Abstract
Objective: This study aimed to investigate the potential of enamel matrix proteins (EMPs) on promoting osteogenic differentiation of porcine bone marrow stromal cells (pBMSCs), as well as new bone formation capabilities, in a tissue‐engineered bone complex scaffold of EMPs, pBMSCs and porous calcium phosphate cement (CPC).
Materials and methods: Effects of EMPs on pBMSCs in vitro was first determined by alkaline phosphatase (ALP) activity, von Kossa staining assay and mRNA expression of ALP, bone sialoprotein (BSP) and osteocalcin (OCN) genes. Next, an ectopic new bone formation test was performed in a nude mouse model with four groups: CPC scaffold alone; CPC scaffold + EMPs; CPC scaffold + pBMSCs; and CPC scaffold + EMPs + pBMSCs, for 2 or 4 weeks.
Results: ALP activity, von Kossa assay and mRNA expressions of ALP, BSP and OCN genes were all significantly higher with 150 μg/ml EMP treatment in vitro. In nude mice, new bone formation was detected only in the CPC scaffold + EMPs + pBMSCs group at 2 weeks. At 4 weeks, in the tissue‐engineered construct there was significantly higher bone formation ability than other groups.
Conclusions: EMPs promoted osteogenic differentiation of pBMSCs, and the tissue‐engineered complex of EMPs, pBMSCs and CPC scaffold may be a valuable alternative to be used in periodontal bone tissue engineering and regeneration.
Introduction
Periodontitis is a chronic inflammatory disease, which undermines the tooth supporting apparatus – including periodontal ligament, cementum and alveolar bone, and leads to odontoseisis and tooth loss (1). The ultimate goal of periodontal therapy is to regenerate supporting tissues destroyed during the disease process. As the breakdown of alveolar bone has a direct correlation with odontoseisis and tooth loss, alveolar bone regeneration is critical for periodontal restoration. Alveolar bone restoration has been deemed the beginning of analyses of problems involved in periodontal tissue engineering (2).
Several clinical techniques have been developed to promote periodontal regeneration, including guided tissue regeneration, bone grafting and use of bioactive agents (2). Among the above mentioned methods, enamel matrix proteins (EMPs) belong to one of the widely studied bioactive agents commercially available. They are derived from the enamel matrix of pre‐erupted porcine tooth germs and are composed of amelogenins, which comprise more than 90% of EMPs, as well as other non‐amelogenin matrix proteins or enzyme components (3, 4). EMPs are largely hydrophobic proteins and share high degrees of homology across different species (5, 6, 7). Due to their important roles in development of the periodontium, EMPs have been used in pre‐clinical and clinical studies to restore periodontal ligament, cementum and alveolar bone. To obtain a better therapeutic efficacy, EMPs have also been used in combination with other materials such as barrier membranes or bone grafts, in recent studies (8, 9, 10), although it is controversial in terms of whether there are additional benefits by combining the proteins with certain materials (11).
Recent advances in tissue engineering and regenerative medicine have provided opportunities for regeneration of various kinds of tissue. This has suggested a new paradigm based on appropriate cells, effective bioactive agents and scaffold materials for periodontal regeneration. For example, ideal cells should be non‐immunogenic, highly proliferative and easy to harvest, and have the ability to differentiate into various cell types with specialized functions (12). Bone marrow stromal cells (BMSCs) which can be easily isolated and expanded in vitro, may be a feasible choice. BMSCs are pluripotent with the ability to differentiate into a variety of phenotypes, which depends on the environment in which they are cultured, and they have been identified as a major source of osteogenic cells (13, 14). It has also been reported that BMSCs could be used successfully to form cementum, periodontal ligament and alveolar bone in vivo after transplantation into periodontal defects in beagles (15). These results suggest that BMSCs could be an important cell source for periodontal regeneration. More importantly, there have been reports that EMPs could promote cell adhesion, proliferation and differentiation, including increase in alkaline phosphatase activity and matrix mineralization of BMSCs (16, 17). These ideas have aroused our interest into whether EMPs could be used together with BMSCs to promote new bone formation in vivo.
In this study, we have explored effects of EMPs on porcine BMSCs (pBMSCs) in vitro, and evaluated the ability of new bone formation by use of a tissue‐engineered bone complex of EMPs, pBMSCs and porous calcium phosphate cement (CPC), in a dorsal subcutaneous model in nude mice. To our knowledge, there seems to be lack of research appearing in the scientific literature of using EMPs, pBMSCs and CPC for tissue‐engineering applications.
Materials and methods
EMP preparation
Porcine EMPs were extracted, according to protocols reported by Moe et al. (18) and Shu Rong et al. (19), from secretory‐stage enamel matrix obtained from 6‐month‐old pig non‐erupted, developing permanent germs. Briefly, unmineralized enamel matrix was scraped from germ surfaces then treated with 0.5 mol/l acetic acid and 20% (w/v) trichloroacetic acid, and finally, lyophilized under −56 °C vacuum to obtain EMP powder. After being analysed using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and western blot analysis, the lyophilized powder was dissolved in 5 mmol/l acetic acid at concentration of 4 mg/ml, sterilized by filtration and stored at −80 °C (20).
