Abstract
Objectives
Human amniotic membrane (HAM) has been widely used in soft tissue engineering both in its fresh form and decellularized; its efficiency to aid treatment of burn injuries is well known. On the other hand, it has been reported clinically by several studies that human adipose‐derived stem cells (hADSC) are a promising cell source for cell therapy for burns. Recently, we have reported a new technique for decellularization of HAM. In this study, potential of prepared decellularized HAM (dHAM) as a viable support for proliferation and delivery of hADSC was investigated.
Materials and methods
Amniotic membranes were collected, decellularized and preserved according to the protocol described in our previously published study. hADSC were obtained from the patients undergoing elective liposuction surgery and cells were then seeded on the decellularized membrane for various times. Efficiency of the decellularized membrane as a delivery system for hADSC was investigated by MTT, LDH specific activity, DAPI staining and SEM.
Results
The results showed that dHAM provided a supporting microenvironment for cell growth without producing any cytotoxic effects. In addition, the cells were spread out and actively attached to the dHAM scaffold.
Conclusion
These results strongly suggest that dHAMs have considerable potential as 3D cell‐carrier scaffolds for delivery of hADSC, in tissue engineering and regenerative medicine applications.
Introduction
Human amniotic membrane (HAM), is a thin tissue that surrounds the developing foetus, and consists of three main components, the epithelial layer, basement layer and connective tissue layer 1, 2. It has favourable characteristics including: inexpensiveness, ease of availability, antibacterial activity, presence of collagen types I, III and IV, laminin, fibronectin and a variety of growth factors. These features make HAM an excellent substance for tissue engineering applications, especially for soft tissues 3, 4, 5. On the other hand, full removal of cells or cell fragments from this tissue profoundly affects its biological and mechanical properties 4, 6. Both fresh and decellularized HAMs (dHAM) have been widely used clinically in regenerative medicine, especially in ophthalmology 7, 8, 9, due to immune privileged properties of the cornea. More recently it has been used as a scaffold for treatment of partial‐thickness burn wound dressings 10. Interestingly, high antibacterial activity of this tissue remarkably prevents nosocomial infections, as one of the major causes of mortality in burn patients 3, 11.
Recently, our research team has developed a novel and simple protocol for decellularization of HAM and has fully characterized biomechanical and biological characteristics of the tissue after denudation 4. In the current study, we aimed to investigate whether decellularized HAM would support proliferation and attachment of human adipose tissue‐derived stem cells, and also serve as a viable delivery system.
Clinically, human adipose‐derived stromal cells (hADSC) are often the choice of cell source for transplantation, in cell‐based therapy, such as burn wound healing 12, 13, 14, 15, 16, 17. In addition, several studies have demonstrated that growth factors secreted by such cells are involved in angiogenesis and injury healing 18, 19, 20, 21, 22, yet a 3D scaffold for expansion and delivery of such cells is still considered to be a largely unmet clinical need. With this concept, dHAM could also serve as a cell‐carrier 3D scaffold for delivery of hADSC, essential in regenerative medicine. The aim of this study was to investigate the potential of dHAM as a viable delivery system for hADSC.
Materials and methods
Preparation and culture of human adipose tissue‐derived stromal cells (hADSC)
Adipose tissues were collected from patients undergoing elective liposuction surgery after obtaining informed consent from them, according to procedures approved by the Ethics Committee at Shahid Beheshti University of Medical Sciences, Tehran, Iran. Samples were rinsed in PBS (PH = 7.4) supplemented with antibiotic and antifungal agents. The cells were prepared and cultured according to the protocol previously described 23 with some modifications. In brief, tissues were treated with 0.2% collagenase II (Sigma, St. Louis, MO, USA) for 30 min at 37 °C, then samples were washed twice in sterile PBS, seeded inT‐75 culture plates containing DMEM supplemented with 10% foetal bovine serum, 1% pen/strep, nystatin and amphotericin B, 2 mm Glutamax, 1 mm L‐glutamine and 1% non‐essential amino acids (all from Gibco, Carlsbad, CA, USA), with a density of 1 × 105cells/ml. They were allowed to proliferate in a cell culture incubator (37 °C and 5% carbon dioxide) until reaching 90% confluence, medium being changed every 3–4 days. Cells harvested from passage 2 were used for this study.
