Abstract
Abstract. This study aims to investigate engraftment of human cord blood and foetal bone marrow stem cells after in utero transplantation via the intracoelomic route in the sheep. Here, we performed transplantation in 14 single and 1 twin sheep foetuses at 40–47 days of development, using a novel schedule for injection. (i) Single injection of CD34+ human cord blood stem cells via the coelomic route (from 10 to 50 × 104) in seven single foetuses. (ii) Single injection of CD34+ foetal bone marrow stem cells via the intracoelomic route with further numbers of cells (20 × 105 and 8 × 105, respectively) in three single and in one twin foetuses. (iii) Double fractioned injection (20–30 × 106) via the coelomic route and 20 × 106 postnatally, intravenously, shortly after birth of CD3‐depleted cord blood stem cells in four single foetuses. In the first group, three single foetuses showed human/sheep chimaerism at 1, 8 and 14 months after birth. In the second group, the twin foetuses showed human/sheep chimaerism at 1 month after birth. In the third group, only two out of four single foetuses that underwent transplantation showed chimaerism at 1 month. While foetal bone marrow stem cells showed good short‐term engraftment (1 month after birth), cord blood stem cells were able to persist longer in the ovine recipients (at 1, 8 and 14 months after birth).
INTRODUCTION
In the particular setting of prenatal transplantation, gestational age of the foetus at transplant seems to be a critical factor to permit the establishment of immune tolerance to the graft, and to take advantage of the high differentiation potential of the embryo. The importance of gestational age is related to various factors, including progressive maturation of the foetal immune system, which becomes increasingly competent and less tolerant to foreign antigens (Cowan et al. 2001), and increase in the number of haematopoietic cells produced by the foetus itself, which occupy the natural sites of the haematopoiesis and compete with transplanted donor cells (Shaaban & Flake 1999). The coelomic cavity seems to be the earliest site for foetal cell transplantation (Noia et al. 2003).
Foetal stem cells can be isolated from foetal bone marrow as well as from other foetal tissues, including the liver. Previous studies have reported that the source of cells injected could be a critical factor for enhancing prospective engraftment. In comparison to adult stem cells, foetal stem cells have advantages of better intrinsic homing and engraftment, greater multipotentiality and lower immunogenicity (O'Donoghue & Fisk 2004). Michejda (2004) recently reported that foetal bone marrow is the best source of stem cells for engraftment and therapeutic reconstitution, due to its high proliferative capacity, low immunogenicity and highest ration of primitive stem/progenitor cells. The aim of the present study was to investigate the possible role of the source of stem cells injected into coelomic cavities on both long‐ and short‐term engraftment.
MATERIALS AND METHODS
Human cord blood cell collection and isolation
Human cord blood cells were obtained from informed mothers undergoing caesarean section delivery of full‐term infants at our hospital. Cord blood collection and the entire study was approved by the Hospital Investigational Review Board. Cord blood collections with final volume higher than 70 ml and showing CD34+ cell frequency higher than 0.2% only were processed for cell isolation. Red cells were removed from cord blood by 90‐min sedimentation using hydroxyethil starch at a ratio of 1 : 8 with cord blood. After washing, mononuclear cells were isolated from red cell‐depleted nucleate cells by centrifugation with Ficoll‐Paque density gradient (1.077 g/ml; Pharmacia LKB, Uppsala, Sweden). Then, isolated mononuclear cells were mixed (at cell concentration of 108 cells/ml) with 0.5 g/ml fluorescein conjugated (FITC) anti‐human CD3 monoclonal antibody (Caltag Laboratories, Burlingame, CA, USA) and were incubated at room temperature for 30 min in the dark. After washing, cells stained by CD3 FITC monoclonal antibody were mixed with 20 µl every 107 cells, MultiSort anti‐FITC microbeads (Miltenyi Biotec, Bergish Gladbach, Germany), were incubated for 15 min at 6–12 °C and then after washing, were loaded on to a VS+ selection column of VarioMACS magnetic instrumentation. CD3−‐depleted cord blood cells were collected prior to elution of the CD3‐positive fraction, following the manufacturer's guidelines. CD3 depletion produced an average 90% (range 70–98%) removal of CD3 cells and post‐immunoselection contaminating CD3 cells contained in CD3−‐depleted grafts averaged 3.5 × 106 (range 0.70–11 × 106). Particularly, CD3−‐depleted cord blood grafts contained, on average, 10% CD3+ cells, 55% monocytes, 15% CD19 cells, 5% CD3−‐negative lymphoid cells, 0.9% CD34+ cells and 13% contaminating band cells and granulocytes. A portion of samples of CD3−‐depleted cord blood mononuclear cells was subsequently incubated with the Miltenyi Multisort anti‐CD34 microbeads (Miltenyi Biotec; 100 µl per 108 cells) and then, after extensive washing, the cells were processed with the VarioMACS magnetic device to remove non‐CD34+ cells, as described by the manufacturer. CD34+ cord blood cells were eluted after removal of unwanted unbound cells by discontinuation of the magnetic fields in the VarioMACS device. All CD34+ immunoselected samples had CD34 cell purity higher than 95% and CD3 contaminating cells were consistently less than 0.1%.
