Abstract
Abstract. Objectives: To characterize mesenchymal stem cell‐like cells isolated from human amniotic fluid for a new source of therapeutic cells. Materials: Fibroblastoid‐type cells obtained from amniotic fluid at the time of birth. Methods: The ability of ex vivo expansion was investigated until senescence, and stem cell‐like characteristics were analyzed by examining differentiation potential, messenger RNA expression and immunophenotypes. Results and Conclusions: A morphologically homogenous population of fibroblastoid‐type (HAFFTs) cells, similar to mesenchymal stem cells from bone marrow (BM‐MSCs), was obtained at the third passage. The cells became senescent after 27 passages over a period of 8 months while undergoing 66 population doublings. Under appropriate culture conditions, by the 8th passage they differentiated into adipocytes, osteocytes, chondrocytes and neuronal cells, as revealed by oil red O, von Kossa, Alcian blue and anti‐NeuN antibody staining, respectively. Immunophenotype analyses at the 17th passage demonstrated the presence of TRA‐1–60; SSEA‐3 and‐4; collagen types I, II, III, IV and XII; fibronectin; α‐SMA; vimentin; desmin; CK18; CD44; CD54; CD106; FSP; vWF; CD31; and HLA ABC. Reverse transcriptase–polymerase chain reaction analysis of the HAFFTs from passages 6–20 showed consistent expression of Rex‐1, SCF, GATA‐4, vimentin, CK18, FGF‐5 and HLA ABC genes. Oct‐4 gene expression was observed up to the 19th passage but not at the 20th passage. HAFFTs showed telomerase activity at the 5th passage with a decreased level by the 21st passage. Interestingly, BMP‐4, AFP, nestin and HNF‐4α genes showed differential gene expression during ex vivo expansion. Taken together, these observations suggest that HAFFTs are pluripotent stem cells that are less differentiated than BM‐MSCs, and that their gene expression profiles vary with passage number during ex vivo expansion.
INTRODUCTION
Stem cells have an extensive capacity to self‐renew and can maintain their population numbers throughout the life of an organism. Under the influence of specific biological signals, they can differentiate into specialized cells that are phenotypically distinct from their precursors. Stem cells are generally classified into two groups: embryonic stem cells (ESCs) if isolated from the inner cell mass of a blastocyst, and adult stem cells if isolated from other tissues.
In the last decade, success in the isolation and culture of human ESCs has created new opportunities with respect to exploring the biological control of these cells and evaluating their potential for use in cell‐based therapies for human disease (Thomson et al. 1998). However, the use of ESCs for research or therapeutic purposes has been constrained by complex social and ethical considerations. In addition, maintaining ESCs in vitro presents significant technical challenges. ESCs require either feeder cells or expensive cytokines to support their growth (Thomson et al. 1998), and they frequently undergo genomic alterations and/or chromosomal aberrations during maintenance in vitro (Hanson & Caisander 2005; Maitra et al. 2005). Most importantly, elimination of undifferentiated cells that could develop malignancy after transplantation of them into the human body, is not yet possible (Fujikawa et al. 2005).
The use of adult stem cells for research or therapy is much less controversial. These cells have the capacity to differentiate into several different cell types, although their differentiation potential is limited compared to that of ESCs. Thus, adult stem cells are regarded as an exciting source for new cell therapies. In particular, human bone marrow‐derived mesenchymal stem cells (BM‐MSCs) are multipotent cells capable of differentiating into diverse lineages, including osteocytes, chondrocytes, adipocytes and cardiomyocytes (Prockop 1997). Morphologically, they are spindle‐shaped and resemble fibroblasts. BM‐MSCs were initially identified in adult bone marrow, but cells resembling BM‐MSCs have also been found in many other tissues, including adult and foetal peripheral blood, foetal liver, foetal spleen, placenta, umbilical cord, umbilical cord blood, amniotic membrane and synovial fluid (Okita et al. 1983; Campagnoli et al. 2001; Hu et al. 2003; Romanov et al. 2003; Bilic et al. 2004).
Bone marrow‐derived mesenchymal stem cells have been widely used in clinical applications. For example, they have been transplanted into children with osteogenesis imperfecta. Representative specimens of trabecular bone taken 3 months after osteoblast engraftment revealed formation of new dense bone, and all patients had increases in total body bone mineral content (Horwitz et al. 1999). Some metabolic diseases, such as Hurler syndrome and metachromatic leukodystrophy, have been corrected by infusion of allogenic and multipotent BM‐MSCs (Koc et al. 2002). The use of adult BM‐MSCs does have some disadvantages, however. In particular, the number of MSCs in adult bone marrow is low, and harvesting them from a patient is an invasive procedure. Therefore, finding alternative sources of MSCs that are useful in clinical applications is an important research goal.
Human amniotic fluid (HAF) obtained during the process of amniocentesis contains a variety of stem cells originating from embryonic and extra‐embryonic tissues (Gosden 1983). Although these cells are routinely used for prenatal diagnosis of a wide range of foetal abnormalities caused by genetic defects, the cell type subsets present in HAF have not been thoroughly characterized. The types and properties of amniotic fluid cells vary with gestational age and if there is foetal pathology.
Based on their morphological and growth characteristics, amniotic fluid cells can be classified into three types: epithelioid, amniotic fluid‐specific and fibroblastoid (Milunsky 1979). Fibroblastoid‐type cells usually appear late during in vitro primary culture and exhibit phenotypes and multilineage differentiation potentials similar to those of BM‐MSCs (In't Anker et al. 2003). Interestingly, HAF has been shown to contain cells expressing Oct‐4 antigen, a specific marker of pluripotent stem cells (Prusa et al. 2003), and these cells display multilineage differentiation potential; depending on the specific culture conditions, they can differentiate into adipocytes, osteocytes or neuronal cells (Tsai et al. 2004). Thus, HAF is intriguing as a possible source of pluripotent stem cells for cell‐based therapeutics, and does not raise the ethical concerns associated with use of ESCs.
However, therapeutic use of HAF‐derived cells will require a much more thorough understanding of their biology. Some important goals include determining the replicative lifespan and cell production potential of the component cell types in culture, characterizing the gene expression profile of these cells, and determining whether the profile changes during ex vivo expansion. In addition, the issue of whether HAF‐derived cells can differentiate into chondrocytes, which are typical mesodermal lineage cells, has not been resolved.
In the present study, we have addressed these issues by further characterizing the in vitro growth kinetics, replicative lifespan and biological properties of HAF‐derived BM‐MSC‐like cells throughout their existence. The techniques used here have included reverse transcriptase–polymerase chain reaction (RT‐PCR), immunocytochemistry, telomerase activity assays and differentiation potential assays.
