Abstract
Abstract. Hepatocyte transplantation would offer an attractive alternative to liver transplantation in the treatment of inborn errors of liver metabolism. However, a major problem in most transplantation studies to date has been the limited growth of transplanted cells in the recipient organ. We performed a strategy for selective proliferation of transplanted cells by interfering with the proliferative capacity of resident hepatocytes, using the pyrrolizidine alkaloid retrorsine and then transplanting liver cells in conjunction with repeated administration of triiodothyronine, an inducer of hepatocyte proliferation in rats. In the present study, foetal and adult syngeneic hepatocyte transplantation into spleen was performed in retrorsine‐treated hyperbilirubinemic Gunn rats. In parallel, repeated injections of triiodothyronine were given to recipients. Rats were sacrificed at 1, 7, 30 and 90 days after transplantation and blood and bile samples were taken to assess the functionality of transplanted cells. The proliferative activity of transplanted hepatocytes was evaluated using proliferating cell nuclear antigen labelling index. In summary, both adult and foetal hepatocyte transplantation were effective in correcting a metabolic abnormality in Gunn rats for as long as 3 months. The RS/T3 model, as a measure to increase graft function, could represent an important advance to future clinical application of hepatocyte transplantation.
INTRODUCTION
Bilirubin is a toxic derivative of the protoporphyrin moiety of haem proteins such as haemoglobin. For its clearance from serum, bilirubin is conjugated with glucuronic acid, and it is generally accepted that UGT1A1 (bilirubin UDP‐glucuronosyltransferase) is the only bilirubin‐glucuronidating UGT1 isoform in humans and rats (Cubero et al. 2001). Genetic variations in the gene encoding UGT1A1 lead to complete or partial inactivation of the glucuronidation of bilirubin, which causes unconjugated bilirubin to accumulate in the serum and can result in hyperbilirubinemic conditions such as the human Crigler–Najjar syndrome (Iyanagi et al. 1998). The Gunn rat is derived from the Wistar parent strain and has constantly elevated concentrations of serum bilirubin, which causes unconjugated hyperbilirubinaemia. The Gunn rat inherently lacks all glucuronidation activities catalysed by the UGT1 isoforms and is therefore used as an animal model for Crigler–Najjar syndrome type I (Chowdhury et al. 1993; Sato et al. 1993).
Repopulation of a chronically diseased liver such as in Crigler–Najjar syndrome type I and in the Gunn rat via hepatocyte transplantation would represent a valuable alternative to whole‐organ transplantation, the method used currently to treat end‐stage liver disease (Gupta & Chowdhury 1992; Wilson 1996; Laconi et al. 1998). However, for therapeutic purposes, a greater magnitude of liver repopulation will be desirable in many situations. This concept has recently been illustrated by a variety of animal studies (Malhi & Gupta 2001). For instance, two experimental models of extensive liver repopulation have been described: the urokinase‐type plasminogen activator transgenic mouse (Rhim et al. 1994) and the fumarylacetoacetate hydrolase (Fah) null mouse (Overturf et al. 1996), the latter serving as a model for hereditary tyrosinaemia type I.
Among other strategies to improve liver repopulation, consideration has been given to enhancing the proliferative capacity of transplanted cells themselves and the inhibition of survival in host hepatocytes. For this approach, retrorsine and triiodothyronine have been used. Retrorsine (RS), a DNA‐binding pyrrolizidine alkaloid, was used to inhibit the proliferative capacity of host hepatocytes (Guo et al. 2002). Retrorsine induces extensive polyploidy in the liver, which refers to the appearance of cells that continue to synthesize excessive amounts of DNA but are unable to divide and are eventually lost. On the other hand, the thyroid hormone (T3) has been shown to provide a recurrent stimulation of hepatocyte proliferation (Oren et al. 1999).
Although the use of RS and other toxins will be hazardous for humans because of their potential for oncogenesis, the principle of inducing damage to native hepatocytes before cells are transplanted must be explored for developing clinically effective strategies. Thus, in the present work we study the proliferation capacity of T3‐stimulated intrasplenic hepatocytes transplanted in hyperbilirubinemic Gunn rats whose indigenous hepatocytes’ regenerative ability has been blocked by RS. Furthermore, we compare adult and foetal cells repopulation in the RS/T3 model. Combining this model with the use of foetal liver cells, highly regenerative and known to grow and function for a long period after intrasplenic transplantation (Kokudo et al. 1995a; Cubero et al. 2001), could provide extensive or complete repopulation of the liver in inborn errors of metabolism such as Crigler–Najjar syndrome type I.
