Abstract
Abstract. CP27 is a gene that has been cloned from an E11 early embryonic library and has been suggested to mediate early organogenesis (Diekwisch et al., 1999, Gene 235, 19). We have hypothesized that CP27 exhibits its effects on organogenesis by affecting individual cell function. Based on the CP27 expression pattern we have selected the CP27 expressing embryonic fibroblast cell line BALB/c 3T3 to determine the effects of CP27 on cell function. CP27 loss of function strategies were performed by adding 5, 12.5 or 25 µg/ml anti‐CP27 antibody to cultured BALB/c 3T3 cells and comparing the results to controls in which identical concentrations of rabbit serum were added to the culture medium. Other controls included an antibody against another extracellular matrix protein amelogenin (negative control) and anti‐CP27 antibodies directed against other areas of the CP27 molecule (positive control). Following cell culture, cell viability, apoptosis, cell proliferation, cell shape, cellular attachment and fibronectin matrix production were assayed using MTT colourimetric assay, BrdU staining, morphometry, immunostaining and western blot analysis. Block of CP27 function using an antibody strategy resulted in the following significant changes: (i) reduced viability, (ii) increased number of apoptotic cells, (iii) reduced proliferation, (iv) alterations in cell shape, (v) loss of attachment, and (vi) reduction in fibronectin matrix production. There was also a redistribution in fibronectin matrix organization demonstrated by immunohistochemistry. We conclude that CP27 plays an important role in the maintance of normal cell function and that CP27 block leads to significant changes in cellular behaviour.
Introduction
Cell–matrix interactions have major effects on phenotypic features such as gene regulation, cytoskeletal structure, differentiation and aspects of cell growth control (Roskelley et al. 1995; Boudreau & Bissell 1998; Lukashev & Werb 1998; Giancotti & Ruoslahti 1999). Many cells are anchorage‐dependent and require adhesion to the extracellular matrix in order to develop and survive (Assoian & Zhu 1997; Howe et al. 1998). Cellular adhesion to extracellular matrices is mediated by cell surface integrins and other cell surface receptors (Hynes 1992; Schwartz et al. 1995), which activate intracellular signalling cascades and cause tension‐dependent changes in cell shape and cytoskeletal structure (Folkman & Moscona 1978; Roskelley et al. 1994; Huang et al. 1998). Recent studies suggest that cell shape may play a critical role in the control of cell cycle progression (Chen et al. 1997). Matrix engagement of specific receptors represents an important control point for determining cell shape, cytoskeletal geometry and the organization of intracellular components as well as initiating the signalling events that lead to cell cycle progression (Guadagno et al. 1993; Fang et al. 1996; Zhu et al. 1996; Chen et al. 1997). One example of a typical extracellular matrix protein that regulates cell shape and controls cell cycle progression is fibronectin (Sechler & Schwarzbauer 1998; Christopher et al. 1999). Another protein that exhibits typical features of an extracellular matrix protein similar to fibronectin is the protein product of our novel gene cp27 (Diekwisch et al. 1999).
cp27 is a gene which has been cloned from an E11 early embryonic library and translates into a protein CP27 with a length of 333 amino acids ( Diekwisch & Marches 1997 ; Diekwisch et al. 1999 ). Northern blot analysis of RNA from multiple mouse tissues demonstrated high levels of expression in developing mouse teeth, heart, lungs, and liver of a single transcript of 1.8 kbp. In situ hybridization using a radioactive RNA probe resulted in distinct signals in the developing neuroepithelium, cerebellum, heart, lungs, liver, teeth, salivary glands and periosteum of developing bone. Immunohistochemical staining of developing mouse tissues detected epitopes specific for CP27 in the mesenchyme surrounding the primary brain vesicles, in basement membranes, in the periosteum, in salivary glands and in the stellate reticulum of teeth. On a cellular level, CP27 was localized in basement membranes, extracellular and pericellular matrices indicating that CP27 might be an extracellular matrix protein ( Diekwisch et al. 1999 ). Recent functional studies have provided additional evidence for our hypothesis that CP27 acts as an extracellular matrix protein and suggested that CP27 is critical molecular factor in mouse embryogenesis ( Diekwisch & Luan 2002 ).
Based on CP27 expression pattern, we have selected the CP27 expressing embryonic fibroblast cell line, mouse BALB/c‐3T3 to determine the effect of CP27 on cell function and observe the interaction between CP27 and fibronectin in regulating cell behaviour. Our studies demonstrated that the blockage of CP27 function exerted a significant antiproliferation effect and induced apoptosis in embryonic fibroblasts. Treatment with anti‐CP27 antibody resulted in a significant decrease in the number of attached cells. These functional differences following CP27 loss of function strategies were paralleled by a down‐regulation of fibronectin synthesis and changes in cell shape. The results demonstrate that CP27 is an important factor to maintain normal cell function in embryonic development.