MATERIALS AND METHODS
Cell isolation and culture
Five millilitres of HAF samples were obtained from patients undergoing amniocentesis for routine prenatal diagnosis at 14–16 weeks of pregnancy. Cells were isolated from the HAF no more than 12 h prior to use in experiments. HAF samples were centrifuged at 300 g for 15 min, and the resulting pellets were washed twice with low‐glucose Dulbecco's modified Eagle's medium (DMEM) (Gibco, Grand Island, NY) to remove blood and cell debris. All of the cells isolated from the 5‐ml sample were plated in a 25‐cm2 culture flask (Nunc, Rochester, MN) containing DMEM supplemented with 100 U/ml penicillin, 0.1 mg/ml streptomycin (Gibco), 3.7 mg/ml sodium bicarbonate, 10 ng/ml epidermal growth factor (EGF) (Peprotech, Princeton, NJ) and 10% foetal bovine serum (FBS) (Gibco). Seven days after the initiation of culture, the medium was replaced with fresh ones, and was subsequently replaced twice a week.
When the cells reached confluence, they were treated with 0.125% trypsin and 1 mm ethylenediamine tetraacetic acid (EDTA) for 3 min. The released cells were collected and re‐plated for subculture. A morphologically homogeneous population of fibroblast‐like cells was obtained after two rounds of subculture. These HAF‐derived fibroblastoid‐type cells were maintained in a humidified atmosphere in an incubator under 5% CO2 at 37 °C.
Informed consent had been obtained from the subjects, and the study protocol was approved by the ethics committee of Ajou University, Korea.
Differentiation potential assay
For differentiation experiments, 8th‐passage cells were cultured in various media with replacement of the medium twice a week. Cells were examined after 6 days in neurogenic medium, after 2 weeks in adipogenic or osteogenic differentiational medium, and after 3 weeks in chondrogenic medium. The adipogenic medium was DMEM supplemented with 10% FBS, 1 µm dexamethasone, 0.5 µm 3‐isobutyl‐1‐methylxanthine, 0.05 mg/l human insulin, and 200 µm indomethacin. After culture, presence of intracellular lipid droplets indicative of adipocyte differentiation was assessed by staining cells with oil red O. The osteogenic medium was DMEM supplemented with 10% FBS, 0.1 µm dexamethasone, 100 mmβ‐glycerol phosphate and 50 µm ascorbic acid‐2‐phosphate. Mineralized calcium indicating osteogenic differentiation was assessed by von Kossa staining.
Chondrogenesis was induced by culturing the cells in chondrogenic medium consisting of high‐glucose DMEM supplemented with 0.1 µm dexamethasone, 50 µg/ml ascorbic acid‐2‐phosphate, 100 µg/ml sodium pyruvate, 40 µg/ml proline, 10 ng/ml transforming growth factor‐β1 (TGF‐β1) (R&D Systems, Minneapolis, MN) and 50 mg/ml ITS premix (insulin, transferrin and selenious acid at 6.25 µg/ml each, 1.35 mg/ml BSA and 5.35 mg/ml linoleic acid; Becton Dickinson, San Jose, CA). Chondrogenic differentiation was assessed by staining with Alcian blue.
For neurogenic differentiation, cells were initially treated overnight with 20% FBS, 20 ng/ml basic fibroblast growth factor (bFGF) (Peprotech) and 20 ng/ml EGF. Neuronal differentiation was then induced by treatment with 2% dimethyl sulfoxide, 200 µm butylated hydroxyanisole, 25 mm KCl, 2 mm valproic acid and 1 µm hydrocortisone in N2 medium (Gibco) plus 1 × N2 supplement (Gibco) for 5 days. Neuronal differentiation was assessed by immunocytochemical staining with mouse monoclonal antihuman Neu N antibody (Chemicon, Temecula, CA).
Immunocytochemistry
HAFFTs at the 17th passage were plated on a Laboratory‐Tek chamber slide (Nunc), fixed with 4% paraformaldehyde in phosphate‐buffered saline (PBS) (Gibco) at 4 °C for 2 h, and were rinsed with PBS. They were then permeabilized with 0.5% Triton X‐100 in PBS for 10 min at room temperature. After several washes with PBS, the cells were incubated in 3% hydrogen peroxidase for 15 min to quench endogenous peroxidase activity. They were then rinsed with PBS and incubated in blocking solution consisting of 2% BSA in PBS for 1 h at room temperature. They were then incubated with a mouse monoclonal or rabbit polyclonal primary antibody for 17 h at 4 °C. The monoclonal antibodies were specific for collagen types I (1 : 50), II (1 : 100), IV (1 : 100) and XII (1 : 500); fibronectin (1 : 100); CD31 (1 : 40); CD44 (1 : 50); CD54 (1 : 200); CD106 (1 : 100); von Willebrand factor (vWF; 1 : 100); CK18 (1 : 50); desmin (1 : 50); vimentin (1 : 200); α‐smooth muscle actin (α‐SMA); TRA‐1–60 (1 : 50); stage‐specific embryonic antigen 3 (SSEA)‐3 and ‐4 (both 1 : 100); fibroblast surface protein (FSP; 1 : 500); HLA ABC (1 : 25); and HLA DR (1 : 50).
After incubation with primary antibody, slides were rinsed three times with PBS and then incubated in biotinylated goat antimouse or antirabbit IgG (Dinona, Seoul, Korea) for 20 min at room temperature. The slides were rinsed with PBS and then incubated with horseradish peroxidase‐conjugated streptavidin (Dinona) for 20 min at room temperature. Immunoreactivity for each protein was visualized using 3,3′‐diaminobenzidine tetrahydrochloride (Dinona) and counterstaining was performed with Mayer's haematoxylin. Finally, the slides were photographed under a Zeiss LSM410 microscope using bright‐field illumination (Carl Zeiss, Oberkochen, Germany).
Telomerase activity assay
Telomerase activity was measured using a telomeric repeat amplification protocol (TRAP) assay kit (Chemicon) with PBS‐washed HAFFT cell pellets that were stored at −70 °C until use. A total of 1 × 106 HAFFTs were resuspended with 200 µl of CHAPS lysis buffer and were incubated on ice for 30 min. The suspension was centrifuged at 12 000 g for 20 min at 4 °C, and 160 µl of the supernatant fraction was removed for the telomerase activity assay. Ten‐microlitre portions of the extract were heat‐treated at 85 °C for 10 min to inactivate telomerase, and the heat‐treated extracts were used in control reactions.
