Abstract
Objectives
Adipose tissue engineering is one of the hottest topics in the field of regenerative medicine. Fat tissue has been considered as an abundant and accessible source of adult stem cells by tissue engineers, since it gives rise to adipose stem cells. However, recent reports have pointed out that adipose tissue, as a secretory and endocrine organ, might secrete cytokines that regulate body functions such as metabolism, infammation and more. In this study, we aim to investigate the adipogenic‐inducing factors secreted by fat tissue.
Materials and methods
Conditioned medium were collected by culturing fat tissue fragments in plastic flasks. Mesenchymal stem cells (MSCs) cultured in conditioned medium (CM) to test the adipogenic‐inducing factors. Oil red O staining, reverse transcription/polymerase chain reaction and immunocytofluorescent staining were performed to examine the differentiation of MSCs in CM.
Results
MSCs cultured in CM of adipose tissue spontaneously differentiated into adipocytes. Furthermore, supplementation of insulin or dexamethasone to CM accelerated the process of lipid accumulation of differentiated MSCs.
Discussion
Results from this study demonstrated that fat tissues secrete small molecules, which induce adipogenic differentiation of MSCs.
Conclusions
Our study provides clues for improving adipose tissue engineering by using fragmented adipose tissue as sources of fat‐inducing factors.
Introduction
Adipose tissue engineering has been developed to restore large‐sized soft tissue defects resulting from trauma, tumour resection and congenital defects, amongst others. Strategies of fat tissue engineering include implantation of pre‐adipocytes or adipocytes together with appropriate scaffolds, to restore the volume of tissue lost at the defect site. Involvement of scaffolds suggests that autologous transplantation of fat tissue obtained from liposuction or aspiration is generally unsuccessful, due to significant absorption of the transplanted tissue over time 1. A hypothesis has been proposed to explain tissue absorption – insufficient angiogenesis of transplanted tissue 2. Aspiration procedures usually damage existing vascular structure of adipose tissue, and mature adipocytes have only limited proliferative capacity to restore vasculature, due to their terminally differentiated status 3. Thus, neither liquefied fat tissue from liposuction nor fully mature adipocytes are a desired cell source for adipose tissue engineering 4.
Stem cells isolated from adipose tissue have the potential to differentiate into multilineages of cells, including osteoblasts, chondrocytes, adipocytes and myoblasts 5, 6, 7. Increasing knowledge concerning adipose stem cells (ASCs) spurs further research to make use of the potential therapeutic applications of ASCs in regenerative medicine 8, 9. Recent reports from several research groups have shed light on the possibility of using ASCs in adipose tissue engineering 10, 11. On the other hand, some researchers have reported that tissues in which stem cells reside, might secrete factors that enhanced differentiation of the stem cells. Some of these factors are produced by paracrine mechanisms, for example, cartilage tissue‐derived factors can suppress collagen X expression 12. Moreover, embryonic stem cells can be induced to differentiate into cardiomyocytes by signalling mediated through cardiac paracrine pathways 13.
Adipose tissue, mesenchymal in origin, is composed of adipocytes, pre‐adipocytes, blood cells, fibroblasts, immune cells and matrix of collagen fibres. The major form of fat in humans is white adipose tissue, which for a long time has been viewed as a passive energy reservoir. This has been changed by the discovery that a number of adipocyte‐derived secretory factors, called (since the 1990s) adipokines 14, 15. Scientists have now started to accept the concept of white adipose tissue as an endocrine organ, which plays a central role in regulating energy homeostasis through adipokine‐mediated regulation of a number of signalling pathways in target tissues. Imbalance of adipokine profiles is a typical symptom of patients suffering from metabolic syndromes 16. A large group of cytokines and bioactive substances are secreted by adipocytes via autocrine, paracrine and endocrine mechanisms, and they work in the surrounding micro‐environment to maintain normal body functions. According to their roles, adipokines can be generally catalogued into four groups 17: metabolism regulating factors; pro‐inflammatory cytokines and acute phase reactants; components of extracellular matrix; and promitogenic and pro‐angiogenic factors. Recently, attention has been paid to the bioactive proteins secreted by adipose tissue, which are able to induce adult stem cells to differentiate into adipocytes 18. This implies that adipose tissue itself might be used to enhance differentiation of the adult stem cells by producing intrinsic supporting factors. Thus, fat tissue might effectively serve as a feeder layer for its own or independent pluripotent stem cells, to eliminate potential variability and contamination caused by feeder cells, significant at the moment for development of tissue regeneration and engineering 19, 20.