Materials and methods
Cell culture and CP27 loss of function strategy
BALB/c 3T3 embryonic fibroblasts were obtaind from the American Type Culture Collection (ATCC, Masasses, VA). The cells were grown in DMEM supplemented with 10% calf serum (Gibco BRL, Grand Island, NY). Cells were seeded at a density of 3 × 105/well in 6‐well plates. After incubation for 24 h at 37 °C in a humidified atmosphere of 5% CO2 in air, the medium was removed and monolayers were washed twice with Hank's buffer. The subconfluent cells then received 5, 12.5 or 25 µg/ml anti‐CP27 antibody treatments. The anti‐CP27 antibody used for block of CP27 function was a polyclonal antibody raised in rabbits that were immunized with peptides directed against a conserved motif of the mouse and human CP27 sequence (Diekwisch et al. 1999; Diekwisch & Luan in press). In order to control for the effect of the rabbit antimouse CP27 antibody, we added an identical percentage of rabbit serum (50 µg/ml) to the control experiment. A further control experiment included the use of an anti antiamelogenin antibody at a concentration of 12.5 µg/ml as a negative control (Simmer et al. 1994).
MTT assay for cell viability
Embryonic fibroblasts were seeded into 96‐well plates at a density of 1 × 104 cells. Twenty‐four hours after incubation, the medium was replaced and CP27 antibody was added in eight replicate wells. The cells were then incubated for a further 24 h, after which MTT (tetrazolium salt 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide) assay stock solution was added to a final concentration of 0.5 µg/ml. To control for background absorbance, eight wells of cells were lysed by the addition of Triton X‐100 to a final concentration of 0.1% v/v immediately prior to the addition of MTT reagent. After incubation for 3 h, the insoluble product was dissolved by addition of 100 µl of cell lysis buffer (20% w/v SDS, 50% v/v dimethylformamide, pH 4.7), and absorbance of the wells was measured at 570 nm using a DU Series 60 Spectrophotometer (Beckman, Fullerton, CA). Cell viability was then calculated by the equation: Percentage cell viability = (absorbance of treated cultures – Absorbance of background control)/(Absorbance of control cultures – Absorbance of background control) × 100.
Cell proliferation assay
Cell proliferation was detected by labelling cells with BrdU. After 24 h treatment with anti‐CP27 antibody, cells were incubated with BrdU (1 : 100) according to the protocol of the manufacturer (ZYMED, San Francisco, CA). Cells were incubated for 1 h in the culture medium and then fixed with 70% ethanol. The incorporated BrdU was detected by the indirect immunoperoxidase method (Amersham, Arlington Heights, IL). Briefly, the culture cells were first incubated for 1 h with Biotin‐Mouse Anti‐BrdU antibody. After washing in TTBS buffer (20 mm Tris, 500 mm NaCl, 0.05% Tween‐20, pH 7.5), the cells were further incubated with Biotinylated goat antimouse immunoglobulins for 10 min. Sections were then washed and incubated with enzyme (peroxidase) conjugate for 10 min at room temperature. Immunoreactivity was revealed by the addition of substrate‐chromogen. The cells were counterstained with haematoxylin and mounted.
Fluorescence microscopy of nuclear morphological analysis
Following 48 h of culture, cells were fixed with 70% ethanol and stained with a solution of 1 µg/ml 4,6‐diamidino‐2‐phenylindole (DAPI) in PBS for 10 min, then mounted in a water‐soluable mounting medium. Cells were examined under a 100 × oil immersion objective using a Leitz DMRB fluorescence microscope (Leica Mikroskopie und Systeme GmbH, Wetzlar, Germany).
Phase contrast morphometry of attached cells
Subconfluent cells were treated with 12.5 µg/ml CP27 antibody or 50 µl/ml rabbit serum. After 72 h, pictures of 10 randomly selected different fields of each well were taken. More than 50 cells were present in every picture. In each picture, the number of adherent cells was counted. The percentage of adherent cells in the experimental group compared to control culture were calculated.
Electron microscopy
Cells were cultured for 24 h in Falcon cell culture inserts (cat #3090) and treated with either 12.5 µg/ml anti‐CP27 antibody or 50 µl/ml rabbit serum. Following culture, cells were fixed in Millonig's buffered osmium to provide optimum contrast for transmission electron microscopy. Specimens were then dehydrated and embedded in Eponate 812 as previously described (1995, 1993). Ultrathin sections were cut at 80 nm, contrasted using uranyle acetate and lead citrate, and analysed using a JEOL electron microscope (JEOL USA, Peabody, MA) (1995, 1993).