Polymerase chain reaction amplification was performed in a 50‐µl reaction volume containing 5 µl of 10 × TRAP reaction buffer, 1 µl of 50 × dNTPs, 1 µl of TS primer, 1 µl of TRAP primer mix, 0.4 µl (2 U) Taq polymerase, 39.6 µl dH2O and 2 µl of HAFFT cell extract. Reactions were incubated at 30 °C for 30 min and then placed in a thermocycler (Perkin Elmer, Boston, MA). Amplification was performed with 30 cycles of a two‐step PCR protocol consisting of 94 °C for 30 s and 59 °C for 30 s. When PCR reactions were complete, the products were mixed with 5 µl of loading dye (0.25% bromophenol blue, 0.25% xylene cyanol, 50% glycerol, 50 mm EDTA) and separated on a 10% non‐denaturing polyacrylamide gel in 0.5 × TBE buffer (89 mm Tris base, 89 mm boric acid, 1 mm EDTA, pH 8.0) at 40 V. After electrophoresis, the gel was stained with SYBR® Gold Nucleic Acid Stain (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. DNA bands in the gel were then visualized under ultraviolet light using a Bioprofile image analysis system (Viber Lourmat, Mame la Vallee, France).
Total RNA isolation and RT‐PCR
All solutions were prepared using distilled water treated with 0.1% diethylpyrocarbonate. HAFFT cell pellets were washed with Ca2+‐ and Mg2+‐free PBS and then transferred to a chilled Eppendorf microcentrifuge tube on ice. Immediately thereafter, 500 µl of Tri‐reagent (Sigma Chemical Co., St. Louis, MO) were added to the tube, which was then stored at −20 °C until use. Total RNA was isolated from the HAFFT cell pellets according to the manufacturer's instructions. The RNA was allowed to stand at 65 °C for 5 min in a heating block, chilled on ice and then quantified spectrophotometrically. Purity of the RNA was assessed by determining the ratio of absorbance at 260 nm to that at 280 nm (> 1.8).
For each sample, 5 µg of total RNA was reverse‐transcribed in a 20‐µl reaction containing 1 × reaction buffer, 1 mm dNTP mixture, 0.5 µg/µl oligo(d)T15, 20 U RNase inhibitor (Takara, Japan) and 20 U M‐MuLV reverse transcriptase (Fermentas, Canada). Reactions were allowed to proceed for 60 min at 42 °C, and the RT products (cDNAs) were used directly in PCR reactions.
Polymerase chain reaction amplification of HAFFT cell cDNA was performed in a GeneAmp PCR system 2400 (Perkin Elmer). The 10‐µl reaction mixtures contained 2 mm MgCl2, 1 × Taq buffer, 0.25 U Taq polymerase (Takara) and 10 pm sense and antisense gene‐specific primers (Table 1). Amplification was performed for 35 cycles of denaturation at 94 °C for 30 s, annealing for 30 s and extension at 72 °C for 30 s. Annealing temperatures were dependent on the primers used and are shown in Table 1. Upon completion of the reactions, the PCR products were mixed with 6 × loading buffer consisting of 0.25% bromophenol blue, 0.25% xylene cyanol and 40% sucrose and then separated on a 2% agarose gel. The gel was stained with ethidium bromide, and DNA in the gel was imaged under ultraviolet light using a Bioprofile image analysis system (Viber Lourmat).
Table 1.
RT‐PCR primer sequences and products size
| Gene | Primer sequence (5′→3′) | Accession number | Size (bp) | Annealing temp. (°C) |
|---|---|---|---|---|
| GAPDH | aca act ttg gta tcg tgg aa aaa ttc gtt gtc ata cca gg | NM_002046 | 456 | 53 |
| Oct‐4 | cgt gaa gct gga gaa gga gaa gct g caa ggg ccg cag ctc aca cat gtt c | AF268617 | 245 | 55 |
| Rex‐1 | atg gct atg tgt gct atg agc cct caa ctt cta gtg cat cc | NM_174900 | 449 | 57 |
| SCF | cca ttg atg cct tca agg ac ctt cca gta taa ggc tcc aa | M59964 | 275 | 55 |
| GATA‐4 | ttc ctc ttc cct cct caa at tca gcg tgt aaa ggc atc tg | NM_002052 | 194 | 60 |
| Vimentin | cct tcg tga ata cca cg acct gc taa tat atc gcc tgc cac tga g | Z19554 | 321 | 56 |
| CK18 | gag atc gag gct ctc aag ga caa gct ggc ctt cag att tc | NM_00024 | 357 | 57 |
| HLA ABC | gta ttt ctt cac atc cgt gtc ccg gtc cgc cgc ggt cca aga gcg cag | L18898 | 394 | 70 |
| FGF‐5 | gct gtg tct cag ggg att gta gga ata tat cca aag cga aac ttg agt ctg ta | NM_004464 | 434 | 55 |
| Brachyury | gag ctc acc aat gag atg at ggc tca tac tta tgc aag ga | NM_002052 | 335 | 57 |
| Pax‐6 | aga ttc aga tga ggc tca aa aat tgg ttg gta gac act gg | AY707088 | 313 | 57 |
| NCAM | gag ggg gaa gat gcc gtg atg tg ata ttc tgc ctg gcc cgg atg gta g | NM_000615 | 269 | 60 |
| BMP‐2 | ttg cgg ctg ctc agc atg tt ttg cga gaa cag atg caa gat g | BC069214 | 315 | 55 |
| HLA DR | ctg atg agc gct cag gaa tca tgg gac tta ctt cag ttt gtg gtg agg gaa g | X06079 | 220 | 60 |
| BMP‐4 | agc cat gct agt ttg ata cc tca ggg atg ctg ctg agg tt | D30751 | 383 | 55 |
| AFP | gtg ctg cac ttc ttc ata tgc tga cag cct caa gtt gtt cc | NM_001134 | 218 | 54 |
| Nestin | cca gaa act caa gca cca c ttt tcc act cca gcc atc c | X65964 | 398 | 54 |
| HNF‐4α | gag cag gaa tgg gaa gaa tg ggc tgt cct ttg gga tga ag | NM_178849 | 205 | 62 |
Antibodies and reagents
Mouse monoclonal antibodies specific for the following human proteins were used: CD31, CK18 and desmin (Dako, Carpinteria, CA), TRA‐1–60 and SSEA‐4 (Chemicon), FSP (Abcam, Cambridge, UK), CD44, CD106, CD54, collagen type II, collagen type IV, vimentin, fibronectin, vWF, α‐SMA, HLA ABC and HLA DR (Novo Castra; Newcastle, UK), collagen type I (Acris Antibodies, Hiddenhausen, Germany), collagen type XII (Kamiya, Seattle, WA) and SSEA‐3 (R&D Systems). A rabbit polyclonal antibody specific for collagen type III was purchased from Abcam. All other reagents that are not specified elsewhere were obtained from Sigma Chemical Co.
