Abstract
Abstract. Cardiovascular disease is a global cause of mortality and morbidity. Current treatments fail to address the underlying scarring and cell loss, which are the causes of ischaemic heart failure. Cellular transplantation can overcome these problems and new impetus has been injected into this field following the isolation of human embryonic and adult stem cells. These cells have shown remarkable ability to produce cardiomyocytes and vascular cells in vitro and in vivo. Initial transplantation studies have demonstrated functional benefits and it is hoped further randomised clinical trials will concur with initial findings. Much basic science remains to be unearthed, such as the signals for homing, differentiation and engraftment of transplanted cells. Further matters of concern are the role of cell fusion and the mechanisms by which transplanted cells improve cardiac function. In spite of initial progress made in stem cell therapy there is still much to be done and we are some way off from achieving the goal of effective cellular regeneration.
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in Europe, of which nearly half is attributable to coronary heart disease (CHD) (British Heart Foundation 2000). Globally, CVD accounted for one‐third of all deaths in 2001 with the World Health Organization estimating 7.2 million from CHD each year (WHO 2002). This situation is expected to become worse, with CVD becoming the leading cause of death in developing countries by 2010 and a predicted 25 million CVD deaths worldwide by 2020 (Chockalingam & Balaguer‐Vintro 1999; WHO 2002). These predictions are made taking into consideration the advances in medical treatment and an overall decline in mortality rate in western countries (Reitsma et al. 1999; Tunstall‐Pedoe et al. 1999; Boersma et al. 2003). Whilst mortality may be decreasing, the morbidity associated with CHD is increasing as more people survive and grow old. One of the most devastating sequelae of CHD is the development of heart failure, a condition characterized by the symptoms of shortness of breath, oedema and disability. The outlook for this condition has improved in recent years, but mortality remains high, especially compared with other diseases. Patients with heart failure have 1‐year survival rates that are comparable, if not worse, than patients suffering with cancer. For example, 1 year from diagnosis of breast cancer 92% of patients are expected to be alive compared with only 62% of patients with a diagnosis of heart failure – even with current treatments (Cowie et al. 2000; MacIntyre et al. 2000; Quinn et al. 2001).
Heart failure in the context of CHD is characterized pathophysiologically by loss of functioning cardiomyocytes secondary to ischaemic injury. As cardiomyocytes have a very low potential for repair and regeneration, the ability of pharmacological agents to improve cardiac function is limited as these agents do not address the fundamental issue of cell loss. This has led to the search for a new approach in the treatment of patients with heart failure.
Currently, there are two therapeutic strategies that overcome the problem of cell loss in heart failure. Heterotropic heart transplantation is able to resolve the problems of heart failure and relieve patients’ symptoms, however, this treatment option is severely limited by donor organ availability. Although the technique has been used for approximately 30 years, the long‐term outcome for such patients is still complicated by problems of organ rejection on the one hand, and contrasted with both infection and development of lymphoproliferative disorders caused by immunosuppressive regimens designed to modulate the rejection process on the other (Miniati & Robbins 2002).
A second approach to the treatment of heart failure that addresses the issue of cell loss, and consequent decrease in heart function, is the concept of ‘cellular transplantation’. Ideally, this process allows the replacement of non‐functional cardiomyocytes and scar tissue with new fully functional contracting cells, improving cardiac function, and relieving the symptoms of heart failure. This ideal was initially realized with the use of cells derived from neonatal or embryonic tissue. Although the results showed great promise in ability to recover heart function, the future of this source of cells was limited due to ethical considerations and their short supply (Soonpaa et al. 1994; Sakai et al. 1999; Muller‐Ehmsen et al. 2002). Recent advances in the understanding of adult stem cell biology have reinstated the concept of cellular transplantation to replace dysfunctional cells.
Adult as well as embryonic stem cells have been shown to differentiate into cardiomyocytes, hence providing a new and viable source of these cells to treat patients. The full potential of each cell type remains to be seen. Embryonic stem cells appear to be truly totipotent, i.e. have the ability to differentiate into any cell type, whereas stem cells from adults appear to have undergone a process of partial differentiation such that they are committed to certain cell lineages. Provisional results have shown that transplantation of either embryonic or adult‐derived stem cells can improve cardiac function to some degree in animal models of myocardial infarction. The precise mechanism for this effect remains unclear and potential explanations will be discussed in the following text.
STEM CELL OVERVIEW
Stem cells are defined by their ability to self renew and to form one or more differentiated cell types. Cells with these characteristics can be usefully categorized in a number of ways; anatomically, functionally or by cell surface markers, transcription factors and proteins they express.
One clear division of the stem cell family is between those isolated from the embryo, and known as embryonic stem (ES) cells, and those found in adult somatic tissue known as adult stem cells. Within these categories, stem cells can be further divided, according to the number of differentiated cell types they can produce. Totipotent stem cells are able to form all fully differentiated cells of the body and trophoblastic cells of the placenta. The embryo, zygote and the descendants of the first two cell divisions are the only cells considered to be totipotent.
Pluripotent cells are able to differentiate into almost all cells that arise from the three germ layers, but are unable to give rise to the placenta and supporting structures. At around 5 days following fertilization, ES cells that form the inner cell mass of the blastocyst are considered pluripotent.
Multipotential stem cells are capable of producing a limited range of differentiated cell lineages appropriate to their location and are usually found in adult tissues. However, the use of the term ‘multipotential’ may be somewhat redundant, as it appears now that certain adult stem cells, removed from their usual location, transdifferentiate into cells that reflect their new environment. Stem cells with the least potential for differentiation are termed unipotential, an example of which is the epidermal stem cell found in the basal skin layer that only produces keratinized squames. From this initial introduction, it can be gleaned that ES cells are initially the most attractive option when considering the use of embryonic or adult stem cells for cellular therapies given their totipotential. However, their use is limited by ethical considerations, and thus, practically, adult stem cells are of more use. The remainder of this review will consider in greater detail the characteristics of ES cells and adult stem cells and their use in cellular therapy to treat heart disease.
EMBRYONIC STEM CELLS
ES cells were initially isolated in mice and more recently in humans (Evans & Kaufman 1981; Thomson et al. 1998; Reubinoff et al. 2000). ES cells are derived from the inner cell mass of the pre‐implantation or peri‐implantation blastocyst‐stage embryo. After mouse derived ES cells have been isolated, they can be cultured for prolonged periods in an undifferentiated state in the presence of leukaemia inhibitory factor (LIF) or mouse embryonic fibroblasts (MEFs). In contrast, human ES (hES) cells remain in an undifferentiated state only when cultured on a feeder layer of mitotically inactivated MEFs.
For the safe use of hES cells in clinical scenarios, an important step in the culture of these cells is the removal of xenoproducts such as MEFs from the process of isolation and culture. To this end, some progress has been made to replace MEFs. Thus far, hES have been shown to remain undifferentiated when grown in the presence of either human fetal fibroblasts, adult epithelial cells, foreskin cells or a matrigel/laminin matrix in media conditioned by MEFs (Xu et al. 2001; Richards et al. 2002; 2003a, 2003b).
Undifferentiated hES can be identified due to characteristic markers. These include stage‐specific embryonic antigens, SSEA‐3, SSEA‐4 and the glycoproteins TRA‐1–60, TRA‐1–81. Undifferentiated ES cells also express alkaline phosphatase, high telomerase activity and the transcription factor Oct‐4.
Human ES cells are pluripotent and can be induced into the differentiation process on removal from feeder layers (see Fig. 1). The multicellular aggregates of differentiated and undifferentiated cells that form as a consequence are termed embryoid bodies (EBs) and resemble early post‐implantation embryos. Within the Ebs, cellular derivatives of all three primary germ layers can be found (Itskovitz‐Eldor et al. 2000).
Figure 1.
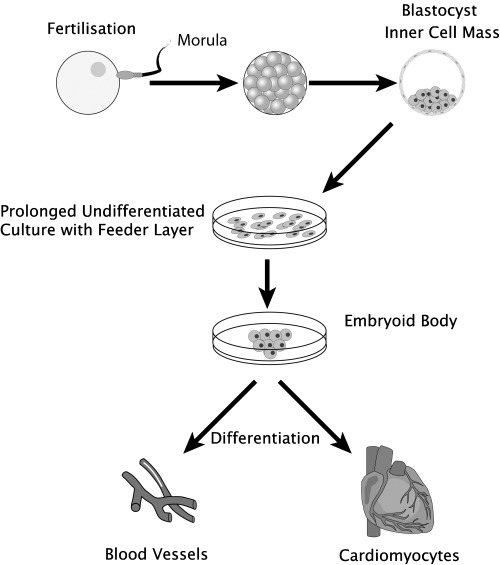
Transdifferentiation of embryonic stem cells into a cardiovascular phenotype. Following fertilised the inner mass from the blastocyst can be cultured ex vivo to form multiple cardiovascular cell types.