MATERIALS AND METHODS
Animals
Hyperbilirubinemic Gunn rats (gg) weighing 130–180 g were used as recipients, and normal syngeneic rats (GG) as donors. Animals were bred in the laboratory in solid‐floored cages containing wood shavings and standard breeder chow in pellet form and water ad libitum. Animals were housed in climate‐controlled (21 °C) room with a 12‐h light–dark cycle. In order to use 21 days of gestation foetuses, gestational age was determined taking the day of sperm‐positive vaginal smear as day 0. The subsequent rapid weight gain of the dam confirmed the success of the pregnancy. Care of these animals complied with the principles of laboratory animal care of the European Union.
Experimental design
To study engraftment and function of transplanted cells, Gunn rats were given two injections of RS (Sigma Chemical Co., St. Louis, MO), 30 mg/kg each, intraperitoneally, 2 weeks apart. Four weeks after the second injection, each animal received 40 × 106 syngeneic hepatocytes. On the same day and every 10 days thereafer, T3 (Sigma; 400 µg/100 g body weight) was injected subcutaneously.
The experimental groups are:
-
•
FHT‐RS + T3: foetal hepatocyte transplantation treated with retrorsine and triiodothyronine
-
•
AHT‐RS + T3: adult hepatocyte transplantation treated with retrorsine and triiodothyronine
-
•
FHT‐RS: foetal hepatocyte transplantation treated with retrorsine
-
•
AHT‐RS: adult hepatocyte transplantation treated with retrorsine
Two control groups of animals transplanted with foetal and adult hepatocytes without any further treatment were also studied. Finally, non‐transplanted controls were included. Animals in the experimental groups were sacrificed at 1, 7, 30 and 90 days. At least 12 rats were included at each experimental time point.
Bilirubin levels were assessed from blood, and at the end of the study, from bile. Immunodetection of proliferating cell nuclear antigen (PCNA) was also carried out in livers and from all groups of rats, and labelling index evaluated.
Hepatocyte isolation
Adult hepatocytes were isolated using a two‐step collagenase perfusion technique described previously (Seglen 1976). The cell suspension was subjected to low‐speed centrifugation three times to yield a final fraction enriched with viable hepatocytes (> 85%, trypan blue exclusion). Foetal livers were taken at 21 days of gestation. Foetal hepatocytes were isolated by using the method described by Berry and Friend (1969) and modified by Arahuetes et al. (2001).
Hepatocyte transplantation
The recipients were subjected to midline laparotomy under halothane anaesthesia. Transplantation consisted of direct injection into the red pulp of the spleen of a suspension containing approximately 40 × 106 hepatocytes. The number and viability of cells were estimated by Trypan blue exclusion. The average hepatocyte yield was reported to be 100 × 106 cells/g of liver. Trypan blue exclusion was 85–90%.
Inhibition of liver mitosis of recipients by RS
Administration of retrorsine (RS) exerts a strong and persistent inhibition of hepatocyte cell division that lasts several months (Laconi et al. 1998). With the purpose of inhibiting recipient liver proliferation and provide a selective advantage for proliferation to the transplanted cells, before transplantation animals were given two injections of RS (Sigma Chemical Co., St. Louis), 30 mg/kg each, intraperitoneally, 2 weeks apart.
Stimulation of hepatocyte proliferation by T3
To study the mechanism(s) underlying T3‐stimulated liver repopulation, we introduced T3 into Gunn rats, on the same day of transplantation and every 10 days thereafter, T3 (Sigma; 400 µg/100 g body weight) was injected subcutaneously.
Assessment of hepatocyte function
Blood and bile samples were taken from normal syngeneic, untreated, and transplanted rats at 1, 7, 30 and 90 days after transplantation. Bilirubin levels were assessed in serum and bile. Blood was withdrawn from the jugular vein before the implant, and at the end of the study. Serum was separated by centrifugation. Bile was collected from the bile duct, at the time of sacrifice.
Levels were measured by the Total and Direct Bilirubin kit obtained from Sigma Co. (St. Louis, Missouri, USA).
