Abstract
Objectives: Poor therapeutic results have been reported for treatment of malignant melanoma; therefore in this study we have investigated inhibitory capacity of ethyl acetate, chloroform (Chl) and methanol extracts from Moricandia arvensis on mouse melanoma (B16‐F0) and human keratinocyte (HaCaT) cell proliferation. Influence of Chl extract on percentage distribution in cell cycle phases and melanogenesis was also studied.
Material and methods: Cell viability was determined at various periods using the MTT assay, and flow cytometry was used to analyse effects of Chl extract on progression through the cell cycle and apoptosis. In addition, amounts of melanin and tyrosinase were measured spectrophotometrically at 475 nm.
Results: Chl extract exhibited significant anti‐proliferative activity after incubation with the two types of tumour skin cells. Morphological changes in B16‐F0 cells, accompanied by increase of tyrosinase activity, and of melanin synthesis were observed, which are markers of differentiation of malignant melanoma cells. Furthermore, cell cycle analysis revealed that B16‐F0 cells treated with Chl extract were arrested predominantly in G1 phase.
Conclusion: Chl extract had the ability to reverse malignant melanoma cells from proliferative to differentiated state, thus providing a new perspective in developing novel strategies for prevention and treatment of malignant melanoma, possibly through consumption of the extract in an appropriate cancer prevention diet. Moreover, there is scope for the extract being introduced into cosmetic products as a natural tanning agent.
Introduction
Treatment of skin infections with topical applications of plant extracts has gained increasing research attention (1). Indeed, traditional use of plants to treat against skin diseases, especially for cosmetic purposes, is a common practice in indigenous medicine of many cultures; thus, extracts may contain compounds with better anti‐pigmentation or hyperpigmentation properties (2). Furthermore, many medicinal plants have served as anti‐cancer pharmaceutical resources, and over 60% of current anti‐cancer drugs (such as vinblastine, topotecan, etoposide and paclitaxel) were originally found as plant‐derived compounds (3). Numbers of studies have suggested that cruciferous vegetables exert a protective consequence against a variety of carcinomas. This beneficial effect may result from exposure to a number of nutritive and non‐nutritive constituents known to inhibit chemically induced carcinogenesis in experimental animals. Some glucosinolates, namely, those possessing an aromatic or indolic side chains, as well as phenolic compounds possessing antioxidant properties (4), have been reported to be associated with anti‐carcinogenic activity (5).
The dominant component of normal human skin colour is provided by melanin (6), although there are four chromophores that contribute to it; haemoglobin, oxyhaemoglobin, carotenoids and melanin itself (7). Melanin plays a major role in skin coloration and in pigmentation of skin cells in the innermost layer of the epidermis. Upon exposure of skin to UV radiation, melanogenesis is initiated by an enzyme called tyrosinase (8). Tyrosinase is a multifunctional copper‐containing enzyme present in fungi and vertebrates; it catalyses the initial step in formation of melanin from tyrosine (9). This process proceeds via 3,4‐dihydroxy phenylalanine (DOPA), which is formed by tyrosinase activity on tyrosine. The next step is oxidation of DOPA into DOPA quinone. These quinones spontaneously polymerize to high molecular weight brown‐pigmented components, known as melanin. Type and amount of melanin synthesized by melanocytes, and its distribution in surrounding keratinocytes determine actual final colour of human skin (9). In recent years, more attention has been paid to use of natural plant extracts in the cosmetics industry (10).
Leaves and stems of Moricandia arvensis (L.) DC. subsp. eu‐arvensis (Brassicacae) have been used in treatment of syphilis and scurvy (11, 12). Our previous studies on M. arvensis leaves showed that total oligomer flavonoids (TOF) and methanol (MeOH) extracts have the highest anti‐radical activity towards both DPPH• and O2 •− (13, 14). In addition, when tested using the SOS Chromotest, chloroform (Chl), TOF, MeOH, petroleum ether (PE) and ethyl acetate (EA) extracts exhibit anti‐genotoxicity towards two mutagens, nitrofurantoin and hydrogen peroxide (13).
Taking into account M. arvensis frequent use in traditional medicine, its important biological activities and its membership in the Brassicacae family known to decrease risk of some types of cancer (5). In this study, we have investigated the potential of M. arvensis leaf extracts for anti‐proliferative and differentiational effects on HaCaT and B16‐F0 cells. To our knowledge, this is the first report on the effect of M. arvensis extracts on skin tumours.
Material and methods
Reagents
Trypsin, sodium dodecyl sulphate (SDS), penicillin, streptomycin, vitamins, sodium pyruvate, Dulbecco’s modified Eagle’s medium (DMEM), non‐essential amino acids (NEA) and foetal bovine serum (FBS) were purchased from Gibco/BRL (Scotland, UK). 3‐(4, 5‐dimethyl‐thiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) and propidium iodide (PI) were purchased from Sigma‐Aldrich (Steinheim, Germany). Ribonuclease A (RNase), Triton ×100, 3,4‐dihydroxy‐l‐phenylalanine (L‐DOPA), Ethylenediaminetetraacetic acid (EDTA) and dimethyl sulphoxide (DMSO) were procured from Sigma (St Louis, MO, USA).