In this study, a secretory mechanism is described concerning adipose tissue's influence on adipogenic differentiation of mesenchymal stem cells. Fundamental properties of secreted factors from adipose tissue were also characterized. Data presented here indicate the potential for using fragmented fat tissue as source for both seeding cells and inducing factors in adipose tissue engineering.
Materials and methods
Isolation and expansion of mesenchymal stem cells
Bone marrow‐derived MSCs were isolated as previously reported 21. Briefly, MSCs were harvested from bone marrow of femurs and tibias of 8‐week‐old BALB/c mice, by inserting a 5‐gauge needle into the shaft of the bone and flushing out marrow with α‐modified Eagle's medium (α‐MEM) containing 10% foetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin (expansion medium). Cells from one mouse were plated into one T25 flask. After 48 h, floating cells were discarded and adherent cells were washed in phosphate‐buffered saline (PBS). MSCs were cultured for 7–10 days in expansion medium to reach confluence and passaged twice before seeding on glass coverslips for adipogenic differentiation. All cell culture reagents were purchased from Gibco (Grand Island, NY, USA) unless otherwise stated. All our protocols relating to animal procedures were approved by Animal Care Committee of West China College of Stomatology, Sichuan University.
Isolation of adipose stem cells and collection of fat tissue‐conditioned medium
Inguinal fat pads were isolated from 8‐week‐old BALB/c mice, then fat tissues were excised and washed extensively in sterile PBS to remove any contaminating blood. Tissues were then cut into small pieces of around 2 mm diameter, as we described previously 22. Fat tissue fragments were placed in tissue‐culture treated T75 flasks and incubated at 37 °C for 30 min to allow them to attach to the bottoms of flasks; then, expansion medium was added carefully to the flasks. After 5 days, fat tissues were removed by washing out with medium. ASCs were cultured for 7 days in expansion medium to reach confluence and passaged twice before seeding on glass coverslips for adipogenic differentiation. To collect conditioned medium of fat tissues, the same procedure was used to isolate and process them. In addition, fat tissue fragments were placed in tissue culture‐treated T75 flasks and incubated at 37 °C for 30 min to allow them attach to the bottoms of flasks. Then, either serum‐free α‐MEM or expansion medium was added to carefully the flasks to make CM without FBS or CM with FBS. After 1 week, both kinds of conditioned media were harvested and stored at 4 °C. CM without FBS was supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin before being used to culture MSCs. Then, ultracentrifugation was used to separate different components in conditioned medium by their molecular weights. Briefly, CM without FBS was first introduced into Amicon® Ultra‐15 Centrifugal Filter Units with Ultracel‐5 membrane (5000 Nominal Molecular Weight Limit, NMWL) and centrifuged at 1968 g for 30 min. This concentrate was called CM > 5k. Other filtrates were introduced into Amicon® Ultra‐15 Centrifugal Filter Units with Ultracel‐3 membrane (3000 NMWL) and centrifuged at 4000 rpm for 30 min. The concentrates and filtrates were called 3k < CM < 5D and CM > 5k respectively. For some experiments, dexamethasone (1 μm) or insulin (10 μm) was supplemented to conditioned medium as indicated in figure legends.
Adipogenic differentiation and oil red‐O staining
For adipogenic differentiation, cells were cultured for 7 days in adipogenic medium containing α‐MEM supplemented with 10% FBS, 1 μm dexamethasone (Sigma, St. Louis, MO, USA), 10 μm insulin (Sigma), 200 μm indomethacin (Sigma), and 0.5 mm isobutyl‐methylxanthine (IBMX; Sigma); medium was replaced every 2 days. After 1 week, MSCs were subjected to oil red‐O staining. Briefly, the MSCs were fixed in 10% formaldehyde solution (Sigma) for 15 min, washed in 60% isopropanol (Sigma), and stained with oil red O solution (in 60% isopropanol) for 5 min followed by repeated washing in PBS. Then, images were taken of the cells using a Nikon microscope (Tokyo, Japan). To quantify fat droplets in cells, pixels of red areas (positively stained) were calculated using software imagej (available on website: http://rsbweb.nih.gov/ij/). Values represent means ± SE of six replicates.
RNA isolation and RT‐PCR
RNA samples of MSCs cultured in expansion medium or adipogenic medium were isolated using Total Tissue/cell RNA Extraction Kit (Watson, China), according to the manufacturer's protocol. One microgram of total RNA was reverse transcribed into cDNA in a 20‐ml reverse transcription system (Fementas, Vilnius, Lithuan) according to the manufacturer's instructions. cDNA samples were amplified using a Pfu PCR kit (Tiangen, Beijing, China); specific primers are listed in Table 1. All PCR products were resolved on 2% agarose gel.
Table 1.