Immunohistochemistry
Immunoreactions were performed as previously described (Diekwisch et al. 1997; Thieberg et al. 1999). Affinity purified antihuman fibronectin was used as primary antibody (Sigma, St. Louis, MO). In previous studies, this antibody cross‐reacted in mice in a tissue‐specific fashion (data not shown). Anti‐rabbit IgG was applied as a secondary antibody. Signals were detected using 3‐amino‐9‐ethylcarbazole (AEC) as a substrate. In between these steps, slides were washed in phosphate buffered saline. The following controls were used to determine the specificity of the antifibronectin antibodies: (i) tissue controls – the specificity of the antibody will be evaluated in tissues with known immunoreactivity, (ii) antibody controls by using a dilution series, (iii) controls with preadsorbed anti‐CP27 antibody to exclude unspecific binding, (iv) controls with preimmune serum to control for binding to serum components and (v) omission of primary antibody as a systemic control.
Western blotting
Cells were lysed directly after washing in PBS with SDS‐PAGE sample buffer (without dye). Aliquots of samples were separated on a 10% SDS‐PAGE gel and transferred to nitrocellulose in a blotting apparatus filled with transfer buffer (25 mm Tris, 190 mm glycine, 20% methanol) for 1 h at 75 mA. Nitrocellulose filters were blocked in 2% BSA overnight at room temperature. The blot was incubated with 1 : 1000 dilution of goat antihuman fibronectin (Sigma) for 2 h, 1 : 2000 dilution of biotinylated rabbit antigoat IgG (Amersham) for 1 h, and then with an avidin‐biotin complex. Controls omitting the primary antibody were all negative.
Results
Loss of CP27 function reduced cell viability and enhances apoptosis
In order to evaluate the effect of CP27 block on cell viability and cell survival, we applied three different techniques which all assayed for different aspects of cell vitality and thus resulted in a multifaceted analysis of the effect of CP27 block on cell life and death regulation. While CP27 block of function using our anti‐CP27 antibody exhibited a strong effect on cultured cells, there was no effect observed using rabbit serum or a control antibody (antiamelogenin) at identical concentrations of 50 µl/ml.
First we used the MTT assay as a method to determine the percentage of viable cells that were able to cause a specific colour reaction. CP27 loss of function strategies resulted in a significant (P < 0.01) loss of cell viability as demonstrated by the MTT assay (percent values of viable cells compared to control). The potency in reducing BALB/c 3T3 cell viability depended on effective block of CP27 function (Fig. 1). Compared to the control group, viable cells were 70.1 ± 5.9% (5 µg/ml anti‐CP27 antibody culture), 66.6 ± 3.4% (12.5 µg/ml anti‐CP27 antibody), and 53.8 ± 1.7% (25 µg/ml anti‐CP27 antibody).
Figure 1.
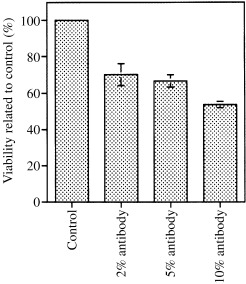
Effect of CP27 function inhibition on cell viability. The graph shows changes in cell viability following treatment with 2, 5 and 10% anti‐CP27 antibody or 10% rabbit serum. The cells were BALB/c 3T3 Cells seeded into 96‐well plates at a density of 10 4 cells/well. Viable cells were detected based on mitochondrial MTT metabolism and measured at 570 n m using a Deneley plate reader. Cell viability was then calculated by the equation: percentage cell viability = (absorbance of treated culture – absorbance of background control)/(absorbance of control culture – absorbance of background control) × 100. Note the extremely significant reduction of viable cells in the anti‐CP27 antibody‐ treated groups as well as the decreased percentage of viable cells with increased antibody concentrations.
As a second method to determine the effect of CP27 block on cells in culture, we used DAPI fluorescence microscopy of nuclear shape and staining. Apoptotic cells contained smaller brightly stained nuclei with chromatin condensed into discrete globular structures while normal nuclei exhibited diffuse, homogeneous DAPI staining. Treatment of the embryonic fibroblasts with 12.5 µg/ml CP27 antibody for 24 h caused the appearance of morphologically apoptotic nuclei (Fig. 2b, arrows). The cells of the control group contained intact nuclei with diffuse homogeneous DAPI staining (Fig. 2a). The proportion of the apoptotic cells was 21.8 ± 0.9% in 12.5 µg/ml CP27 treated culture and only 2.1 ± 0.3% in control culture. The treatment with CP27 antibody resulted in a significant increase in the number of apoptotic cells (P < 0.05).
Figure 2.