Immunohistochemical detection of PCNA
Livers and spleens from recipient rats were removed after the animals were sacrificed. Tissues were fixed in fresh 4% formaldehyde buffered in PBS and embedded in paraffin. Standard haematoxiline and eosin staining was performed.
Anti‐PCNA immunohistochemical staining was carried out in the livers of animals within all study groups, control non‐transplanted and hepatocyte‐tranplanted, in order to evaluate the proliferative potential of transplanted hepatocytes. After deparaffinization and rehydration, 5‐µm sections of livers were incubated in 3% hydrogen peroxidase for 10 min. Normal goat serum was applied for blocking for 10 min. The slides were incubated with anti‐PCNA antibody (Biomeda; US) at a 1/400 dilution at 4 °C for 2 h, and then incubated for a further 30 min with a biotinylated goat antimouse IgG (Dako, Denmark) at a 1/400 dilution at room temperature. Finally, sections were developed with 0.02% diaminobenzine tetrahydrochloride.
In each slide, the proliferation index is determined from positive and negative hepatocytes, counted in 10 fields randomly chosen.
Statistical analysis
After testing for normality and homogeneity of variances, anova of repeated measures and Student paired t‐test was used for statistical intergroup and intragroup comparison of values expressed as mean ± SD. A value of P < 0.05 was considered to be significant.
RESULTS
Change in total serum bilirubin
Pre‐transplantation total serum bilirubin (TSB) was compared to TSB obtained after transplantation in each experimental group and day of study, considering the great variability of basal levels of bilirubin in the Gunn rat. No statistical differences were found between transplanted animals and RS‐pre‐treated transplanted animals. After foetal hepatocyte transplantation in the RS‐pre‐treated group (FHT‐RS), TSB significantly decreased at the 7‐day point, until the end of the study (Fig. 1a). However, when the antimitotic RS was administered in conjunction with T3 (FHT‐RS + T3), TSB considerably decreased from the 24 h point until the end of the experimental period (Fig. 1b). After transplantation of adult liver cells in RS‐pretreated animals (AHT‐RS), a significant decline in TSB levels was also observed, although in this case, the decrease was noted from the 1‐day point to a maximum of 30 days following transplantation (Fig. 2a). When T3 was administered in conjunction with RS, however, TSB levels also diminished from the 24‐h point until the end of the experiment. (Fig. 2b). When both adult and foetal hepatocyte transplantations were statistically compared, no differences were found between both types of transplantation (FHT‐RS versus AHT‐RS, and FHT‐RS + T3 versus AHT‐RS + T3, respectively).
Figure 1.
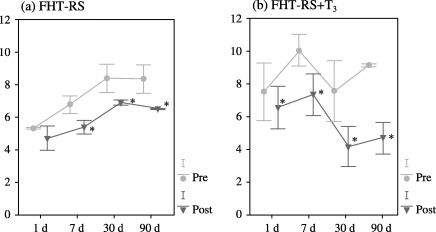
(a) Total serum bilirubin expressed as mg/dL throughout the times of study after foetal hepatocyte transplantation in RS‐pretreated rats (n = 20). (b) Total serum bilirubin expressed as mg/dL throughout the study after foetal hepatocyte transplantation and thyroid hormone administration in previously RS‐pretreated rats (n = 19). (pre = total serum bilirubin before transplantation; post = total serum bilirubin after transplantation; *P < 0.05).
Figure 2.
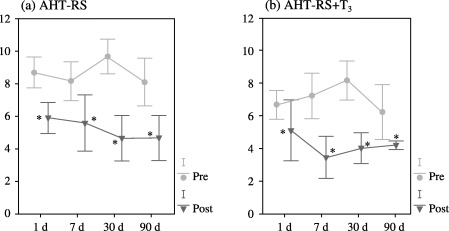
(a) Total serum bilirubin expressed as mg/dL from 24 h to 90 days before and after adult hepatocyte transplantation in RS‐pretreated rats (n = 19). (b) Total serum bilirubin expressed as mg/dL from 1 day to 90 days before and after adult hepatocyte transplantation and thyroid hormone administration in previously RS‐pretreated rats (n = 18). (Pre = Total serum bilirubin before transplantation; Post = Total serum bilirubin after transplantation; *P < 0.05).