Foetal bone marrow cell collection and isolation
Foetal bone marrow cells* were donated by Dr Maria Michejda (International Center for Interdisciplinary Studies of Immunology, Georgetown University Hospital, Washington, DC, USA). Cells were thawed to 37 °C, were resuspended and were washed in complete PBS (0.5% albumin, 100 U/ml DNAse) and a second washing was made in simple PBS. An aliquot of cells was countered in presence of trypan blue.
Foetal sheep recipients
Fifteen female comiso sheep (Ovis aries Comisana) were purchased and were transferred to the Center for Animal Breeding and Use, Catholic University of Rome. The animals grazed and were fed with legume hay together with a vitamin/mineral supplement. Oestrus was induced with polyurethane vaginal suppositories impregnated with fluorogestone acetate (40 mg), and these were left in place for 14 days. Mating occurred and pregnancy was diagnosed between 25th and 28th day after mating by means of a transvaginal ultrasonographic scan with a 6.5 MHz transducer. Animals were selected between day 40 and day 45 of gestation. Gestational age was determined by foetal growth curves, as we have previously described (Noia et al. 2002). For each transplantation, the pregnant ewe was anaesthetized with 5 ml of ketamine. The coelomic cavity was visualized by ultrasound, and human stem cells were injected under aseptic conditions via the transabdominal route. A 20‐gauge needle was used in a free‐hand double‐operator technique, for injection of stem cells in a fixed volume of 1.5 ml saline solution. In all cases, foetal heartbeat was checked immediately after transplant and at weekly intervals thereafter until birth or if intrauterine death occurred.
Schedule of injection
In our series, 14 single and 1 twin sheep foetus underwent transplantation in utero with different schedules of injection as follows in three groups (individual details shown in Table 1):
Table 1.
Cumulative data and outcome in 16 sheep foetuses
| Animal no. | Gestational age | No. of HSC injected (in utero) | Delivery | No. of HSC injected (postnatal) | Postnatal evaluation | Engraftment | ||
|---|---|---|---|---|---|---|---|---|
| CF | RT–PCR | IC | ||||||
| 072759 | 46 days | 1 × 105 CD34+ (CB) | SD | – | 1 month | Yes (bone marrow) | No | No |
| 072487 | 46 days | 5 × 105CD34+ (CB) | SD | – | 8 months | Yes (liver) | No | No |
| 072436 | 45 days | 4 × 105CD34+ (CB) | SD | – | 14 months | Yes(liver) | No | No |
| 787655 | 45 days | 20 × 106 CD3− (CB) | Miscarriage | |||||
| 956106 | 45 days | 30 × 106 CD3− (CB) | Miscarriage | |||||
| 72778 | 45 days | 18.5 × 106 CD3− (CB) | Miscarriage | |||||
| 915296 | 45 days | 30 × 106 CD3− (CB) | Miscarriage | |||||
| 217148 | 47 days | 0.8 × 105 CD34+ (FBM) | SD | – | 1 month | No | No | No |
| 217545 | 45 days | 2 × 105 CD34+ (FBM) | SD | – | 1 month | No | Yes (liver) | No |
| 9 months | No | No | No | |||||
| 217546 a | – | – | SD | – | 1 month | No | Yes (brain, liver, heart) | No |
| 217122 | 45 days | 2 × 105 CD34+ (FBM) | Miscarriage | |||||
| 217123 | 45 days | 0.8 × 105 CD34+ (FBM) | Miscarriage | |||||
| 72491 | 44 days | 20 × 106 CD3− (CB) | SD | 20 × 106 CD3− (CB) | 1 month | No | No | No |
| 217125 | 46 days | 30 × 106 CD3− (CB) | SD | 20 × 106 CD3− (CB) | 1 month | No | No | No |
