in stem cell properties during serial passage. Cytotherapy 13, 582–593. [DOI] [PubMed] [Google Scholar]
- 17. Jang JH, Jung JS, Im YB, Kang KS, Choi JI, Kang SK (2011) Crucial role of nuclear Ago2 for hUCB‐MSCs differentiation and self‐renewal via stemness control. Antioxid. Redox Signal. 16, 95–111. [DOI] [PubMed] [Google Scholar]
- 18. Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA (2000) Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 28, 1456–1462. [DOI] [PubMed] [Google Scholar]
- 19. Di X, Shiu RP, Newsham IF, Gewirtz DA (2009) Apoptosis, autophagy, accelerated senescence and reactive oxygen in the response of human breast tumor cells to adriamycin. Biochem. Pharmacol. 77, 1139–1150. [DOI] [PubMed] [Google Scholar]
- 20. Zhuang W, Li B, Long L, Chen L, Huang Q, Liang Z (2011) Induction of autophagy promotes differentiation of glioma‐initiating cells and their radiosensitivity. Int. J. Cancer 129, 2720–2731. [DOI] [PubMed] [Google Scholar]
- 21. Rajawat YS, Hilioti Z, Bossis I (2009) Aging: central role for autophagy and the lysosomal degradative system. Ageing Res. Rev. 8, 199–213. [DOI] [PubMed] [Google Scholar]
- 22. De Martinet W, Meyer GR (2009) Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ. Res. 104, 304–317. [DOI] [PubMed] [Google Scholar]
- 23. Eskelinen EL (2005) Maturation of autophagic vacuoles in Mammalian cells. Autophagy 1, 1–10. [DOI] [PubMed] [Google Scholar]
- 24. Scherz‐Shouval R, Elazar Z (2011) Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 36, 30–38. [DOI] [PubMed] [Google Scholar]
- 25. Chen DF, Li X, Xu Z, Liu X, Du SH, Li H et al (2010) Hexadecanoic acid from Buzhong Yiqi decoction induced proliferation of bone marrow mesenchymal stem cells. J. Med. Food 13, 967–970. [DOI] [PubMed] [Google Scholar]
- 26. Potu BK, Bhat KM, Rao MS, Nampurath GK, Chamallamudi MR, Nayak SR et al (2009) Petroleum ether extract of Cissus quadrangularis (Linn.) enhances bone marrow mesenchymal stem cell proliferation and facilitates osteoblastogenesis. Clinics (Sao Paulo) 64, 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu X, Chen Z, Mao X, Tang S (2011) Effects of phenytoin and Echinacea purpurea extract on proliferation and apoptosis of mouse embryonic palatal mesenchymal cells. J. Cell. Biochem. 112, 1311–1317. [DOI] [PubMed] [Google Scholar]
- 28. Park R, Kim MS, So HS, Jung BH, Moon SR, Chung SY et al (2000) Activation of c‐Jun N‐terminal kinase 1 (JNK1) in mistletoe lectin II‐induced apoptosis of human myeloleukemic U937 cells. Biochem. Pharmacol. 60, 1685–1691. [DOI] [PubMed] [Google Scholar]
- 29. Choi SH, Lyu SY, Park WB (2004) Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch. Pharm. Res. 27, 68–76. [DOI] [PubMed] [Google Scholar]
- 30. Park JH, Hyun CK, Shin HK (1998) Cytotoxicity of heat‐treated Korean mistletoe. Cancer Lett. 126, 43–48. [DOI] [PubMed] [Google Scholar]
- 31. Thies A, Dautel P, Meyer A, Pfuller U, Schumacher U (2008) Low‐dose mistletoe lectin‐I reduces melanoma growth and spread in a scid mouse xenograft model. Br. J. Cancer 98, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gabius HJ, Darro F, Remmelink M, Andre S, Kopitz J, Danguy A et al (2001) Evidence for stimulation of tumor proliferation in cell lines and histotypic cultures by clinically relevant low doses of the galactoside‐binding mistletoe lectin, a component of proprietary extracts. Cancer Invest. 19, 114–126. [DOI] [PubMed] [Google Scholar]