Animal model
Three 3 month old male healthy crossbred pigs, weight 20–25 kg, and eight 5‐week‐old male nude Balb/c mice weight 20 ± 2 g were used in the study. All procedures concerning animals were approved by the Animal Research Committee of the Ninth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine (JYLL‐10016).
Cell culture
Under general anaesthesia with ketamine (10 mg/kg) and xylazine (3 mg/kg), in the region of 4 ml bone marrow was aspirated from ilium of a single pig, with a 16‐gauge bone marrow aspiration needle, and then was transferred into a pre‐heparinized centrifuge tube. Bone marrow specimens were flushed out with Dulbecco’s modified Eagle’s medium (DMEM, high glucose; Gibco, Grand Island, NY, USA) and pBMSCs were cultured in DMEM with 10% foetal bovine serum (Hyclone, Logan, UT, USA), 100 units/ml penicillin and 100 units/ml streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C, in an atmosphere with 95% humidity and 5% CO2. Media were changed after 24 h to remove non‐adherent cells and was then renewed every 3 days. When confluence of 80–90% was reached pBMSCs were released from the culture substratum using trypsin/EDTA (0.25% w/v trypsin, 0.02% EDTA), and were seeded in fresh petri dishes (10 cm in diameter) at density of 1.0 × 105 cells/ml. Cells at passage 2–3 were used for the following study.
EMP treatment
pBMSCs were detached with trypsin/EDTA, and then seeded in six‐well plates at 1.0 × 105 cells/ml density. They were then incubated in culture media containing 50 or 150 μg/ml EMP, as experimental groups, and in media without EMPs as control group. All culture media were changed every 3 days.
Alkaline phosphatase activity and von Kossa assay
All groups of pBMSCs were cultured for a further 14 days, and alkaline phosphatase (ALP) activity was evaluated by ALP staining kit according to manufacturer’s instructions. Briefly, cells were fixed for 10 min at 4 °C and incubated in a mixture of naphthol AS‐MX phosphate and fast blue BB salt (ALP kit; Hongqiao, Shanghai, China) (21). Areas stained purple were designated as ALP positive. After being treated with EMP for 21 days, mineralized nodules of all groups were assayed by von Kossa staining. Cells were fixed in 70% ethanol and stained with 5% silver nitrate and put under ultraviolet light for 10 min, then treated with 5% NaS2O3 for 2 min and washed in distilled water (22).
RNA extraction and real‐time PCR analysis
Total RNA was isolated using Trizol reagent (Invitrogen) plus RNA purification kit (Invitrogen) according to manufacturer’s instructions at 3 and 6 days after EMP treatment. Quality and quantity of RNA extracted were analysed using an Rneasy Mini kit (Qiagen, Hilden, Germany). Following RNA extraction, reverse transcription was carried out with PrimeScriptTM RT reagent kit (TaKaRa Bio, Otsu, Japan). A quantity of 20 μl reverse transcription reaction volume contained 1 μg total RNA according to the manufacturer’s recommendations. Sequences of primers for alkaline phosphatase (ALP), bone sialoprotein (BSP), osteocalcin (OCN) and glyceraldehyde‐3‐phosphatedehydrogenase (GAPDH) as the calibrator gene for normalization, were synthesized commercially (Shengong Co. Ltd., Shanghai, China) from known porcine sequences (Table 1). Real‐time PCR analysis of osteogenic marker genes was performed using MyiQTM Single Color Real‐time PCR Detection System (Bio‐Rad, Hercules, CA, USA). Briefly, 10 μl SYBR Premix Ex TaqTM, 0.4 μl forward, 0.4 μl reverse primer and 2.0 μl cDNA template were added to a final reaction volume of 20 μl. All samples were assayed in triplicate (n = 3) and three independent experiments were performed. The 2−ΔΔCt method was used to calculate gene expression levels relative to GAPDH and control group.
Table 1.
The sequences of primers for GAPDH, ALP, BSP and OCN
| Genes | Primer sequence (forward/reverse) | Product size (bp) | Annealing temperature (°C) | Accession number |
|---|---|---|---|---|
| GAPDH | 5′‐GGTCGGAGTGAACGGATTTGGC‐3′ | 172 | 57 | AF017079.1 |
| 5′‐AGCCTTGACTGTGCCGTGGAAT‐3′ | ||||
| ALP | 5′‐CAAAGGCTTCTTCTTGCTGGTG‐3′ | 185 | 58 | AY145131.1 |
| 5′‐CAAAGGTAAAGACGTGGGAGTGG‐3′ | ||||
| BSP | 5′‐ACCAGCACC AACAGCACAGAGG‐3′ | 169 | 59 | L10363.1 |
| 5′‐GTTCAAGCCCACCATTCGGAGA‐3′ | ||||
| OCN | 5′‐GGCGCTTCTATGGCATAGCCT‐3′ | 139 | 60 | AY150038.1 |
| 5′‐GGGATGATGGGGACCTTACACTT‐3′ |
Preparation of EMPs/pBMSCs/CPC construct
CPC scaffolds were manufactured and provided by Rebone Biomaterial Co. Ltd. (Shanghai, China). Average diameter of scaffold pores was 400 μm and average porosity was 70% (Fig. 1). In this study, CPC granules of dimensions of Ø 4 × 2 mm were used. CPC scaffolds were dropped into 150 μg/ml EMP solution for 3 h at 37 °C, then flash frozen and lyophilized (20, 23). pBMSCs were seeded on to CPC scaffolds according to the method reported by Maniatopoulos et al. (24). Briefly, pBMSCs were released from culture substratum using trysin/EDTA (0.25% w/v trypsin, 0.02% EDTA), centrifuged to remove supernatant, then resuspended in serum‐free medium at a density of 2 × 107 cells/ml. Cell suspensions were added to the CPC scaffolds to final saturation. Surgical procedures were performed immediately after seeding saturation was reached.