| 217142 | 42 days | 20 × 106 CD3− (CB) | SD | 20 × 106 CD3−(CB) | 1 month | No | No | Yes (liver) |
| 217143 | 42 days | 20 × 106 CD3− (CB) | SD | 20 × 106 CD3−(CB) | 1 month | No | No | Yes (liver) |
CB, cord blood‐derived; CF, cytofluorimetry; FBM, foetal bone marrow‐derived; HSC, haematopoietic stem cells; IC, immunohistochemistry; RT–PCR, reverse transcriptase–polymerase chain reaction; SD, spontaneous delivery.
Twin foetus not directly transplanted.
-
1
Seven single foetuses that underwent transplantation with a single injection of varied amounts of CD34+ cord blood haematopoietic stem cells.
-
2
Three single foetuses underwent transplantation in utero via the intracelomic route with two different amounts of CD34+ foetal bone marrow cells. One of the twin foetuses (217545) received the same quantity of foetal bone marrow stem cells, but the other foetus (217546) did not receive a transplant, to evaluate whether cells injected would be able to migrate from one twin foetus to the other.
-
3
Four further foetuses received double transplantation with a first intracoelomic injection of T‐cell‐depleted Cord Blood Mononuclear cells (CB‐MNC), followed by a second injection of 20 × 106 T‐cell‐depleted CB‐MNC administered intravenously 3 days after birth.
Fate of the animals
Four single pregnancies in the first group and two single pregnancies in the second group failed soon after transplantation. All 10 surviving foetuses were spontaneously delivered at term. The lambs were pre‐anaesthetized with atropine 0.02 mg/kg + ketamine 10 mg/kg + diazepam 0.1 mg/kg and anaesthetized with O2 + isofluorane 0.8–1% at 1 month, at 8 months and at 14 months after birth and tissue samples of liver and bone marrow were drawn under aseptic conditions. Lamb no. 217146 died 24 days after birth and brain, liver and heart were analysed (Table 1).
Analysis of human cell engraftment
Flow cytometry
Flow cytometry was performed on tissues with relevant haematopoietic activity (liver, bone marrow) to detect haematopoietic cells expressing human surface markers. Tissue samples had been stored in saline solution immediately after collection and were processed according to standard protocols. Single cell suspensions were prepared by mincing samples with scissors and scalpel in a Petri dish. Cells were centrifuged at 200 g for 10 min and were resuspended in isotonic buffer supplemented with 5% foetal calf serum; the entire procedure was carried out at 4 °C. The cell suspension was stained with saturating amounts of FITC anti‐human CD45, phycoerythrin‐conjugated anti‐human CD19, phycoerythrin anti‐human CD11b, FITC anti‐human CD3, peridinin chlorophyll protein (PerCP)‐conjugated anti‐human CD34 and PerCP anti‐human CD45 (all reagents from Caltag Laboratories, except anti‐CD34 and CD45‐PerCP which were from Becton Dickinson, San Jose, CA, USA), to evaluate human/sheep chimaerism. After a 30‐min incubation on ice, cell suspensions were thoroughly washed in cold Hank's balanced salt solution, were resuspended in the same medium, and were subjected to flow cytometric analysis. Background fluorescence was established using isotype‐matched phycoerythrin‐, PerCP‐ or FITC‐irrelevant monoclonal antibodies. All cytofluorimetric analyses were carried out using a FACScan (Becton Dickinson) flow cytometer.