Conjugated bilirubin in the bile
As mentioned before, bile was withdrawn through a catheter from the bile duct. Following this procedure, the animals died. For this reason, basal data of non‐transplanted Gunn rats was compared to the levels of those transplanted. In normal rats, bile conjugated bilirubin (BCB) represents the major form of bilirubin in bile, reaching 85% of the total bilirubin. In contrast, Gunn rats, incapable of conjugating bilirubin, have less BCB, typically around 45% of the total bilirubin. No statistical differences were found between transplanted animals and RS‐preated transplanted animals.
In the FHT‐RS group, clearance of bilirubin starts by day 7 post‐transplantation, and BCB was the major form of bilirubin in bile until the end of the study. Meanwhile, in the FHT‐RS + T3 group, BCB was the major form of bilirubin in bile from 24 h after transplantation. Comparison of BCB in both groups transplanted with foetal hepatocytes shows that clearance of bilirubin was significantly greater in the FHT‐RS + T3 animals at all times of study (Fig. 3a).
Figure 3.
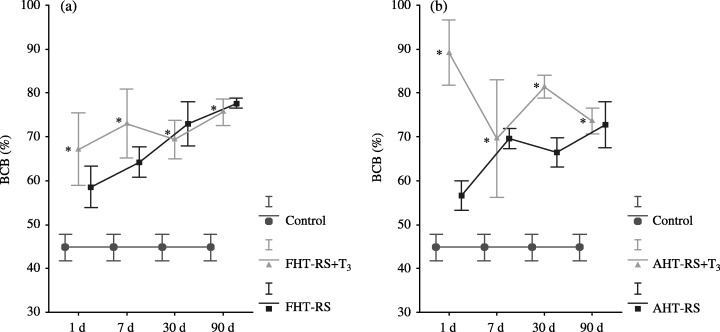
(a) Percentage of bile conjugated bilirubin in the experimental groups FHT‐RS (foetal hepatocyte transplantation in RS‐pretreated rats, n = 12) and FHT‐RS + T3 (foetal hepatocyte transplantation + RS + T3, n = 12) and in the control group (Gunn rats, n = 64) along the study. (b)Percentage of conjugated bilirubin in the experimental groups AHT‐RS (adult hepatocyte transplantation in RS‐pretreated rats, n = 20) and AHT‐RS + T3 (adult hepatocyte transplantation + RS + T3, n = 21) and in the control group (Gunn rats, n = 64) along the study. (BCB: bile conjugated bilirubin; *P < 0.05).
BCB resembles the conjugated fraction of bilirubin in normal rats from 24 h to 90 days after AHT in RS‐pre‐treated Gunn rats. In the AHT‐RS + T3 group, clearance of bilirubin is also efficient at all points of study (Fig. 3b). When percentage of BCB of both groups was compared, though, the clearance of bilirubin was notably greater in the AHT‐RS + T3 animals throughout the study.
Finally, statistical comparison of clearance of bilirubin between foetal and adult hepatocyte transplantation was carried out. In bile, the transplantation of foetal hepatocytes into RS‐pre‐treated animals provoked a significant increase of BCB 1 week after the implants, which remained until the end of the study. In contrast, adult liver cell transplantation in the AHT‐RS group resulted in a significant decrease of BCB from the first 24 h after the implantation to the 7‐day mark, after which BCB levels rose. There were no statistical differences between both experimental groups, AHT and FHT, treated with T3 and RS at any time during the study.
PCNA labelling index in the livers of Gunn rats after hepatocyte transplantation
Right after intrasplenic transplantation hepatocytes migrate to the liver of the recipient via the vena porta. Afterwards, they enter the hepatic parenchyma and integrate into it.
The results of PCNA labelling index (LI) are depicted in Table 1.
Table 1.