RESULTS
Ex vivo expansion of HAFFTs
When the cells isolated from HAF were observed during primary culture, they were somewhat heterogeneous but consisted primarily of two types of cells: one was similar to fibroblasts and the other was flat and circular, resembling epithelial cells. Fibroblast‐like cells sometimes appeared as colonies in the culture flask during the primary culture (Fig. 1a,b). After 16 days of culture, the cells were confluent and heterogeneous (Fig. 1c,d).
Figure 1.
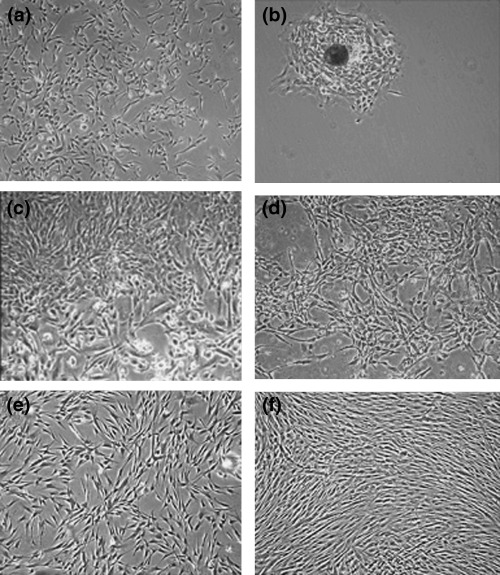
Morphological appearance of HAFFTs during culture. (a) Seven days after initiation of the primary culture, many isolated cells were visible. (b) A cell colony in the same culture flask as in (a). (c) At 16 days, a heterogeneous population of cells was present in the same culture flask as in (a). (d–f) The same culture flask as in (a) is shown at 27 days (d), 51 days (at third passage; e), and 123 days (at 10th passage; f) after initiation of the primary culture, respectively. Magnification: × 40.
At 80% confluence, cells were treated with EDTA for 2 or 5 min, but no cells were isolated. Cells were then treated with both trypsin and EDTA for 5 min, which resulted in the detachment of a group of cells from the bottom of the flasks. These separated cells were designated as HAFFTs and were used in all subsequent experiments. Other cells that remained attached after this treatment were not used as they were phenotypically heterogeneous and grew slowly, not stem cell characteristics.
At the 3rd passage, the HAFFT cells became morphologically homogeneous, and they consistently exhibited fibroblast‐like morphology until the end of the culture period (Fig. 1e,f). At the 27th passage, the HAFFTs ceased proliferating and became large and flat, as is typical for senescent cells. Expansion factor and doubling number of the HAFFTs were calculated according to the period of culture (Fig. 2a,b). Average doubling time from passages 3–26 was 3.6 days. Over the course of the 8‐month experiment, the HAFFTs were successively passaged 27 times, undergoing around 66 cell doublings. The cumulative cell number was thus estimated as 7.7 × 1023.
Figure 2.
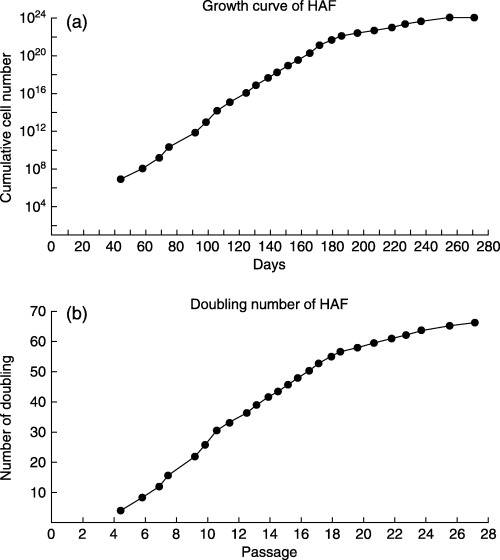
Growth curve and doubling number of HAFFTs. (a) HAFFT growth curve observed over 271 days of culture. (b) HAFFT population doubling number during the same culture period.
Differentiation potential of HAFFTs
We examined the ability of HAFFTs to differentiate into mesodermal lineage cells (adipocytes, osteocytes and chondrocytes) and into ectodermal lineage cells (neuronal cells). When HAFFTs at the 8th passage (Fig. 3a) were cultured under adipogenic conditions for 2 weeks, they exhibited intense cytoplasmic staining with oil red O, signifying the accumulation of lipid vacuoles (Fig. 3b). When cultured in osteogenic medium for 2 weeks, the HAFFTs differentiated into osteoblasts, as shown by positive von Kossa staining (Fig. 3c) and when the cells were cultured under chondrogenic conditions for 3 weeks, they exhibited positive Alcian blue staining (Fig. 3d). When cultured for 5 days in neurogenic induction medium, the cells expressed distinct immunoreactivity for the neuronal marker NeuN (Fig. 3c,d).
Figure 3.
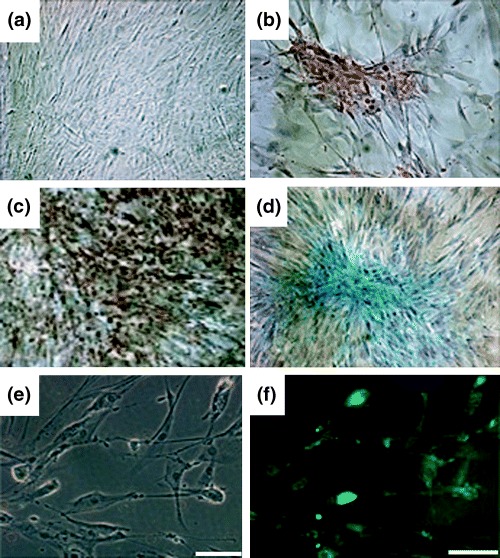
Mesodermal and neuronal differentiation of HAFFTs. HAFFTs at eighth passage were cultured in control (a), adipogenic (b), osteogenic (c) or chondrogenic (d) medium for 2–3 weeks. The differentiation capabilities of each group of HAFFTs were determined using tissue‐specific staining and haematoxylin counterstaining. Magnification: × 100. (e) HAFFTs after culture in neurogenic medium. (f) Fluorescence image of cells shown in (e) after immunostaining with antibody against the neurone marker NeuN. Note the distinct staining of HAFFTs. Bar = 100 µm.