Reverse transcriptase–polymerase chain reaction
All ovine samples from liver, brain, muscle, heart and bone marrow were frozen in liquid nitrogen after collection and were stored at –80 °C. Total RNA was extracted using RNeasy Miny Kit (QIAGEN) according to manufacturer's protocol, from tissues homogenized for 2 min at maximum speed using ULTRA‐TURRAX T 25 (IKAWERK Labortechnik. Staufen i. Br.). RNA concentration of each sample was measured by spectrophotometry (Beckman spectrophotometer DU640, Palo Alto, CA, USA). RNA samples were frozen at –80 °C until reverse transcriptase–polymerase chain reaction (RT–PCR) analysis. Two microgram of total RNA were reverse transcribed with 25 U of Moloney murine leukaemia virus reverse transcriptase (PE Applied Biosystems, Foster City, CA, USA) at 42 °C for 30 min in the presence of random hexamers. Two microlitres of cDNA products were amplified with 1 U of AmpliTaq Gold (PE Applied Biosystems) in the presence of specific primers for the RNA of interest. Reactions were conducted in the PTC‐0200 DNA Engine (MJ Research, Waltham, MA, USA). Amplification of human myoglobin mRNA was achieved by 35 cycles of 45 s at 57 °C and 1 min at 72 °C in the presence of 1 mm MgCl2. Four microlitres of DNA product were re‐amplified using the same conditions to augment sensitivity. The following primers were used for amplification of human myoglobin (GI 44955876) mRNA: 5′‐GGGTGTTGAAGTTGGCTTTG‐3′ and 5′‐CAGACAATTGCTACTGTCAG‐3′. Amplification of GAPDH mRNA was achieved by 28 cycles of 45 s at 57 °C and 1 min at 72 °C in the presence of 1 mm MgCl2. The following primers were used for amplification of GAPDH (GI 7669491) mRNA: 5′‐TGACATCAAGAAGGTGGTGA‐3′ and 5′‐TCCACCACCCTGTTGCTGTA‐3′. These nucleotide primers were synthesized by M‐Medical (Florence, Italy). Amplification of human troponin I (GI 37427) mRNA was achieved by 35 cycles of 45 s at 61.8 °C and 1 min at 72 °C in the presence of 1 mm MgCl2. Four microlitres of DNA product were re‐amplified using the same conditions to augment sensitivity. The following primers were used for amplification of human troponin I mRNA: 5′‐AACTACCGCGCTTATGCCAC‐3′ and 5′‐GTGTCCTCCTTCTTCACCTG‐3′. These nucleotide primers were synthesized by MWG (Florence, Italy). PCR products were analysed on 2% agarose gel stained with ethidium bromide.
Immunohistochemistry
To detect the engraftment of the transplanted human umbilical cord blood stem cells, we also performed immunohistochemistry on ovine liver. Tissue samples were formalin‐fixed and paraffin‐embedded according to standard protocols. Human cells were detected by immunostaining using monoclonal antibodies against human CD34 and against human hepatocyte‐specific antigen. Specimens were counterstained with haematoxylin and were visualized using diaminobenzidine (brown) as the chromogen.
RESULTS
Individual details are shown in Table 1.
-
1
In the first group, flow cytometry performed on lambs that underwent transplantation of human CD34+ cord blood stem cells showed human engraftment in the liver 1, 8 and 14 months after birth (animals 072487, 072759 and 072436), yet RT–PCR and immunohistochemistry techniques showed no human engraftment.
-
2
In the second group, RT–PCR analysis performed on liver of the lamb (217148) injected with foetal bone marrow CD34+ cells was negative. In the twin pregnancy, RT–PCR analysis was positive in the liver of the foetus that underwent transplantation (217145) 1 month after birth; the twin lamb (217146) died 24 days after birth and RT–PCR analysis indicated the presence of human DNA in the brain, liver and heart (Fig. 1), yet flow cytometry and immunohistochemistry were unsuccessful. No human engraftment was observed 9 months later in animal 217145.