Forward (F) and Reverse (R) primer pairs used for RT‐PCR to detect gene expression pattern in MSCs
| Gene name | Primer sequence | Product size | GenBank no. |
|---|---|---|---|
| PPARγ |
F: 5′ GACCACTCGCATTCCTTT 3′ R: 5′ CCACAGACTCGGCACTCA 3′ |
266 | NM_011146 |
| LPL |
F: 5′ AGGGTGAGGAATCTAATG 3′ R: 5′ CAGGTGTTTCAACCGCTA 3′ |
270 | NM_008509 |
| aP2 |
F: 5′ CATCAGCGTAAATGGGGATT 3′ R: 5′ TCGACTTTCCATCCCACTTC 3′ |
182 | NM_024406 |
| GAPDH |
F: 5′ ACCACAGTCCATGCCATCAC 3′ R: 5′ TCCACCACCCTGTTGCTGTA 3′ |
492 | NM_001001303 |
The amplicons obtained for both PPAR‐γ and GAPDH were quantified according to their signal intensities on agarose gel, by using imagej Software; this is defined as densitometric reading for each gene. Then, relative levels of PPAR‐γ for each sample were estimated by using ratio of densitometric readings of PPAR‐γ and GAPDH.
Immunofluorescence staining
MSCs cultured either in expansion medium or adipogenic medium, were plated on to glass coverslips in six‐well plates, 24 h before staining. Cells were washed briefly in PBS, fixed in 4% paraformaldehyde for 30 min at room temperature, then permeabilized and blocked in 0.5% Triton‐X 100 and 0.5% bovine serum albumin (BSA) for 15 min at room temperature. Samples were subsequently incubated overnight at 4 °C in rabbit polyclonal antibody against PPAR‐γ (AbCam, Cambridge, MA, USA) conjugated to rhodamine (Pierce, Rockford, IL, USA), and nuclei were stained with DAPI (Molecular Probes, Eugene, OR, USA). After rinsing in PBS, cells were observed and images were produced using a DMi 6000 B fluorescence microscope (Leica, Wetzlar, Germany).
Statistical analysis
Three independent sets of all experiments were performed. Three replicates were performed for qualitative experiments, and six replicates for quantitative experiments. Data shown as means ± SD, and were analysed by paired analysis of variance (ANOVA). P‐values are described in figures; P < 0.05 was considered statistically significant.
Results
Adipose‐derived stromal cells that migrated spontaneously from fat tissue pieces differentiated into adipocytes
We have previously reported that ASCs can be obtained by culturing fat tissue fragments in culture flasks 23. These cells were able to differentiate into a variety of cell types such as adipocytes, osteoblasts and chondrocytes. Here, as early as the 3rd day, there were cells growing out of fat tissue pieces (Fig 1a). Normally, fat would be washed away at day 5 and ASCs expanded to confluence. However, these ASCs started to accumulate lipid droplets if fat tissues were not removed until day 10. At day 14, more lipid droplets could be seen in the ASCs (Fig 1a). Another interesting finding was that the closer the ASCs were to the parent tissue, the more fat droplets could be found in the cells. As adipocytes are fully differentiated mature cells, unable to migrate out of tissue, the fat‐containing cells had differentiated into fat cells spontaneously after migrating from the tissue fragments. Then, we hypothesized that there are soluble factors secreted by adipose tissue that induce adipogenic differentiation of ASCs. Therefore, conditioned medium was collected from adipose tissue and tested for its adipogenic potential, by culturing ASCs for 1 week. As shown in Fig. 1b, medium conditioned with FBS induced adipogenic differentiation of ASCs.
Figure 1.