The technique of feeder layer or LIF withdrawal, and formation of EBs has been successfully used to obtain cells with a cardiomyocytic phenotype from mouse and human ES cells (Doetschman et al. 1985; Kehat et al. 2001). After the formation of an EB, most ES cell lines will spontaneously differentiate into a wide variety of cell lineages that include spontaneously beating cells. These beating cells have been extensively characterized and carry a cardiomyocytic phenotype. Morphology and ultrastructure of these cells is organized with sarcomeric structures, formation of intercalated discs, desmosomes and gap junctions characteristic of cardiomyocytes (Westfall et al. 1997; Kehat et al. 2001). These gap junctions have been demonstrated to function by transference of Lucifer yellow dye between cells and microelectrode array mapping, demonstrating the presence of a functional syncitium with action potential propagation (Westfall et al. 1997; Kehat et al. 2002). Immunohistochemistry and in situ hybridization have demonstrated the presence of appropriate proteins in cardiomyocytes and, furthermore, these are expressed in a time course that is analogous to that seen in cardiomyocytes developing in vivo (Guan et al. 1999 ). Normal functioning of the cells is suggested by their spontaneous contraction, but also by their electrophysiological profile and response to pharmacological agents (Wobus et al. 1991; Maltsev et al. 1999).
Cardiomyocytes formed in EBs are heterogeneous. By examining the electrophysiological profiles of spontaneously contracting cells, three distinct cell lines have been isolated; atrial, ventricular and purkinje cells (He et al. 2003). This finding underlines the pluripotential nature of ES cells, however, this attractive property of stem cells is also a hindrance to their potential clinical application. Therapeutic transplantation of cells that retain pluripotential capability can lead to undesirable effects such as oncogenesis. Equally, transplantation of pre‐differentiated cells, such as atrial or non‐cardiac lineage cells, into the ventricle could act to destabilize the electrical milieu and lead to arrythymogenesis. Isolation and expansion of specific cells types from EBs is crucial for a therapeutic role to be realized. Two approaches can be applied to this problem, either solely or in combination. One approach, often described as enrichment, involves addition of growth factors or other metabolically active agents to the culture medium to preferentially direct differentiation of ES cells to a cardiac lineage. Several factors alone and in combination have been shown to enrich cardiac differentiation – hepatocyte growth factor (HGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), transforming growth factor b1 (TGFb1), platelet‐derived growth factor (PDGF), sphingosine‐1‐phosphate, retinoic acid, 5‐azacytidine, vitamin C and over expression of GATA‐4 (Grepin et al. 1997; Schuldiner et al. 2000; Xu et al. 2002; Sachinidis et al. 2003; Takahashi et al. 2003). Besides addition of factors to culture medium, co‐culture with visceral‐endoderm‐like cells helps direct hES cells to differentiate into cardiomyocytes (Mummery et al. 2003). Other variables that may also be altered to enhance cardiomyocyte formation from ES cells are the use of different cell lines, culture mediums or feeder layers, timing of EB plating and altering the initial number of cells plated (Wobus et al. 2002).
The process of enrichment is yet to yield a collection of cardiomyocytes pure enough to consider for use in clinical therapy. An alternative avenue that promises a purity of cell line necessary for clinical application is to isolate cells after they have differentiated down a cardiac lineage by using a cell marker and separation technique. The key to this process is to use a marker that is specific for the cardiac lineage and ideally able to distinguish ventricular cardiomyocytes from atrial or purkinje cells. One such method involves the transfection of ES cells with a fusion gene of a αMHC promoter linked to a cDNA encoding aminogylcoside phosphotransferase. Following differentiation, selection of cells of cardiac lineage is possible due to expression of the antibiotic resistant gene. Using this protocol, up to a 99% pure cardiomyocyte sample has been obtained (Klug et al. 1996). Another method, used to isolate ventricular type cells only, involves the transfection of ES cells with enhanced green fluorescent protein under transcriptional control of ventricular‐specific myosin light chain‐2v. This reporter gene based approach, in conjunction with fluorescent assisted cell sorting, has allowed isolated collection of ventricular cardiomyocytes (Muller et al. 2000). These methods of purification are highly effective, but both insert a foreign epitope into the ES cells that may lead to immunological rejection following transplantation. An alternative strategy that circumvents this problem, and has been applied to hES cells, is the use of a Percoll gradient that separates cells based on their density. This technique has produced a 4‐fold enrichment of cardiomyocyte yield, with no undifferentiated hES cells detected following separation (Xu et al. 2002). Using these strategies to purify and enrich cardiomyocyte cultures, animal work has been conducted to establish the effect of these ES cell‐derived cells.
Transplanted ES cell‐derived cardiomyocytes have been shown to engraft in infarcted myocardium and improve cardiac function (see Table 1). Differentiated cardiomyocytes, derived from mES cells, have been transplanted using epicardial injection in myocardial infarction models and in animals with a muscular dystrophy. Results from these studies consistently show a significant improvement in cardiac function using echocardiography or left ventricular pressure transduction. Engraftment of transplanted cardiomyocytes has been confirmed by donor‐cell tracking, using transfection with green fluorescent protein, and immunohistochemistry (Klug et al. 1996; Behfar et al. 2002; Min et al. 2002b; Yang et al. 2002).
Table 1.
Stem cell transplantation experiments in man and animal models grouped by donor cell phenotype
| Reference | Host | Injury | Donor cell phenotype | Route of administration | Time after injury | Number of cells | Outcome | Follow‐up |
|---|---|---|---|---|---|---|---|---|
| Yang et al. 2002 | Friend leukaemia virus strain B mice | LAD ligation | mES (ES‐D3)‐derived early differentiated cardiomyo‐ cytes, transfected with GFP and ± transfected with VEGF | Epicardial into BZ and infarct 3 × 10 µl | 15 min | 3 × 105 | Significant increase in function & capillary density. Stem cells transfected with VEGF > increase than stem cells alone > than controls | 6 weeks |
| Behfar et al. 2002 | Sprague‐Dawley/Wistar | LAD ligation | mES (CGR8)‐derived cardiomyocytes | Epicardial into BZ 27G needle | 4 weeks | 3 × 105 | Significant increase in LVEF on echocardiography | 5 weeks |
| Min et al. 2000b | Wistar rats | LAD ligation | mES (ES‐D3)‐derived cardiomyocytes, transfected with GFP | Epicardial into BZ and infarct 3 × 10 µl | 30 min | 3 × 104 | Significant functional improvement, compared with controls. Engrafted GFP +ve cardiomyocytes seen | 6 weeks |
| Klug et al. 1996 | Mdx adult mice | None | mES (ES‐D3 transfected MHC‐neor/pGK‐transgene) derived cardiomyocytes | Epicardial 30G tuberculin syringe | N/A | 1 × 104 | Cardiomyocytes engrafted, no measures of function | N/A |
| Perin et al. 2003 | Humans | Congestive cardiac failure/IHD | Autologous BM, Mononuclear cells separated, avg 2.4%CD45lowCD34+ | Percutaneous transendocardial catheter | N/A | 25.5 ± 6.3 ×106 cells | NYHA, CCS improved, EF significantly improved 20–29%, SPECT less stress defects | 2 & 4 months |
| Tse et al. 2003 | Humans | IHD | Autologous BM, Mononuclear cells separated 3.2%CD34+ 27G needle, 16 injections @11 sites | NOGA mapping, Endomyocardial injection via catheter | N/A | 1.4 × 107 | Less angina attacks, reduced GTN tablet requirement, MRI: improved target wall thickening & motion | 3 months |