- 33. Lyu SY, Park WB (2006) Mistletoe lectin (Viscum album coloratum) modulates proliferation and cytokine expressions in murine splenocytes. J. Biochem. Mol. Biol. 39, 662–670. [DOI] [PubMed] [Google Scholar]
- 34. Vehmeyer K, Hajto T, Hostanska K, Konemann S, Loser H, Saller R et al (1998) Lectin‐induced increase in clonogenic growth of haematopoietic progenitor cells. Eur. J. Haematol. 60, 16–20. [DOI] [PubMed] [Google Scholar]
- 35. Lyu SY, Park SM, Choung BY, Park WB (2000) Comparative study of Korean (Viscum album var. coloratum) and European mistletoes (Viscum album). Arch. Pharm. Res. 23, 592–598. [DOI] [PubMed] [Google Scholar]
- 36. Lee MJ, Jung J, Na KH, Moon JS, Lee HJ, Kim JH et al (2010) Anti‐fibrotic effect of chorionic plate‐derived mesenchymal stem cells isolated from human placenta in a rat model of CCl(4)‐injured liver: potential application to the treatment of hepatic diseases. J. Cell. Biochem. 111, 1453–1463. [DOI] [PubMed] [Google Scholar]
- 37. Ballas CB, Zielske SP, Gerson SL (2002) Adult bone marrow stem cells for cell and gene therapies: implications for greater use. J. Cell. Biochem. Suppl. 38, 20–28. [DOI] [PubMed] [Google Scholar]
- 38. Reiser J, Zhang XY, Hemenway CS, Mondal D, La Pradhan L, Russa VF (2005) Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin. Biol. Ther. 5, 1571–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wei X, Shen CY (2011) Transcriptional regulation of oct4 in human bone marrow mesenchymal stem cells. Stem Cells Dev. 20, 441–449. [DOI] [PubMed] [Google Scholar]
- 40. Pierantozzi E, Gava B, Manini I, Roviello F, Marotta G, Chiavarelli M et al (2011) Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT‐4 and SOX‐2. Stem Cells Dev. 20, 915–923. [DOI] [PubMed] [Google Scholar]
- 41. Go MJ, Takenaka C, Ohgushi H (2008) Forced expression of Sox2 or Nanog in human bone marrow derived mesenchymal stem cells maintains their expansion and differentiation capabilities. Exp. Cell Res. 314, 1147–1154. [DOI] [PubMed] [Google Scholar]
- 42. Pera MF, Tam PP (2010) Extrinsic regulation of pluripotent stem cells. Nature 465, 713–720. [DOI] [PubMed] [Google Scholar]
- 43. Jang JH, Jung JS, Choi JI, Kang SK (2011) Nuclear Ago2/HSP60 contributes to broad spectrum of hATSCs function via Oct4 regulation. Antioxid. Redox Signal. 16, 383–399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44. Li XD, Wang JS, Chang B, Chen B, Guo C, Hou GQ et al (2011) Panax notoginseng saponins promotes proliferation and osteogenic differentiation of rat bone marrow stromal cells. J. Ethnopharmacol. 134, 268–274. [DOI] [PubMed] [Google Scholar]
- 45. Parisuthiman D, Singhatanadgit W, Dechatiwongse T, Koontongkaew S (2009) Cissus quadrangularis extract enhances biomineralization through up‐regulation of MAPK‐dependent alkaline phosphatase activity in osteoblasts. In Vitro Cell Dev. Biol. Anim. 45, 194–200. [DOI] [PubMed] [Google Scholar]
- 46. Pae HO, Seo WG, Shin M, Lee HS, Kim SB, Chung HT (2000) Protein kinase A or C modulates the apoptosis induced by lectin II isolated from Korean mistletoe, Viscum album var. Coloratum, in the human leukemic HL‐60 cells. Immunopharmacol. Immunotoxicol. 22, 279–295. [DOI] [PubMed] [Google Scholar]