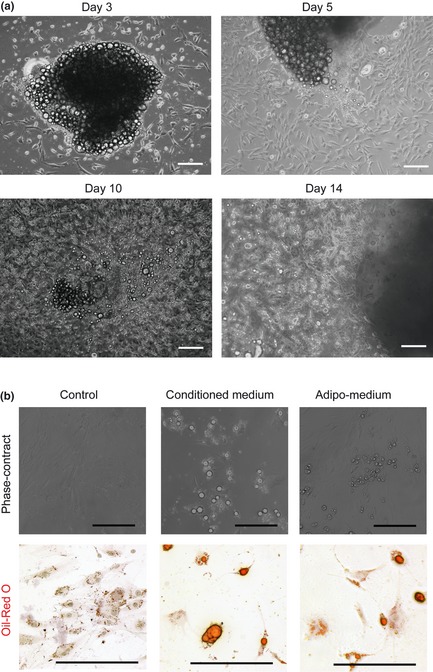
Spontaneous differentiation of adipose‐derived stromal cells. (a) Fat tissue isolated from the groin fat pad, cut into small pieces and cultured in flasks, for up to 2 weeks. Adipose stromal cells migrated out of fat tissue fragments and gradually turned into fully mature adipocytes. Bar = 50 μm (b) Conditioned medium was formed by culturing fat tissue with DMEM containing 10% FBS for 1 week. Bone marrow MSCs cultured in conditioned medium for 1 week differentiation into adipocytes. Upper panel – pictures with no staining, while lower panel shows images of oil‐red O staining. Bar = 50 μm
Conditioned medium induced adipogenic differentiation of bone marrow MSCs
As we have previously reported, freshly isolated ASCs contain a minor fraction of pre‐adipocytes 6 and it is true that some tiny fat droplets could be found in cells cultured in expansion medium only (Fig 1b). Thus too avoid contamination by pre‐existing adipocytes, we needed an adipogenic differentiation model using cells derived from another source rather than from fat tissue itself. Thus, a model using bone marrow MSCs was set up to test the conditioned medium's potential to induce adipogenic differentiation. Serum‐free medium was used to condition fat tissue, as serum could be an obstacle in the subsequent study of analysing active components of conditioned medium. As shown in Fig. 2, MSCs cultured in CM with FBS differentiated into adipocytes, and expressed adipogenic genes such as for PPAR‐γ, aP2 and LPL. CM‐without FBS induced MSCs to accumulate fat drops (Fig. 2a) and express adipogenic genes (Fig. 2b). Immunofluorescence staining showed that PPAR‐γ was expressed by BMSCs cultured either in CM with FBS or CM without FBS. Nuclei were counterstained with DAPI (Fig. 2c).
Figure 2.
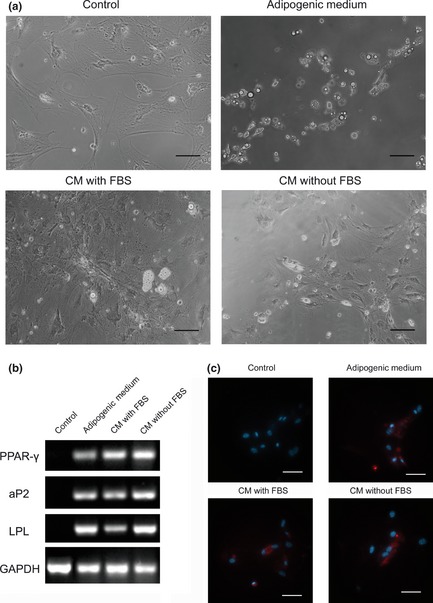
Serum‐free conditioned medium from adipose tissue, induces adipogenic differentiation of bone marrow MSC s. (a) CM with FBS was obtained by culturing fat tissue with DMEM containing 10% FBS for 1 week. CM without FBS was obtained by culturing fat tissue with serum‐free medium for 1 week and then supplemented with 10% FBS. Both CM with FBS and CM without FBS induced adipogenic differentiation of MSCs after 1 week's culture. Bar = 50 μm. (b) RT‐PCR analysis of adipogenic genes on MSCs, cultured in different media. (c) Imunofluorescence staining showed that PPAR‐γ was expressed by MSCs cultured either in CM with FBS or in CM without FBS. Nuclei counterstained with DAPI. Bar = 10 μm.
Dexamethasone or insulin accelerated lipid accumulation of MSCs cultured in conditioned medium
To enhance adipogenic differentiation of MSCs cultured in conditioned medium, dexamethasone (1 μm) or insulin (10 μm) were added to expansion medium and CM‐without FBS. As shown in Fig. 3a, both dexamethasone and insulin enhanced fat‐drop accumulation in MSCs cultured in conditioned medium, while dexamethasone or insulin alone only induced few MSCs to generate fat droplets. Then, oil‐red O staining was performed and pixels of positive areas were calculated using imagej to quantify amounts of lipid droplets formed in MSCs. As shown in Fig. 3b, MSCs cultured in CM supplemented with insulin accumulated most droplets. Results of semi‐quantitative RT‐PCR also indicated that CM supplemented with insulin induced the strongest expression of PPAR‐γ mRNA (Fig. 3c,d). Expression of PPAR‐γ at protein level was detected by immunofluorescence staining (Fig. 3e).
Figure 3.