Immunocytochemical demonstration of HAFFTs expression profile
Immunocytochemical staining demonstrated that HAFFT cells at the 17th passage expressed collagen types I, II, III, IV and XII; fibronectin; CD44 (homing cell adhesion molecule, HCAM), CD54 (intercellular cell adhesion molecule‐1, ICAM‐1), CD31 (platelet/endothelial adhesion molecule‐1, PECAM‐1), CD106 (vascular cell adhesion molecule‐1, VCAM‐1), α‐SMA (alpha‐smooth muscle actin), CK18, desmin, vimentin, vWF, TRA‐1–60, SSEA‐3 and ‐4, FSP and HLA ABC proteins. Staining was particularly strong with the anti‐CD44, ‐vimentin, ‐FSP and ‐HLA ABC antibodies. Staining for collagen types I, II and III, fibronectin, α‐SMA, TRA‐1–60 and SSEA‐4 was moderate, and no staining was observed with anti‐HLA DR antibody. The immunocytochemical staining results are shown in Fig. 4.
Figure 4.
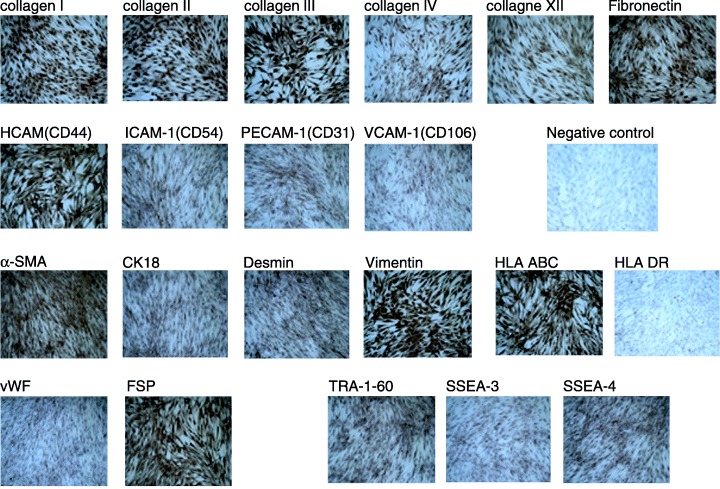
Immunocytochemical analysis of HAFFTs. HAFFTs at 17th passage exhibited distinct immunoreactivity with antibodies against collagen types I, II, III, IV and XII; fibronectin, HCAM (CD44); ICAM‐1 (CD54); VCAM‐1 (CD106); CK18; α‐SMA; vimentin; FSP; desmin; TRA‐1–60; SSEA‐3 and ‐4; vWF; PECAM‐1 (CD31); and HLA ABC. Note the absence of staining for the anti‐HLA DR antibody and the negative control. Nuclei were counterstained with Mayers haematoxylin. Magnification: × 40.
Telomerase activity of HAFFTs
Telomerase is a ribonucleoprotein that synthesizes telomeric repeats and directs them onto the 3′ end of existing telomeres. Expression of telomerase activity is limited to immortal cells, such as malignant cells and germ cells. When the telomerase activity of HAFFTs was examined at passages 5 and 21, cells of both passages yielded distinct TRAP‐ladder bands (Fig. 5, lanes 1 and 3), and disappearance of the uppermost band was observed only for HAFFTS at the 21st passage. When HAFFT cell extracts were heated before the TRAP assay reaction, the telomerase activity disappeared, confirming that the ladder bands indeed appeared as a result of the activity of heat‐sensitive telomerase (Fig. 5, lanes 2 and 4).
Figure 5.
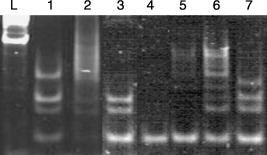
Telomerase activity in HAFFT extracts at the 5th and 21st passages. L, DNA ladder; lane 1, HAFFT extract at 5th passage; lane 2, heat‐inactivated HAFFT extract (control for lane 1); lane 3, HAFFT extract at 21st passage; lane 4, heat‐inactivated HAFFT extract (control for lane 3); lane 5, negative control; lane 6, positive control; lane 7, TSR8 (control template). Note the disappearance of the uppermost band in lane 3 compared to lane 1.
Gene expression profiles of HAFFTs
Results of RT‐PCR analysis showed that HAFFT cells at passages 6, 9, 16, 19 and 20 consistently expressed the Rex‐1, SCF, GATA‐4, vimentin, CK18, HLA ABC and FGF‐5 genes. In contrast, expression of genes coding for Brachyury, Pax‐6, NCAM, BMP‐2 and HLA DR was not observed throughout the culture period (Table 2). The Oct‐4 gene, coding for the transcription factor unique to pluripotent stem cells, was expressed until the 19th passage and was not expressed at the 20th passage. Interestingly, the expression patterns of some of the genes varied with passage number (Fig. 6). BMP‐4, AFP and nestin genes were not expressed at the 6th passage but were expressed at the 16th and 20th passages, whereas the HNF‐4α gene was not expressed at the 16th passage but was expressed at the 20th.
Table 2.
RT‐PCR analysis of gene expression by HAFFTs
| Gene | Passage number | ||||
|---|---|---|---|---|---|
| 6 | 9 | 16 | 19 | 20 | |
| Oct‐4 | + | + | + | + | – |
| Rex‐1 | + | + | + | + | + |
| SCF | + | + | + | + | + |
| GATA‐4 | + | + | + | + | + |
| Vimentin | + | + | + | + | + |
| CK18 | + | + | + | + | + |
| HLA ABC | + | + | + | + | + |
| FGF‐5 | + | + | + | + | + |
| Brachyury | – | – | – | – | – |
| Pax‐6 | – | – | – | – | – |
| NCAM | – | – | – | – | – |
| BMP‐2 | – | – | – | – | – |
| HLA DR | – | + | – | – | – |
+, expressed; –, not expressed.
Figure 6.
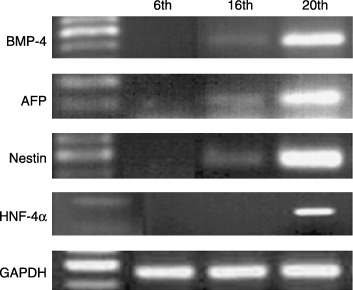
Expression profile of selected HAFFT genes. HAFFTs were collected at 6th, 16th and 20th passages, and their expression levels of BMP‐4, AFP, nestin and HNF‐4α mRNAs were compared to the level of GAPDH as a control using RT‐PCR. Right column represents a part of DNA ladder.