Human amniotic membrane collection and decellularization
HAMs were collected and decellularized according to the protocol described in our previously published studies 3, 4. For this purpose, tissue was obtained from consenting mothers after their caesarean‐section deliveries. All donors were screened serologically for human immunodeficiency virus type II, syphilis, gonorrhoea, toxoplasmosis, human hepatitis virus types B and C, and cytomegalovirus 6. Amniotic membranes were separated from the chorion and washed three times in antibiotic‐supplemented PBS. Tissues were then treated with 0.2% EDTA for 30 min at 37 °C, followed with 0.2 mm NaOH for 30 s. Cells were separated from membranes by scraping it while embedded in ammonium chloride.
Characterization of dHAM
Decellularization was confirmed by haematoxylin and eosin (H&E) staining of the samples and histological observation. Samples were then fixed in 10% natural‐buffered formalin, dehydrated through an increasing series of graduated ethanols, embedded in paraffin wax then sectioned at 4 μm. Samples were viewed by light microscopy after staining with H&E.
hADSC seeding on and responses to dHAM 3D scaffolds
dHAM 3D scaffolds were sterilized by gamma irradiation (25 kGy) then placed in closed Petri dishes; they were then left to dry under a laminar flow hood for 3 h. Living/dead cell ratios were determined using trypan blue staining and the hADSC were seeded on the dHAM scaffold for following examination.
Cell viability and cytotoxicity
Cell viability and cytotoxicity were determined by MTT and lactate dehydrogenase (LDH) specific activity assays respectively, and performed as described in detail in our previously published works 3, 4. To determine effects of the tissue on viability of hADSC, cells were seeded on dHAM at 2 × 104 cells/cm2, and incubated in a cell culture incubator for 1, 2, 3 and 7 days. After the predetermined time points, the cells were washed twice in PBS (7.4) and treated with tetrazolium salt (MTT, 3‐(4, 5‐Dimethyl‐2‐thiazolyl)‐2, 5‐diphenyl‐2Htetrazolium bromide) for 2 h at 37 °C. Then, they were washed again in PBS and treated with DMSO for 15 min, in the dark. Optical density (OD) values of living cells were measured by ELISA (enzyme‐linked immunosorbent assay) assay, read at 590 nm, with 620 nm reference filter (ODs). MTT results for each sample were compared to controls (cells cultured on plain plastic surfaces of flasks), for each incubation time.
Lactate dehydrogenase is a cytoplasmic enzyme that converts lactate to pyruvate in the presence of the NAD+ as a co‐enzyme. This enzyme is released from cells after injury or cell death. Thus, determination of LDH in the medium directly contributes to cell damage in vitro 24. The kit used for LDH assay is based on conversion of p‐nitro phenyl phosphate to p‐nitro phenol. UV absorbance of NADH, representing concentration of NADH, was quantitated on a Biotek EL800 absorbance plate reader at 490 nm 25. LDH specific activity in the medium in which the cells were exposed to dHAM for the different times, 1, 2, 3 and 7 days, was measured using LDH kit (ZistShimi kits, Co No: 10‐503 and 10‐533‐1, Iran). LDH results for each sample were compared to controls 4.
Cell/dHAM morphology
Cell/dHAM interaction was investigated by SEM (scanning electron microscopy) and DAPI (4ʹ, 6‐diamidino‐2‐phenylindole) staining, after culturing hADSCs on dHAM for 72 hours.
Morphology of the cells spread out on dHAM was observed by SEM. They were cultured on the tissue for 72 h, then prepared for SEM observation according to previously published protocols 26. Briefly, cells/dHAM constructs were rinsed in PBS, fixed in 2.5% glutaraldehyde (GA) solution and dehydrated using acetone (Merck). Samples were then treated with 1% osmium tetroxide (OsO4) (Sigma‐Aldrich, St. Louis, MO, USA) and freeze dried. To provide SEM micrographs, samples were sputter coated with gold and viewed under the microscope (Philips XL30, Eindhoven, the Netherlands) at an acceleration voltage of 15 kV.