Figure 1.

SEM observation of microstructure of the three‐dimensional porous structure of CPC scaffold (×50).
Surgical procedures
Eight 5‐week‐old male nude Balb/c mice were enrolled in this study. Each mouse received 4 constructs from the following different groups: ➀ CPC scaffold alone; ➁ CPC scaffold + 150 μg/ml EMPs; ➂ CPC scaffold + pBMSCs and ➃ CPC scaffold + 150 μg/ml EMPs + pBMSCs, and all constructs were harvested at 2 or 4 weeks with four samples for each group at both time points. All constructs were implanted under dorsal subcutaneous skin with a distance of more than 5 mm between each implant as adopted by Tsuda et al. (25). Briefly, animals were anaesthetized by intraperitoneal injection of pentobarbital (Nembutal 3.5 mg/100 g) after light ether inhalation. Mid‐longitudinal skin incisions were made in the back of each mouse and subsequently four separate subcutaneous pockets were created with two on each flank, by blunt dissection through the incision. Four constructs were placed into the subcutaneous pockets then skin incisions were sutured. At the time of surgery, location information of all the implants was recorded accurately.
Implant harvest and histological analysis
Mice were sacrificed at either 2 or 4 weeks post‐surgery by intraperitoneal overdose injection of pentobarbital, and implants were harvested for histological analysis. Briefly, after fixation in 10% neutral buffered formalin, implants were decalcified in 10% EDTA for 2 weeks, then embedded in paraffin wax. Five micrometre serial sections were cut parallel to cross sections, for haematoxylin and eosin (H&E) staining. Percentage of new bone formation in each implant was calculated by mean value of three sections selected from each of the three equally divided parts, cut parallel to the cross section, by complete random sampling method, which was further used to calculate mean value for each group. For each section, H&E staining result was observed and recorded using an OLYMPUS BX51 microscope, then computer‐assisted histomorphometric measurement of new bone formation was performed using a PC‐based image analysis system (Image‐Pro Plus System; Media Cybernetics, Silver Spring, MD, USA). New bone formation in each section was expressed as percentage of new bone area in observed areas per whole implant.
Statistical analysis
Statistically significant differences (P < 0.05) between various groups were determined using ANOVA and SNK post hoc, for real‐time PCR analysis and Independent Samples t‐test for new bone area assay. All statistical analyses were carried out using SPSS 16.0 statistical software package (Chicago, IL, USA). All data are presented as mean ± standard deviation.
Results
Cell culture and EMP treatment
Cell morphology in each group is shown in Fig. 2. pBMSCs grew well after being treated with 50 μg/ml EMP (Fig. 2b,e) or 150 μg/ml EMP (Fig. 2c,f), without obvious observed cell death. More importantly, pBMSCs at 3 days after being treated with 50 μg/ml EMP and 150 μg/ml EMP, displayed spindle and polygonal morphology as shown in Fig. 2b,c. When treatment time was extended to 6 days, pBMSCs almost reached confluence and gradually showed morphology of polygonal to square shape (Fig. 2e,f). This trend of change to cell morphology was more pronounced in pBMSCs treated with 150 μg/ml EMPs, whereas pBMSCs untreated with EMPs always maintained short fibroblasts‐like morphology.
Figure 2.
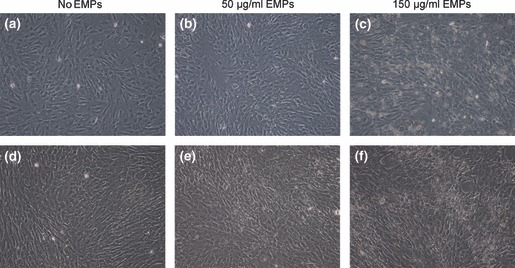
Cell culture and EMP treatment. Cell morphology of pBMSCs treated with 0, 50 and 150 μg/ml EMPs at 3 days (a, b, c) and 6 days (d, e, f) respectively. pBMSCs without being treated with EMPs always maintained short fibroblasts‐like morphology, while pBMSCs treated with EMPs displayed spindle and polygonal morphology at day 3, and showed a morphology of polygonal or squarish by day 6 (reverse phase contrast microscope, ×100).
Alkaline phosphatase activity and von Kossa assay
Fourteen days after EMP treatment, alkaline phosphatase staining was stronger and more intense in 150 μg/ml EMP‐treated pBMSCs (Fig. 3c) than in those treated with 50 μg/ml EMPs (Fig. 3b) or untreated pBMSCs (Fig. 3a). In addition, pBMSCs 21 days after EMP treatment revealed more pronounced von Kossa staining of mineralized nodules in 150 μg/ml EMP treated pBMSCs (Fig. 3f), compared to 50 μg/ml EMP treated (Fig. 3e) or untreated ones (Fig. 3d).