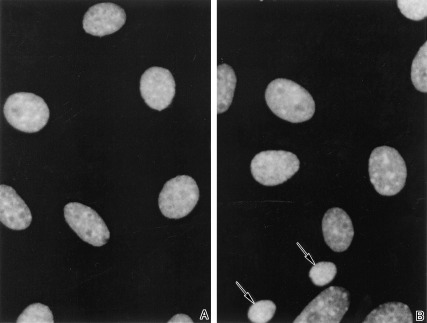
DAPI fluorescence microscopy of cultured embryonic fibroblast nuclei in a control group treated with 50 µl/ml rabbit serum (a) and an experimental group treated with 12.5 µg/ml anti‐CP27 antibody (b). The experimental group in which CP27 was blocked contained a high number of condensed nuclei indicative of apoptosis (b) compared to the control group (a).
Electron microscopy was used as a third method to determine how CP27 function block modulates cell survival. In the cells of the control culture, the structure of the nucleus was apparently normal (Fig. 3a). In the cells of CP27 antibody‐treated group, however, numerous nuclei were invaginated and pinched off, while crescent‐shaped spaces (CSS) were found around the nuclear envelope of these apoptotic cells (Fig. 3b).
Figure 3.
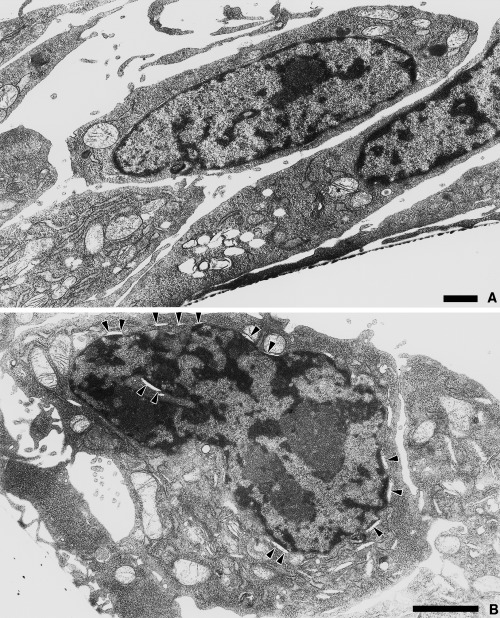
Ultrastructural analysis of the effects of CP27 loss of function on BALB/c 3T3 embryonic fibroblasts (b) in comparison with a nontreated control group (a). Subconfluent cells were treated with 12.5 µg/ml anti‐CP27 antibody for 24 h. All cells were fixed in Millonig's buffered fixative and processed for electron microscopy. The arrowheads indicate crescent‐shaped spaces indicative of apoptosis in the nuclear envelope of the anti‐CP27 antibody‐treated cells. The magnification is 5000 × (a) and 8500 × (b). The bar indicates 1 µm.
CP27 block resulted in a reduction of cell proliferation
To confirm that CP27 antibody impacts fibroblast proliferation, the percentage of cells that entered the S phase of the cell cycle was assessed by using BrdU labelling. Cells were cultured for 24 h, either in the presence of 50 µl/ml rabbit serum or in the presence of 12.5 µg/ml anti‐CP27 antibody. The number of BrdU labelled cells in the anti‐CP27 antibody‐treated group (Fig. 4b) was significantly lower than the number of BrdU labelled cells in the control group (Fig. 4a). Using the BrdU assay, 30.9 ± 2.5% of CP27 antibody‐treated cells stained (Fig. 4b), compared with 50.8 ± 1.7% of the control cells (Fig. 4a). The significant reduction in the percentage of proliferating cells following anti‐CP27 antibody treatment is apparent in the diagram (Fig. 4c).
Figure 4.
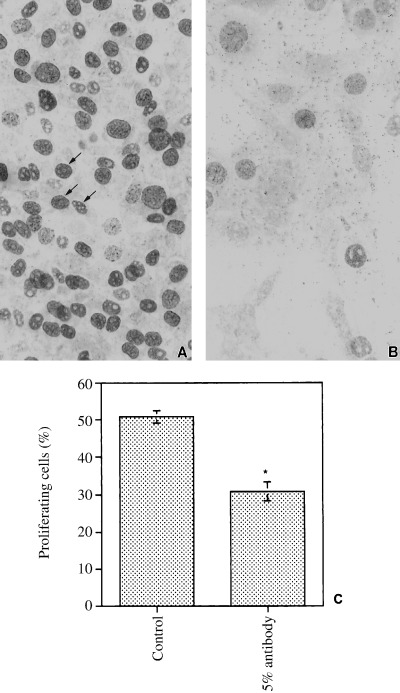
Effect of CP27 block on cell proliferation as determined using BrdU staining. The number of BrdU labelled cells in the anti‐CP27 antibody‐treated group (b) was significantly lower than the number of BrdU labelled cells in the control group (arrows, a). The graph in (c) illustrates the significant reduction in the percentage of proliferating cells following anti‐CP27 antibody treatment.