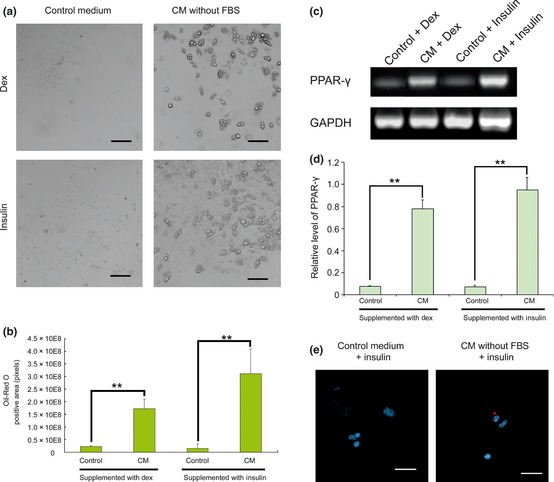
Conditioned medium supplemented with dexamethasone or insulin induced stronger adipogenic differentiation of MSC s. (a) Control medium (DMEM+10%FBS) and CM without FBS (collected as described before) were supplemented either with dexamethasone (Dex) or insulin, and these media were used to culture MSCs for 1 week. CM without FBS supplemented with insulin induced strongest lipid accumulation. Bar = 100 μm. (b) MSCs were then fixed and stained with oil‐red O. Pixels of positive area were calculated using imagej, to show amounts of lipid formed in MSCs. **P < 0.01. (c) Expression of PPAR‐γ was detected by RT‐PCR. GAPDH was used as internal control. (d) Signal intensities of PPAR‐γ and GAPDH on agarose gel were quantified to estimate relative levels of PPAR‐γ. **P < 0.01. (e) Expression of PPAR‐γ was visualized by secondary antibodies conjugated to rhodamine. Nuclei were counterstained with DAPI. Bar = 10 μm.
Molecular weight of active components was between 3 and 5 kDa
Subsequently, ultracentrifugation was used to study molecular weights of active components of conditioned medium (CM without FBS). With combination of two cut‐offs (3 and 5 kDa), conditioned medium was separated into three fractions: CM < 3k (molecules smaller than 3 kDa), 3k < CM < 5k (molecules between 3 and 5 kDa) and CM > 5k (molecules bigger than 5 kDa). Only MSCs cultured in 3k < CM < 5k accumulated fat droplets (Fig. 4a); pixels of positive areas were calculated by imagej to show amounts of lipid formed in MSCs. Quantification of oil‐red O staining confirmed that positively stained areas of MSCs cultured in 3k < CM < 5k were significantly larger than those in CM < 3k or CM > 5k (Fig. 4b). Results of semi‐quantitative RT‐PCR indicated that MSCs culture in 3k < CM < 5k expressed more PPAR‐γ mRNA than MSCs in CM < 3k and CM > 5k (Fig. 4c,d), and immunocytofluorescence showed 3k < CM < 5k‐induced PPAR‐γ expression in MSCs (Fig. 4e).
Figure 4.
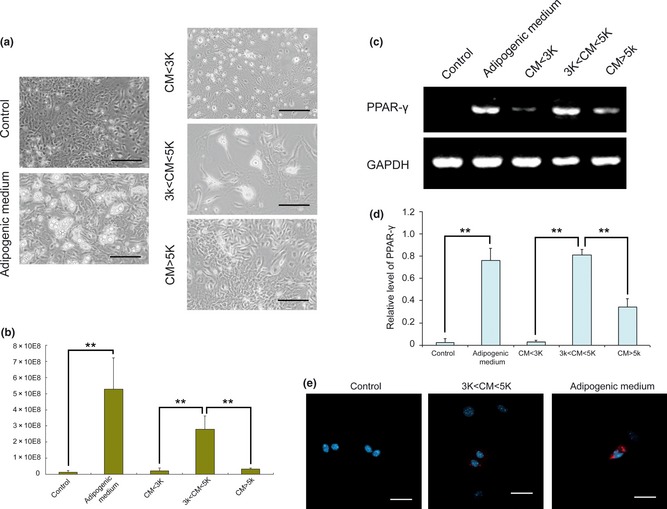
Active components of conditioned medium, between 3 and 5 kDa. (a) Different components of CM without FBS were separated by ultra centrifugation. All three components, as well as controls, were supplemented with 10% FBS and insulin. MSCs cultured in these media for 1 week showed different levels of adipogenic differentiation. Bar = 50 μm (b) MSCs were then fixed and stained in oil‐red O. Pixels of positive areas were calculated using imagej, to show amounts of lipid formed in MSCs. **P < 0.01. (c) Expression of PPAR‐γ was detected by RT‐PCR. GAPDH was used as internal control. (d) Signal intensities of PPAR‐γ and GAPDH on agarose gel were quantified to estimate relative levels of PPAR‐γ. **P < 0.01. (e) Expression of PPAR‐γ was visualized using secondary antibodies conjugated to rhodamine. Nuclei were counterstained with DAPI. Bar = 10 μm.