DISCUSSION
Previous studies have shown that second trimester HAF contains cells expressing the gene for Oct‐4, an embryonic stem cell marker (Tsai et al. 2004), and that HAF is a source of foetal MSCs that are phenotypically similar to BM‐MSCs (In't Anker et al. 2003). Oct‐4‐expressing MSCs of HAF were subsequently shown to differentiate into adipocytes, osteocytes and neuronal cells (Prusa et al. 2004; Tsai et al. 2006). In the present study, we have demonstrated that HAFFTs possess immunophenotypes and gene expression profiles that are largely characteristic of undifferentiated cells. Hence, HAFFTs may have therapeutic potential that is even greater than that of BM‐MSCs. In addition, we have shown that HAFFT cells exhibit gene expression profiles that change with passage number during ex vivo expansion.
HAFFTs share many antigenic properties and gene expression profiles with BM‐MSCs. Of the immunophenotypes expressed by HAFFTs, HCAM‐1, ICAM‐1, VCAM‐1, vWF and α‐SMA (Conget & Minguell 1999); HLA‐ABC (Majumdar et al. 2003); collagen types I, III and IV and fibronectin (Chichester et al. 1993); and vimentin (Kadner et al. 2002) are also expressed by BM‐MSCs. The RT‐PCR analyses performed in the present study showed that the genes for stem cell factor (SCF) (Majumdar et al. 2000), CK18 (Lee et al. 2004), nestin (Vogel et al. 2003) and HLA‐ABC are expressed in HAFFTs, as they are in BM‐MSCs. Our immunocytochemical and RT‐PCR analyses showed no expression of HLA‐DR in HAFFTs in common with BM‐MSCs (Majumdar et al. 2003). Indeed, we have shown that HAFFTs, like BM‐MSCs, exhibited the potential to differentiate into a variety of cell types, including neuronal cells and three typical mesodermal‐lineage cells (adipocytes, chondrocytes and osteocytes).
Despite these similarities, many genes that are expressed in HAFFTs have not been shown to be expressed in BM‐MSCs. Our immunophenotype analysis showed that HAFFTs express antigens of desmin, collagen type II, TRA‐1–60, SSEA‐3 and SSEA‐4 that are not expressed in BM‐MSCs (Ogueta et al. 2002; Xu et al. 2004). Furthermore, our RT‐PCR analyses showed that HAFFTs also express genes for Rex‐1, GATA‐4 and FGF‐5 throughout the culture period, and they express genes for BMP‐4, nestin, AFP and HNF‐4α at later passages; expression of these genes has not been reported in BM‐MSCs (Lee et al. 2004).
The TRA‐1–60, SSEA‐3 and SSEA‐4 antigens are typical markers of embryonal carcinoma cells and ESCs (Andrews et al. 1987; Xu et al. 2001). Oct‐4 and Rex‐1 expression is also a marker of these cells (Peter & Donovan 2001). Our finding that these markers of undifferentiated cells are also expressed in HAFFTs leads us to conclude that HAFFTs may be less differentiated than most BM‐MSCs, and may more closely resemble pluripotent ESCs. This hypothesis is supported by the superior replicative lifespan of HAFFTs. In our experiment, they exhibited an average doubling time of 3.6 days from passages 3–26. Over the 8 months before the onset of senescence, the HAFFTs doubled their numbers 66 times, with a cumulative yield of approximately 7.7 × 1023 cells. Typical BM‐MSCs usually achieve 40–50 doublings during their lifespans (Stenderup et al. 2003), and reach senescence after 197.4 days and 10 passages, when they are obtained from subjects 0–18 years old (Baxter et al. 2004). HAFFTs could therefore generate more cells during ex vivo expansion than could BM‐MSCs, which is an advantage in cell‐based therapeutics.
D’Ippolito et al. (2004) have reported that BM‐MSCs contain a unique population of cells that, like HAFFTs, express Oct‐4, Rex‐1 and telomerase. The population doubling time for these BM‐MSCs was 36–72 h, and they have been expanded in culture for more than 50 doublings. They have been differentiated into bone‐forming osteoblasts, cartilage‐forming chondrocytes, fat‐forming adipocytes and neural cells. An intriguing possibility is that these BM‐MSCs, termed human marrow‐isolated adult multilineage‐inducible cells, are the same type of cells as HAFFTs.
In adult organisms, AFP is normally produced by the foetal gut, yolk sac and liver, and is known as a hepatocyte marker (Engelhardt et al. 1984). HNF‐4α is well known for its role in liver development (Parviz et al. 2003), and its expression has been observed in other tissues as well, including the kidney, intestine and endocrine cells of the pancreas (Miquerol et al. 1994). CK18 is one of the endodermal lineage‐specific genes and a marker of hepatic differentiation (Wells et al. 1997). Therefore, together with the consistent expression of CK18, the onset of expression of the AFP and HNF‐4α genes at later passages suggests that as HAFFTs age in vitro, they seem to differentiate easily into hepatocyte‐like cells. BM‐MSCs also exhibit spontaneous changes in their gene expression pattern during ex vivo expansion, but their progressive ageing leads to a commitment to osteogenic differentiation (Banfi et al. 2002).
HAFFTs expressed the Oct‐4 gene throughout the culture period until the 19th passage, ceasing to express it at the 20th passage. Because HAFFTs reached senescence at the 27th passage, the disappearance of Oct‐4 expression at the 20th passage may be related to the onset of ageing at this time, resulting in senescence by the 27th passage. Furthermore, that telomere shortening plays an important part in the molecular ageing process has been well established (Wright & Shay 2002). When we compared telomerase activity of HAFFTs at the 5th and 21st passages, we found that its activity significantly decreased by the 21st passage. These results coincide with a previous report that HAF‐derived cells exhibit a decrease in both telomerase activity and telomere length during ageing in vitro (Mosquera et al. 1999). Similarly, Oct‐4 and telomerase expression in ESCs decreases during differentiation in vitro (Lebkowski et al. 2001). In contrast, most BM‐MSCs do not exhibit telomerase activity at all, and their telomere length decreases during in vitro expansion (Banfi et al. 2002).
Of the proteins expressed by HAFFTs, desmin is well known to play an important role in cardiac and skeletal muscle function (Goldfarb et al. 1998), and GATA‐4 is a cardiac‐specific member of the GATA family of zinc‐finger transcription factors (Grepin et al. 1997). FGF‐5 regulates neurone differentiation and survival (Lindholm et al. 1994), and is also involved in cardiac function (Suzuki et al. 2005). Nestin, a specific marker for neural stem cells (Lendahl et al. 1990), is also found in other differentiating cells, including muscle and myocardium (Sjoberg et al. 1994). Knockout of BMP‐4 by homologous recombination results in embryonic lethality, and BMP‐4 appears to play a role in chondrogenesis and articular cartilage repair as well as in bone formation (Kuroda et al. 2006), and collagen type II is a marker of cartilaginous tissue (Aigner et al. 1993). Thus, the spontaneous expression of all of these genes and their proteins suggests that HAFFTs have the potential to readily differentiate into cardiomyocytes as well as other mesodermal derivatives, in addition to neural cells. In BM‐MSCs, only prolonged treatment with hepatocyte growth factor induces the expression of the cardiac‐specific markers GATA‐4, MEF2C, TEF1, desmin, α‐MHC, β‐MHC and nestin (Forte et al. 2006).