CP27 block caused loss of attachment and changes in cell shape
We used phase contrast microscopy to determine the effect of CP27 function inhibition on the number of attached cells and on cell shape. Cells were cultured for 48 h and analysed by phase contrast microscopy. There was an extremely significant reduction in the number of attached cells in the anti‐CP27 antibody‐treated group (29.5 ± 0.6%) (Fig. 5b) compared to the control group (100%) (Fig. 5a). In comparison to the control, the cells in the antibody‐treated group were elongated and contained long and thin processes. The antibody‐treated dish also contained a high number of free floating cells which had a rounded appearance and were aggregated in groups. The remaining adherent cells were less birefringent than the cells of the control group (comparison not visible on micrographs). The dimensions of empty intercellular spaces between cells were increased in the antibody‐treated group (Fig. 5b). The arrows indicate typical attached cells with altered morphology in the anti‐CP27 antibody‐treated group (Fig. 5b). These results suggest that the loss of CP27 function leads to disruption of normal cell architecture.
Figure 5.
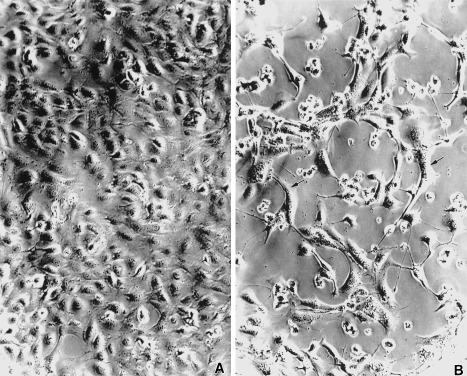
Loss of attachment and modulation of cell shape following CP27 function inhibition. Cells were cultured for 48 h and analysed by phase contrast microscopy. In the anti‐CP27 antibody‐treated group (b) the number of attached cells was significantly reduced compared to the control group (a). In comparison to the control, the cells in the antibody‐treated group were elongated and contained long and thin processes. The antibody‐treated dish also contained a high number of free floating cells which had a rounded appearance and were aggregated in groups. The arrows indicate typical attached cells with altered morphology in the anti‐CP27 antibody‐treated group.
CP27 block reduced levels of fibronectin gene expression
Fibronectin gene expression as a result of CP27 block was assayed both using immunohistochemistry and western blot. The immunohistochemical analysis allowed us to determine the effects of CP27 function inhibition on fibronectin distribution (Fig. 6). In the control group, the fibronectin extracellular matrix detected by the antifibronectin antibody followed the contours of the cells and was homogeneously distributed in cell plasm and cell processes (Fig. 6a). In the anti‐CP27 antibody‐treated group, the overall intensity of the fibronectin staining was greatly reduced (Fig. 6b). There was however, some distinct antifibronectin staining in aggregated granules mostly in the cell processes (Fig. 6b).
Figure 6.
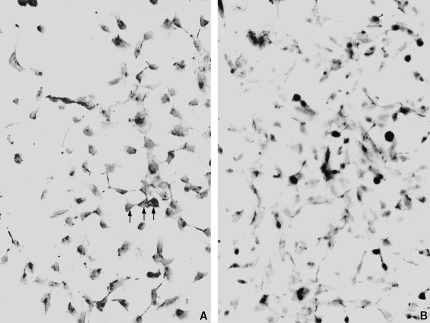
Effect of CP27 function inhibition on fibronectin matrix formation. Cells were either treated with 50 µl/ml rabbit serum (a, control) or anti‐CP27 antibody (b, experimental group) for 8 h. Immunohistochemical staining for fibronectin revealed homogeneous staining of cells and processes in the control group. In the anti‐CP27 antibody‐treated group, the overall intensity of the fibronectin staining was greatly reduced. However, in the anti‐CP27 antibody‐treated group, the antifibronectin antibody intensely reacted with numerous granules in the cell processes.
The western blot technique, in combination with densitometry, allowed for a quantitative analysis of relative levels of fibronectin in the anti‐CP27 antibody‐treated group compared to the control group (Fig. 7). In both groups, the antihuman fibronectin antibody recognized a band of 200 kDa, which was in keeping with the size of the fibronectin monomer. The treatment with anti‐CP27 resulted in a significant reduction of the 200 kDa band indicative of down‐regulated fibronectin synthesis (Fig. 7b). Densitometric analysis demonstrated that the reduction of fibronectin in the antibody‐treated group compared to the control group was 24.9%. Together, these results suggest that CP27 function inhibition reduced fibronectin synthesis and induced changes in the architecture of fibronectin matrix and cell shape.
Figure 7.