Attachment of hADSC to dHAM was investigated by DAPI staining of the cells. Nuclei of cells cultured on the tissue were stained by DAPI (Sigma‐Aldrich) and viewed using ultraviolet (UV) light 4. DAPI‐stained nuclei were revealed as light blue granular organelles.
Statistical analysis
Distribution of data was assessed using the Kolmogorov–Smirnov test, and independent sample t‐testing was used to analyse the data (mean ± SD). P value of ≤0.05 was defined as level of significance. Graphs were plotted using SigmaPlot 11.0 θ software (Systat Software Inc, London, UK).
Results
Haematoxylin and eosin staining of scaffolds
Micrographs of H&E staining of both fresh and decellularized HAM is shown in Fig. 1. Cells were successfully removed from the tissue by denudation.
Figure 1.
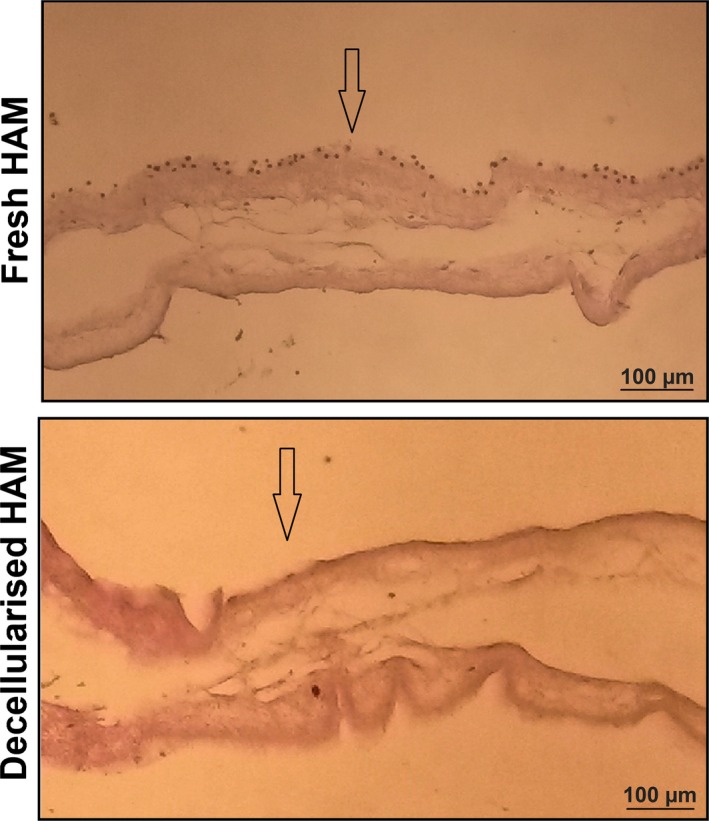
H&E staining of fresh and decellularized HAM s. Cells were successfully removed from the tissue by decellularization. Arrows indicate apical surfaces of the tissues.
Cell viability and cytotoxicity
Viability of hADSC cultured on dHAM was determined based on viability of living cells in reduction of tetrazolium salt and formation of crystals within their mitochondria 27. In this study, cell viability was examined at a variety of incubation time points up to 1 week after seeding, and OD of each sample was compared to control. MTT results are shown in Fig. 2a, and according to these results dHAM increased cell viability, although differences were not significant up to 3 days. However, cell viability significantly increased (approximately 111%) after one week incubation at 37 °C (P < 0.05).
Figure 2.