Discussion
The concept of regarding white adipose tissue as an endocrine organ began in 1995 when Halaas and colleagues discovered leptin and its wide ranging biological functions 24. Aiding maintenance of normal body functions, adipocytes secrete diverse cytokines and other bioactive substances into their surrounding environment. Some of these secreted factors have been demonstrated to induce differentiation of mesenchymal stem cells into adipocytes 18. Study of secretory profiles of adipose tissue can be useful for fat tissue regeneration 25.
In this study, we first confirmed that cells are able to migrate out of fat tissue pieces, as we have previously reported. Then we found that these cells accumulated lipid droplets if their parent fat tissue fragments were not removed from the culture flasks. It is very unlikely that fully differentiated mature adipocytes (which contain large fat droplets) migrate out of fat tissue. Therefore, these cells containing lipid droplets must have been ASCs or pre‐adipocytes, induced into mature adipocytes by bioactive factors secreted from the adipose tissue. Then, conditioned medium of adipose tissue was collected and shown to be adipogenic/inductive, by culturing ASCs in it. To avoid interference of pre‐existing adipocytes, bone marrow MSCs were used to test adipogenic inductivity of conditioned medium. MSCs cultured in CM differentiated into adipocytes and expressed adipogenic genes for PPAR‐γ, aP2 and LPL, as endorsed by RT‐PCR. Furthermore, influence of serum was eliminated by using serum‐free medium to formulate conditioned medium of fat tissue. CM without FBS induced MSCs to accumulate fat droplets and induced expression of adipogenic genes. Results further indicate that adipogenic inductivity of CM could be greatly enhanced by supplementation of dexamethasone or insulin. Finally, the molecular weights of active compounds was refined to be between 3 and 5 kDa.
Dexamethasone or insulin was added to enhance adipogenic inductivity of conditioned medium. Both are important components of well‐established adipogenic differentiation media 26. It is generally known that insulin plays important roles in glycaemic control 27, glucose transport 28 and glycogen synthesis 29. Moreover, insulin also functions as metabolic regulator by participating in cell proliferation and differentiation 30, lipid accumulation in adipocyte 31 and lipolysis regulation 32. Dexamethasone is a synthetic glucocorticoid, mainly used to treat severe inflammatory diseases 33. However, it is also frequently used in the laboratory, to accelerate adipogenic differentiation of mesenchymal stem cells, by promoting PPAR‐γ2 expression 34 and depressing that of Runx 2 35. Data from the present study confirms that the process of lipid accumulation in MSCs could be greatly accelerated by supplementation of dexamethasone or insulin to the conditioned medium. These results indicate that active molecules in conditioned medium could be synergized by dexamethasone and insulin.
So far, most relevant studies have put emphasis on proteins secreted by whole adipose tissues. Some of these proteins are inflammatory cytokines such as interleukins, TNF‐α and more 36; some proteins such as adiponectin, adipsin, apelin, apolipoprotein E, insulin‐like growth factor 1, leptin, lipoprotein lipase, and more, play crucial roles in lipid metabolism, while others are involved in vascular haemostasis or the complement system 17. Very few studies have paid attention to small molecules secreted by adipose tissue, however, it is widely known that animal tissues (especially those of the central nerve system) produce many small molecules that regulate body functions 37, 38. On the basis of that, the fraction of the conditioned medium between 3 and 5 kDa induced most adipogenic differentiation; we believe that a group of small molecules that tune differentiation of adult stem cells, could be found in this adipose tissue secretome. Therefore, the results of present study open a window for us to commit to research of small molecules secreted by adipose tissues.
In our previous studies, a group of cells derived from adipose tissue was shown to have characteristics of both osteogenic and adipogenic progenitors 23. These cells could be enriched in the Sca‐1 negative population by fluorescence‐activated cell sorting, and expressed RUNX2 and PPAR‐γ proteins. When cultured in adipogenic medium, PPAR‐γ moves to nuclei and the cells differentiate into adipocytes. Conversely, when RUNX2 moves to nuclei and cells differentiate into osteoblasts, when cultured in osteogenic medium. Now, in this study, small molecules have also been discovered to be secreted by adipose tissue, to induce fat accumulation in MSCs. Thus, fat tissue fragments may not only be used as sources of seed cells but also could be used to provide inducing factors in adipose tissue engineering.
In summary, we found that adipose tissue fragments can secrete factors that trigger adipogenic differentiation of mesenchymal stem cells. We have determined molecular weight of the active components in conditioned medium to be between 3 and 5 kDa. We believe that the results of the present study has enriched our understanding of adipogenic differentiation of MSCs, demonstrating that adipose tissue, as an endocrine organ, plays an important role in increasing adipogenic differentiation of stem cells. Further studies may pave the way for applying fragmented fat tissue as the source for both seed cells and inducing factors, in adipose tissue engineering.