-
3
In the third group, no human engraftment was observed at birth and/or 2 months later by means of flow cytometry, immunohistochemistry or RT–PCR in animals 72491 and 217125. Animals 217142 and 217143 showed liver engraftment by immunocytochemistry. As shown in Fig. 2, immunohistochemical signals of positive human cell engraftment seemed increased in the second sample and with broader localization around blood vessels. No human cell engraftment was observed 9 months later.
Figure 1.
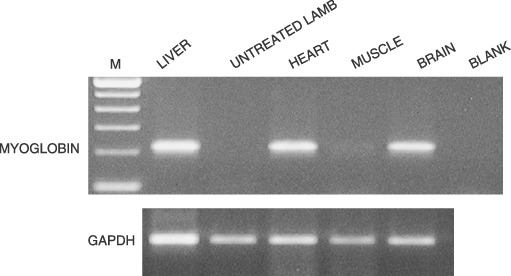
RT–PCR analysis of human myoglobin of transplanted lamb with foetal bone marrow stem cells (lamb no. 217546).
Figure 2.
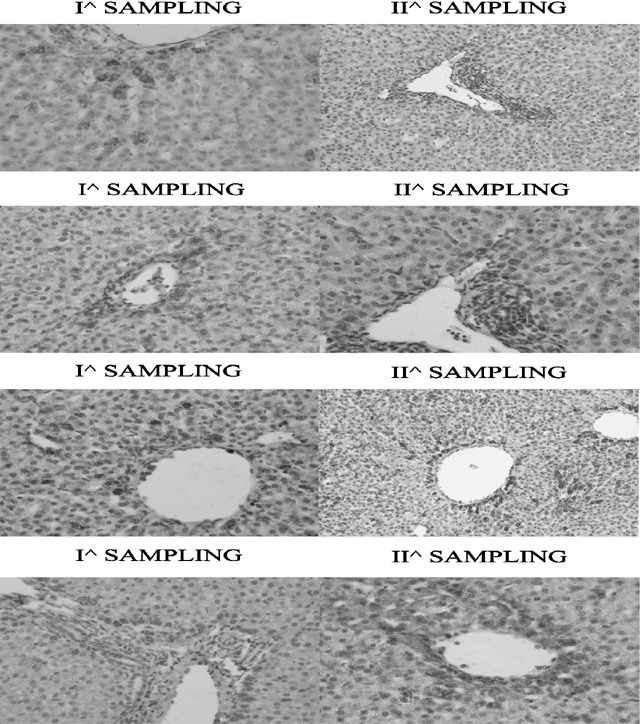
Immunohistochemical signals of positive human cells engraftment seem to increase in the second sample and with wider localization around the vessels.
DISCUSSION
Human stem cell transplantation in immunocompromised, pre‐immune or normal adult animals could represent reliable in vivo models to assess stem cell activity and/or tissue replacement capacity after experimental organ injury (Zanjani et al. 1992; Bhatia et al. 1997; Chen et al. 2001). While in adult animals experimentally provoked organ damage can be partially repaired by syngeneic or xenogenic stem cell transplantation (Lagasse et al. 2000; Chen et al. 2001), in pre‐immune sheep foetuses receiving human stem cells, human chimaerism in bone marrow at birth has been demonstrated as well as the presence of human non‐haematopoietic terminally differentiated cells in organs of endodermic origin (Almeida‐Porada et al. 2001).
In a recent study, we reported for the first time that intracoelomic injection of human stem cells into sheep foetuses at early gestational age is feasible and is associated with a high degree of engraftment in haematopoietic tissues (6–32%) (Noia et al. 2003, 2004). These results are no lower than those reported for the intraperitoneal route, which is described to produce 0.1–10% human chimaerism (Shimizu et al. 1998). Our data suggest that the source of cells for in utero transplantation plays a critical role for the engraftment of stem cells. As previously reported, foetal tissues are useful donor sources for stem cell transplantation due to their lower immunogenicity and higher clonogenic potential (Diukman et al. 1992). In particular, foetal bone marrow seems to be richer in CD34+ cells compared to adult bone marrow, peripheral blood mononuclear cells and cord blood, and show very low capacity for immunoreactivity, in comparison to those other sources (Wu 1999, Michejda 2004). In addition, foetal bone marrow showed highest levels of expression of selected cytokines such as CSF, IL‐6 and IL‐11 when compared to the other sources (Michejda 2004). We can speculate that these special characteristics of foetal bone marrow are responsible for good short‐term engraftment in foetuses of the second group; on the other hand, the rapid decline in chimaerism could be related to the lack of CD3 cells. Shields et al. (2003) have shown a statistical correlation between the level of chimaerism and number of CD3 cells, when injected in primate models.