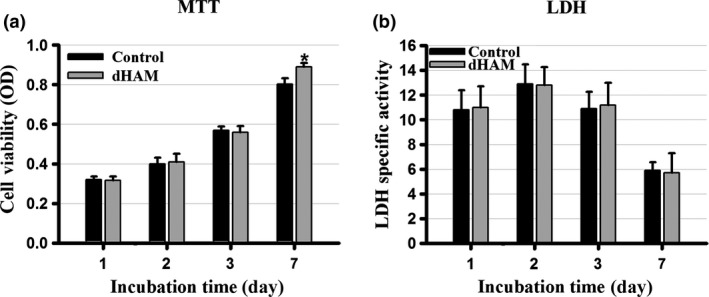
(a) There was no significant difference between cell viabilities measured with MTT obtained from dHAM samples compared to controls (independent sample t ‐test, P > 0.05) from days 1, 2 and 3. However, cell viability significantly increased in dHAM samples by day 7 in comparison with controls (independent sample t‐test, P < 0.05). (b) Lactate dehydrogenase (LDH) specific activity assay, representing potential cytotoxic properties of dHAM, showed no significant cytotoxic activity compared to controls up to 7 days incubation time (independent sample t‐test, P > 0.05) *indicates the significant difference with control.
Cytotoxicity effects of decellularized tissue on the tested cells were determined by assessing amounts of LDH in the cell culture media after the chosen incubation time points. As can be seen, no significant difference was observed in LDH level of the samples at all time points compared to controls (Fig. 2b).
Cell/tissue morphology
Attachment of cells to dHAM was determined by both SEM and DAPI staining.
SEM observation
Cell morphology on the tissue was observed by SEM after sample preparation 4 and is shown in Fig. 3. hADSCs were well attached and expanded on the tissue, indicating good adhesion properties of the decellularized HAM for the examined cells.
Figure 3.
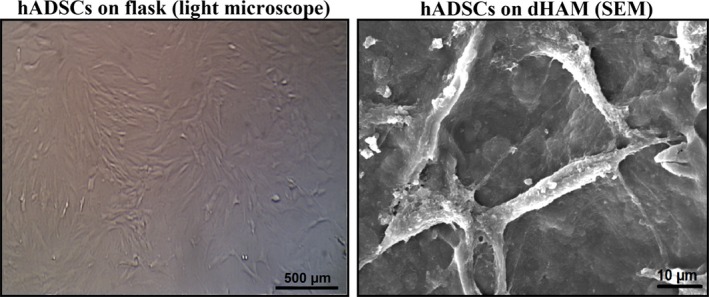
Typical SEM images of human adipose‐derived stromal cells ( hADSC s) cultured on plain plastic flasks as control, and on decellularized human amniotic membrane ( dHAM ).
DAPI staining
Attachment of hADSC to dHAM was determined by DAPI staining. DAPI stains nuclei of both living and dead cells in light blue. However, damaged or dead cells loose their attachment and their nuclei were not stained. Thus, light blue organelles seen by UV light microscopy indicate the presence of viable cells adhered to the membrane. As shown in Fig. 4, hADSC were actively attached to dHAM after 72 h incubation time
Figure 4.
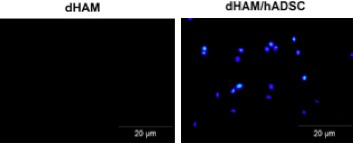
Typical DAPI staining of dHAM samples. Left dHAM without cells and right hADSC on dHAM. dHAM, decellularized human amniotic membrane; hADSC, human adipose‐derived stromal cells.
Discussion
The human amniotic membrane has widely been used as a soft tissue scaffold, especially for wound dressings 4, 5, 10, 28. In addition to immunomodulatory effects, HAM has many favourable characteristics such as antibacterial properties 3, low antigenicity, ready availability and positive effects on cell proliferation and adhesion 3, 4, 5; these have made it an excellent substance for tissue engineering applications. It can be considered to be a support for cell growth or as a delivery system 4. On the other hand, it is very important to note that full removal of original cells from the HAM may profoundly affect both its biomechanical and biological properties 4, 6. An ideal tissue engineering scaffold requires a biocompatible surface to provide a microenvironment for cell proliferation and adhesion 29, 30. Decellularization not only eliminates immunogenicity of HAM but also exposes its extracellular matrix proteins that eventually improve its biological properties 4, 28.