Acknowledgements
This work was funded by National Natural Science Foundation of China (81071273, 31170929), National Natural Science Foundation of Hainan Province (30635), Foundation for the Author of National Excellent Doctoral Dissertation of China (FANEDD 200977) and Program for New Century Excellent Talents in University (NCET‐08‐0373), Funding for Distinguished Young Scientists in Sichuan (2010JQ0066).
Ling Wu and Tao Wang contribute equally to this work.
References
- 1. Gomillion CT, Burg KJ (2006) Stem cells and adipose tissue engineering. Biomaterials 27, 6052–6063. [DOI] [PubMed] [Google Scholar]
- 2. Patrick CW Jr (2001) Tissue engineering strategies for adipose tissue repair. Anat. Rec. 263, 361–366. [DOI] [PubMed] [Google Scholar]
- 3. Beahm EK, Walton RL, Patrick CW Jr. (2003). Progress in adipose tissue construct development. Clin. Plast. Surg. 30, 547–558, viii. [DOI] [PubMed] [Google Scholar]
- 4. Tremolada C, Palmieri G, Ricordi C (2010) Adipocyte transplantation and stem cells: plastic surgery meets regenerative medicine. Cell Transplant. 19, 1217–1223. [DOI] [PubMed] [Google Scholar]
- 5. Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL et al (2001) Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue‐derived stromal cells. Tissue Eng. 7, 729–741. [DOI] [PubMed] [Google Scholar]
- 6. Lin Y, Liu L, Li Z, Qiao J, Wu L, Tang W et al (2006) Pluripotency potential of human adipose‐derived stem cells marked with exogenous green fluorescent protein. Mol. Cell. Biochem. 291, 1–10. [DOI] [PubMed] [Google Scholar]
- 7. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ et al (2001) Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng. 7, 211–228. [DOI] [PubMed] [Google Scholar]
- 8. Levi B, Longaker MT (2011) Concise review: adipose‐derived stromal cells for skeletal regenerative medicine. Stem Cells 29, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen JF, Sugawara A, Yamashita J, Ogura H, Sato S (2011) Dedifferentiated fat cells: an alternative source of adult multipotent cells from the adipose tissues. Int. J. Oral Sci. 3, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jing W, Lin Y, Wu L, Li X, Nie X, Liu L et al (2007) Ectopic adipogenesis of preconditioned adipose‐derived stromal cells in an alginate system. Cell Tissue Res. 330, 567–572. [DOI] [PubMed] [Google Scholar]
- 11. Tsuji W, Inamoto T, Yamashiro H, Ueno T, Kato H, Kimura Y et al (2009) Adipogenesis induced by human adipose tissue‐derived stem cells. Tissue Eng. A 15, 83–93. [DOI] [PubMed] [Google Scholar]
- 12. Grassel S, Ahmed N (2007) Influence of cellular microenvironment and paracrine signals on chondrogenic differentiation. Front Biosci. 12, 4946–4956. [DOI] [PubMed] [Google Scholar]
- 13. Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A et al (2002) Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 16, 1558–1566. [DOI] [PubMed] [Google Scholar]
- 14. Coppack SW, Pinkney JH, Mohamed‐Ali V (1998) Leptin production in human adipose tissue. Proc. Nutr. Soc. 57, 461–470. [DOI] [PubMed] [Google Scholar]
- 15. Mohamed‐Ali V, Pinkney JH, Coppack SW (1998) Adipose tissue as an endocrine and paracrine organ. Int. J. Obes. Relat. Metab. Disord. 22, 1145–1158. [DOI] [PubMed] [Google Scholar]
- 16. Leow MK, Addy CL, Mantzoros CS (2003) Clinical review 159: human immunodeficiency virus/highly active antiretroviral therapy‐associated metabolic syndrome: clinical presentation, pathophysiology, and therapeutic strategies. J. Clin. Endocrinol. Metab. 88, 1961–1976. [DOI] [PubMed] [Google Scholar]
- 17. Deng Y, Scherer PE (2010) Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 1212, E1–E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stillaert F, Findlay M, Palmer J, Idrizi R, Cheang S, Messina A et al (2007) Host rather than graft origin of Matrigel‐induced adipose tissue in the murine tissue‐engineering chamber. Tissue Eng. 13, 2291–2300. [DOI] [PubMed] [Google Scholar]