Plant materials
Moricandia arvensis subsp. eu‐arvensis was collected freshly from Oued Ghezran in Gafsa, a region situated in southern Tunisia, in December 2005. Its identification was carried out by Pr. M. Cheieb (Department of Botany, Faculty of Sciences, University of Sfax, Tunisia), according to Flora of Tunisia (15). A voucher specimen (M.a‐12.05) has been retained in our laboratory for future reference. Leaves were shade‐dried, powdered and stored for further use.
Preparation of plant extracts
The fresh leaves of M. arvensis were dried at room temperature and coarsely powdered. One hundred grams of powdered leaves were sequentially extracted in Soxhlet apparatus (AM Glassware, Aberdeen, Scotland, UK) with chloroform, ethyl acetate and methanol solvents (1 l each), to obtain the respective extracts; yields were 1.612%, 0.156% and 18.13% respectively. These extracts, with different polarities, were concentrated to dryness and residues were kept at 4 °C, and then dissolved in DMSO for use. Concentrations tested for anti‐proliferation assay were 50, 200, 400, 800 and 1000 μg/ml. The extract which showed most important anti‐proliferative effect, was chosen to test its direct effect on cell progression through the cell cycle. Doses of Chl extract tested for flow cytometry assay were 800 and 400 μg/ml (doses corresponding respectively to 1.6 × IC50 and 0.8 × IC50), for malignant melanoma cells B16‐F0, and 400 and 200 μg/ml (doses corresponding respectively to 1.33 × IC50 and 1.5 × IC50) for HaCaT cells. To evaluate tyrosinase activity and melanin content, highest tested dose of Chl extract was chosen for the assay.
Cell lines and culture conditions
B16‐F0, a mouse melanoma cell line (purchased from ATCC Catalogue No: CRL‐6322) was cultured in DMEM supplemented with 10% heat‐inactivated FBS, 1% NEA (100×), 1% de l‐glutamine (200 mm), 1% vitamins (100×), 1% penicillin (10 000 U/ml)–streptomycin (10 000 μg/ml) and 1% sodium pyruvate 100 mm. Cells were grown at 37 °C in a humidified atmosphere containing 5% CO2. The spontaneously immortalized human keratinocyte cell line, HaCaT, was cultured at 37 °C in DMEM supplemented with 1% of penicillin–streptomycin and 10% FBS.
Viability test
Effects of tested extracts on viability of B16‐F0 and HaCaT cells were determined using the MTT assay. Tetrazolium salt has the property to be reduced to blue crystals of formazan by mitochondrial succinate dehydrogenase. This enzyme, which plays an important role in the Krebs cycle, catalyses dehydrogenation of succinate to fumarate. It is a flavoprotein located on internal mitochondrial membranes of viable cells. Activity of this enzyme is measured by reduction of the MTT. Toxicity of doses to a given cell population is determined by spectrophotometry. Absorbance is directly related to activity of succinate dehydrogenase, itself related to cell toxicity or to number of viable cells.
MTT colorimetric assay was performed in 96‐well plates. B16‐F0 and HaCaT cells were seeded respectively, at concentration of 2 × 103 and 5 × 103 cells/well and incubated for 24 h at 37 °C. After 24 h incubation, various concentrations ranging from 50 to 1000 μg/ml of each extract from M. arvensis (100 μl), were added to cultures and incubated for additional 24, 48 and 72 h at 37 °C. Controls were performed with the same final DMSO concentrations in media as in samples. Then, MTT solution (5 mg/ml in PBS, pH = 7.4) was added (10 μl/well) to cultures. After 4 h incubation at 37 °C, 100 μl of SDS 10%–HCl 1% (0.01 N) was added to each well, and plates were incubated for an additional 3 h at 37 °C. Amounts of purple formazan formed were determined by measuring absorbance at 540 nm (16). Data were obtained from wells in triplicate. Inhibition percentage (IP %) was calculated according to the following formula:
where OD of treated cells was optical density of cells treated with different amounts of tested extract and OD of control cells was optical density of cells treated with vehicle solution.
Cell cycle analysis using flow cytometry
Human HaCaT keratinocytes (5 × 105) and B16‐F0 (2 × 105) were seeded into a 50 cm2 culture dish and incubated for 24 h. Cells were treated with different concentrations of Chl extract (800 and 400 μg/ml) for 48 h, trypsinized and washed twice in PBS (pH = 7.4). Cells were harvested and 106 were resuspended in solution containing 30% cold PBS (pH = 7.4) and 70% cold ethanol. Then, they were stored at 4 °C until analysis. On day of analysis, cells were incubated 15 min at room temperature and washed twice in cold PBS (pH = 7.4). After treatment with RNase A (10 μg/ml) for 30 min at room temperature and staining with 50 μl propidium iodide (1 mg/ml) for 10 min, cell cycle analysis was conducted using FACS system (BD Biosciences, San Jose, CA, USA). Percentages of cells in each phase of the cell cycle were calculated.