A number of studies have been conducted to develop an efficient method for decellularization and preservation of HAM, almost all of them using chemical or enzymatic agents, for example by Wilshaw et al. 6. Although these successfully remove cells and cell fragments from the membrane, the decellularization methods were time consuming and expensive. In our previously published study, a simple and cost‐effective procedure has been developed for decellularization and preservation of HAM 4. The main novelty and advantage of our developed method compared to other decellularization procedures is the use of chemical and mechanical techniques rather than enzymes, making it simple, cost‐effective and safe. In clinical practice, efficiency of sterilization of biological products is crucially important; we used GLP gamma irradiation. This is a recommended technique used by many research and clinical teams worldwide, including by us for clinical purposes. The gamma‐irradiated amnion is an efficient, cost‐effective and easy to use biological dressing for skin tissue engineering and wound healing 31, 46, 47, 48, 49. Following that study, after full characterization of the dHAM in terms of mechanical and biological responses, antibacterial activity of samples has been evaluated against multi‐drug resistant bacteria isolated from burn patients 3.
hADSCs are a favourable source of stem cells for autologous transplantation in cell therapy 32, 33. Easy availability, high differentiation capability into a variety of differentiated cell phenotypes, as well as simple isolation and culture, have made such cells a promising source 34, 35, 36, 37. They have been widely used in both the clinic and in research.
It is well documented that development of a favourable delivery system for such cells is worthy 16, 38, 39. To this aim, HAM was decellularized, and this was confirmed by observation of H&E staining. hADSC were seeded on the decellularized membrane for selected time points and efficiency of dHAM as a carrier and cell growth supporter for the hADSC was investigated. Both MTT and LDH assays strongly revealed that dHAM had no cytotoxic effects on the tested cells even after 1 week. These results are confirmed by our previously published paper and other studies in this research line 4, 5, 6. Cell adhesion properties of tissue engineering scaffolds are amonsgt the most critical characteristics that could profoundly affect its efficiency in tissue engineering applications. For this purpose, morphology of hADSCs cultured on dHAM was observed and studied by SEM. Furthermore, presence of cells on membranes was confirmed by DAPI staining. All data obtained from SEM revealed that the hADSC were actively attached to the dHAM. Additionally, DAPI staining confirmed distribution of the cells on the dHAM. This finding is consistent with our previously published article indicating that dHAM acts as a favourable cell adhesion supporter, although further quantitative examinations are required to confirm this matter. High expansion and adhesion of cells was clearly seen, indicating excellent biocompatibility and adhesion properties of the dHAM, confirmed by the data obtained from MTT and LDH in this study. Furthermore, these characteristics of dHAM have also been confirmed by other studies that used different other cell types 4, 40, 41. Both HAM and hADSC have been used for soft tissue engineering, especially in patients with skin burns, necrotising fasciitis, corneal defects and wound healing 3, 42, 43, 44, 45. Although further in vivo investigation is required to fully characterize the hADSC‐seeded dHAM, such constructs can be considered as a promising choice in regeneration of burn injuries in the clinic.
In this study, we demonstrated that dHAM can serve as a cell‐carring 3D scaffold for delivery of hADSC. Our study strongly suggests the potential of dHAM as a viable delivery system for hADSC in tissue engineering applications. hADSC have low proliferation properties and differentiate into various cell types after first passage in appropriate media 16. The most important limitation of this study was isolation and culture of the hADSC for clinical application.
In conclusion, in our previously published study, we showed that we had developed a novel decellularization method for HAM. Here, we have found that the denuded membrane had favourable attachment properties for human adipose tissue‐derived stem cells and showed no cytotoxic activity. All the results suggested that HAM prepared by our proposed method of decellularization was a potential carrier for hADSCs for soft tissue engineering applications.