| Pak et al. 2003 | Pigs | LAD distal occlussion | MSC & BM or BM only | Direct epicardial, injection, × 15 | 1 month | 1.5 × 108 MSC, BM N/A | Higher nerve density in MSC group | 60 & 96 days |
| Nishida et al. 2003 | Dark agouti syngenic rats | LAD ligation | Aspirated BM | Direct epicardial injection, 6 × 10 µl, 26G needle | Stat | 6 × 107 | Increase in FS, microvessel density & blood flow & 90 days | 7, 30, 60 |
| Strauer et al. 2002 | Humans | Acute myocardial infarction | Autologus mononuclear cells from BM aspiration, Ficoll gradient, 2.1%CD34+, 0.6% AAC133+ | IC catheter, 6–7 injections 2–3 mls per injection, each 1.5–4 × 106 cells | 5–9 days | 18 × 106 cells | Repeat cardiac catheter significant reduction in hypo/dys/akinetic segments, improved perfusion on thallium, no significant increase in EF | 3 months |
| Hamano et al. 2002 | Dogs | LAD ligation, permanent | Autologus BM, density centrifugation | Direct epicardial, 6 injections, 0.1 ml each 27G needle | 30 days | 12 × 107 | Improved wall thickening & density of microvessels in marginal area | 30 days some up to 240 |
| Hamano et al. 2001 | Humans | IHD | Autologus mononulcear cells separated from BM aspiration | Endocardial at CABG, avg 11 injections 0.1 mL each, 26G needle | N/A | 5 × 108−1 × 109 cells | 60% improved stress tests | 1 year |
| Kobayashi et al. 2000 | Inbred dark agouti rats | LAD ligation | Unsorted BM | Direct epicardial injection, 6 × 10 µl PBS | 1 h | 5 × 106 | Siginificant increase in No. of vessels | 1, 3 & 7 days |
| Tomita et al. 1999 | Sprague‐Dawley rats | LV cryoinjury | BM separated with Percoll gradient, fresh, cultured 7 days or 5‐azacytidine treated | Direct epicardial injection, 50 µl into centre of scar, tuberculin syringe | 3 weeks | 106 | All grps significant increase capillaries, significant increase peak systolic & developed pressure only in BM treated with azacytidine | 5 weeks |
| Orlic et al. 2001a | C57BL/6 mice | LAD ligation | Aspirated BM, sorted for Lin−c‐kit+, from male transgenic eGFP mice | Direct epicardial injection, 2.5 µl into border zones | 3–5 h | 1.5 × 104–1 × 105 | Improved LV haemodynamics, 68% of infarct engrafted with transplanted cells | 9 days |
| Orlic et al. 2001b | C57BL/6 mice | LAD ligation | None | G‐CSF & SCF 5 days prior & 3 days post injury | N/A | N/A | Increase in survival, EF, & regenerating myocardium seen | 27 days |
| Jackson et al. 2001 | Irradiated mice | LAD ligation 60 mins | SP cells separated from Rosa C57BL/16 mice, CD34−/low, c‐kit+ Sca‐1+ | BM transplant | 10 weeks prior | 2000 for transplant | Engrafted cells of which, 0.02% cardiomyocytes, 3.3% endothelial cells | 2 & 4 weeks |
| Mangi et al. 2003 | Sprague‐Dawley rats | LAD ligation | MSCs CD117+, CD90+, CD34−, transfected with GFP, LacZ or Akt | Direct epicardil injection, × 5 injections into border zone | 60 min | 2.5 or 5 × 106 | MSC with Akt group complete normalization of function, 80–90% regeneration of myocardium | 2 weeks |
| Thompson et al. 2003 | Pigs | None | Aspirated BM, adherent cells, transfected with GFP | Transendocardial, 15 injections | N/A | N/A | GFP + ve cells found in all animals, no adverse outcome | 0–28 days |
| Gojo et al. 2003 | C3H/HeJ adult mice | None | BM stromal cells, treated with 5‐Azacytidine, CD34low/– c‐kit+,CD140a+ | Direct epicardial injection, 31G needle, 10 µl in ventricle or IVC | N/A | 106 | 0.25% of cells engrafted | 1, 4, 8 & 12 weeks |
| Shake et al. 2002 | Pigs | LAD ligation, 60 mins | Aspirated BM, MSC separated | Direct epicardial, 6 injections, 0.5 ml each 30G needle | 2 weeks | 6 × 107 cells | Significant increase systolic function, Transdifferentiation | 2 & 4 weeks |
| Min et al. 2002a | Pigs | LAD ligation | Human MSCs & human Fetal cardiomyocytes | Endocardial injection, BZ | 5 min | 7 × 106 cells | Transdifferentiation, improved haemodynamics & improved blood flow (microspheres) | 6 weeks |
| Toma et al. 2002 | CB17 SCID/ Beige mice | None | hMSC, transfected with LacZ | Transdiaphragm epicardial injection, 100 µls 32G needle | N/A | 0.5–1 × 106 | Up to 0.44% engraftment, transdifferentiation | 30 mins, 4, 14, 21, 30 & 60 days |
| Stamm et al. 2003 | Humans | Myocardial infarction | Autologous, BM aspiration, AC133+ cells separated by MACS | Endocardial at CABG, × 10 injections 0.2 mls, 22G needle | 10 days 3 months | 1 × 106 | No long‐term adverse effects, Improved NYHA, Improved LVEF (minimal). Improved perfusion on SPECT | avg 6.5 months |
| Assmus et al. 2002 | Humans | Acute myocardial infarction | BM mononuclear cells, 90% endothelial characteristics VEGFR2, CD105, PECAM‐ 1, vWF, VE‐Cadherin & CD146 | Intracoronary, ballon inflated for 3 ×, 3, 3 mls per patient | 4 days | 245 × 106, 7 × 106CD34+/CD45+ | EF improved significant, Reduced WMA, improved CFR, improved viability FDG‐PET | 4 months |
| Kocher et al. 2001 | Athymic nude rats Sprague‐ Dawley | LAD ligation permnt | BM aspiration CD34+ve separated, Dil labelled | Tail vein injection | 48 h | 2 × 106 cells | Significant improvement EF | 2 & 15 week |
| Kawamoto et al. 2001 | Athymic nude rats, Hsd: RH‐rnv | LAD ligation, Permnt | Peripheral blood MNCs, Dil labelled | Tail vein injection | 3 h | 106 | Significant increase in FS & capillary density, improved regional wall motion, transdifferentiation | 28 days |
| Kamihata et al. 2001 | ePigs | LAD ligation | Aspirated BM, MNC separated, transfected with GFP | Epicardial injection, BZ & infarct, 25 × 0.02 mls | 1 h | 108 cells | Significant increase EF, blood flow, vessels on angio & histology, decreased perfusion defects | 3 weeks & 12 week |
| Fuchs et al. 2001 | Pigs | LCx ameroid implant | Aspirated BM, MNC separated | Transendocardial injection, 10–12 injections 0.2 mls each | 4 week | N/A | Improved regional contractility, perfusion & EF | 1, 3, 7 & 21 days |
BM, bone marrow; BZ, border zone (of infarct); CABG, coronary artery bypass graft; CCS, Canadian cardiovascular score (for angina); EF, ejection fraction; FS, fractional shortening; GFP, green fluorescent protein; IC, intra‐coronary; IHD, ischaemic heart disease; LAD, left anterior descending artery; LV, left ventricle; Mdx, muscular dystrophy; mES, murine embryonic stem cell; MNC, mononuclear cell; MRI, magnetic resonance imaging; NYHA, New York Heart Association (score for heart failure); PBS, phosphate‐buffered saline; PET, positron emission tomography; SPECT, single photon emission computed tomography; VEGF, vascular endothelial growth factor.
In the failing heart, in addition to the replenishment of cardiomyocytes by ES derived cells, a simultaneous increase in the blood supply is necessary for optimal and prolonged engraftment. Hence, it is of interest that ES cells differentiate to all cell lines necessary for formation of new blood vessels. Both mES and hES cells spontaneously differentiate to form endothelial and smooth muscle cells in vitro, and blood vessel‐like structures can be seen within EBs (Vittet et al. 1996; Yamashita et al. 2000; Levenberg et al. 2002). ES cell‐derived endothelial cells express endothelial cell markers, including CD31, Flk‐1, VE‐cadherin, PECAM, Tie‐1 and Tie‐2. They also take up acetylated‐LDL, a functional characteristic of the endothelial cell phenotype. Enrichment and purification strategies, similar to those applied to cardiomyocytes, have been developed for endothelial cells (Yamashita et al. 2000; Marchetti et al. 2002). A range of transplant studies demonstrate that these cells can form vessels in vivo (Yamashita et al. 2000; Levenberg et al. 2002; Marchetti et al. 2002). Encouragingly, human ES cell‐derived endothelial cells have been shown to anastomose with host tissue following transplantation in immunodeficient mice and the structures so formed have been found to contain red blood cells (Levenberg et al. 2003).
Despite the progress made in transplantation, enrichment, purification, and eradication of xenoproducts in the derivation and culture of human stem cells, several issues still remain. The issue of rejection of donor cells still necessitates the use of immunosuppressive therapy with the accompanying hazards. A way round the issue of immunogenicity of the donor cell may be achieved using techniques such as somatic cell nuclear transfer (SCNT) or parthenogenesis.