Figure 3.
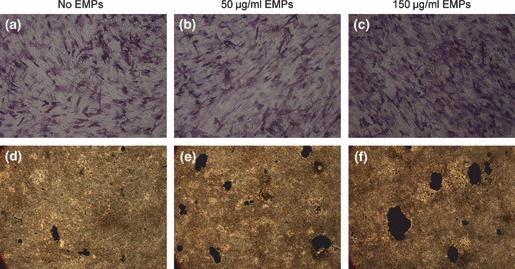
In vitro analysis of osteogenic differentiation of pBMSCs with or without EMP treatment. (a, b, c) Alkaline phosphatase expression of pBMSCs treated with 0, 50 and 150 μg/ml EMPs for 14 days (reverse phase contrast microscope, ×100). (d, e, f) von Kossa staining for mineralized nodules of pBMSCs treated with 0, 50 and 150 μg/ml EMPs for 21 days (reverse phase contrast microscope, ×16).
Real‐time PCR analysis of osteogenic markers
Real‐time PCR analysis of osteogenic differentiation markers was performed to compare differential gene expression at 3 and 6 days, after pBMSCs were treated with 50 μg/ml EMPs, 150 μg/ml EMPs or left untreated. Compared to expression of ALP gene in three groups, expression level in 150 μg/ml EMP treated pBMSCs showed dramatic up‐regulation than 50 μg/ml EMP treated or untreated pBMSCs at both 3 and 6 days; however, no significant difference was found between 50 μg/ml EMP treated and untreated pBMSCs (Fig. 4a). Also, it was revealed that gene expression of BSP was significantly enhanced in 150 μg/ml EMP treated pBMSCs compared to untreated pBMSCs both at 3 and 6 days, although there was less notable difference found in 50 μg/ml EMP treated pBMSCs than untreated ones at day 3 (Fig. 4b). Finally, gene expression level of OCN in pBMSCs treated with 150 μg/ml EMPs showed significant up‐regulation compared to 50 μg/ml EMP treated or untreated cells at both 3 and 6 days. Gene expression in pBMSCs treated with 50 μg/ml EMP was also significantly enhanced at 6 days compared to untreated pBMSCs (Fig. 4c). Taken together, these data support the notion that EMPs enhanced osteogenic differentiation of pBMSCs and the effect was dose‐dependent and related to time in culture.
Figure 4.
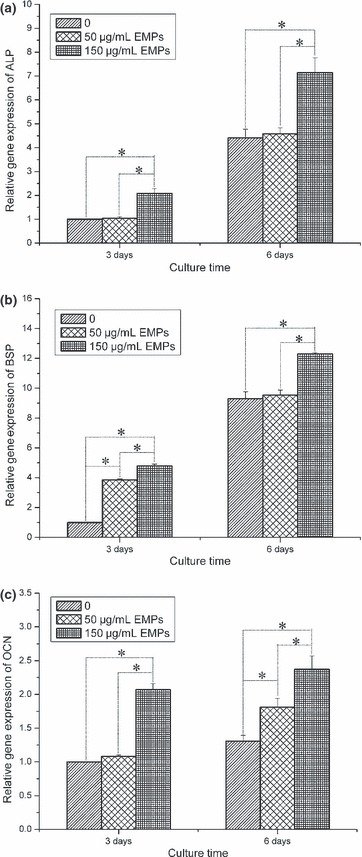
Real‐time PCR analysis of osteogenic differentiation gene expression in pBMSCs treated with 0, 50 and 150 μg/ml EMPs at 3 and 6 days. (a) alkaline phosphatase (ALP), (b) bone sialoprotein (BSP), (c) osteocalcin (OCN). All values normalized to GAPDH (*P < 0.05).
Histological analysis of new bone formation
Two weeks after surgery, half the implants were harvested and no inflammation was found in any group. No new bone formation was found in CPC scaffold alone, CPC scaffold + 150 μg/ml EMPs, and CPC scaffold + pBMSCs groups. However, a hint of new bone formation was noted in CPC scaffold + 150 μg/ml EMPs + pBMSCs group (Fig. 5). Four weeks post‐operation, all remaining implants were harvested and again no inflammation was found in all groups. New bone formation was observed in CPC scaffold + pBMSCs group, and CPC scaffold + 150 μg/ml EMPs + pBMSCs group; there was no obvious new bone formation observed in CPC scaffold alone or CPC scaffold + 150 μg/ml EMPs groups. Percentage of new bone areas was 18.52 ± 1.03% in CPC scaffold + 150 μg/ml EMPs + pBMSCs group compared to 11.63 ± 0.95% in CPC scaffold + pBMSCs group – this was statistically different (P < 0.05) (Fig. 6). These results indicate that EMPs had enhanced bone formation ability using this tissue‐engineering method, in vivo.
Figure 5.