Determination of melanin content
Melanin release by cells was measured, as described previously (17). Briefly, B16‐F0 cells (2 × 105) were seeded into a 50 cm2 culture dish with 10 ml of culture medium, and incubated for 24 h. Then, cells were treated with Chl extract (800 μg/ml) for 48 h. After treatment, melanogenesis activity (closely related to the amount of produced melanin), was estimated from amount of melanin secreted into cultured medium (extracellular melanin) and melanin retained in cells (intracellular melanin). Adherent cells were detached by incubation in trypsin (2.5%)/ethylenediamine tetraacetic acid (0.11%); 106 cells in tubes were solubilized in 1 ml of Triton X100 (0.1%). Spectrophotometric absorbance of extra‐ and intracellular melanin content was measured at 475 nm. Absorbance was compared against a standard curve of known concentration of synthetic melanin and amounts estimated.
Tyrosinase activity
Tyrosinase enzyme activity was estimated by measuring rate of L‐DOPA oxidation, as described previously (18) with slight modification. Briefly, cells (2 × 105) were treated with 800 μg/ml of Chl extract for 48 h, 106 cells were then solubilized in phosphate buffer (0.1 m; pH 6.8) containing 0.1% Triton X100. Lysate was clarified by centrifugation at 17 500 g for 10 min at 4 °C; 400 μl of supernatant was mixed with 400 μl of L‐DOPA (0.15%), and absorbance was measured spectrophotometrically at 475 nm, every minute for 10 min, after addition of the substrate (L‐DOPA).
Statistical analysis
All tests were carried out in triplicate and results were presented as mean ± SD. Data were tested for statistical differences by one‐way ANOVA followed by Duncan’s multiple comparison test using statistica (Version 6.0; Statsoft Inc.,Tulsa, OK, USA) to compare data of control (untreated cells) with those of cells treated with different extracts. Statistical differences were determined at P < 0.05.
Results
Viability testing
Relationship between concentration of extracts and their anti‐proliferative effects on B16‐F0 and HaCaT cells was investigated by MTT assay. MTT is a yellow, water‐soluble tetrazolium salt. Metabolically active cells are able to convert the dye to water‐insoluble dark blue formazan by reductive cleavage of the tetrazolium ring. As shown in 1, 2, all tested extracts (except MeOH extract), exhibited significant anti‐proliferative effects in a dose‐ and time‐dependent manner (P < 0.05), for both B16‐F0 and HaCaT cells. Results of this experiment demonstrated that Chl extract exhibited the highest anti‐proliferative effect on both B16‐F0 and HaCaT cells when compared to other tested extracts. Chl extract at concentrations of 50 and 1000 μg/ml decreased proliferation of B16‐F0 cells by 21.55% and 96.86%, respectively, after 72 h of incubation. Inhibition of proliferation of HaCaT cells reached maximum of 79.87% at concentration of 1000 μg/ml after 72 h incubation. IC50 values of B16‐F0 and HaCaT cell proliferation after 72 h incubation were 190 and 165 μg/ml respectively (Table 1). EA extract exhibited less anti‐proliferative effect on both B16‐F0 and HaCaT cells with inhibition percentage of 58.53% and 57.33% (at the same concentration of 1000 μg/ml), respectively, and IC50 values of 805 and 875 μg/ml after 72 h of incubation. MeOH extract showed weakest anti‐proliferative effect when compared to Chl and EA extracts. Inhibition percentages of B16‐F0 cells were almost stable at 24, 48 and 72 h incubation (6.63%, 6.87% and 7.15% respectively) after exposure to 1000 μg/ml MeOH extract. In contrast, MeOH extract inhibited proliferation of HaCaT cells in a dose‐dependent manner. Inhibition percentages of cell viability at 1000 μg/ml were 20.55%, 28.27% and 34.68%, respectively, after 24, 48 and 72 h incubation.
Figure 1.
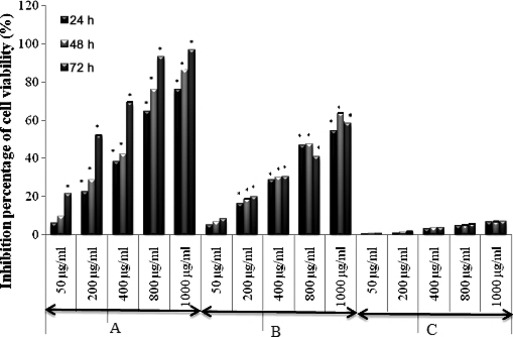
Anti‐proliferative effect of Chl, EA and MeOH extracts on melanoma cells B16‐F0. Percentage of cell viability was measured by MTT assay. Data represent mean ± SD of three independent experiments. *P < 0.05 means significant difference between untreated and treated cells. A: Chloroform extract (Chl); B: Ethyl acetate extract (EA) and C: Methanol extract (MeOH).
Figure 2.
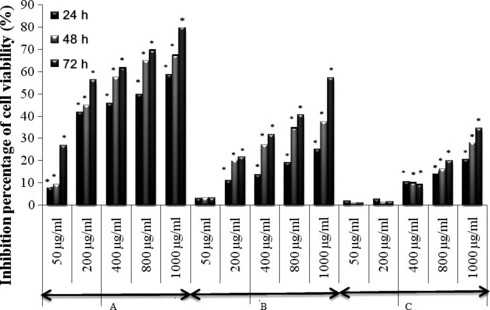
Anti‐proliferative effect of Chl, EA and MeOH on HaCaT cells. Percentage of cell viability was measured by MTT assay. Data represent mean ± SD of three independent experiments. *P < 0.05 means significant difference between untreated and treated cells. A: Chloroform extract (Chl); B: Ethyl acetate extract (EA) and C: Methanol extract (MeOH).