SCNT, also known as therapeutic cloning, involves introduction of an adult cell nucleus from the potential recipient, into an enucleated oocyte to generate a cloned embryo (Hochedlinger & Jaenisch 2003). Embryonic stem cells may then be isolated from the resultant blastocyst. ES cells isolated in this way are pluripotent, and differentiated cells such as cardiomyocytes can be obtained (Kawase et al. 2000; Munsie et al. 2000). Viability of cardiac tissue produced by SCNT has been demonstrated in vivo (Lanza et al. 2002 ). The benefit of this process is that cells derived by nuclear transfer are genetically identical to the recipient cells (which would be obtained from the patient requiring treatment), thus eliminating the risk of organ rejection and requirement for immunosuppressive therapy. Alternatively, a similar technique that has the same benefits of SCNT is ‘parthenogenesis.’ This technique allows formation of an embryo from an egg without sperm. Understanding of this process is not as advanced as SCNT, but pluripotent ES cells have nevertheless been produced. Cardiomoyocytes have been produced in vitro, whilst in vivo teratomas containing tissue from all three germ layers have formed following implantation in immunodeficient mice (Cibelli et al. 2002).
The greatest obstacle to the use of stem cells derived from embryonic tissue relates to the ethical considerations involved. The combined scientific and social barriers preventing hES use in clinical therapy to date have led to consideration of other cell sources for therapeutic transplantation. Recent years have seen adult stem cells take centre stage due to their previously unrealized potential to produce a range of cell types outside of their expected lineage restriction.
ADULT STEM CELLS
Adult stem cells are found in many tissues and organs where they have the capacity to replenish cells that are lost during physiological homeostasis. Unearthing of a hitherto unknown property of some adult stem cells in which they appear to undergo a process of transdifferentiation or exhibit plasticity has led to significant interest in these cells. Plasticity describes a property of adult stem cells whereby they are able to produce specialized cells that are outside of their normal lineage commitment (Poulsom et al. 2002). In vitro and in vivo studies have demonstrated that these cells can transdifferentiate into brain, gut, lung, liver, pancreas, kidney and cardiac cells when placed under specific conditions (Makino et al. 1999; Pittenger et al. 1999; Alison et al. 2000; Mezey et al. 2000; Krause et al. 2001; Poulsom et al. 2001; Deb et al. 2003; Ianus et al. 2003) (see Fig. 2). Within cardiovascular research, most progress in the use of adult stem cells for cellular transplantation and end organ recovery has been seen with haematopoietic stem cells (HSC), mesenchymal stem cells (MSC) and endothelial progenitor cells (EPC).
Figure 2.
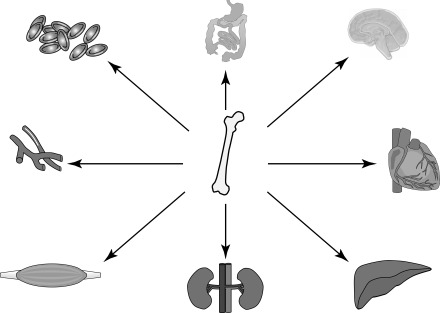
Adult stem cells plasticity, bone marrow derived stem cells have been shown to engraft and transdifferentiate into a variety of cell types other than haematopoietic lineages.
HAEMATOPOIETIC STEM CELLS
Haematopoietic stem cells are functionally defined as cells capable of reconstituting and maintaining all blood lineages. For practical purposes, these cells are usually defined with respect to cell surface markers, or ability to efflux Hoechst dye. Cells expressing certain combinations of markers can behave as HSCs. To date, cells expressing CD34+ alone or the combinations Thy‐1.1loSca‐1hiLineage−/lo, Lineage−/loSca‐1+c‐kit+, Lineage−/loSca‐1+c‐kit+CD34−, and Lineage−/loSca‐1+c‐kit+CD38+ have been used successfully in repopulation assays (Spangrude et al. 1988; 1996a, 1996b; Randall et al. 1996). Using the relevant antibody and fluorescence‐activated cell sorting (FACS) or magnetic‐assisted cell sorting (MACS) these cells lines can be isolated for research purposes or transplantation (Thomas et al. 1999).
So far, in vitro studies have been unable to demonstrate the potential for HSC transdifferentiation to a cardiac cell lineage. However, in vivo studies have shown transdifferentiation of HSCs to cardiomyocytes and to vascular structures (see Table 1). These studies have also demonstrated improvement in cardiac function. Side population cells, expressing CD34−/LOWc‐kit+Sca‐1+ have been shown to differentiate into cells that bear a cardiomyocytic and endothelial cell phenotype in a mouse model of myocardial infarction (Jackson et al. 2001). Furthermore, Lin−c‐kit+ HSCs are able to engraft and significantly improve ventricular function following myocardial infarction (Orlic et al. 2001a). This potential regenerative capacity has also been seen with human HSCs in a rat model of myocardial infarction. In this model, intravenous injection of human CD34+ cells lead to a significant reduction in scar tissue formation, improved neovascularization and resulted in an overall improvement in cardiac function (Kocher et al. 2001).
An alternative method for HSC delivery to the damaged heart, is to enhance the process of migration and homing from the bone marrow. Mobilization of HSCs using stem cell factor (SCF) and granulocyte colony stimulating factor (G‐CSF) before and after myocardial infarction in a mouse model was seen to significantly increase survival and cardiac function. Sections of heart from treated mice revealed newly formed myocytes and blood vessels (Orlic et al. 2001b). A second study, in non‐human primates, that infused SCF and G‐CSF 4 hours post‐MI, was able to demonstrate regeneration of vascular structures, with a significant increase in the number of capillaries and arterioles. Correspondingly, a significant increase in blood flow to the infarcted territory was seen in vivo, as measured by positron emission topography. In contrast to the former study, using the mouse model, formation of myocytes was not witnessed and there was no benefit to cardiac function (Norol et al. 2003). The first human trial of stem cell mobilization to treat CAD used a protocol of intra‐coronary granulocyte‐macrophage colony‐stimulating factor followed by 2 weeks of subcutaneous administration. Treated patients had a significant increase in coronary collateral flow, suggesting new vessel formation (Seiler et al. 2001). Further studies of stem cell mobilization are in progress, but results are yet to be published. It is also not clear which of the adult cell lineage is responsible for these changes.
The ability of mobilized stem cells to home to the infarcted heart necessitates a signalling mechanism to attract and retain the cells. Stromal derived factor‐1 (SDF‐1) has been suggested as a candidate for promoting homing of stem cells. SDF‐1 has proven to be essential in HSC homing to bone marrow for reconstitution in irradiated animals (Lapidot & Kollet 2002). Endothelial precursor homing also entails SDF‐1 – local injection of SDF‐1, in an animal model of limb ischaemia led to significantly increased vasculogenesis and blood flow (Yamaguchi et al. 2003). In the heart, SDF‐1 is essential to development and SDF‐1 expression is significantly up‐regulated post‐MI (McGrath et al. 1999; Pillarisetti & Gupta 2001). More evidence for the role of SDF‐1 in stem cell homing has been obtained from an experiment in which SDF‐1‐expressing cardiac fibroblasts were transplanted into the infarct region of rat hearts 8 weeks after MI. Stem cells were then mobilized with G‐CSF. The results showed a significant homing of CD117+ (c‐kit) cells, thought to represent endothelial progenitors, to the injured myocardium and a greater improvement in cardiac function when compared with control animals (Askari et al. 2003). This suggests that local expression of SDF‐1 can be used to enhance adult stem cell homing in animal models of myocardial infarction. The future of this approach in man remains to be seen.
MESENCHYMAL STEM CELLS
Mesenchymal stem cells (MSCs) can be found in bone marrow, muscle, skin and adipose tissue. MSCs are characterized by the potential to differentiate into muscle, fibroblasts, bone, tendon, ligament and adipose tissue (Caplan 1991). MSCs can be obtained from vigorous washing of cells from bone marrow aspirates in culture as MSCs differentially adhere to the plastic culture dish. This technique has been used to isolate MSCs from humans, rats and mice (Krebsbach et al. 1997; Colter et al. 2000; Barbash et al. 2003). Removing cells with HSC or EPC markers by FACS or MACS prior to plating initial cultures further enhances the specificity of this method.