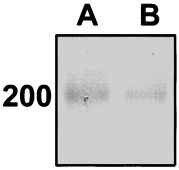
Western blot analysis of the total protein extract of CP27 antibody treated (right lane) and untreated (left lane) BALB/c 3T3 embryonic fibroblasts using fibronectin as primary antibody. The western blot indicates a significant reduction of fibronectin extracellular matrix in the anti‐CP27 antibody treated group. Densitometric analysis demonstrated a 24.9% reduction of fibronectin in the antibody‐treated group compared to the control group.
Discussion
In the present study we have used the embryonic fibroblast cell line BALB/c 3T3 as a model to test the effect of CP27 function inhibition in vitro. The BALB/c 3T3 cell line was chosen based on the CP27 gene expression pattern and on preliminary expression studies. We have used a polyclonal anti‐CP27 antibody recognizing a single epitope as a primary reagent to test the effect of CP27 loss of function. In our experiments, the anti‐CP27 antibody provided a powerful tool to inhibit and block CP27 functional activity. The anti‐CP27 antibody also exhibited its effect in comparison to normal rabbit serum, demonstrating that any inhibitory effects caused by this antibody were not due to the effects of a species‐specific serum. In control experiments using two other anti‐CP27 antibodies directed against other antigenic sites, all three antibodies had identical effects. Other control experiments using an antiamelogenin antibody did not result in any effects on cell functions and morphology. We therefore conclude that the anti‐CP27 antibody was a specific tool to determine the effect of CP27 function block in cell culture. In our experiments, this antibody‐based CP27 loss of function strategy in BALB/c 3T3 embryonic fibroblast cell culture resulted in highly significant effects on cell functions and morphology.
Using our antibody‐based loss‐of‐function strategy, we detected that CP27 block reduced cell viability and enhanced apoptosis. Cell viability and apoptosis were determined using several strategies including MTT assay, DAPI fluorescence microscopy of apoptotic nuclei and electron microscopy. The MTT assay is a colourimetric assay that measures the reduction of 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5,‐diphenyl tetrazolium bromide (MTT) by mitochondrial succinate dehydrogenase. Inside of the cells, the MTT is reduced to a colour precipitate. The addition of an organic solvent (isopropanol) allows for spectrophotometric measurements of the colour precipitate. As reduction of MTT can only occur in metabolically active cells, the level of activity is a measure of the viability of the cells (Berridge & Tan 1993). The significant reduction of MTT staining in BALB/c 3T3 embryonic fibroblasts treated with CP27 antibody indicated that CP27 block reduced cell viability. There was a dose‐dependency of the percentage of viable cells, correlating reduced numbers of viable cells with an increase in antibody concentration. Cell viability assays measure the number of viable cells and establish an indirect estimate for cellular survival and death. In contrast, apoptosis assays determine the number of apoptotic cells using criteria for apoptotic cell death. Here, we have chosen both DAPI fluorescence microscopy and electron microscopy to determine whether typical indicators of apoptosis were detected following CP27 block. Our results demonstrated that characteristic signs of apoptosis, including nuclear compaction and detachment from the nuclear envelope, were frequently observed in the anti‐CP27 treated group, while they were absent in the control groups. Our data indicate that CP27 loss of function results in a decrease of cell viability and an increase in apoptosis. We therefore postulate that CP27 function is essential for normal cell survival. In previous studies (Diekwisch et al. 1999), we have localized CP27 in the extracellular matrix and the effectiveness of the antibody‐based blocking strategy supports the concept of CP27 as an extracellular matrix protein. Based on the known pathways by which extracellular matrix proteins exert control on cell life and death, we suggest that CP27 exercises its control over cell survival via integrin‐based signal transduction pathways that have been recently established for many extracellular matrix proteins (Giancotti & Ruoslahti 1999).
CP27 block induced programmed cell death, suggesting that CP27 may have effects on aspects of cell growth control. To understand the response of the embryonic fibroblasts to anti‐CP27 antibody and relate it to cell cycle control, we determined the effect of anti‐CP27 antibody on cell proliferation Using BrdU as a marker for S phase cell entry (Arita et al. 1998; Sechler & Schwarzbauer 1998; Shankland et al. 1999), we documented that the CP27 antibody significantly decreased BrdU incorporation into DNA and resulted in a reduced number of cells achieving S phase entry in embryonic fibroblasts. Similar to apoptosis, cell growth and proliferation are closely regulated by extracellular matrix molecules via integrin‐based mechanisms (Giancotti & Ruoslahti 1999). We therefore suggest that CP27 as an extracellular matrix molecule affects cell proliferation via similar mechanisms as other extracellular matrix molecules using integrin‐controlled pathways and that the pathways by which CP27 affects apoptosis and proliferation are closely linked to each other.