Table 1.
IC50 of different extracts from Moricandia arvensis on the proliferation of B16‐F0 and HaCaT cells
| Extracts | Time of incubation (h) | IC50 (μg/ml) (B16‐F0) | IC50 (μg/ml) (HaCaT) |
|---|---|---|---|
| Chl | 24 | 580 | 815 |
| 48 | 500 | 300 | |
| 72 | 190 | 165 | |
| EA | 24 | 875 | – |
| 48 | 815 | – | |
| 72 | 805 | 875 | |
| MeOH | 24 | – | – |
| 48 | – | – | |
| 72 | – | – |
Chl, chloroform extract; EA, ethyl acetate extract; MeOH, methanol extract.
Cell cycle analysis
To study the anti‐proliferative activity of Chl extract, distribution of cell cycle phases was examined by flow cytometry. B16‐F0 and HaCaT cells were treated respectively, with 400 and 800 μg/ml, and 200 and 400 μg/ml of Chl extract, and cell cycle distribution was examined after 48 h treatment. Table 2 shows proportions of B16‐F0 cells in G1 phase have markedly increased after treatment with increasing concentrations of Chl extract. Percentage of cells arrested in G1 was 80.70% and 88.13%, respectively, after treatment with 400 and 800 μg/ml Chl extract, versus 65.50% in untreated cells. However, treatment of HaCaT cells with Chl extract showed very low progressive accumulation of cells in G1 (Table 3). G1 phase percentage was only 35.15% and 36.33%, respectively, at doses of 200 and 400 μg/ml, versus 32.54% in untreated cells. Morphological changes in B16‐F0 cells induced by Chl extract from M. arvensis were detected by microscopy. As shown in Fig. 3, B16‐F0 cells rapidly acquired elongated morphology after 48 h treatment with Chl extract compared to control cells. However, Chl extract strongly stimulated cell dendricity, which is a morphological feature of differentiated melanocytes and the first observable parameter of melanoma cell differentiation (19).
Table 2.
Cell cycle distribution of B16‐F0 cells after treatment with 400 and 800 μg/ml of Chl extract
| Cells | Phases of cell cycle | Control | Chl 400 μg/ml | Chl 800 μg/ml | |
|---|---|---|---|---|---|
| Percentage of distribution of cells in different phases of cell cycle | B16‐F0 | G1 | 65.50 | 80.70 | 88.13 |
| G2‐M | 11.56 | 7.320 | 5.65 | ||
| S | 22.94 | 11.98 | 6.22 | ||
| Apoptosis | 0.02 | 2.08 | 1 |
Table 3.
Cell cycle distribution of HaCaT cells after treatment with 400 and 800 μg/ml of Chl extract
| Cells | Phases of cell cycle | Control | Chl 200 μg/ml | Chl 400 μg/ml | |
|---|---|---|---|---|---|
| Percentage of distribution of cells in different phases of Cell cycle | HaCaT | G1 | 32.54 | 35.17 | 36.33 |
| G2‐M | 17.27 | 19.37 | 19.98 | ||
| S | 50.18 | 45.45 | 43.69 | ||
| Apoptosis | 1.54 | 0.60 | 1.07 |
Figure 3.
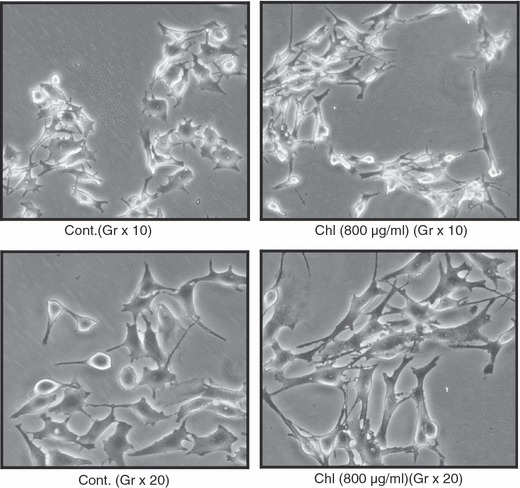
Morphological changes in B16‐F0 cells incubated after 48 h with Chl extract (800 μg/ml). Chl, chloroform extract; Cont, Control.
Effect of Chl extract on melanin synthesis and tyrosinase activity
Highest tested dose of Chl extract was black in colour in the supernatant of treated cells, which was darker than that obtained from untreated ones. This observation allowed us to hypothesize that this concentration induced melanogenesis. Hence, we evaluated the ability of Chl extract to stimulate melanogenesis by assaying tyrosinase activity, which is known to be a rate‐limiting enzyme in melanin synthesis. Results presented in Fig. 4 indicate that Chl extract significantly stimulated production of intra‐ and extracellular melanin (87.45 μg/106 adherent cells and 10.61 μg/ml of supernatant, respectively) when compared to untreated cells (54.68 μg/106 adherent cells and 2.12 μg/ml of supernatant respectively), as well as tyrosinase activity in B16‐F0 cells, in a time‐dependent manner (Fig. 5).