Platelet/endothelial adhesion molecule‐1 is a member of the immunoglobulin superfamily that is expressed by leucocytes, platelets and endothelial cells, and it primarily participates in homophilic binding between adjacent cells (Newton et al. 1997). VCAM‐1 regulates leucocyte migration from the blood circulation into tissues; its expression is induced on endothelial cells during inflammatory bowel disease, atherosclerosis, allograft rejection, infection and asthmatic responses (Matheny et al. 2000). The endothelial cell marker vWF is a multimeric adhesive protein that has an important function in primary haemostasis and, as a carrier of factor VIII, it plays a pivotal role in thrombogenesis (Nieswandt & Watson 2003). The presence of these antigens as well as ICAM‐1 leads to the intriguing speculation that HAFFTs share their origin with haematopoietic stem cells. However, the discrepancies between the antigenic profiles of HAFFTs and the previously described HAF‐derived cells (In't Anker et al. 2003; Fauza 2004) might be explained by different origins for the two cell types. Because amniotic fluid contains a heterogeneous population of cells originating from embryonic and/or extra‐embryonic tissues (Gosden 1983), this explanation seems plausible.
The results of the experiments described in this report demonstrate that HAFFTs can differentiate into adipocytes, osteocytes, chondrocytes and neuronal cells, can express many pluripotent stem cell‐specific genes, exhibit telomerase activity and proliferate well during ex vivo expansion. In particular, we have found that HAFFT gene expression profiles change with the cells ages in vitro. Based on these unique properties, we conclude that HAFFTs are pluripotent stem cells that are probably less differentiated than BM‐MSCs, and that thus, they have considerable potential for use in cell‐based therapeutics.
ACKNOWLEDGEMENTS
This work was supported by the grant from the 2005 Research Program of the HurimBioCell Inc Seoul, Korea.
REFERENCES
- Aigner T, Bertling W, Stoss H, Weseloh G, Von Der Mark K (1993) Independent expression of fibril‐forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J. Clin. Invest. 91, 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW, Fenderson B, Hakomori S (1987) Human embryonic carcinoma cells and their differentiation in culture. Int. J. Androl. 10, 95–104. [DOI] [PubMed] [Google Scholar]
- Banfi A, Bianchi G, Notaro R, Luzzatto L, Cancedda R, Quarto R (2002) Replicative aging and gene expression in long‐term cultures of human bone marrow stromal cells. Tissue Eng. 8, 901–910. [DOI] [PubMed] [Google Scholar]
- Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I (2004) Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 22, 675–682. [DOI] [PubMed] [Google Scholar]
- Bilic G, Ochsenbein‐Kolble N, Hall H, Huch R, Zimmermann R (2004) In vitro lesion repair by human amnion epithelial and mesenchymal cells. Am. J. Obstet. Gynecol. 190, 87–92. [DOI] [PubMed] [Google Scholar]
- Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM (2001) Identification of mesenchymal stem/progenitor cells in human first‐trimester fetal blood, liver, and bone marrow. Blood 98, 2396–2402. [DOI] [PubMed] [Google Scholar]
- Chichester CO, Fernandez M, Minguell JJ (1993) Extracellular matrix gene expression by human bone marrow stroma and by marrow fibroblasts. Cell Adhes. Commun. 1, 93–99. [DOI] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ (1999) Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J. Cell Physiol. 181, 67–73. [DOI] [PubMed] [Google Scholar]
- D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC (2004) Marrow‐isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J. Cell Sci. 15, 2971–2981. [DOI] [PubMed] [Google Scholar]
- Donovan PJ (2001) High‐octane fuel powers the stem cell. Nat. Genet. 29, 246–247. [DOI] [PubMed] [Google Scholar]
- Engelhardt NV, Baranov VN, Lazareva MN, Goussev AI (1984) Ultrastructural localization of alpha‐feto (AFP) in regenerating mouse liver poisoned with CCL‐4.1. Reexpression of AFP in differentiated hepatocytes. Histochemistry 80, 401–409. [DOI] [PubMed] [Google Scholar]
- Fauza D (2004) Amniotic fluid and placental stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 18, 877–891. [DOI] [PubMed] [Google Scholar]
- Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, Devito P, Baldini PM, Prat M, Di Nardo P (2006) Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells 24, 23–33. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE (2005) Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell derived insulin‐producing cells. Am. J. Pathol. 166, 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb LG, Park KY, Cervenakova L, Gorokhova S, Lee HS, Vasconcelos O, Nagle JW, Semino‐Mora C, Sivakumar K, Dalakas MC (1998) Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat. Genet. 19, 402–403. [DOI] [PubMed] [Google Scholar]
- Gosden CM (1983) Amniotic fluid cell types and culture. Br. Med. Bull. 39, 348–354. [DOI] [PubMed] [Google Scholar]
- Grepin C, Nemer G, Nemer M (1997) Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA‐4 transcription factor. Development 124, 2387–2395. [DOI] [PubMed] [Google Scholar]
- Hanson C, Caisander G (2005) Human embryonic stem cells and chromosome stability. APMIS. 113, 751–755. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpartrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK (1999) Transplantability and therapeutic effects of bone marrow‐derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 5, 309–313. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liao L, Wang Q, Ma L, Ma G, Jiang X, Zhao RC (2003) Isolation and identification of mesenchymal stem cells from human fetal pancreas. J. Lab. Clin. Med. 141, 342–349. [DOI] [PubMed] [Google Scholar]
- In't Anker PS, Scherjon SA, Kleijburg‐van der Keur C, Noort WA, Class FH, Willemze R, Fibbe WE, Kanhai HH (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102, 1548–1549. [DOI] [PubMed] [Google Scholar]
- Kadner A, Hoerstrup SP, Zund G, Eid K, Maurus C, Melnitchouk S, Grunenfelder J, Turina MI (2002) A new source for cardiovascular tissue engineering: human bone marrow stromal cells. Eur. J. Cardiothorac. Surg. 21, 1055–1060. [DOI] [PubMed] [Google Scholar]
- Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W (2002) Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS‐IH). Bone Marrow Transplant. 30, 215–222. [DOI] [PubMed] [Google Scholar]
- Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, Cummins J, Fu FH, Huard J (2006) Cartilage repair using bone morphogenetic protein 4 and muscle‐derived stem cells. Arthritis Rheum. 54, 433–442. [DOI] [PubMed] [Google Scholar]
- Lebkowski JS, Gold J, Xu C, Funk W, Chiu CP, Carpenter MK (2001) Human embryonic stem cells: culture, differentiation, and genetic modification for regenerative medicine applications. Cancer J. 2, 583–593. [PubMed] [Google Scholar]
- Lee KD, Kuo TK, Whang‐Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK (2004) In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 40, 1275–1284. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60, 585–595. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Harikka J, Da Penha Berzaghi M, Castren E, Tzimagiorgis G, Hughes RA, Thoenen H (1994) Fibroblast growth factor‐5 promotes differentiation of cultured rat septal cholinergic and raphe serotonergic neurons: comparison with the effects of neurotrophins. Eur. J. Neurosci. 1, 244–252. [DOI] [PubMed] [Google Scholar]
- Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, Sui G, Cutler DJ, Liu Y, Brimble SN, Noaksson K, Hyllner J, Schulz TC, Zeng X, Freed WJ, Crook J, Abraham S, Colman A, Sartipy P, Matsui S, Carpenter M, Gazdar AF, Rao M, Chakravarti A (2005) Genomic alterations in cultured human embryonic stem cells. Nat. Genet. 37, 1099–1102. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL (2000) Human marrow‐derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long‐term hematopoiesis when differentiated toward stromal and osteogenic lineages. J. Hematother. Stem Cell Res. 9, 841–848. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Keane‐Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD (2003) Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J. Biomed. Sci. 10, 228–241. [DOI] [PubMed] [Google Scholar]
- Matheny HE, Deem TL, Cook‐Mills JM (2000) Lymphocyte migration through monolayers of endothelial cell lines involves VCAM‐1 signaling via endothelial cell NADPH oxidase. J. Immunol. 164, 6550–6559. [DOI] [PubMed] [Google Scholar]
- Milunsky A (1979) Amniotic fluid cell culture Genetic Disorder of the Fetus. New York: Plenum Press, 75. [Google Scholar]
- Miquerol L, Lopez S, Cartier N, Tulliez M, Raymondjean M, Kahn A (1994) Expression of the 1‐type pyruvate kinase gene and the hepatocyte nuclear factor 4 transcription factor in exocrine and endocrine pancreas. J. Biol. Chem. 269, 8944–8951. [PubMed] [Google Scholar]
- Mosquera A, Fernandez JL, Campos A, Goyanes VJ, Ramiro‐Diaz J, Gosalvez J (1999) Simultaneous decrease of telomere length and telomerase activity with ageing of human amniotic fluid cells. J. Med. Genet. 36, 494–496. [PMC free article] [PubMed] [Google Scholar]
- Newton JP, Buckley CD, Jones EY, Simmons DL (1997) Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites of PECAM‐1/CD31. J. Biol. Chem. 15, 20555–20563. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Watson SP (2003) Platelet–collagen interaction: is GPVI the central receptor? Blood 102, 449–461. [DOI] [PubMed] [Google Scholar]
- Ogueta S, Munoz J, Obregon E, Delgado‐Baeza E, Garcia‐Ruiz JP (2002) Prolactin is a component of the human synovial liquid and modulates the growth and chondrogenic differentiation of bone marrow‐derived mesenchymal stem cells. Mol. Cell. Endocrinol. 25, 51–63. [DOI] [PubMed] [Google Scholar]
- Okita JR, Sagawa N, Casey ML, Snyder JM (1983) Comparison of human tissue and amnion cells in primary culture by morphological and biochemical criteria. In Vitro 19, 117–126. [DOI] [PubMed] [Google Scholar]
- Parviz. F, Matullo C, Garrison WD, Savatski L, Adamson JW, Ning G, Kaestner KH, Rossi JM, Zaret KS, Duncan SA (2003) Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat. Genet. 34, 292–296. [DOI] [PubMed] [Google Scholar]
- Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschlager M (2003) Oct‐4 expressing cells in human amniotic fluid: a new source for stem cell research? Hum. Reprod. 18, 1489–1493. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, Bernaschek G, Hengstschlager M (2004) Neurogenic cells in human amniotic fluid. Am. J. Obstet. Gynecol. 191, 309–314. [DOI] [PubMed] [Google Scholar]
- Romanov YA, Svintsitskaya VA, Smirnov VN (2003) Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC‐like cells from umbilical cord. Stem Cells 21, 105–110. [DOI] [PubMed] [Google Scholar]
- Sjoberg G, Jiang WQ, Ringertz NR, Lendahl U, Sejersen T (1994) Colocalization of nestin and vimentin/desmin in skeletal muscle cells demonstrated by three‐dimensional fluorescence digital imaging microscopy. Exp. Cell Res. 214, 447–458. [DOI] [PubMed] [Google Scholar]
- Stenderup K, Justesen J, Clusen C, Kassem M (2003) Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33, 919–926. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Lee TC, Fallavollita JA, Canty JM Jr (2005) Adenoviral gene transfer of FGF‐5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ. Res. 96, 705–707. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Lee JL, Chang YJ, Hwang SM (2004) Isolation of human multipotent mesenchymal stem cells from second‐trimester amniotic fluid using a novel two‐stage culture protocol. Hum. Reprod. 19, 1450–1457. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Hwang SM, Tsai YL, Cheng FC, Lee JL, Chang YJ (2006) Clonal amniotic fluid‐derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol. Reprod. 74, 545–551. [DOI] [PubMed] [Google Scholar]
- Vogel W, Grunebach F, Messam CA, Kanz L, Brugger W, Buhring HJ (2003) Heterogeneity among human bone marrow‐derived mesenchymal stem cells and neural progenitor cells. Haematologica 88, 126–133. [PubMed] [Google Scholar]
- Wells MJ, Hatton MW, Hewlett B, Podor TJ, Sheffield WP, Blajchman MA (1997) Cytokeratin 18 is expressed on the hepatocyte plasma membrane surface and interacts with thrombin‐antithrombin complexes. J. Biol. Chem. 272, 28574–28581. [DOI] [PubMed] [Google Scholar]
- Wright WE, Shay JW (2002) Historical claims and current interpretations of replicative aging. Nat. Biotechnol. 20, 682. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK (2001) Feeder‐free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 19, 971–974. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y (2004) Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp. Biol. Med. 229, 623–631. [DOI] [PubMed] [Google Scholar]