MSCs that have been used in cardiac research are mainly from the heterogeneous population of cells derived from adherent bone marrow cultures as described above. Several studies have demonstrated that these cells can transdifferentiate into cardiomyocytes and vascular‐like structures (Makino et al. 1999; Min et al. 2002a; Shake et al. 2002; Toma et al. 2002; Gojo et al. 2003; Planat‐Benard et al. 2003; Thompson et al. 2003). Unlike ES cells, MSCs do not spontaneously form cardiomyocytes in vitro, but require stimulation to proceed along a cardiomyocytic lineage. Thus far, treatment of MSCs with 5‐azacytidine, has successfully lead to the development of cells that express a cardiomyocytic phenotype. This phenotype not only displays ultrastructural characteristics of cardiomyocytes, but also the localization of various cardiac‐specific markers, including the cytoplasmic proteins troponin, myosin, actin, atrial naturetic factor, as well as transcriptional factors such as GATA4, MEF‐2 and Nkx2.5. Spontaneous contraction and action potentials are observed, as are muscarinic, α and β adrenergic receptors. Adaptive functionality is suggested by the appropriate response of these cells to agonists and antagonists with a corresponding rise in intracellular molecules and change in contraction rate (Makino et al. 1999; Hakuno et al. 2002; Rangappa et al. 2002; Planat‐Benard et al. 2003).
MSCs have been shown to differentiate into cardiomyocytes and endothelial cells in vivo when transplanted to the heart in both non‐injury and myocardial infarction models. The cells have been strictly characterized by immunohistochemistry and positively stain for cardiac and endothelial specific markers, as well as gap junction proteins. (Wang et al. 2000; Rangappa et al. 2002; Shake et al. 2002; Toma et al. 2002; Gojo et al. 2003). Myocardial function and capillary formation are significantly increased in experimental groups treated with MSCs when compared with controls (see Table 1) (Mangi & Dzau 2002; Tomita et al. 2002; Davani et al. 2003). The ability of MSCs to transdifferentiate into specialized cells that improve function of the failing heart makes MSCs a realistic option for cellular transplantation. This is further underlined by the relative ease by which they can be maintained and expanded in culture. The ability to grow MSCs in culture also makes them a promising target for gene transduction. The value of combining gene therapy with cellular transplantation has already been demonstrated in a rat myocardial infarction model. Rat MSCs were isolated, expanded ex vivo and retrovirally transduced to over‐express Akt1, a mediator of survival signals and glucose metabolism. These cells were then transplanted into the heart of the recipient animal one hour following myocardial infarction. When compared with transplantation with standard MSCs, there was a significant decrease in collagen formation and inflammation – processes that may well be detrimental to recovery of cardiac function. Furthermore, 80–90% of lost myocardium was regenerated and function was completely normalized (Mangi et al. 2003). Such combination therapy has yet to be used in a clinical setting.
However, the use of bone marrow stem cells has already been tested in the setting of human heart disease. Unlike animal studies, in which attempts have been made to study specific cell lines, early clinical trials have tended to use a mononuclear cell fraction isolated from bone marrow aspirates. This fraction contains HSCs, MSCs and EPCs, and this should be kept in mind when considering the results of clinical studies. Mononuclear cells have been delivered via several routes, including intra‐coronary alone or combined with angioplasty and stenting following acute MI. So far, small numbers of patients have been studied with limited control groups. However, the therapy appears to be safe and functional benefits have been recorded, with reduction in wall motion abnormalities and improvements in left ventricular ejection fraction and myocardial perfusion (Strauer et al. 2002). Similar results have been generated by mononuclear cell administration via direct endocardial injection concomitant with coronary artery bypass (Hamano et al. 2001). A third technique – transendocardial injection of bone marrow‐derived mononuclear cells – has been used to treat patients with chronic severe CAD and heart failure. This method has also produced encouraging results, with improved cardiac function and patients experiencing less symptoms, as well as being able to reduce their cardiac medications (Perin et al. 2003; Tse et al. 2003). All trials published to date have been open label and small in size. The promise of these initial findings must be confirmed by larger randomised controlled trials before routine use of adult stem cell therapy can begin.
ENDOTHELIAL PROGENITOR CELLS
Successful cellular therapy for cardiac regeneration will require transplantation of functioning cardiomyocytes and concomitant generation of an adequate blood supply. Endothelial progenitors cells (EPCs) can contribute to tissue revascularization and can be isolated from adult bone marrow or from the peripheral circulation (termed circulating endothelial progenitor cells – CEPs). Adult‐derived EPCs and CEPs can be distinguished from mature endothelial cells by a functional in vitro assay due to their high proliferation rate. Temporally, they form late‐outgrowth colonies in which the cells are mainly EPCs and CEPs, and can form colony‐forming unit‐endothelial cells (Lin et al. 2000; Rafii & Lyden 2003).
Mature endothelial cells, EPCs and CEPs share several endothelial specific markers. However, only EPCs and CEPs express AC133 (CD133) (Miraglia et al. 1997; Yin et al. 1997). Cells expressing CD133+VEGFR2+ can proliferate in vitro to form mature endothelial cells (Gehling et al. 2000; Peichev et al. 2000). Human CEPs have also shown potential to differentiate to cardiomyocytes. When co‐cultured with neonatal rat cardiomyocytes, human CEPs formed cells with a cardiomyocytic phenotype, as defined by positive staining for cardiac specific markers such as troponin, atrial naturetic peptide and MEF‐2. Functional gap junctions were also demonstrated with transfer of Lucifer yellow dye and calcein between the cells (Badorff et al. 2003).
EPCs and CEPs play an important role in neovascularization in vivo (Asahara et al. 1999). Circulating EPCs are mobilized in response to organ ischemia, trauma and acute myocardial infarction (Takahashi et al. 1999; Gill et al. 2001; Shintani et al. 2001). The increase in CEPs post‐MI is mirrored by a rise in the growth and migratory cytokine VEGF‐A, and suggests a role for this factor in the mobilization of progenitor cells (Rabbany et al. 2003). Also, 3‐hydroxy‐3‐methylglutaryl co‐enzyme A (HMG‐CoA) reductase inhibitors (statins) have been shown to augment mobilization of EPCs. This important observation may provide an alternative mechanism by which statins decrease morbidity and mortality in patients with ischaemic heart disease (Dimmeler et al. 2001).
Transplantation of EPCs and CEPS has been shown to promote neovascularization of the ischaemic heart and improve function (see Table 1). Transdifferentiation to endothelial cells, smooth muscle cells and cardiomyocytes has been characterized by immunohistochemistry (Kawamoto et al. 2001; Kocher et al. 2001; Yeh et al. 2003). In animal models of MI, transplantation of EPCs or CEPs causes a significant increase in capillary density, regional blood flow, and collateral formation in the ischaemic heart. In addition, cardiac function is also significantly improved following transplantation (Kawamoto et al. 2001; Kocher et al. 2001; Kamihata et al. 2002; Kawamoto et al. 2003). Encouraging results such as these have led to human clinical studies of EPC transplantation. Two studies have been completed to date; EPCs or CEPs were transplanted either intra‐coronary following acute MI or endocardially at the time of coronary bypass surgery. There have been no adverse outcomes as yet, and both studies were able to show an increase in cardiac function with improved myocardial perfusion (Assmus et al. 2002; Stamm et al. 2003).
WHERE NOW?
Adult and embryonic stem cells are showing great potential for the treatment of cardiovascular disease, in particular ischaemic heart disease and heart failure. Results from animal studies and initial human trials are encouraging and have prompted the development of larger clinical trials. However, important aspects of stem cell biology and the transplantation process remain unresolved.
Currently, one main area of debate is the role of cell fusion in adult stem cell transdifferentiation. The possibility of stem cells acquiring a new phenotype by fusion with a differentiated cell type rather than by transdifferentiation into the new cell type by themselves has been demonstrated in vitro (Terada et al. 2002; Ying et al. 2002). Recently, this phenomenon has been used to explain the development of the hepatocyte phenotype from bone marrow‐derived progenitor cells (Vassilopoulos et al. 2003; Wang et al. 2003). This finding begs the question as to whether cell fusion is the process that explains progenitor‐derived cells expressing cardiomyocytic and endothelial cell phenotypes as discussed in this review. The possibility of cell fusion has not been addressed in the majority of stem cell transplant studies and future publications must examine this with rigour if the mechanisms of cellular transplantation are to be understood.