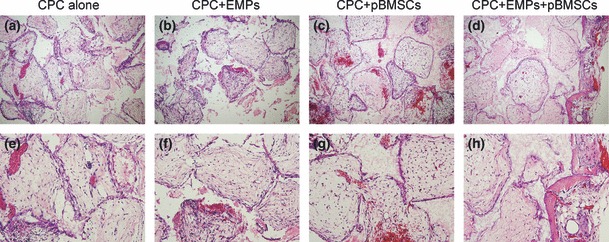
Representative histological images of implants at 2 weeks post‐surgery. (a) and (e) CPC alone group, (b) and (f) CPC + 150 μg/ml EMPs group, (c) and (g) CPC + pBMSCs group, (d) and (h) CPC + 150 μg/ml EMPs + pBMSCs group (a, b, c, d ×100; e, f, g, h ×200). A slight amount of new bone formation was found in the CPC + 150 μg/ml EMPs + pBMSCs group, whereas no new bone was found in other groups.
Figure 6.
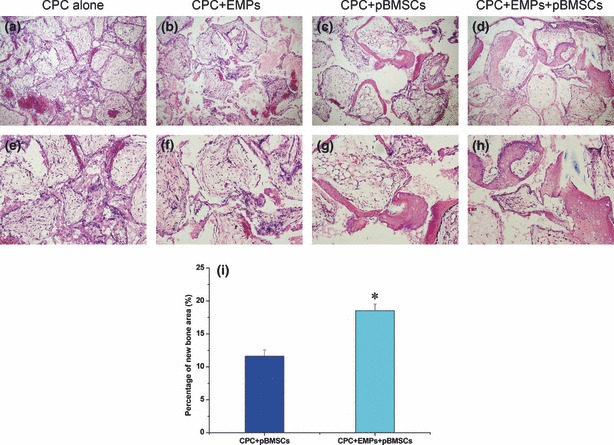
Representative histological image and new bone area assessment of implants at 4 weeks post‐surgery. (a) and (e) CPC alone group, (b) and (f) CPC + 150 μg/ml EMPs group, (c) and (g) CPC + pBMSCs group, (d) and (h) CPC + 150 μg/ml EMPs + pBMSCs group (a, b, c, d ×100, e, f, g, h ×200). Apparent new bone formation was observed in CPC + pBMSCs group and CPC + 150 μg/ml EMPs + pBMSCs group, but still no new bone was found in CPC alone and CPC + 150 μg/ml EMPs groups. (i) Percentage of new bone area in CPC + 150 μg/ml EMPs + pBMSCs group was significantly higher than that in CPC + pBMSCs group by histomorphometric analysis (n = 4, *P < 0.05).
Discussion
Bone tissue‐engineering methods are generally centred on delivery of osteoinductive growth factors, implantation of osteogenic cells and combination with osteoconductive scaffolds, to promote bone regeneration.
EMPs, secreted and synthesized by cells of the Hertwig’s epithelial root sheath, are actively involved in embryogenesis of cementum, periodontal ligament and periodontal supporting bone (26) EMPs can be extracted from developing porcine tooth germs, the major component of which is amelogenin and its cleavage products, a family of hydrophobic proteins that make up over 90% of the total protein content (27). The remaining components of EMPs include ameloblastin (also called amelin or sheathlin), amelotin, tuftelin and enamelin. Amelogenins can self‐aggregate into supramolecular aggregates, so‐called nanospheres, and play a crucial role in regulating initiation and growth of hydroxyapatite crystals during formation of enamel (28, 29). Besides, in the context of periodontal tissue regeneration, it is clear that EMPs have functions other than enamel biomineralization. For example, EMPs have been available as a therapeutic agent (brand name Emdogain), since 1997. Emdogain consists of an enamel matrix derivative, water, and a carrier, propylene glycol alginate. Clinically, Emdogain is used for periodontal regeneration of teeth affected by periodontitis, root coverage procedures and tooth replantation, although mechanism of its function is still regarded to be obscure (30).
For the success of bone tissue engineering, porous scaffolds which act as a temporary carriers for anchorage‐dependent cells are also an important factor. Ideal scaffolds require three‐dimensional interconnected porous structures to provide sufficient space for cell migration, adhesion, proliferation and for new bone tissue ingrowth (31). Suitable pore size and porosity is beneficial for cell infiltration, bone ingrowth and internal mineralized bone formation (32, 33). CPC adopted in this study has composition and structure close to natural bone minerals, excellent bone biocompatibility, osteoconductivity and bioresorbability, and is easy to shape (34, 35, 36, 37). Average pore size of 400 μm and average porosity of 70% are suitable for cell infiltration and bone ingrowth. As in vitro morphological features of cells cultured on scaffolds partially reflects biocompatibility of the scaffolds, pBMSCs need to be able to attach, spread and proliferate well on the CPC scaffold, indicating whether the scaffold would well support good cell outcome. Thus, CPC was considered to be an ideal material for BMSCs to construct tissue‐engineered bone in the current study.
According to the study of Song et al. (17) as well as our previous experimental results, EMPs with concentration of 50 and 150 μg/ml were used here to explore the effect of EMPs on pBMSCs in osteogenic differentiation in vitro. ALP activity and mineralized nodule formation, makers of osteogenic differentiation and commitment of pBMSCs towards the osteoblastic phenotype, were observed at 14 and 21 days after EMP treatment, respectively. 150 μg/ml EMP‐treated pBMSCs showed strong expression of ALP and a significant increase in mineralized nodules when compared to 50 μg/ml EMP treated, or untreated cells. However, less apparent differences were clearly observed between 50 μg/ml EMP treated and untreated pBMSCs. These findings indicated that pBMSCs treated with 150 μg/ml EMP had been directed towards, and specifically enhanced, osteogenic differentiation, and that the effect of EMPs was dose‐dependent, which results were further confirmed by real‐time PCR analysis of osteogenic makers.