Nevertheless, significant disadvantages are present for using foetal tissue. Difficulties in obtaining human foetal bone marrow stem cells during legal abortion could lead to impaired cell viability or viral, bacterial and fungal contamination. Moreover, from a single aborted human foetus, we can obtain only a small number of cells, thus several foetal donors would be required to perform just one transplantation. In addition, ethical implications concerning the clinical use of human foetal tissues from abortion limit their use (Surbek et al. 1999). Cord blood stem cells persist longer, but our data are not univocal because of the small number of foetuses that underwent transplantation to date.
Except for the biological aspects, few ethical issues have become a concern for transplantation teams who use cord blood. Cord blood is a source of a limited number of stem cells, but it is easy to collect. Blood that remains in the delivered placenta also can be safely and easily collected and stored. The ‘naïve’ nature of Umbilical Cord Blood (UCB) lymphocytes may explain lower incidence and severity of graft‐versus‐host disease encountered in UCB transplantation compared to allogeneic bone marrow cell transplantation (Bojanic et al. 2006). In contrast, stem cells recovered postnatally from the umbilical cord, including umbilical cord blood cells, amnionplacenta, umbilical cord vein, or umbilical cord matrix cells, are a readily available and are an inexpensive source of cells capable of differentiating into many different cell types (multipotentiality) (Weiss & Trover 2006).
Important factors that could interfere with transplantation were also found: (i) when injection was performed outside the foetus; (ii) when injection was performed into the coelomic fluid, where total molar amino acid concentration is high (Jauniaux et al. 1998); (iii) amino acid concentration could interfere with stem cells migration; and (iv) graft rejection by the recipient in xenotransplantation. Previous studies have assessed that a postnatal boost injection enhances engraftment (Milner et al. 1999; Almeida‐Porada et al. 2000) and our results also demonstrate such an increase in donor cell engraftment.
CONCLUSION
Our study has had many limits due to the use of different quantities of cells injected. Nevertheless, it seems that impact of the source of cells injected has shown discordant results in term of long‐ and short‐term engraftment. Soon after birth (0–40 days) source of the cells favoured the importance of foetal bone marrow origin, but long‐term engraftment evaluation showed that cord blood haematopoietic stem cells CD34+ had a better capacity to persist after birth. The meaning of this discrepancy could not be completely clarified due to many biases involved in postnatal checking, but the ovine model has demonstrated that nevertheless, when injection was performed outside the foetus, engraftment was particularly evident in haematopoietic over non‐haematopoietic tissues and even with a declining trend, persistence of engraftment lasted 14 months.
ACKNOWLEDGEMENT
This work was partly supported by the cord blood stem cell project, ‘Fondazione Cassa di Risparmio’, Rome, Italy.
All authors declare no conflicts of interest.
Sampled from spontaneous abortions after parental consensus and ethical Committee approval.