Recent studies have started to try and resolve the issues of cell fusion versus differentiation, using techniques such as a cre/lox recombination system to detect cell fusion. This method has been used in a mouse model, in the presence of ischaemia, where the overall engraftment of transplanted stem cells to the heart was about 3%. Half of the engrafted cells were the result of cell fusion events, the other half were felt to represent transdifferentiation and expressed appropriate cardiomyocytic markers. In the absence of ischaemia in this model, stem cell fusion with cardiomyocytes was seen at a very low level (Alvarez‐Dolado et al. 2003; Oh et al. 2003). However, in contrast to these findings, quantification of DNA failed to reveal cell fusion in a rat myocardial infarction model following stem cell transplantation (Beltrami et al. 2003). Human heart sections from female patients who had received male bone marrow transplants were found to contain cells that carried the Y chromosome suggesting donor origin. These donor cells displayed a cardiac phenotype which was shown to result from a process of transdifferentiation rather than cell fusion (Deb et al. 2003). Interpretation of these conflicting results is difficult and it remains to be seen what the true extent of cell fusion is and whether it is beneficial or detrimental. It is also of note that several of these studies have postulated the existence of a native adult cardiac stem cell. This was first suggested after demonstration of replicating cells in the adult heart in pathological states (Kajstura et al. 1998; Beltrami et al. 2001). Two potential sources for native cardiac stem cells have been suggested. Firstly, these cells may have their origins in the bone marrow from which they are released and engraft in the heart, either as a low‐level process of ongoing renewal, or in response to injury (Deb et al. 2003). Alternatively, it has been suggested that these cells represent a local cardiac stem cell population. The latter theory has been proposed following the isolation of lin−c‐kit+ cells and Sca‐1+ cells from heart tissue which were found to be self‐renewing, clonogenic and multipotent. When transplanted into an animal myocardial infarction model, these cells appeared to recover heart function. Although these ‘cardiac stem cells’ did not express markers suggesting a haematopoietic or endothelial lineage, there is still the possibility these cells were derived from other sources such as the bone marrow (Beltrami et al. 2003; Oh et al. 2003). Isolation of a truly local pluripotent cardiac stem cell would change our understanding of the native homeostatic processes in the heart and may provide us with the ultimate cell type for therapeutic cardiac regeneration.
For therapeutic cellular transplantation to occur, many clinical problems remain to be resolved. We must define which patient groups are suitable for this therapy and which stem cell or cell types are the most effective given the underlying pathology. The optimum timing and method of delivery still remain to be determined and may have a significant bearing on the beneficial outcome of cellular transplantation. Long‐term side‐effects of treatment are unknown as most of the clinical studies are very recent.
Pressing scientific problems are also unresolved. The mechanism by which transplanted stem cells improve cardiac function is not understood. It was initially assumed that stem cells might improve cardiac function by undergoing differentiation into replacement cardiomyocytes or vascular cells to take on the role of non‐functioning tissue. Stem cell‐derived cardiomyocytes would therefore improve function by contributing mechanically to contraction, whilst stem cell‐derived endothelial cells would improve blood supply by a process of neoangiogenesis. However, direct evidence for these assumptions is poor and provides sufficient doubt to allow alternative theories for the mechanism of functional benefit. These include cell fusion and other mechanisms such as release of local factors modulating cell division, apoptosis, extra cellular matrix homeostasis or metabolic efficiency. The cellular signals and mechanisms involved in homing, engraftment and differentiation of stem cells are partly established for ES cells, but on the whole remain undiscovered for adult stem cells. Understanding of these processes will increase the potential benefits of stem cell therapy. Finally, as mentioned earlier, immunomodulation strategies and methods of removing xenoproducts from the culture and isolation of stem cells are in their infancy, and need more work.
In summary, in spite of the exponential growth of interest and initial progress made in stem cell therapy for treatment of cardiovascular disease, there is much to be done and we are still some way off from achieving the ultimate goal of effective cellular regeneration.
REFERENCES
- Abeel A, Mangi VJD (2002) Isolation, expansion, and genetic modification of bone marrow mesenchymal stem cells for in‐vivo repair of damaged myocardium. J Am. Coll. Cardiol. XX, 445A. [Google Scholar]
- Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA (2000) Hepatocytes from non‐hepatic adult stem cells. Nature 406, 257. [DOI] [PubMed] [Google Scholar]
- Alvarez‐Dolado M, Pardal R, Garcia‐Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez‐Buylla A (2003) Fusion of bone‐marrow‐derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968. [DOI] [PubMed] [Google Scholar]
- Amit M, Margulets V, Segev H, Shariki K, Laevsky I, Coleman R, Itskovitz‐Eldor J (2003a) Human feeder layers for human embryonic stem cells. Biol. Reprod. 68, 2150. [DOI] [PubMed] [Google Scholar]
- Amit M, Shariki C, Margulets V, Itskovitz‐Eldor J (2003b) Feeder and serum‐free culture of human embryonic stem cells. Biol. Reprod. Nov 2003, DOI 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 85, 221. [DOI] [PubMed] [Google Scholar]
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, Dicorleto PE, Topol EJ, Penn MS (2003) Effect of stromal‐cell‐derived factor 1 on stem‐cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362, 697. [DOI] [PubMed] [Google Scholar]
- Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM (2002) Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE‐AMI). Circulation 106, 3009. [DOI] [PubMed] [Google Scholar]
- Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S (2003) Transdifferentiation of blood‐derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation 107, 1024. [DOI] [PubMed] [Google Scholar]
- Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J (2003) Systemic delivery of bone marrow‐derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108, 863. [DOI] [PubMed] [Google Scholar]
- Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M (2002) Stem cell differentiation requires a paracrine pathway in the heart. Faseb J. 16, 1558. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal‐Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P (2001) Evidence that human cardiac myocytes divide after myocardial infarction. N. Engl. J. Med. 344, 1750. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal‐Ginard B, Anversa P (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763. [DOI] [PubMed] [Google Scholar]
- Boersma E, Mercado N, Poldermans D, Gardien M, Vos J, Simoons ML (2003) Acute myocardial infarction. Lancet 361, 847. [DOI] [PubMed] [Google Scholar]
- British Heart Foundation (2000) European cardiovascular disease statistics. Rayner M & Petersen S. London: BHF.
- Caplan AI (1991) Mesenchymal stem cells. J. Orthop Res. 9, 641. [DOI] [PubMed] [Google Scholar]
- Chockalingam A, Balaguer‐Vintro I (1999) Impending global pandemic of cardiovascular diseases: challenges and opportunities for the prevention and control of cardiovascular diseases in developing countries and economies in transition. World Heart Federation. Barcelona: Prous Science. [PubMed] [Google Scholar]
- Cibelli JB, Grant KA, Chapman KB, Cunniff K, Worst T, Green HL, Walker SJ, Gutin PH, Vilner L, Tabar V, Dominko T, Kane J, Wettstein PJ, Lanza RP, Studer L, Vrana KE, West MD (2002) Parthenogenetic stem cells in nonhuman primates. Science 295, 819. [DOI] [PubMed] [Google Scholar]
- Colter DC, Class R, Digirolamo CM, Prockop DJ (2000) Rapid expansion of recycling stem cells in cultures of plastic‐adherent cells from human bone marrow. Proc. Natl Acad. Sci. USA 97, 3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie MR, Wood DA, Coats AJ, Thompson SG, Suresh V, Poole‐Wilson PA, Sutton GC (2000) Survival of patients with a new diagnosis of heart failure: a population based study. Heart 83, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, Etievent JP, Kantelip JP (2003) Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation 108, II253. [DOI] [PubMed] [Google Scholar]
- Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM (2003) Bone marrow‐derived cardiomyocytes are present in adult human heart: a study of gender‐mismatched bone marrow transplantation patients. Circulation 107, 1247. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Aicher A, Vasa M, Mildner‐Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM (2001) HMG‐CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3‐kinase/Akt pathway. J. Clin. Invest. 108, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R (1985) The in vitro development of blastocyst‐derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morph. 87, 27. [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154. [DOI] [PubMed] [Google Scholar]
- Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, Weissman NJ, Leon MB, Epstein SE, Kornowski R (2001) Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J. Am. Coll. Cardiol. 37, 1726. [DOI] [PubMed] [Google Scholar]
- Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W (2000) In vitro differentiation of endothelial cells from AC133‐positive progenitor cells. Blood 95, 3106. [PubMed] [Google Scholar]
- Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S (2001) Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ. Res. 88, 167. [DOI] [PubMed] [Google Scholar]
- Gojo S, Gojo N, Takeda Y, Mori T, Abe H, Kyo S, Hata J, Umezawa A (2003) In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp. Cell Res. 288, 51. [DOI] [PubMed] [Google Scholar]
- Grepin C, Nemer G, Nemer M (1997) Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA‐4 transcription factor. Development 124, 2387. [DOI] [PubMed] [Google Scholar]
- Guan K, Furst DO, Wobus AM (1999) Modulation of sarcomere organization during embryonic stem cell‐derived cardiomyocyte differentiation. Eur. J. Cell Biol. 78, 813. [DOI] [PubMed] [Google Scholar]
- Hakuno D, Fukuda K, Makino S, Konishi F, Tomita Y, Manabe T, Suzuki Y, Umezawa A, Ogawa S (2002) Bone marrow‐derived regenerated cardiomyocytes (CMG cells) express functional adrenergic and muscarinic receptors. Circulation 105, 380. [DOI] [PubMed] [Google Scholar]
- Hamano K, Nishida M, Hirata K, Mikamo A, Li TS, Harada M, Miura T, Matsuzaki M, Esato K (2001) Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: clinical trial and preliminary results. Jpn Circ J. 65, 845. [DOI] [PubMed] [Google Scholar]
- Hamano K, Li TS, Kobayashi T, Hirata K, Yano M, Kohno M, Matsuzaki M (2002) Therapeutic angiogenesis induced by local autologous bone marrow cell implantation. Ann. Thorac. Surg. 73, 1210. [DOI] [PubMed] [Google Scholar]
- He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ (2003) Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ. Res. 93, 32. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Jaenisch R (2003) Nuclear transplantation, embryonic stem cells, and the potential for cell therapy. N. Engl. J. Med. 349, 275. [DOI] [PubMed] [Google Scholar]
- Ianus A, Holz GG, Theise ND, Hussain MA (2003) In vivo derivation of glucose‐competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J. Clin. Invest. 111, 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskovitz‐Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N (2000) Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 6, 88. [PMC free article] [PubMed] [Google Scholar]
- Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA (2001) Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. 107, 1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P (1998) Myocyte proliferation in end‐stage cardiac failure in humans. Proc. Natl Acad. Sci. USA 95, 8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Amano K, Iba O, Imada T, Iwasaka T (2002) Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler. Thromb. Vasc. Biol. 22, 1804. [DOI] [PubMed] [Google Scholar]
- Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T (2001) Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 104, 1046. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T (2001) Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 103, 634. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T (2003) Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 107, 461. [DOI] [PubMed] [Google Scholar]
- Kawase E, Yamazaki Y, Yagi T, Yanagimachi R, Pedersen RA (2000) Mouse embryonic stem (ES) cell lines established from neuronal cell‐derived cloned blastocysts. Genesis 28, 156. [PubMed] [Google Scholar]
- Kehat I, Kenyagin‐Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz‐Eldor J, Gepstein L (2001) Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest. 108, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Gepstein A, Spira A, Itskovitz‐Eldor J, Gepstein L (2002) High‐resolution electrophysiological assessment of human embryonic stem cell‐derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ. Res. 91, 659. [DOI] [PubMed] [Google Scholar]
- Klug MG, Soonpaa MH, Koh GY, Field LJ (1996) Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J. Clin. Invest. 98, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Hamano K, Li TS, Katoh T, Kobayashi S, Matsuzaki M, Esato K (2000) Enhancement of angiogenesis by the implantation of self bone marrow cells in a rat ischemic heart model. J. Surg. Res. 89, 189. [DOI] [PubMed] [Google Scholar]
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S (2001) Neovascularization of ischemic myocardium by human bone‐marrow‐derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat. Med. 7, 430. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ (2001) Multi‐organ, multi‐lineage engraftment by a single bone marrow‐derived stem cell. Cell 105, 369. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG (1997) Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation 63, 1059. [DOI] [PubMed] [Google Scholar]
- Lanza RP, Chung HY, Yoo JJ, Wettstein PJ, Blackwell C, Borson N, Hofmeister E, Schuch G, Soker S, Moraes CT, West MD, Atala A (2002) Generation of histocompatible tissues using nuclear transplantation. Nat. Biotechnol. 20, 689. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Kollet O (2002) The essential roles of the chemokine SDF‐1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune‐deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 16, 1992. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Golub JS, Amit M, Itskovitz‐Eldor J, Langer R (2002) Endothelial cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA 99, 4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz‐Eldor J, Langer R (2003) Differentiation of human embryonic stem cells on three‐dimensional polymer scaffolds. Proc. Natl Acad. Sci. USA 100, 12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey A, Hebbel RP (2000) Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 105, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre K, Capewell S, Stewart S, Chalmers JW, Boyd J, Finlayson A, Redpath A, Pell JP, McMurray JJ (2000) Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation 102, 1126. [DOI] [PubMed] [Google Scholar]
- Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S (1999) Cardiomyocytes can be generated from marrow stromal cells in vitro . J. Clin. Invest 103, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Ji GJ, Wobus AM, Fleischmann BK, Hescheler J (1999) Establishment of β‐adrenergic modulation of 1‐type Ca2+ current in the early stages of cardiomyocyte development. Circ. Res. 84, 136. [DOI] [PubMed] [Google Scholar]
- Mangi AA, Dzau VJ (2002) Isolation, expansion, and genetic modification of bone marrow mesenchymal stem cells for in‐vivo repair of damaged myocardium. J. Am. Coll. Cardiol. 39(5) Suppl, abstract 410–3. [Google Scholar]
- Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ (2003) Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 9, 1195. [DOI] [PubMed] [Google Scholar]
- Marchetti S, Gimond C, Iljin K, Bourcier C, Alitalo K, Pouyssegur J, Pages G (2002) Endothelial cells genetically selected from differentiating mouse embryonic stem cells incorporate at sites of neovascularization in vivo . J. Cell Sci. 115, 2075. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J (1999) Embryonic expression and function of the chemokine SDF‐1 and its receptor, CXCR4. Dev. Biol. 213, 442. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR (2000) Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290, 1779. [DOI] [PubMed] [Google Scholar]
- Min JY, Sullivan MF, Yang Y, Zhang JP, Converso KL, Morgan JP, Xiao YF (2002a) Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann. Thorac. Surg. 74, 1568. [DOI] [PubMed] [Google Scholar]
- Min JY, Yang Y, Converso KL, Liu L, Huang Q, Morgan JP, Xiao YF (2002b) Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J. Appl. Physiol. 92, 288. [DOI] [PubMed] [Google Scholar]
- Miniati DN, Robbins RC (2002) Heart transplantation: a thirty‐year perspective. Annu. Rev. Med. 53, 189. [DOI] [PubMed] [Google Scholar]
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW (1997) A novel five‐transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood 90, 5013. [PubMed] [Google Scholar]
- Muller M, Fleischmann BK, Selbert S, Ji GJ, Endl E, Middeler G, Muller OJ, Schlenke P, Frese S, Wobus AM, Hescheler J, Katus HA, Franz WM (2000) Selection of ventricular‐like cardiomyocytes from ES cells in vitro . FASEB J. 14, 2540. [DOI] [PubMed] [Google Scholar]
- Muller‐Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L (2002) Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J. Mol. Cell Cardiol. 34, 107. [DOI] [PubMed] [Google Scholar]
- Mummery C, Ward‐Van Oostwaard D, Doevendans P, Spijker R, Van Den Brink S, Hassink R, Van Der Heyden M, Opthof T, Pera M, De La Riviere AB, Passier R, Tertoolen L (2003) Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm‐like cells. Circulation 107, 2733. [DOI] [PubMed] [Google Scholar]
- Munsie MJ, Michalska AE, O'Brien CM, Trounson AO, Pera MF, Mountford PS (2000) Isolation of pluripotent embryonic stem cells from reprogrammed adult mouse somatic cell nuclei. Curr. Biol. 10, 989. [DOI] [PubMed] [Google Scholar]
- Nishida M, Li TS, Hirata K, Yano M, Matsuzaki M, Hamano K (2003) Improvement of cardiac function by bone marrow cell implantation in a rat hypoperfusion heart model. Ann. Thorac. Surg. 75, 768. [DOI] [PubMed] [Google Scholar]
- Norol F, Merlet P, Isnard R, Sebillon P, Bonnet N, Cailliot C, Carrion C, Ribeiro M, Charlotte F, Pradeau P, Mayol JF, Peinnequin A, Drouet M, Safsafi K, Vernant JP, Herodin F (2003) Influence of mobilized stem cells on myocardial infarct repair in a nonhuman primate model. Blood 102, 4361. [DOI] [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD (2003) Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl Acad. Sci. USA 100, 12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal‐Ginard B, Bodine DM, Leri A, Anversa P (2001a) Bone marrow cells regenerate infarcted myocardium. Nature 410, 701. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal‐Ginard B, Bodine DM, Leri A, Anversa P (2001b) Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl Acad. Sci. USA 98, 10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H (1996a) Long‐term lymphohematopoietic reconstitution by a single CD34‐low/negative hematopoietic stem cell. Science 273, 242. [DOI] [PubMed] [Google Scholar]