In the present study we further established that CP27 block caused loss of attachment and changes in cell shape. Our data revealed that cells treated with CP27 antibody assumed distinct morphologies characterized by more stretched (attached cells) or round (floating cells) shapes when compared to the stellate cells of the control group. Interestingly, the more stretched cells showed reduced cell proliferation while the rounded cells were associated with programmed cell death in corresponding data sets. As a consequence, we believe that the inhibition of CP27 function did not only lead to changes in cell shape but also indirectly affected cell growth and cell survival. Cell shape appears to be fundamental to growth control in all anchorage‐dependent cells (Huang et al. 1998). By culturing adherent cells on a defined concentration and area of different ECM ligands, Chen and colleagues demonstrated that cell spreading alone was associated to proliferation, whereas cellular rounding was associated with apoptosis (Chen et al. 1997). The localization of the CP27 receptor on the cell surface might provide further insights into how CP27 affects embryonic fibroblast morphogenesis and function. The mechanism by which CP27 antibody causes change in cell function appears to be related to specific growth traits known to be influenced by deformation of fibronectin matrix, or by change in cell shape (Folkman & Moscona 1978; Chen et al. 1997; Huang et al. 1998; Boudreau & Jones 1999).
In the present study, we documented that anti‐CP27 antibody can change the expression pattern and decrease the level of fibronectin matrix. The reduction in total fibronectin following CP27 block was documented using both western blots and immunohistochemistry. This reduction of fibronectin might be a key mechanism by which CP27 block exerts its effect on cell proliferation and cell death, as numerous studies have shown the close relationship between cell growth, death and differentiation as modulated by extracellular matrix molecules such as fibronectin (Frisch & Ruoslahti 1997; Howe et al. 1998). As an integral component of extracellular matrices, fibronectin matrix interacts with integrin receptors (Hynes 1992; Schwartz et al. 1995) and controls cell cycle progression (Sechler & Schwarzbauer 1998; Christopher et al. 1999; Manabe et al. 1999). The second interesting finding related to the effect of CP27 block on fibronection was the change of fibronection distribution from homogeneous to granular and from the cell body to the cell processes. The polymerizatin of fibronectin is a cell‐dependent process which occurs on the surface of most adherent cell types (Rebres et al. 1995; Christopher et al. 1999). Recent studies have demonstrated that some agents such as cytochalasin D profoundly alter cell morphology and change the organization and distribution of cell surface fibronectin (Christopher et al. 1999). We believe that the redistribution of fibronectin following CP27 block is closely linked to the alteration in cell function caused by the CP27 block.
In summary, we have demonstrated that CP27 function inhibition was associated with changes in key features associated with cell function, including reduced cell viability, increased apoptosis, reduced cell proliferation, loss of attachment, changes in cell shape, and a reduced fibronectin matrix synthesis. It might be assumed that CP27 interacts with CP27 receptor, integrin or other extracellular matrix proteins such as fibronectin to modulate cell shape and attachment, which in turn affect other functions of cell behaviour.
Acknowledgement
Support for these studies by NIH grant DE13095 to TGHD is gratefully acknowledged.
References
- Arita J, Hashi A, Hoshi K, Mazawa S, Suzuki S (1998) D2 dopamine‐receptor‐mediated inhibition of proliferation of rat lactotropes in culture is accompanied by changes in cell shape. Neuroendocrinology 68, 163. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Zhu X (1997) Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr. Opin. Cell Biol. 9, 93. [DOI] [PubMed] [Google Scholar]
- Berridge MV, Tan AS (1993) Characterization of the cellular reduction of 3‐(4,5‐dimethythiazol‐2yl‐) 2,5‐diphenytetrazolium bromide (MTT): subcellular localization substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophysics 303, 474. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Bissell MJ (1998) Extracellular matrix signalling: integration of form and function in normal and malignant cells. Current Opinion Cell Biol. 10, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau NJ, Jones PL (1999) Extracellular matrix and integrin signalling: the shape of things to come. Biochem. J. 339, 481. [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang SV, Aiitesides GM, Ingber DE (1997) Geometric control of cell life and death. Science 276, 1425. [DOI] [PubMed] [Google Scholar]