REFERENCES
- Almeida‐Porada G, Porada C, Tran N, Zanjani ED (2000) Transplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boost cell levels in bone marrow at later time points after transplantation. Blood 95, 3620–3627. [PubMed] [Google Scholar]
- Almeida‐Porada G, Porada C, Zanjani ED (2001) Adult stem cell plasticity and methods of detection. Rev. Clin. Exp. Hematol. 5, 26–41. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE (1997) Purification of primitive human hematopoietic cells capable of repopulating immune‐deficient mice. Proc Natl Acad Sci USA 94, 5320–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojanic I, Golubic Cepulic B (2006) Umbilical cord blood as a source of stem cells. Acta Med. Croatica 60, 215–225. [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez‐Ramos J, Chopp M (2001) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32, 2682–2688. [DOI] [PubMed] [Google Scholar]
- Cowan MJ, Chou SH, Tarantal AF (2001) Tolerance induction post in utero stem cell transplantation. Ernst. Schering. Res. Found. Workshop 33, 145–171. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Gulbis B, Gerlo E, Rodeck C (1998) Free amino acid distribution inside the first trimester human gestational sac. Early Hum. Dev. 51, 159–169. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al‐Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo . Nat. Med. 6, 1229–1234. [DOI] [PubMed] [Google Scholar]
- Michejda M (2004) Which stem cells should be used for transplantation? Fetal. Diagn. Ther. 19, 2–8. [DOI] [PubMed] [Google Scholar]
- Milner R, Shaaban A, Kim HB, Fichter C, Flake AW (1999) Postnatal booster injection increase engraftment after in utero stem cell transplantation. J. Surg. Res. 83, 44–47. [DOI] [PubMed] [Google Scholar]
- Noia G, Pierelli L, Bonanno G, Monego G, Perillo A, Rutella S, Cavaliere AF, Straface G, Fortunato G, Cesari E, Scambia G, Terzano M, Iannace E, Zelano G, Michetti F, Leone G, Mancuso S (2004) The intracoelomic route: a new approach for in utero human cord blood stem cell transplantation. Fetal. Diagn. Ther. 19, 13–22. [DOI] [PubMed] [Google Scholar]
- Noia G, Pierelli L, Bonanno G, Perillo A, Rutella S, Cavaliere AF, De Santis M, Ligato MS, Fortunato G, Scambia G, Terzano GM, Iannace E, Zelano G, Michetti F, Leone G (2003) A novel route of transplantation of human cord blood stem cells in preimmune fetal sheep: the intracelomic cavity. Stem Cells 21, 638–646. [DOI] [PubMed] [Google Scholar]
- Noia G, Romano D, Terzano MG, De Santis M, Di Domenico M, Cavaliere AF, Ligato MS, Petrone A, Fortunato G, Filippetti F, Caruso A, Mancuso S (2002) Ovine fetal growth curves in twin pregnancy: ultrasonographic assessment. Clin. Exp. Obstet. Gynecol. 24, 251–256. [PubMed] [Google Scholar]
- O'Donoghue K, Fisk NM (2004) Fetal stem cell. Best Pract. Res. Clin. Obstet. Gynaecol. 18, 853–875. [DOI] [PubMed] [Google Scholar]
- Shaaban AF, Flake AW (1999) Fetal hematopoietic stem cell transplantation. Semin. Perinatol. 23, 515–523. [DOI] [PubMed] [Google Scholar]
- Shields LE, Gaur LK, Gough M, Potter J, Sieverkropp A, Andrews RG (2003) In utero hematopoietic stem cells transplantation in nonhuman primates: the role T cells. Stem Cells 21, 304–314. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Ogawa M, Kobayashi M, Almeida‐Porada G, Zanjani ED (1998) Engraftment of cultured human hematopoietic cells in sheep. Blood 91, 3688–3692. [PubMed] [Google Scholar]
- Surbek DV, Gratwohl A, Holzegreve W (1999) In utero hematopoietic stem cell transfer: current status and future strategies. Eur. J. Obstet. Gynecol. Reprod. Biol. 85, 109–115. [DOI] [PubMed] [Google Scholar]
- Weiss ML, Trover DL (2006) Stem cells in umbilical cord. Stem Cell Rev. 2, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AG, Michejda M, Mazumder A, Mechan KR, Menerdez FA, Tchabo JG, Slack R, Johnson MP, Bellanti JA (1999) Analysis and characterization of hematopoietic progenitor cells from fetal bone marrow, adult bone marrow, peripheral blood and cord blood. Pediatr Res. 46, 163–169. [DOI] [PubMed] [Google Scholar]
- Zanjani ED, Pallavicini MG, Ascensao JL (1992) Engraftment and long‐term expression of human fetal hemopoietic stem cells in sheep following transplantation in utero. J. Clin. Invest. 89, 1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]