Proliferation of liver from non‐transplanted and transplanted animals throughout the study (mean ± SE)
| Time | PCNA labelling index | PCNA labelling index | ||||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 7 d | 30 d | 90 d | 24 h | 7 d | 30 d | 90 d | |
| C | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| C + RS | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.7 ± 0.2 | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.7 ± 0.2 |
| C + T3 | 2.8 ± 0.4 | 2.3 ± 0.2 | 2.9 ± 0.3 | 2.9 ± 0.2 | 2.8 ± 0.4 | 2.3 ± 0.2 | 2.9 ± 0.3 | 2.9 ± 0.2 |
| Time | Foetal hepatocytes | Adult hepatocytes | ||||||
| 24 h | 7 d | 30 d | 90 d | 24 h | 7 d | 30 d | 90 d | |
| Tx | 3.4 ± 0.2 | 3.4 ± 0.1 | 3.5 ± 0.3 | 2.8 ± 0.5 | 2.7 ± 0.2 | 2.8 ± 0.7 | 3.0 ± 0.8 | 3.2 ± 0.7 |
| Tx + RS | 3.5 ± 0.5 | 3.6 ± 0.2 | 3.6 ± 0.4 | 2.7 ± 0.2 | 3.0 ± 0.4 | 3.2 ± 0.9 | 3.1 ± 0.2 | 3.3 ± 0.7 |
| Tx + T3 | 4.1 ± 0.3 | 4.5 ± 0.4 | 4.7 ± 0.6 | 4.7 ± 0.3 | 4.0 ± 0.4 | 4.3 ± 0.7 | 4.43 ± 0.3 | 4.5 ± 1.0 |
| Tx + RS + T3 | 4.0 ± 0.2 | 4.5 ± 0.2 | 4.6 ± 0.3 | 4.8 ± 0.3 | 4.3 ± 0.5 | 4.3 ± 0.3 | 4.6 ± 0.2 | 4.7 ± 0.3 |
The upper part of the table shows LI from non‐transplanted Gunn rats: C (non‐treated rats, n = 6), C + RS (RS‐pre‐treated rats, n = 6), C + T3 (T3‐stimulated rats, n = 6). The lower part of the table shows the LI from Gunn rats transplanted with fetal (left) and adult (right) hepatocytes: Tx (non‐treated rats), Tx + RS (RS‐pre‐treated rats, n = 6), Tx + T3 (T3‐stimulated rats, n = 6) and Tx + RS + T3 (treated with RS and T3, n = 6).
The LI of normal quiescent hepatocytes in the host liver was 1.0% ± 0.1%. The injection of the thyroid hormone stimulated proliferation up to 2.9% ± 0.3%.
The LI of Gunn rat liver, after foetal hepatocyte transplantation (FHT) was as high as 3.4% ± 0.2% 24 h after transplantation, and it gradually decreased to 2.8% ± 0.5% 3 months after transplantation. In RS‐pre‐treated animals transplanted with foetal hepatocytes, LI was similar to the previous group. Administration of T3 increased proliferation of hepatocytes in the liver up to a value of 4.7% ± 0.3% 3 months after transplantation. When RS was administered in conjunction with T3, the PCNA LI was similar, ranging from 4.0% ± 0.2% 24 h after transplantation to 4.8% ± 0.3% 3 months after transplantation.
The LI of Gunn rat liver, after adult hepatocyte transplantation (AHT) was 2.7% ± 0.2% 24 h following transplantation, and it gradually increased to 3.2% ± 0.7% 3 months after transplantation. When the adult hepatocytes were transplanted into RS‐pre‐treated Gunn rats, the PCNA LI of livers was similar to that of non‐treated animals, increasing from 3.0% ± 0.4% 24 h to 3.3% ± 0.7% 3 months after transplantation. Adult hepatocytes transplanted into T3‐stimulated animals increased the proliferation activity reaching a maximum value of 4.5% ± 1.0%. This situation was repeated when RS was administered in conjunction with T3; the PCNA LI was 4.3% ± 0.5% at 24 h, and progressively augmented to 4.7% ± 0.3% at 3 months.
Some of the previously mentioned findings are illustrated in Fig. 4, in which a control liver without any treatment (4a), and the liver of an animal transplanted with foetal hepatocytes and treated with RS and T3 (4b).
Figure 4.
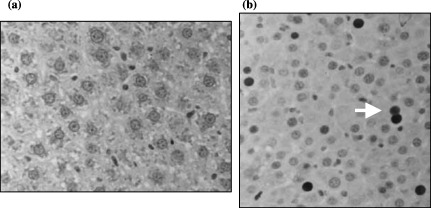
(a) Anti‐PCNA immunohistochemical staining of a control Gunn rat liver treated with RS (40×). (b) Anti‐PCNA immunohistochemical staining of the host liver of a Gunn rat transplanted with foetal hepatocytes, treated with RS and T3, 30 days after transplantation (40×) (arrow: positively stained nucleus). Each picture shows a part of a field. As explained in Material and Methods the LI is determined from 10 fields randomly chosen in each slide.