EMP treated pBMSCs presented a defined sequence of gene expression during osteogenic differentiation and maturation. ALP, an early maker of osteogenic differentiation, was significantly up‐regulated in 150 μg/ml EMP treated pBMSCs compared to untreated cells at both 3 and 6 days, while no obvious up‐regulation was observed in the 50 μg/ml EMP treated group. Results were consistent with those of ALP staining. Besides, BSP was considered to be intermediate osteogenic differentiation maker (38), expression of which, similar to that of ALP, was also significantly increased in the 150 μg/ml EMP treated group at 3 and 6 days, although a less significant increase was found in the 50 μg/ml EMP treated group only at 3 days. Finally, OCN, which was reported to be a later marker of osteogenic differentiation corresponding with matrix deposition and mineralization (21), also showed clear increases in the 150 μg/ml EMP treated group at 3 and 6 days, in comparison with the control group. In contrast, expression of OCN gene showed less significant up‐regulation at 6 days in the 50 μg/ml EMP treated pBMSCs. These results further indicated that osteogenic differentiation effects of EMPs on pBMSCs was both dose‐dependent and time‐dependent. This implies that EMPs might have played an important role in commitment of mesenchymal progenitor cells to the osteogenic lineage. Similarly, there have been reports that EMPs could significantly enhance ALP activity and in vitro mineralized nodule formation in rat BMSCs (16). Likewise, Iwata et al. fractionated enamel matrix extracts from developing porcine teeth and found that an osteoinductive fraction (OFE) containing mainly 20, 23 and 25 kDa proteins could enhance ALP activity and in vitro mineralized nodule formation, as well as lead to up‐regulated ALP, BSP and OCN mRNA expression in ST2 cells, a mouse bone marrow stromal cell line (39). However, other studies have reported an inhibitory effect of EMPs on expression of OCN (40, 41). Guida et al. have shown that EMPs can down‐regulate type I collagen synthesis and ALP activity of human BMSCs, whereas decrease in OCN synthesis was not statistically significant (42). In a heterogeneous cell population from rat bone marrow, EMPs had no significant effect on cell proliferation, ALP activity and mRNA expression of type I collagen, OCN and ALP (43). Discrepancies between different reports may be due to differences in cell types, culture conditions, methods used, concentration and timing of EMP addition to cultures (44). Nevertheless, according to the current in vitro experimental results, gene expression of ALP, BSP and OCN in 150 μg/ml EMP treated group were all significantly increased at both time points, thus 150 μg/ml was considered to be the effective protein concentration in inducing osteoblastic differentiation and thus was adopted for our further in vivo investigation.
In our study, we used an ectopic osteogenesis model in nude mouse to investigate bone formation by implantation of porous CPC scaffolds combined with 150 μg/ml EMPs and seeded with pBMSCs, without extra osteogenic medium induction. Two weeks post‐surgery, new bone formation was observed only in the CPC scaffold + EMPs + pBMSCs group. Four weeks post‐surgery, a small amount of bone formation was also detected in the CPC scaffold + pBMSCs group, while there was no indication of new bone formation in the remaining CPC scaffold alone or the CPC scaffold + EMPs group. Formation of small amounts of new bone in the CPC scaffold + pBMSCs group may be attributed to self‐osteogenic differentiation tendency of BMSCs, while significantly more newly formed bone in the group of CPC scaffolds + 150 μg/ml EMPs + pBMSCs was found compared to the group with CPC scaffold + pBMSCs. Results revealed that the tissue‐engineering technique by implantation of the bone complex of EMPs, pBMCs and CPC scaffold can accelerate new bone formation and augment the amount of new bone. While CPC alone or simply combining CPC with EMPs was not sufficient enough to induce new bone formation under the current formulation here.
It can thus be concluded from our study that EMPs promote osteogenic differentiation of pBMSCs in vitro and benefit new bone formation in vivo when combined with pBMSCs and a CPC scaffold. The results suggested that a tissue‐engineered complex of EMPs, pBMSCs and CPC scaffold will be a valuable alternative to be used in periodontal bone tissue engineering and regeneration.
Acknowledgements
The authors appreciate Prof. Changshen Liu (East China University of Science and Technology, Shanghai, China) for providing the CPC scaffolds, Xiuli Zhang (Oral Bioengineering Lab, Ninth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine) for assistance with experiments, and Prof. Qi Zhu (Department of English, Shanghai Jiao Tong University School of Medicine) for his modification of the manuscript. This work was funded by National Nature Science Foundation of China 30670555, 30973342; Shanghai Leading Academic Discipline Project S30206, T0202; Program for New Century Excellent Talents in University NCET‐08‐0353; Science and Technology Commission of Shanghai Municipality 0952nm04000, 10430710900, 10dz2211600.