Figure 4.
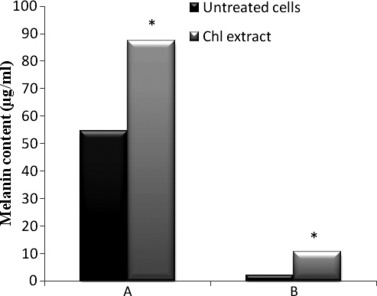
Effect of Chl extract (800 μg/ml) on melanin content in B16‐F0 cells after 48 h incubation.*P < 0.05 means significant difference between untreated and treated cells. (A) Intracellular melanin content (μg/106 adherent cells). (B) Extracellular melanin (μg/ml). Chl, chloroform extract.
Figure 5.
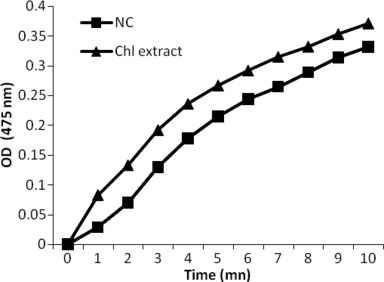
Effect of Chl extract (800 μg/ml) on tyrosinase activity in B16‐F0 cells after 48 h incubation. Chl, chloroform extract; NC, negative control (untreated cells); OD, optical density.
Discussion
EA, MeOH and Chl extracts possessed an inhibitory effect on both HaCaT and B16‐F0 cell proliferation. Anti‐proliferative activity of both MeOH and EA extracts may be attributed to presence of specific components such as flavonoids and polyphenols, as reported previously (13). However, minor components may act synergistically and contribute to anti‐proliferative effect of tested extracts, as described by others (20). Beside this, some studies have shown that flavonoids exhibit anti‐proliferative effects on various human (21) and malignant cells (22). Some tannin‐related compounds induced an anti‐proliferative effect against HL‐60 cells (23); likewise, some polyphenols have been described with inhibiting growth effect. It has been reported previously that polymethoxylated flavonoids, nobiletin and tangeretin, markedly inhibited cell growth of human squamous carcinoma cell line (HTB43) (24), whereas gallic acid and 1,2,3,4,6‐pentagalloylglucose isolated from Pistacia lentiscus, exhibited anti‐proliferative effects on K562 cells (25), and quercetin and quercetin‐7,4′‐dimethylether exerted anti‐proliferative effects on MT‐4 cells (26).
Anti‐proliferative activity exhibited by Chl extract may be attributed to its polyphenol, sterol, tannin (13) and iridoid (27) contents, and previous studies have also reported anti‐proliferative effects of iridoids (28, 29). Among iridoids, penta‐acetyl geniposide inhibits population growth of C6 glioma cells (30). Sterols, which are the main constituents of Chl extract, are also widely described for their anti‐proliferative effect (31), for example, schottenol and spinasterol (32). We believe that anti‐proliferative effects of these tested extracts may be ascribed to presence of mixtures of different compounds, which act synergistically; chemical study of these extracts has revealed presence of well‐studied compounds (such as squalene, fatty acids and more) in low quantities (data not shown), which did not exhibit any interesting biological effects when tested alone. In confirmation of our hypothesis, Smith et al. (33) demonstrated that squalene by itself was a weak inhibitor of cell proliferation, but it has been shown to prevent or arrest tumour growth in combination with anti‐cancer drugs in various experimental tumour models. For instance, when applied topically to mouse skin along with a fatty acid (oleic acid), squalene inhibits benzo(a)pyrene‐induced skin carcinogenicity (34). These data as well as those reported by Haruenkit et al. (35) and Campbell et al. (36) confirm that combinations of flavonoids are more effective in cell population growth inhibition than individual flavonoids.
In this study, we have demonstrated that Chl extract inhibited population growth of B16‐F0 and HaCaT cell lines in a dose‐dependent manner and induced B16‐F0 cell cycle arrest in G1 phase of the cell cycle. Many reports have demonstrated that enhanced anti‐proliferative activity is associated with compounds blockade processes (37). In addition, it is now established that blockage of the cell cycle is often linked to cell differentiation (38). The dendritic phenotype acquired by melanoma cells after differentiation and induced by several compounds (39), was also observed in B16‐F0 cells treated with Chl extract. Thus, in the present study, we provide evidence that a single treatment with Chl extract is able to induce differentiation of B16‐F0 melanoma cells reverting them from proliferating to the differentiated state.
Alesiani et al. (40) demonstrated that melanoma population growth reduction was linked to differentiation processes detected by monitoring some specific markers: (i) morphological changes with development of dendrite‐like projections from the cell surface; and (ii) melanin synthesis. Chl extract exposure effectively stimulates expression of differentiation markers of melanoma cells, such as tyrosinase activity and melanin content. These observations confirm that tested extract in our study triggered a cell differentiation programme. In agreement with our observations, Zhao et al. (41) demonstrated that treatment of B16‐F0 cells by an extracellular glycolipid, mannosylerythritol lipid (MEL) induced a significant increase in expression of melanin and activity of tyrosinase, which are considered to be molecular markers of cell differentiation. Typical morphological changes during induced pigmentation of B16‐F0 melanoma line answer the differentiation description (42).