DISCUSSION
The proliferative capacity of transplanted hepatocytes has attracted the attention of various groups trying to overcome the limitations of orthotopic liver transplantation. Current research on hepatocyte transplantation into the spleen focuses on optimizing parameters that relate to the limited expansion of donor cells in the recipient liver, given that hepatocytes transplanted into spleen migrate to the liver through the portal vein (Rhim et al. 1994; Overturf et al. 1996; Maganto 1997; Yazigi et al. 1997; Malhi & Gupta 2001; Laconi 2002). The main rate limiting component towards better clinical results of hepatocyte transplantation is the low level of proliferation of transplanted cells in the host liver. To this end, an important strategy has been considered to enhance the proliferation capacity of transplanted cells themselves. This has been introduced with the development of model system wherein donor hepatocytes are transplanted into a pre‐conditioned host. Laconi et al. (1998) developed a rat model of enhanced liver repopulation by pretreating rats with RS. This alkaloid exerts a strong and persistent block of native hepatocyte proliferation and does not affect donor cells that are transplanted two or more weeks after drug administration (Laconi et al. 1999; Guo et al. 2002). In that study, partial hepatectomy was performed to provoke a regenerative environment. Interestingly, partial hepatectomy can be substituted in this regime by repeated administration of T3. The thyroid hormone has been shown to be a specific inducer of hepatocyte proliferation in rats (Oren et al. 1999).
Thus, we combined the use of a recipient liver cell cycle inhibitor, RS, with T3 to promote preferential proliferation of transplanted cells in the Gunn rat. The Gunn rat, a model of hyperbilirubinemia, has widely been used in experimental hepatocyte transplantation, monitoring serum bilirubin levels as an indicator of graft function (Borel‐Rinkes et al. 1994; Cubero et al. 2001).
In this study, we have shown that serum total bilirubin (TSB) levels of Gunn rats were significantly decreased after both adult and foetal liver transplanted in similar manners. These improvements were noticed after foetal hepatocyte transplantation into RS‐pre‐treated Gunn rats (FHT‐RS) two weeks after the treatment and lasted throughout the experimental period. However, after adult hepatocyte transplantation into RS‐pre‐treated Gunn rats (AHT‐RS), the decrease in TSB was noticed 24 h after the implants and lasted until the end of the first month of study. When, T3 was administered in RS‐pre‐treated animals both foetal and adult cells transplantation resulted in an improvement of TSB at all times of the study. There have been several reports on the functionality of hepatocytes after transplantation in Gunn rats (Kokudo et al. 1995a; Cubero et al. 2001). Coincident to the results obtained by Kokudo et al. (1995b) the improvement in serum hyperbilirubinaemia was not statistically different between foetal and adult hepatocyte transplantation. Furthermore, after FHT the decrease in TSB is more remarkable from 3 months on after transplantation (Kokudo et al. 1995a). However, when liver cell transplantation in the Gunn rat is carried out in conjunction with the preconditioning of the host with RS and the stimulation of transplanted cells with T3, both foetal and adult hepatocytes show a similar short‐ and long‐term functionality as we have proved in this report.
The metabolic improvement was also demonstrated by increased bile conjugated bilirubin (BCB) after both foetal and adult hepatocyte transplantation. BCB was increased in both the RS‐pre‐treated and in the RS + T3 groups. However, percentage of BCB was higher in the RS + T3 injected animals transplanted with foetal hepatocytes. Our findings are coincidental but slightly greater than Kokudo's et al. (1995b) who observed considerable amounts of bilirubin glucuronides in the bile from Gunn rats 4 months after FHT (36.0%) and AHT (27.0%). We have reported glucuronidation percentages around 66% after FHT in our preliminary report (Cubero et al. 2001). In this study we have found BCB around 70–80% after either FHT or AHT in both the RS‐pre‐treated and the RS + T3 groups. This can be explained by the greater number of cells that we transplant, 40 × 106 in contrast to Kokudo et al. (1995b) who only transplanted 10 × 106. Recently, RS pre‐treatment alone has been demonstrated to induce repopulation of a liver by transplanted cells (Laconi et al. 2001). This could also explain the improvement of serum and bile hyperbilirubinaemia found in the RS‐pre‐treated groups.