References
- 1. Esposito M, Grusovin MG, Papanikolaou N, Coulthard P, Worthington HV (2009) Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review. Eur. J. Oral Implantol. 2, 247–266. [PubMed] [Google Scholar]
- 2. Ripamonti U, Reddi AH (1997) Tissue engineering, morphogenesis, and regeneration of the periodontal tissues by bone morphogenetic proteins. Crit. Rev. Oral Biol. Med. 8, 154–163. [DOI] [PubMed] [Google Scholar]
- 3. Tan J, Leung W, Moradian‐Oldak J, Zeichner‐David M, Fincham AG (1998) Quantitative analysis of amelogenin solubility. J. Dent. Res. 77, 1388–1396. [DOI] [PubMed] [Google Scholar]
- 4. Simmer JP, Lau EC, Hu CC, Aoba T, Lacey M, Nelson D et al. (1994) Isolation and characterization of a mouse amelogenin expressed in Escherichia coli . Calcif. Tissue Int. 54, 312–319. [DOI] [PubMed] [Google Scholar]
- 5. Fincham AG, Moradian‐Oldak J, Simmer JP (1999) The structural biology of the developing dental enamel matrix. J. Struct. Biol. 126, 270–299. [DOI] [PubMed] [Google Scholar]
- 6. Toyosawa S, O’HUigin C, Figueroa F, Tichy H, Klein J (1998) Identification and characterization of amelogenin genes in monotremes, reptiles, and amphibians. Proc. Natl. Acad. Sci. USA 95, 13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Tannukit S, Zhu D, Snead ML, Paine ML (2005) Enamel matrix protein interactions. J. Bone Miner. Res. 20, 1032–1040. [DOI] [PubMed] [Google Scholar]
- 8. Prata CA, Lacerda SA, Brentegani LG (2007) Autogenous bone graft associated with enamel matrix proteins in bone repair. Implant Dent. 16, 413–420. [DOI] [PubMed] [Google Scholar]
- 9. Kuru B, Yilmaz S, Argin K, Noyan U (2006) Enamel matrix derivative alone or in combination with a bioactive glass in wide intrabony defects. Clin. Oral Investig. 10, 227–234. [DOI] [PubMed] [Google Scholar]
- 10. Reichert C, Al‐Nawas B, Smeets R, Kasaj A, Gotz W, Klein MO (2009) In vitro proliferation of human osteogenic cells in presence of different commercial bone substitute materials combined with enamel matrix derivatives. Head Face Med. 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tu YK, Woolston A, Faggion CM Jr (2010) Do bone grafts or barrier membranes provide additional treatment effects for infrabony lesions treated with enamel matrix derivatives? A network meta‐analysis of randomized‐controlled trials. J. Clin. Periodontol. 37, 59–79. [DOI] [PubMed] [Google Scholar]
- 12. Otsuka T, Kasai H, Yamaguchi K, Nishihara T (2005) Enamel matrix derivative promotes osteoclast cell formation by RANKL production in mouse marrow cultures. J. Dent. 33, 749–755. [DOI] [PubMed] [Google Scholar]
- 13. Bianco P, Robey PG (2001) Stem cells in tissue engineering. Nature 414, 118–121. [DOI] [PubMed] [Google Scholar]
- 14. Tremain N, Korkko J, Ibberson D, Kopen GC, DiGirolamo C, Phinney DG (2001) MicroSAGE analysis of 2,353 expressed genes in a single cell‐derived colony of undifferentiated human mesenchymal stem cells reveals mRNAs of multiple cell lineages. Stem Cells 19, 408–418. [DOI] [PubMed] [Google Scholar]
- 15. Hasegawa N, Kawaguchi H, Hirachi A, Takeda K, Mizuno N, Nishimura M et al. (2006) Behaviour of transplanted bone marrow‐derived mesenchymal stem cells in periodontal defects. J. Periodontol. 77, 1003–1007. [DOI] [PubMed] [Google Scholar]
- 16. Keila S, Nemcovsky CE, Moses O, Artzi Z, Weinreb M (2004) In vitro effects of enamel matrix proteins on rat bone marrow cells and gingival fibroblasts. J. Dent. Res. 83, 134–138. [DOI] [PubMed] [Google Scholar]
- 17. Song ZC, Shu R, Zhang XL (2010) Cellular responses and expression profiling of human bone marrow stromal cells stimulated with enamel matrix proteins in vitro. Cell Prolif. 43, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moe D, Salling E, Kirkeby S (1984) Extraction of enamel proteins. Scand. J. Dent. Res. 92, 503–507. [DOI] [PubMed] [Google Scholar]
- 19. Shu R, Li C, Liu Z (1999) The extraction and N‐terminal amino acid sequence analysis of porcine enamel matrix proteins. J. Comprehensive Stomatol. 15, 67–68. [Google Scholar]
- 20. Song AM, Shu R, Xie YF, Song ZC, Li HY, Liu XF et al. (2007) A study of enamel matrix proteins on differentiation of porcine bone marrow stromal cells into cementoblasts. Cell Prolif. 40, 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang X, Zhao J, Wang S, Sun X, Zhang X, Chen J et al. (2009) Mandibular repair in rats with premineralized silk scaffolds and BMP‐2‐modified bMSCs. Biomaterials 30, 4522–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aghaloo T, Jiang X, Soo C, Zhang Z, Zhang X, Hu J et al. (2007) A study of the role of nell‐1 gene modified goat bone marrow stromal cells in promoting new bone formation. Mol. Ther. 15, 1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheridan MH, Shea LD, Peters MC, Mooney DJ (2000) Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J. Control. Release 64, 91–102. [DOI] [PubMed] [Google Scholar]