Chl extract led to dose‐dependent reduction in cell population growth and increase in melanin synthesis and tyrosinase activity. It is generally accepted that population growth and differentiation are antinomic effects, that is, cells cannot proliferate and differentiate at the same time. This hypothesis is supported by reverse correlation between cell population growth and melanogenesis, observed in this study and in several other reports (43). Differentiation processes exhibited by Chl extract are very important as progression to malignant melanoma is characterized by loss of cell differentiation and by increased proliferation ability; thus in this context, it appears extremely attractive to achieve tumour reversion using differentiation‐inducing compounds in anti‐tumour therapy. Recent experimental approaches have used agents able to modify tumour growth by inducing terminal differentiation, a process termed ‘differentiation therapy’ (44).
Many studies have demonstrated that melanin acts as photoprotector against radiation in organisms and cells, particularly against UV radiation (45) by absorbing UV sunlight. Moreover, melanin plays an important role in removing reactive oxygen species (ROS), and scavenging toxic drugs and chemicals (46). Previous studies have indicated that elongation and extension of dendritic process and increased melanin synthesis by melanocytes are the consequence of tanning induced by UVA and UVB radiation (47). Indeed, increase in melanin production by Chl extract can result in its use in cosmetology and also as a therapeutic arsenal, that is, as a photoprotector against UV radiation and as a tanning product. Tanning or UV‐induced melanogenesis is another photoprotective response of human skin in which epidermal melanin increases gradually over several days, rendering skin less vulnerable to subsequent UV damage. It is known that tanned skin has a higher threshold for sunburn and skin that tans well is far more resistant to photodamage than skin that tans poorly (48). In addition, a dark natural tan offers unparalleled protection against skin cancer, and for these reasons, scientists are involved in developing compounds that trigger tanning.
In summary, this study suggests that Chl extract has a potential to be used as a natural product for tanning in cosmetic applications. We also report that Chl extract markedly inhibited population growth of mouse melanoma B16‐F0 cells in a dose‐dependent manner by inducing G1 cell cycle arrest. Further investigations are required to understand molecular mechanisms by which Chl extract affects the cell cycle, before drawing final conclusions concerning mechanisms of the probable anti‐cancer effects of the tested extract. Moreover, exposure of B16‐F0 cells to Chl extract stimulated expression of markers of melanoma cell differentiation such as tyrosinase activity and enhanced production of melanin, indicating that the tested extract triggered the cell differentiation programme. Although further studies are necessary to understand molecular mechanisms underlying the differentiating effect induced by Chl extract on B16‐F0 cells, our results provide a new perspective for development of novel strategies for prevention and treatment of melanoma.
Acknowledgements
We acknowledge the financial support from ‘Ministry of Higher Education, Scientific Research and Technology, Tunisia’, for this study and the ‘Ministry of Foreign Affairs, France (Action Intégrée de Coopération Inter universitaire Franco‐Tunisienne, CMCU 07 G0836 PAR)’. We acknowledge the laboratory of “Biomolecules and antitumoral therapy, Faculty of Pharmacy, Limoges, France” for all cell culture manipulations. The authors acknowledge Mr. Boukataya Samir (English teacher at the Faculty of Dental Medicine, Tunisia) for linguistic editing of the manuscript.
References
- 1. Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Inagaki K et al. (2005) Suppression of lipopolysaccharide‐induced cytokine production of gingival fibroblasts by a soybean, Kunitz trypsin inhibitor. J. Periodontal. Res. 40, 461–468. [DOI] [PubMed] [Google Scholar]
- 2. Pieroni A, Cassandra L, Villanelli M, Mangino P, Sabbatini G, Boccetti T et al. (2004) Ethnopharmacognostic survey on the natural ingredients in folk cosmetics, cosmeceuticals and remedies skin disease in the inland Marches, Central‐Eastern Italy. J. Ethnopharmacol. 91, 331–344. [DOI] [PubMed] [Google Scholar]
- 3. Cragg GM, Newman DJ (2005) Plants as a source of anticancer agents. J. Ethnopharmacol. 100, 72–79. [DOI] [PubMed] [Google Scholar]