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1. Bourne G (1960) The microscopic anatomy of the human amnion and chorion. Am. J. Obstet. Gynecol. 79, 1070. [DOI] [PubMed] [Google Scholar]
- 2. Mohamad H (2001) Chapter 10. Anatomy and embryology of human placenta, amnion and chorion In: Nather A. The Scientific Basis of Tissue Transplantation. Advanced in Tissue Banking Vol. 5. 2001. pp. 139–148. Singapore: Published by World Scientific Publishing Co. Pte. Ltd. [Google Scholar]
- 3. Gholipourmalekabadi M, Bandehpour M, Mozafari M, Hashemi A, Ghanbarian H, Sameni M et al (2015) Decellularized human amniotic membrane: more is needed for an efficient dressing for protection of burns against antibiotic‐resistant bacteria isolated from burn patients. Burns 2, S0305‐4179(15)00126‐6. [DOI] [PubMed] [Google Scholar]
- 4. Gholipourmalekabadi M, Mozafari M, Salehi M, Seifalian A, Bandehpour M, Ghanbarian H et al (2015) Development of a cost‐effective and simple protocol for decellularization and preservation of human amniotic membrane as a soft tissue replacement and delivery system for bone marrow stromal cells. Adv. Healthc. Mater. 6, 918–926. [DOI] [PubMed] [Google Scholar]
- 5. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM (2008) Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cell. Mater. 15, 88–99. [DOI] [PubMed] [Google Scholar]
- 6. Wilshaw S‐P, Kearney JN, Fisher J, Ingham E (2006) Production of an acellular amniotic membrane matrix for use in tissue engineering. Tissue Eng. 12, 2117–2129. [DOI] [PubMed] [Google Scholar]
- 7. Sorsby A, Haythorne J, Reed H (1947) Further experience with amniotic membrane grafts in caustic burns of the eye. Br. J. Ophthalmol. 31, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dua HS, Gomes JA, King AJ, Maharajan VS (2004) The amniotic membrane in ophthalmology. Surv. Ophthalmol. 49, 51–77. [DOI] [PubMed] [Google Scholar]
- 9. Tseng SC, Prabhasawat P, Lee S‐H (1997) Amniotic membrane transplantation for conjunctival surface reconstruction. Am. J. Ophthalmol. 124, 765–774. [DOI] [PubMed] [Google Scholar]
- 10. Mohammadi AA, Jafari SMS, Kiasat M, Tavakkolian AR, Imani MT, Ayaz M et al (2013) Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns 39, 349–353. [DOI] [PubMed] [Google Scholar]
- 11. Tehrani FA, Ahmadiani A, Niknejad H (2013) The effects of preservation procedures on antibacterial property of amniotic membrane. Cryobiology 67, 293–298. [DOI] [PubMed] [Google Scholar]
- 12. De Francesco F, Ricci G, D'Andrea F, Nicoletti GF, Ferraro GA (2015) Human Adipose Stem Cells (hASCs): from bench to bed‐side. Tissue Eng. 24, 2142–2157. [DOI] [PubMed] [Google Scholar]
- 13. Shingyochi Y, Orbay H, Mizuno H (2015) Adipose‐derived stem cells for wound repair and regeneration. Expert Opin. Biol. Ther. 5, 1–8. [DOI] [PubMed] [Google Scholar]
- 14. Naderi N, Wilde C, Haque T, Francis W, Seifalian AM, Thornton CA et al (2014) Adipogenic differentiation of adipose‐derived stem cells in 3‐dimensional spheroid cultures (microtissue): Implications for the reconstructive surgeon. J. Plast. Reconstr. Aesthet. Surg. 67, 1726–1734. [DOI] [PubMed] [Google Scholar]