- 47. Lyu SY, Park WB, Choi KH, Kim WH (2001) Involvement of caspase‐3 in apoptosis induced by Viscum album var. coloratum agglutinin in HL‐60 cells. Biosci. Biotechnol. Biochem. 65, 534–541. [DOI] [PubMed] [Google Scholar]
- 48. Plaisant M, Giorgetti‐Peraldi S, Gabrielson M, Loubat A, Dani C, Peraldi P (2011) Inhibition of hedgehog signaling decreases proliferation and clonogenicity of human mesenchymal stem cells. PLoS One 6, e16798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ryu JM, Lee MY, Yun SP, Han HJ (2010) High glucose regulates cyclin D1/E of human mesenchymal stem cells through TGF‐beta1 expression via Ca2+/PKC/MAPKs and PI3K/Akt/mTOR signal pathways. J. Cell. Physiol. 224, 59–70. [DOI] [PubMed] [Google Scholar]
- 50. Gagari E, Rand MK, Tayari L, Vastardis H, Sharma P, Hauschka PV et al (2006) Expression of stem cell factor and its receptor, c‐kit, in human oral mesenchymal cells. Eur. J. Oral Sci. 114, 409–415. [DOI] [PubMed] [Google Scholar]
- 51. Park SB, Seo KW, So AY, Seo MS, Yu KR, Kang SK et al (2011) SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf‐1 and c‐MYC. Cell Death Differ. 19, 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oguro H, Iwama A (2007) Life and death in hematopoietic stem cells. Curr. Opin. Immunol. 19, 503–509. [DOI] [PubMed] [Google Scholar]
- 53. Efferth T (2006) Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr. Drug Targets 7, 407–421. [DOI] [PubMed] [Google Scholar]
- 54. Veal EA, Day AM, Morgan BA (2007) Hydrogen peroxide sensing and signaling. Mol. Cell 26, 1–14. [DOI] [PubMed] [Google Scholar]
- 55. Jang JH, Jung JS, Im YB, Kang KS, Choi JI, Kang SK (2012) Crucial role of nuclear Ago2 for hUCB‐MSCs differentiation and self‐renewal via stemness control. Antioxid. Redox Signal. 16, 95–111. [DOI] [PubMed] [Google Scholar]
- 56. Zhao J, Chen J, Lu B, Dong L, Wang H, Bi C et al (2008) TIP30 induces apoptosis under oxidative stress through stabilization of p53 messenger RNA in human hepatocellular carcinoma. Cancer Res. 68, 4133–4141. [DOI] [PubMed] [Google Scholar]
- 57. Ribeiro IR, Olliaro P (1998) Safety of artemisinin and its derivatives. A review of published and unpublished clinical trials. Med. Trop. (Mars) 58, 50–53. [PubMed] [Google Scholar]
- 58. Jing K, Song KS, Shin S, Kim N, Jeong S, Oh HR et al (2011) Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild‐type p53. Autophagy 7, 1348–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang Z, Klionsky DJ (2007) Permeases recycle amino acids resulting from autophagy. Autophagy 3, 149–150. [DOI] [PubMed] [Google Scholar]
- 60. Sanchez CG, Penfornis P, Oskowitz AZ, Boonjindasup AG, Cai DZ, Dhule SS et al (2011) Activation of autophagy in mesenchymal stem cells provides tumor stromal support. Carcinogenesis 32, 964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang C, Han LO (2010) Knockdown of Beclin 1 inhibits vitamin K3 induced autophagy, but promotes apoptosis of human hepatoma SMMC‐7721 cells. Mol. Med. Report 3, 801–807. [DOI] [PubMed] [Google Scholar]
- 62. Lyu SY, Choi SH, Park WB (2002) Korean mistletoe lectin‐induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch. Pharm. Res. 25, 93–101. [DOI] [PubMed] [Google Scholar]