- 4. Jayaprakasha GK, Girennavar B, Patil BS (2008) Radical scavenging activities of Rio Red grapefruits and sour orange fruit extracts in different in vitro model systems. Bioresour. Technol. 99, 4484–4494. [DOI] [PubMed] [Google Scholar]
- 5. Belkhiri A, Lockwood BG (1989) An indole derivative and glucosinolates from Moricandia arvensis . Phytochemistry 29, 1315–1316. [DOI] [PubMed] [Google Scholar]
- 6. Launey EW, Land AW (1984) Principles and practice of dermatology In: Disorders of Hyperpigmentation, 2nd edn Sydney: Butterworths, chapter 6. [Google Scholar]
- 7. Summers B (2006) A lightening tour of skin‐brightening options. Pharm. Cosmet. Rev. 33, 29–30. [Google Scholar]
- 8. Parvez S, Kang M, Chung H, Cho C, Hong M, Shin M et al. (2006) Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 20, 921–934. [DOI] [PubMed] [Google Scholar]
- 9. Kim YJ, Uyama H (2005) Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol. Life Sci. 62, 1707–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Momtaz S, Mapunya BM, Houghton PJ, Edgerly C, Hussein AA, Naidoo S et al. (2008) Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 119, 507–512. [DOI] [PubMed] [Google Scholar]
- 11. Le Floch E (1983) Contribution à une Etude Ethnobotanique de la Flore Tunisienne. Imprimerie Officielle de la République Tunisienne, Tunisia: Ministère de L’Enseignement Supérieur et de la Recherche Scientifique, p.106. [Google Scholar]
- 12. Cheieb M, Boukhris M (1998) Flore succinite et illustré des zones arides et sahariennes de la Tunisie. Tunis: L’or du temps, p.170. [Google Scholar]
- 13. Skandrani I, Ben Sgheiyer M, Neffati A, Boubaker J, Bouhlel I, Kilani S et al. (2007) Antigenotoxic and free radical scavenging activities of extracts from Moricandia arvensis . Drug Chem. Toxicol. 30, 361–382. [DOI] [PubMed] [Google Scholar]
- 14. Skandrani I, Bouhlel I, Limem I, Boubaker J, Bhouri W, Neffati A et al. (2009b) Moricandia arvensis extracts protect against DNA damage, mutagenesis in bacteria system and scavenge the superoxide anion. Toxicol. In vitro 23, 166–175. [DOI] [PubMed] [Google Scholar]
- 15. Pottier‐Alapetite G (1979) Flore de la Tunisia: Angiospermes, Dicotyledones, Apetales, Dialypetales. Imprimerie Officielle de la République Tunisienne, Tunisia: Ministère de L’Enseignement Supérieur et de la Recherche Scientifique, p. 210. [Google Scholar]
- 16. Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpam N, Neungton N (2004) Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J. Ethnopharmacol. 90, 161–166. [DOI] [PubMed] [Google Scholar]
- 17. Siegrist W, Eberle AN (1986) In Situ melanin assay for MSH using mouse B16 melanoma cells in culture. Anal. Biochem. 159, 191–197. [DOI] [PubMed] [Google Scholar]
- 18. Takahashi H, Parsons PG (1992) Rapid and reversible inhibition of tyrosinase activity by glucosidase inhibitors in human melanoma cells. J. Invest. Dermatol. 98, 482–487. [DOI] [PubMed] [Google Scholar]
- 19. Serafino A, Sinibaldi P, Lazzarino VG, Tavazzi B, Rasi G, Pierimarchi P et al. (2004) Differentiation of human melanoma cells induced by cyanidin‐3‐O‐β‐glucopyranoside. FASEB J. 18, 1940–1942. [DOI] [PubMed] [Google Scholar]
- 20. Yu JQ, Lei JQ, Yu HD, Cai X, Zou GL (2004) Chemical composition and antimicrobial activity of essential oil of Scutellaria barbata . Phytochemistry 65, 881–884. [DOI] [PubMed] [Google Scholar]
- 21. Hirano T, Gotoh M, Oka K (1994) Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL‐60 cells. Life Sci. 55, 1061–1069. [DOI] [PubMed] [Google Scholar]
- 22. Kuntz S, Wenzel U, Daniel H (1999) Comparative analysis of effects of flavonoids on proliferation, antiproliferativeity and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 38, 133–142. [DOI] [PubMed] [Google Scholar]
- 23. Amit K, Taraphdar R, Madhumita R, Bhattacharya RK (2001) Natural products as inducers of apoptosis: implication for cancer therapy and prevention. Curr. Sci. 80, 10–11. [Google Scholar]
- 24. Kandaswami C, Perkins E, Soloniuk DS, Drzewiecki G, Middleton E (1991) Antiproliferative effects of citrus flavonoids on a human squamous cell carcinoma in vitro . Cancer Lett. 56(2), 147–152. [DOI] [PubMed] [Google Scholar]
- 25. Abdelwahed A, Bouhlel I, Skandrani I, Valenti K, Kadri M, Guiraud P et al. (2007) Study of antimutagenic and antioxidant activities of Gallic acid and 1,2,3,4,6‐pentagalloylglucose from Pistacia lentiscus Confirmation by microarray expression profiling. Chem. Biol. Interact. 165, 1–13. [DOI] [PubMed] [Google Scholar]
- 26. Cimanga RK, Kambu K, Tona L, Hermans N, Apers S, Tott′e J et al. (2006) Cytotoxicity and in vitro susceptibility of Entamoeba histolytica to Morinda morindoides leaf extracts and its isolated constituents. J. Ethnopharmacol. 107, 83–90. [DOI] [PubMed] [Google Scholar]