- 19. Sugii S, Kida Y, Kawamura T, Suzuki J, Vassena R, Yin YQ et al (2010) Human and mouse adipose‐derived cells support feeder‐independent induction of pluripotent stem cells. Proc. Natl. Acad. Sci. USA 107, 3558–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F et al (2009) Feeder‐free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc. Natl. Acad. Sci. USA 106, 15720–15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu L, Cai X, Dong H, Jing W, Huang Y, Yang X et al (2010) Serum regulates adipogenesis of mesenchymal stem cells via MEK/ERK‐dependent PPARgamma expression and phosphorylation. J. Cell Mol. Med. 14, 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu L, Wu Y, Lin Y, Jing W, Nie X, Qiao J et al (2007) Osteogenic differentiation of adipose derived stem cells promoted by overexpression of osterix. Mol. Cell. Biochem. 301, 83–92. [DOI] [PubMed] [Google Scholar]
- 23. Lin YF, Jing W, Wu L, Li XY, Wu Y, Liu L et al (2008) Identification of osteo‐adipo progenitor cells in fat tissue. Cell Prolif. 41, 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D et al (1995) Weight‐reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546. [DOI] [PubMed] [Google Scholar]
- 25. Zvonic S, Lefevre M, Kilroy G, Floyd ZE, DeLany JP, Kheterpal I et al (2007) Secretome of primary cultures of human adipose‐derived stem cells: modulation of serpins by adipogenesis. Mol. Cell. Proteomics 6, 18–28. [DOI] [PubMed] [Google Scholar]
- 26. Chen JJ, London IM (1981) Hemin enhances the differentiation of mouse 3T3 cells to adipocytes. Cell 26, 117–122. [DOI] [PubMed] [Google Scholar]
- 27. Duran C, Tuncel E, Ersoy C, Ercan I, Selimoglu H, Kiyici S et al (2009) The investigation of the efficacy of insulin glargine on glycemic control when combined with either repaglinide or acarbose in obese Type 2 diabetic patients. J. Endocrinol. Invest. 32, 69–73. [DOI] [PubMed] [Google Scholar]
- 28. Chang L, Chiang SH, Saltiel AR (2004) Insulin signaling and the regulation of glucose transport. Mol. Med. 10, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yokoo H, Nemoto T, Yanagita T, Satoh S, Yoshikawa N, Maruta T et al (2007) Glycogen synthase kinase‐3beta: homologous regulation of cell surface insulin receptor level via controlling insulin receptor mRNA stability in adrenal chromaffin cells. J. Neurochem. 103, 1883–1896. [DOI] [PubMed] [Google Scholar]
- 30. Arufe MC, Lu M, Lin RY (2009) Differentiation of murine embryonic stem cells to thyrocytes requires insulin and insulin‐like growth factor‐1. Biochem. Biophys. Res. Commun. 381, 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hennige AM, Sartorius T, Tschritter O, Preissl H, Fritsche A, Ruth P et al (2006) Tissue selectivity of insulin detemir action in vivo. Diabetologia 49, 1274–1282. [DOI] [PubMed] [Google Scholar]
- 32. Garcia‐Escobar E, Rodriguez‐Pacheco F, Haro‐Mora JJ, Gomez‐Zumaquero JM, Rubio‐Martin E, Gutierrez‐Repiso C et al (2011) Effect of insulin analogues on 3t3‐l1 adipogenesis and lipolysis. Eur. J. Clin. Invest. 41, 979–986. [DOI] [PubMed] [Google Scholar]
- 33. Lian JB, Shalhoub V, Aslam F, Frenkel B, Green J, Hamrah M et al (1997) Species‐specific glucocorticoid and 1,25‐dihydroxyvitamin D responsiveness in mouse MC3T3‐E1 osteoblasts: dexamethasone inhibits osteoblast differentiation and vitamin D down‐regulates osteocalcin gene expression. Endocrinology 138, 2117–2127. [DOI] [PubMed] [Google Scholar]
- 34. Shi XM, Blair HC, Yang X, McDonald JM, Cao X (2000) Tandem repeat of C/EBP binding sites mediates PPARgamma2 gene transcription in glucocorticoid‐induced adipocyte differentiation. J. Cell. Biochem. 76, 518–527. [DOI] [PubMed] [Google Scholar]
- 35. Li X, Jin L, Cui Q, Wang GJ, Balian G (2005) Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporos. Int. 16, 101–108. [DOI] [PubMed] [Google Scholar]
- 36. Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89, 2548–2556. [DOI] [PubMed] [Google Scholar]
- 37. Kumar R (2009) Phosphate sensing. Curr. Opin. Nephrol. Hypertens. 18, 281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Droin N, Hendra JB, Ducoroy P, Solary E (2009) Human defensins as cancer biomarkers and antitumour molecules. J. Proteomics 72, 918–927. [DOI] [PubMed] [Google Scholar]