- Osawa M, Nakamura K, Nishi N, Takahasi N, Tokuomoto Y, Inoue H, Nakauchi H (1996b) In vivo self‐renewal of c‐Kit+ Sca‐1+ Lin (low/–) hemopoietic stem cells. J. Immunol. 156, 3207. [PubMed] [Google Scholar]
- Pak HN, Qayyum M, Kim DT, Hamabe A, Miyauchi Y, Lill MC, Frantzen M, Takizawa K, Chen LS, Fishbein MC, Sharifi BG, Chen PS, Makkar R (2003) Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a swine model of myocardial infarction. J. Cardiovasc. Electrophysiol. 14, 841. [DOI] [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S (2000) Expression of VEGFR‐2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95, 952. [PubMed] [Google Scholar]
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT (2003) Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 21, 21. [DOI] [PubMed] [Google Scholar]
- Pillarisetti K, Gupta SK (2001) Cloning and relative expression analysis of rat stromal cell derived factor‐1 (SDF‐1) 1: SDF‐1 α‐mRNA is selectively induced in rat model of myocardial infarction. Inflammation 25, 293. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143. [DOI] [PubMed] [Google Scholar]
- Planat‐Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia‐Verdugo J‐M, Penicaud L, Casteilla L (2003) Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ. Res. Dec 2003, DOI 10.1161/01. RES. 0000109792. 43271.47. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Forbes SJ, Hodivala‐Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA (2001) Bone marrow contributes to renal parenchymal turnover and regeneration. J. Pathol. 195, 229. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Alison MR, Forbes SJ, Wright NA (2002) Adult stem cell plasticity. J. Pathol. 197, 441. [DOI] [PubMed] [Google Scholar]
- Quinn M, Babb P, Brock A, Kirby L, Jones J (2001) Cancer trends in England and Wales 1950–99 In: Jones J. ed. Studies on Medical and Population Subjects, 6. London: The Stationery Office. [Google Scholar]
- Rabbany SY, Heissig B, Hattori K, Rafii S (2003) Molecular pathways regulating mobilization of marrow‐derived stem cells for tissue revascularization. Trends Mol. Med. 9, 109. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D (2003) Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 9, 702. [DOI] [PubMed] [Google Scholar]
- Randall TD, Lund FE, Howard MC, Weissman IL (1996) Expression of murine CD38 defines a population of long‐term reconstituting hematopoietic stem cells. Blood 87, 4057. [PubMed] [Google Scholar]
- Rangappa S, Reddy VG, Ariff B, Lee EH, Sim EKW (2002) Transformation of the adult mesenchymal stem cells into cardiomyocyte‐like cells in vivo . Cardiovascular Engineering 2, 7. [Google Scholar]
- Reitsma JB, Dalstra JA, Bonsel GJ, Van Der Meulen JH, Koster RW, Gunning‐Schepers LJ, Tijssen JG (1999) Cardiovascular disease in the Netherlands, 1975–95: decline in mortality, but increasing numbers of patients with chronic conditions. Heart 82, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A (2000) Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro . Nat. Biotechnol. 18, 399. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A (2002) Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 20, 933. [DOI] [PubMed] [Google Scholar]
- Sachinidis A, Gissel C, Nierhoff D, Hippler‐Altenburg R, Sauer H, Wartenberg M, Hescheler J (2003) Identification of platelet‐derived growth factor‐BB as cardiogenesis‐inducing factor in mouse embryonic stem cells under serum‐free conditions. Cell Physiol. Biochem. 13, 423. [DOI] [PubMed] [Google Scholar]
- Sakai T, Li RK, Weisel RD, Mickle DA, Jia ZQ, Tomita S, Kim EJ, Yau TM (1999) Fetal cell transplantation: a comparison of three cell types. J. Thorac. Cardiovasc. Surg. 118, 715. [DOI] [PubMed] [Google Scholar]
- Schuldiner M, Yanuka O, Itskovitz‐Eldor J, Melton DA, Benvenisty N (2000) Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA 97, 11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler C, Pohl T, Wustmann K, Hutter D, Nicolet PA, Windecker S, Eberli FR, Meier B (2001) Promotion of collateral growth by granulocyte‐macrophage colony‐stimulating factor in patients with coronary artery disease: a randomized, double‐blind, placebo‐controlled study. Circulation 104, 2012. [DOI] [PubMed] [Google Scholar]
- Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ (2002) Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann. Thorac. Surg. 73, 1919. [DOI] [PubMed] [Google Scholar]
- Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T (2001) Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103, 2776. [DOI] [PubMed] [Google Scholar]
- Soonpaa MH, Koh GY, Klug MG, Field LJ (1994) Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science 264, 98. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL (1988) Purification and characterization of mouse hematopoietic stem cells. Science 241, 58. [DOI] [PubMed] [Google Scholar]
- Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schumichen C, Nienaber CA, Freund M, Steinhoff G (2003) Autologous bone‐marrow stem‐cell transplantation for myocardial regeneration. Lancet 361, 45. [DOI] [PubMed] [Google Scholar]
- Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P (2002) Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106, 1913. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T (1999) Ischemia‐ and cytokine‐induced mobilization of bone marrow‐derived endothelial progenitor cells for neovascularization. Nat. Med. 5, 434. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT (2003) Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation 107, 1912. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW (2002) Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416, 542. [DOI] [PubMed] [Google Scholar]
- Thomas TE, Miller CL, Eaves CJ (1999) Purification of hematopoietic stem cells for further biological study. Methods 17, 202. [DOI] [PubMed] [Google Scholar]
- Thompson CA, Nasseri BA, Makower J, Houser S, McGarry M, Lamson T, Pomerantseva I, Chang JY, Gold HK, Vacanti JP, Oesterle SN (2003) Percutaneous transvenous cellular cardiomyoplasty. A novel nonsurgical approach for myocardial cell transplantation. J. Am. Coll. Cardiol. 41, 1964. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105, 93. [DOI] [PubMed] [Google Scholar]
- Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ (1999) Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100, II247. [DOI] [PubMed] [Google Scholar]
- Tomita S, Mickle DA, Weisel RD, Jia ZQ, Tumiati LC, Allidina Y, Liu P, Li RK (2002) Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J. Thorac. Cardiovasc. Surg. 123, 1132. [DOI] [PubMed] [Google Scholar]
- Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP (2003) Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 361, 47. [DOI] [PubMed] [Google Scholar]
- Tunstall‐Pedoe H, Kuulasmaa K, Mahonen M, Tolonen H, Ruokokoski E, Amouyel P (1999) Contribution of trends in survival and coronary‐event rates to changes in coronary heart disease mortality: 10‐year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet 353, 1547. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR, Russell DW (2003) Transplanted bone marrow regenerates liver by cell fusion. Nature 422, 901. [DOI] [PubMed] [Google Scholar]
- Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin‐Sisteron H, Uzan G, Dejana E (1996) Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood 88, 3424. [PubMed] [Google Scholar]
- Wang JS, Shum‐Tim D, Galipeau J, Chedrawy E, Eliopoulos N, Chiu RC (2000) Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J. Thorac. Cardiovasc. Surg. 120, 999. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al‐Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M (2003) Cell fusion is the principal source of bone‐marrow‐derived hepatocytes. Nature 422, 897. [DOI] [PubMed] [Google Scholar]
- Westfall MV, Pasyk KA, Yule DI, Samuelson LC, Metzger JM (1997) Ultrastructure and cell–cell coupling of cardiac myocytes differentiating in embryonic stem cell cultures. Cell Motil. Cytoskel. 36, 43. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Wallukat G, Hescheler J (1991) Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation 48, 173. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Guan K, Yang HT, Boheler KR (2002) Embryonic stem cells as a model to study cardiac, skeletal muscle, and vascular smooth muscle cell differentiation. Meth. Mol. Biol. 185, 127. [DOI] [PubMed] [Google Scholar]
- WHO (2002) World health report: reducing risks, promotiny healthy life. In: Murray C, Lopez A, eds. France: World Health Organization. [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK (2001) Feeder‐free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 19, 971. [DOI] [PubMed] [Google Scholar]
- Xu C, Police S, Rao N, Carpenter MK (2002) Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ. Res. 91, 501. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch‐Marce M, Masuda H, Losordo DW, Isner JM, Asahara T (2003) Stromal cell‐derived factor‐1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 107, 1322. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K (2000) Flk1‐positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408, 92. [DOI] [PubMed] [Google Scholar]
- Yang Y, Min JY, Rana JS, Ke Q, Cai J, Chen Y, Morgan JP, Xiao YF (2002) VEGF enhances functional improvement of postinfarcted hearts by transplantation of ESC‐differentiated cells. J. Appl. Physiol. 93, 1140. [DOI] [PubMed] [Google Scholar]
- Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z (2003) Transdifferentiation of human peripheral blood CD34+‐enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo . Circulation 108, 2070. [DOI] [PubMed] [Google Scholar]
- Yin AH, Miraglia S, Zanjani ED, Almeida‐Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW (1997) AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90, 5002. [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG (2002) Changing potency by spontaneous fusion. Nature 416, 545. [DOI] [PubMed] [Google Scholar]