- 24. Maniatopoulos C, Sodek J, Melcher AH (1988) Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 254, 317–330. [DOI] [PubMed] [Google Scholar]
- 25. Tsuda H, Wada T, Yamashita T, Hamada H (2005) Enhanced osteoinduction by mesenchymal stem cells transfected with a fiber‐mutant adenoviral BMP2 gene. J. Gene Med. 7, 1322–1334. [DOI] [PubMed] [Google Scholar]
- 26. Hammarstrom L (1997) Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 24, 658–668. [DOI] [PubMed] [Google Scholar]
- 27. Gestrelius S, Andersson C, Johansson AC, Persson E, Brodin A, Rydhag L et al. (1997) Formulation of enamel matrix derivative for surface coating. Kinetics and cell colonization. J. Clin. Periodontol. 24, 678–684. [DOI] [PubMed] [Google Scholar]
- 28. Bartlett JD, Ganss B, Goldberg M, Moradian‐Oldak J, Paine ML, Snead ML et al. (2006) Protein‐protein interactions of the developing enamel matrix. Curr. Top. Dev. Biol. 74, 57–115. [DOI] [PubMed] [Google Scholar]
- 29. Margolis HC, Beniash E, Fowler CE (2006) Role of macromolecular assembly of enamel matrix proteins in enamel formation. J. Dent. Res. 85, 775–793. [DOI] [PubMed] [Google Scholar]
- 30. Bosshardt DD (2008) Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 35, 87–105. [DOI] [PubMed] [Google Scholar]
- 31. Wu C, Chang J, Zhai W, Ni S, Wang J (2006) Porous akermanite scaffolds for bone tissue engineering: preparation, characterization, and in vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 78, 47–55. [DOI] [PubMed] [Google Scholar]
- 32. Xu HH, Simon CG Jr (2004) Self‐hardening calcium phosphate cement‐mesh composite: reinforcement, macropores, and cell response. J. Biomed. Mater. Res. A 69, 267–278. [DOI] [PubMed] [Google Scholar]
- 33. Karageorgiou V, Kaplan D (2005) Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26, 5474–5491. [DOI] [PubMed] [Google Scholar]
- 34. Xu HH, Carey LE, Simon CG Jr (2007) Premixed macroporous calcium phosphate cement scaffold. J. Mater. Sci. Mater. Med. 18, 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calafiori AR, Di Marco G, Martino G, Marotta M (2007) Preparation and characterization of calcium phosphate biomaterials. J. Mater. Sci. Mater. Med. 18, 2331–2338. [DOI] [PubMed] [Google Scholar]
- 36. Khairoun I, Magne D, Gauthier O, Bouler JM, Aguado E, Daculsi G et al. (2002) In vitro characterization and in vivo properties of a carbonated apatite bone cement. J. Biomed. Mater. Res. 60, 633–642. [DOI] [PubMed] [Google Scholar]
- 37. Pan Z, Jiang P (2008) Assessment of the suitability of a new composite as a bone defect filler in a rabbit model. J. Tissue Eng. Regen. Med. 2, 347–353. [DOI] [PubMed] [Google Scholar]
- 38. Ganss B, Kim RH, Sodek J (1999) Bone sialoprotein. Crit. Rev. Oral Biol. Med. 10, 79–98. [DOI] [PubMed] [Google Scholar]
- 39. Iwata T, Morotome Y, Tanabe T, Fukae M, Ishikawa I, Oida S (2002) Noggin blocks osteoinductive activity of porcine enamel extracts. J. Dent. Res. 81, 387–391. [DOI] [PubMed] [Google Scholar]
- 40. Tokiyasu Y, Takata T, Saygin E, Somerman M (2000) Enamel factors regulate expression of genes associated with cementoblasts. J. Periodontol. 71, 1829–1839. [DOI] [PubMed] [Google Scholar]
- 41. Hakki SS, Berry JE, Somerman MJ (2001) The effect of enamel matrix protein derivative on follicle cells in vitro. J. Periodontol. 72, 679–687. [DOI] [PubMed] [Google Scholar]
- 42. Guida L, Annunziata M, Carinci F, Di Feo A, Passaro I, Oliva A (2007) In vitro biologic response of human bone marrow stromal cells to enamel matrix derivative. J. Periodontol. 78, 2190–2196. [DOI] [PubMed] [Google Scholar]
- 43. van den Dolder J, Vloon AP, Jansen JA (2006) The effect of Emdogain on the growth and differentiation of rat bone marrow cells. J. Periodontal. Res. 41, 471–476. [DOI] [PubMed] [Google Scholar]
- 44. Takayama T, Suzuki N, Narukawa M, Tokunaga T, Otsuka K, Ito K (2005) Enamel matrix derivative stimulates core binding factor alpha1/Runt‐related transcription factor‐2 expression via activation of Smad1 in C2C12 cells. J. Periodontol. 76, 244–249. [DOI] [PubMed] [Google Scholar]