- 27. Skandrani I, Boubaker J, Bhouri W, Limem I, Soumaya K, Ben Sghaier M et al. (2009a) Leaf extracts from Moricandia arvensis promote antiproliferation of human cancer cells, induce apoptosis, and enhance antioxidant activity. Drug Chem. Toxicol. 33, 20–27. [DOI] [PubMed] [Google Scholar]
- 28. Fukuyama Y, Minashima Y, Kishimoto Y, Chen I, Takahashi H, Kahashi H et al. (2004) Iridoid glucosides and p‐coumaroyl iridoids from Viburnum luzonicum and their antiproliferativeity. J. Nat. Prod. 67, 1833–1838. [DOI] [PubMed] [Google Scholar]
- 29. Kumarasamy Y, Nahar L, Sarker SD (2003) Bioactivity of gentiopicroside from the aerial parts of Centaurium erythraea . Fitoterapia 74, 151–154. [DOI] [PubMed] [Google Scholar]
- 30. Chang YC, Tseng TH, Lee MJ, Hsu JD, Wang CJ (2002) Induction of apoptosis by penta‐acetyl geniposide in rat C6 glioma cells. Chem. Biol. Interact. 141, 243–257. [DOI] [PubMed] [Google Scholar]
- 31. Samina N, Russell GK, Ramaswamy N (2000) New antiproliferative epoxysecosterols from Pseudopterogorgia americana . Tetrahedron Lett. 41, 6035–6040. [Google Scholar]
- 32. Bennani H, Drissi A, Giton F, Kheuang L, Fiet J, Adlouni A (2007) Antiproliferative effect of polyphenols and sterols of virgin argan oil on human prostate cancer cell lines. Cancer Detect. Prev. 31, 64–69. [DOI] [PubMed] [Google Scholar]
- 33. Smith TJ (2000) Squalene: potential chemopreventive agent. Expert Opin. Investig. Drugs 9, 1841–1848. [DOI] [PubMed] [Google Scholar]
- 34. Van Duuren BL, Goldschmidt BM (1976) Co‐carcinogenic and tumor‐promoting agents in tobacco carcinogenesis. J. Natl. Cancer Inst. 56, 1237–1242. [DOI] [PubMed] [Google Scholar]
- 35. Haruenkit R, Poovarodom S, Vearasilp S, Namiesnik J, Sliwka‐Kaszynska M, Park YS et al. (2010) Comparison of bioactive compounds, antioxidant and antiproliferative activities of Mon Thong durian during ripening. Food Chem. 118, 540–547. [Google Scholar]
- 36. Campbell JK, King JL, Harmston M, Lila MA, Erdman JW Jr (2006) Synergistic effects of flavonoids on cell proliferation in Hepa‐1c1c7 and LNCaP cancer cell lines. J. Food Sci. 71, S358–S363. [Google Scholar]
- 37. Carlson B, Lahusen T, Singh S, Loaiza‐Perez A, Worland PJ, Pestell R et al. (1999) Downregulation of cyclin D1 by transcriptional repression in MCF‐7 human breast cancer cells induced by flavopiridol. Cancer Res. 59, 4634–4641. [PubMed] [Google Scholar]
- 38. Loercher AE, Tank EMH, Delston RB, Harbour JW (2004) MITF links differentiation with cell cycle arrest in melanocytes by transcriptional activation of INK4A. J. Cell Biol. 168, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buscà R, Berlotto C, Ortonne JP, Ballotti R (1996) Inhibition of the phosphatidylinositol 3‐kinase pathway induces B16 melanoma cell differentiation. J. Biol. Chem. 271, 31824–31830. [DOI] [PubMed] [Google Scholar]
- 40. Alesiani D, Cicconi R, Mattei M, Montesano C, Bei R, Canini A (2008) Cell cycle arrest and differentiation induction by 5,7‐dimethoxycoumarin in melanoma cell lines. Int. J. Oncol. 32(2), 425–434. [PubMed] [Google Scholar]
- 41. Zhao X, Wakamatsu Y, Shibahara M, Nomura N, Geltinger C, Nakahara T et al. (1999) Mannosylerythritol lipid is a potent inducer of apoptosis and differentiation of mouse melanoma cells in culture. Cancer Res. 59, 482–486. [PubMed] [Google Scholar]
- 42. Bennett DC (1989) Mechanisms of differentiation in melanoma cells and melanocytes. Environ. Health Perspect. 80, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bellei B, Flori E, Izzo E, Maresca V, Picardo M (2008) GSK3β inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell. Signal. 20, 1750–1761. [DOI] [PubMed] [Google Scholar]
- 44. Waxman S (2000) Differentiation therapy in acute myelogenous leukemia (non‐APL). Leukemia 14, 491–496. [DOI] [PubMed] [Google Scholar]
- 45. Smit N, Vink A, Kolb R, Van den Berg P, Van Nieuwpoort F, Pavel S (2001) Melanin offers protection against induction of cyclobutane pyrimidine dimers and 6‐4 photoproducts by UVB in cultured human melanocytes. Photochem. Photobiol. 74, 424–430. [DOI] [PubMed] [Google Scholar]
- 46. Yaar M, Wu C, Park HY, Panova I, Schutz G, Gilchrest BA (2006) Bone morphogenetic protein‐4, a novel modulator of melanogenesis. J. Biol. Chem. 281, 25307–25314. [DOI] [PubMed] [Google Scholar]
- 47. Richard M, Kumar V, Abbas A, Fausto N (2006) Pocket Companion to Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier, Saunders, p. 231. [Google Scholar]
- 48. Arad S, Konnikov N, Goukassian DA, Gilchrest BA (2006) T‐oligos augment UV‐induced protective responses in human skin. FASEB J. 20, 1895–1897. [DOI] [PubMed] [Google Scholar]