- Christopher RA, Judge SF, Vincent PA, Higgins PJ, McKeown‐Longo PJ (1999) The amino terminal matrix assembly domain of fibronectin stabilizes cell shape and prevents cell cycle progression. J. Cell Sci. 112, 3225. [DOI] [PubMed] [Google Scholar]
- Diekwisch TGH, Berman BJ, Gentner S, Slavkin HC (1995) Initial enamel crystals are spatially not associated with mineralized dentine. Cell Tissue 279, 149. [DOI] [PubMed] [Google Scholar]
- Diekwisch T, David S, Bringas P, Santos V, Slavkin HC (1993) Antisense inhibition of AMEL translation demonstrates supramolecular controls for enamel HAP crystal growth during embryonic mouse molar development. Development 117, 471. [DOI] [PubMed] [Google Scholar]
- Diekwisch TGH, Luan X (2002) CP27 function is necessary for cell survival and differentiation during tooth morphogenesis in organ culture. Gene 287, 141. [DOI] [PubMed] [Google Scholar]
- Diekwisch TGH, Marches F (1997) CP27 novel gene expression during craniofacial development. J. Dent. Res. 76, 27. [Google Scholar]
- Diekwisch TGH, Marches F, Williams A, Luan X (1999) Cloning, gene expression, and characterization of CP27, a novel gene in mouse embryogenesis. Gene 235, 19. [DOI] [PubMed] [Google Scholar]
- Diekwisch TGH, Ware J, Fincharn AG, Zeichner‐David M (1997) Immunohistochemical similarities and differences between amelogenin and tuftelin gene products during tooth development. J. Histochem. Cytochem. 45, 859. [DOI] [PubMed] [Google Scholar]
- Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E (1996) Dependance of cyclin E‐CDK2 kinase activity on cell anchorage. Science 271, 499. [DOI] [PubMed] [Google Scholar]
- Folkman J, Moscona A (1978) Role of cell shape in growth control. Nature 273, 345. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E (1997) Integrin and anoikis. Curr. Opin. Cell Biol. 9, 1. [DOI] [PubMed] [Google Scholar]
- Giancotti F, Ruoslahti E (1999) Integrin signaling. Science 285, 1028. [DOI] [PubMed] [Google Scholar]
- Guadagno TM, Ohstubo M, Roberts JM, Assoian RK (1993) A link between cyclin A expression and adhesion‐dependent cell cycle progression. Science 262, 1572. [DOI] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL (1998) Integrin signaling and cell growth control. Curr. Opin. Cell Biol. 10, 220. [DOI] [PubMed] [Google Scholar]
- Huang S, Chen CS, Ingber DE (1998) Control of cyclin DI, p27Kipl, and cell cycle progression in human capillary endothelial cells by cell shape and cytokeletal tension. Mol. Biol. Cell 9, 3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO (1992) Integrin, versatility, modulation, and signaling in cell adhesion. Cell 69, 11. [DOI] [PubMed] [Google Scholar]
- Lukashev ME, Werb Z (1998) ECM signalling: orchestrating cell behavior and misbehavior. Trends Cell Biol. 8, 437. [DOI] [PubMed] [Google Scholar]
- Manabe RM, Oh‐E N, Sekiguchi K (1999) Alternatively spliced EDA segment regulatesfibronectin dependent cell cycel progression and mitogenic signal transduction. J. Biol. Chem. 274, 5919. [DOI] [PubMed] [Google Scholar]
- Rebres RA, McKeown‐Longo PJ, Vincent PA, Cho E, Saba TM (1995) Extracellular matrix incorporation of normal and NEM‐alkylated fibronectin: liver and spleen deposition. Am. J. Physiol. 269, G902. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ (1994) Extracellular matrix‐dependent tissue‐specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl Acad. Sci. USA 91, 1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ (1995) A hierarchy of ECM‐mediated signalling regulates tissue‐specific gene expression. Current Opinion Cell Biol. 7, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH (1995) Integrins: emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. Sci. 11, 549. [DOI] [PubMed] [Google Scholar]
- Sechler JL, Schwarzbauer JE (1998) Control of cell cycle progression by fibronectin matrix architecture. J. Biol. Chem. 273, 25533. [DOI] [PubMed] [Google Scholar]
- Shankland SJ, Pippin JW, Couser WG (1999) Complement (C5b‐9) induces glomerular epithelial cell DNA synthesis but not proliferation in vitro . Kidney Int. 56, 538. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Lau EC, Hu CC, Aoba T, Lacey M, Nelson D, Zeichner‐David M, Snead ML, Slavkin HC, Fincham AG (1994) Isolation and characterization of a mouse amelogenin expressed in Escherichia coli . Calcif. Tissue Int. 54, 312. [DOI] [PubMed] [Google Scholar]
- Thieberg RH, Yamauchi M, Satchell PG, Diekwisch TGH (1999) Sequential distribution of keratan sulfate and chondrotin sulfate epitopes during ameloblast differentiation. Histochem. J. 31, 573. [DOI] [PubMed] [Google Scholar]
- Zhu X, Ohtsubo M, Bohmer RM, Roberts JM, Assoian RK (1996) Adhesion‐dependent cell cycle progression linked to the expression of cyclin D, activation of cyclin E‐cdK2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 133, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]