- 15. Griffin M, Kalaskar DM, Butler PE, Seifalian AM (2014) The use of adipose stem cells in cranial facial surgery. Stem Cell Rev. 10, 671–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson A, Butler PE, Seifalian AM (2011) Adipose‐derived stem cells for clinical applications: a review. Cell Prolif. 44, 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou C, Cai X, Fu Y, Wei X, Fu N, Xie J et al (2015) Tetraploid complementation proves pluripotency of induced pluripotent stem cells derived from adipose tissue. Cell Prolif. 48, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cun X, Xie J, Lin S, Fu N, Deng S, Xie Q et al (2015) Gene profile of soluble growth factors involved in angiogenesis, in an adipose‐derived stromal cell/endothelial cell co‐culture, 3D gel model. Cell Prolif. 48, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ardeshirylajimi A, Mossahebi‐Mohammadi M, Vakilian S, Langroudi L, Seyedjafari E, Atashi A et al (2015) Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic‐coated electrospun poly (L‐lactide) nanofibres. Cell Prolif. 48, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu Y, Li R, Zhong J, Fu N, Wei X, Cun X et al (2014) Adipogenic differentiation potential of adipose‐derived mesenchymal stem cells from ovariectomized mice. Cell Prolif. 47, 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou C, Cai X, Grottkau B, Lin Y (2013) BMP4 promotes vascularization of human adipose stromal cells and endothelial cells in vitro and in vivo. Cell Prolif. 46, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu L, Wang T, Ge Y, Cai X, Wang J, Lin Y (2012) Secreted factors from adipose tissue increase adipogenic differentiation of mesenchymal stem cells. Cell Prolif. 45, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guasti L, Vagaska B, Bulstrode NW, Seifalian AM, Ferretti P (2014) Chondrogenic differentiation of adipose tissue‐derived stem cells within nanocaged POSS‐PCU scaffolds: a new tool for nanomedicine. Nanomedicine 10, 279–289. [DOI] [PubMed] [Google Scholar]
- 24. Shachar M, Tsur‐Gang O, Dvir T, Leor J, Cohen S (2011) The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 7, 152–162. [DOI] [PubMed] [Google Scholar]
- 25. Rostami A, Mozafari M, Gholipourmalekabadi M, Caicedo HH, Lasjerdi Z, Sameni M et al (2015) Optimization of fluoride‐containing bioactive glasses as a novel scolicidal agent adjunct to hydatid surgery. Acta Trop. 148, 105–114. [DOI] [PubMed] [Google Scholar]
- 26. Gholipourmalekabadi M, Mozafari M, Bandehpour M, Salehi M, Sameni M, Caicedo HH et al (2014) Optimization of nanofibrous silk fibroin scaffolds as a delivery system for bone marrow adherent cells. Biotechnol. Appl. Biochem. 62, 785–794 (In press). [DOI] [PubMed] [Google Scholar]
- 27. Gholipourmalekabadi M, Mozafari M, Gholipourmalekabadi M, Nazm Bojnordi M, Hashemi‐soteh MB, Salimi M et al (2015) In vitro and in vivo evaluations of three‐dimensional hydroxyapatite/silk fibroin nanocomposite scaffolds. Biotechnol. Appl. Biochem. 62, 441–450. [DOI] [PubMed] [Google Scholar]
- 28. Riau AK, Beuerman RW, Lim LS, Mehta JS (2010) Preservation, sterilization and de‐epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 31, 216–225. [DOI] [PubMed] [Google Scholar]
- 29. Mobini S, Solati‐Hashjin M, Peirovi H, Osman NAA, Gholipourmalekabadi M, Barati M et al (2013) Bioactivity and Biocompatibility Studies on Silk‐Based Scaffold for Bone Tissue Engineering. J. Med. Biol. Eng. 33, 207–213. [Google Scholar]
- 30. Nigam R, Mahanta B (2014) An Overview of Various Biomimetic Scaffolds: Challenges and Applications in Tissue Engineering. J. Tissue Sci. Eng. 5, 1–5. [Google Scholar]