The immunohistochemical detection of PCNA has been found to depend on the proliferative potential of a number of cell types including hepatocytes (Kokudo et al. 1995b). We therefore applied this method to estimate hepatocyte proliferation in the liver. After the implantation of hepatocytes into the spleen, cells migrate via porta to the liver, as previous work performed by us and some other authors has demonstrated (Gupta et al. 1999; Cubero et al. 2001). This migration represents a proliferative stimulus that increases cell proliferation by itself, or in conjunction with RS when using both foetal and adult hepatocytes. As is known in the RS model, RS disrupts the cell cycle, and mitosis does not occur. However, we have observed a long‐term gradual decrease in the PCNA labelling index of the liver after foetal hepatocyte transplantation that may be the result of cell maturation or loss of local growth stimulants including TGFα or HGF. Some data indicates that transplanted repopulating liver cells arise both from mature donor hepatocytes and from small hepatocytes or non‐parenchymal cells (Gupta et al. 1999). Guo et al. (2002) support the hypothesis that liver repopulation results from the active proliferation of a small percentage of engrafted cells. In consistence with the well‐known fact that thyroid hormone is considered a strong mitogen that causes a high level of hepatocyte proliferation (Francavilla et al. 1994; Forbes et al. 1998), the PCNA labelling index, after administration of T3 increases as compared to control samples. Oren et al. (1999) found a slower rate of liver repopulation in the first month following T3 administration. Our findings agree with those of Oren who found that repeated subcutaneous injections of T3 stimulated transplanted hepatocytes in RS‐treated animals to undergo multiple rounds of cell division in 2 months. Repopulation of the liver by transplanted cells might result from direct increase in proliferation of transplanted cells suggesting that T3 initially triggers a wave of hepatocyte proliferation, followed by apoptosis, although the extent to which the latter contributes to liver repopulation is presently not clear.
In summary, both adult and foetal hepatocyte transplantation into the spleen were effective in correcting a metabolic abnormality in Gunn rats for as long as 3 months. This longevity of transplanted hepatocytes with significant specific function and some proliferative activity was accomplished by choosing the spleen as the site of implants. The RS/T3 model as a measure to increase graft function could represent an important advance to future clinical application of hepatocyte transplantation.
ACKNOWLEDGEMENTS
We wish to thank to the Animalario de la Facultad de Ciencias Biológicas de la Universidad Complutense de Madrid and the Animalario del Hospital Universitario Puerta de Hierro de Madrid for the care and maintenance of the animals, Dr Isabel Millán for the statistical analysis and Ms Shenna Kevorkian for her editorial assistance. This work was financed by grant FIS 01/0001‐01 and 02 from the Spanish Social Security Funds for Research.
REFERENCES
- Arahuetes RM , Sierra E , Codesal J , García‐Barrutia MS , Arza E , Cubero J , Ortiz A , Maganto P (2001) Optimization of the technique to isolate foetal hepatocytes and assessment of their functionality by transplantation. Life Sci, 68 (7), 763. [DOI] [PubMed] [Google Scholar]
- Berry MN , Friend DJ (1969) High yield preparation of isolated rat liver parenchymal cell: a biochemical and fine structural study. J Cell Biol, 43, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel‐Rinkes IHM , Bijma A , Kazemier G , Sinaasappel M , Valerio D , Terpstra OT (1994) Proliferative response of hepatocytes transplanted into spleen or solid support. J Surg Res, 56, 417. [DOI] [PubMed] [Google Scholar]
- Chowdhury JR , Kondapalli R , Chowdhury NR (1993) Gunn rat: a model for inherited deficiency of bilirubin glucuronidation. Adv Vet Sci Comp Med, 37, 149. [PubMed] [Google Scholar]