- 31. Yue Y, Yang X, Wei X, Chen J, Fu N, Fu Y et al (2013) Osteogenic differentiation of adipose‐derived stem cells prompted by low‐intensity pulsed ultrasound. Cell Prolif. 46, 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khan W, Adesida A, Tew S, Longo U, Hardingham T (2012) Fat pad‐derived mesenchymal stem cells as a potential source for cell‐based adipose tissue repair strategies. Cell Prolif. 45, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lo Cicero V, Montelatici E, Cantarella G, Mazzola R, Sambataro G, Rebulla P et al (2008) Do mesenchymal stem cells play a role in vocal fold fat graft survival? Cell Prolif. 41, 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taha M, Valojerdi M, Mowla S (2006) Effect of Bone Morphogenetic Protein‐4 (BMP‐4) on Adipocyte Differentiation from Mouse Embryonic Stem Cells. Anat. Histol. Embryol. 35, 271–278. [DOI] [PubMed] [Google Scholar]
- 35. Fu N, Yang X, Ba K, Fu Y, Wei X, Yue Y et al (2013) Low‐intensity pulsed ultrasound induced enhanced adipogenesis of adipose‐derived stem cells. Cell Prolif. 46, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oh H, Park E, Lee S, Soh J, Kong I, Choi S et al (2012) Comparison of cell proliferation and epigenetic modification of gene expression patterns in canine foetal fibroblasts and adipose tissue‐derived mesenchymal stem cells. Cell Prolif. 45, 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balwierz A, Czech U, Polus A, Filipkowski R, Mioduszewska B, Proszynski T et al (2008) Human adipose tissue stromal vascular fraction cells differentiate depending on distinct types of media. Cell Prolif. 41, 441–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE et al (2005) Multipotential differentiation of adipose tissue‐derived stem cells. Keio J. Med. 54, 132–141. [DOI] [PubMed] [Google Scholar]
- 39. Chua K, Raduan F, Wan Safwani W, Manzor N, Pingguan‐Murphy B, Sathapan S (2013) Effects of serum reduction and VEGF supplementation on angiogenic potential of human adipose stromal cells in vitro. Cell Prolif. 46, 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koizumi N, Fullwood NJ, Bairaktaris G, Inatomi T, Kinoshita S, Quantock AJ (2000) Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest. Ophthalmol. Vis. Sci. 41, 2506–2513. [PubMed] [Google Scholar]
- 41. Meller D, Pires R, Tseng S (2002) Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br. J. Ophthalmol. 86, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burd A, Ahmed K, Lam S, Ayyappan T, Huang L (2007) Stem cell strategies in burns care. Burns 33, 282–291. [DOI] [PubMed] [Google Scholar]
- 43. Hassan WU, Greiser U, Wang W (2014) Role of adipose‐derived stem cells in wound healing. Wound Repair Regen. 22, 313–325. [DOI] [PubMed] [Google Scholar]
- 44. Nanda S, Chakraborty S, Ray A, Muddin I (2011) Healing of cervical necrotizing fasciitis using amniotic membrane as a dressing material. Nath. J. Maxillofac. Surg. 2, 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilshaw S‐P, Kearney JN, Fisher J, Ingham E (2008) Biocompatibility and Potential of Acellular Human Amniotic Membrane to Support the Attachment and Proliferation of Allogeneic Cells. Tissue Eng. 4, 463–472. [DOI] [PubMed] [Google Scholar]
- 46. Gajiwala K, Gajiwala AL (2004) Evaluation of lyophilised, gamma‐irradiated amnion as a biological dressing. Cell Tissue Banking 5, 73–80. [DOI] [PubMed] [Google Scholar]
- 47. Singh R, Purohit S, Chacharkar MP, Bhandari PS, Bath AS (2007) Microbiological safety and clinical efficacy of radiation sterilized amniotic membranes for treatment of second‐degree burns. Burns 33, 505–510. [DOI] [PubMed] [Google Scholar]
- 48. Nguyen H, Morgan DA, Forwood MR (2007) Sterilization of allograft bone: Is 25 kGy the gold standard for gamma irradiation? Cell Tissue Bank 8, 81–91. [DOI] [PubMed] [Google Scholar]
- 49. Lim LS, Poh RW, Riau AK, Beuerman RW, Tan D, Mehta JS (2010) Biological and ultrastructural properties of acelagraft, a freeze‐dried γ‐irradiated human amniotic membrane. Arch. Ophthalmol. 128, 1303–1310. [DOI] [PubMed] [Google Scholar]