- Cubero FJ , Arza E , Maganto P , García‐Barrutia MS , Mula N , Ortiz A (2001) Expression of bilirubin‐UDP glucuronosyltransferase (bUGT) throughout foetal development: Intrasplenic transplantation into Gunn rats to correct enzymatic deficiency. Dig Dis Sci, 46 (12), 2762. [DOI] [PubMed] [Google Scholar]
- Forbes SJ , Themis M , Alison MR , Selden C , Coutelle C , Hodgson HJ (1998) Retroviral gene transfer to the liver in vivo during tri‐iodothyronine induced hyperplasia. Gene Ther, 5, 552. [DOI] [PubMed] [Google Scholar]
- Francavilla A , Carr BI , Azzarone A , Polimeno L , Wang Z , Van Thiel DH , Subbotin V (1994) Hepatocyte proliferation and gene expression induced by tri‐iodothyronine in vivo and in vitro . Hepatology, 20, 1237. [PubMed] [Google Scholar]
- Guo D , Fu T , Nelson JA , Superina RA , Soriano HE (2002) Repopulation after cell transplantation in mice treated with retrorsine and carbon tetrachloride. Transplant, 73 (11), 1818. [DOI] [PubMed] [Google Scholar]
- Gupta S , Chowdhury RJ (1992) Hepatocyte transplantation: back to the future. Hepatology, 15, 156. [DOI] [PubMed] [Google Scholar]
- Gupta S , Gorla GR , Irani AN (1999) Hepatocyte transplantation: emerging insights into mechanisms of liver repopulation and their relevance to potential therapies. J Hepatol, 30 (1), 162. [DOI] [PubMed] [Google Scholar]
- Iyanagi T , Emi Y , Ikushiro S (1998) Biochemical and molecular aspects of genetic disorders of bilirubin metabolism. Biochim Biophys Acta, 1407, 173. [DOI] [PubMed] [Google Scholar]
- Kokudo N , Otsu I , Okazaki T , Takahashi S , Sanjo K , Adachi Y (1995a) Long‐term effects of intrasplenically transplanted hepatocytes and foetal liver in hyperbilirubinemic Gunn rats. Transpl Int, 8, 262. [DOI] [PubMed] [Google Scholar]
- Kokudo N , Ohashi K , Takahashi S , Bandai Y , Sanjo K , Idezuki Y , Nozawa M (1995b) Proliferative activity of rat hepatocytes transplanted into the spleen. Cell Transplant, 4 (1), S37. [DOI] [PubMed] [Google Scholar]
- Laconi S , Curreli F , Diana S (1999) Liver regeneration in response to partial hepatectomy in rats treated with retrorsine: a kinetic study. J Hepatology, 31 (6), 1069. [DOI] [PubMed] [Google Scholar]
- Laconi E (2002) Principles of hepatocyte repopulation. Cell Dev Biol, 13, 433. [DOI] [PubMed] [Google Scholar]
- Laconi E , Oren R , Mukhopadhyay DK , Hurston E , Laconi S , Pani P , Daveba MD , Shafritz DA (1998) Long‐term, near‐total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am. J. Pathol 153, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi S , Pillai S , Porcu PP , Shafritz DA , Pani P , Laconi E (2001) Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am J Pathol, 158 (2), 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganto P (1997) Hepatocyte Transplantation into Spleen. In: Hepatocyte Transplantation 34.
- Malhi H , Gupta S (2001) Hepatocyte transplantation: new horizons and challenges. J Hepatobiliary Pancreat Surg, 8, 40. [DOI] [PubMed] [Google Scholar]
- Oren R , Dabeva MD , Karnezis AN , Petkov PM , Roseneramtz. R , Sandhu JP (1999) Role of thyroid hormone in stimulating liver repopulation in the rat by transplanted hepatocytes. Hepatology, 30 (4), 903. [DOI] [PubMed] [Google Scholar]
- Overturf K , Al‐Dhalimy M , Tanguay R , Brantly M , Ou CN , Finegold M , Grompe M (1996) Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinemia type 1. Nat Gene, 12 (3), 266. [DOI] [PubMed] [Google Scholar]
- Rhim JA , Sandgren EP , Degen JL , Palmiter RD , Brinster RL (1994) Replacement of diseased mouse liver by hepatic cell transplantation. Science 263, 1149. [DOI] [PubMed] [Google Scholar]
- Sato H , Aono S , Koiwai O (1993) Genetic defect of the hiperbilirubinemic Gunn rat, a model for Crigler–Najjar syndrome type I. Nippon Rinsho, 51 (2), 501. [PubMed] [Google Scholar]
- Seglen PO (1976) Preparation of isolated rat liver cells. Cell Biol. 18, 29. [DOI] [PubMed] [Google Scholar]
- Wilson JM (1996) Round two for liver gene therapy. Nat Genet 12, 232. [DOI] [PubMed] [Google Scholar]
- Yazigi NA , Carnick TL , Bucuvalas JC , Schmidt CS , Balistreri WF , Bezerra JA (1997) Expansion of transplanted hepatocytes during liver regeneration. Transplant, 64 (6), 816. [DOI] [PubMed] [Google Scholar]


