Abstract
Objectives
The lung‐gut axis is known to be involved in the pathogenesis of Staphylococcus aureus pneumonia. However, the underlying mechanisms remain unclear. We examined the role of pulmonary mast cells (MCs) in the regulation of the lung‐gut axis during S. aureus pneumonia.
Materials and Methods
We created a mouse model of S. aureus pneumonia using MC‐deficient mice (Kit W‐sh/W‐sh) and examined the level of inflammation, bacterial burden, expression of cathelicidin‐related antimicrobial peptide (CRAMP) and composition of the gut microbiota. We further evaluated anti‐bacterial immunity by administering bone marrow MCs (BMMCs) or CRAMP into the lungs of Kit W‐sh/W‐sh mice.
Results
After S. aureus challenge, the MC‐deficient mice, compared with wild‐type (WT) mice, displayed attenuated lung inflammation, decreased expression of CRAMP, higher bacterial lung load and disturbance of the intestinal microbiota. Adoptive transfer of BMMCs into the lung effectively reconstituted the host defence against S. aureus in Kit W‐sh/W‐sh mice, thus resulting in recovery of S. aureus pneumonia‐induced intestinal dysfunction. Similarly, exogenous administration of CRAMP significantly enhanced anti‐bacterial immunity in the lungs of MC‐deficient mice.
Conclusions
This study provides evidence for the involvement of MCs in the regulation of the lung‐gut axis during S. aureus pneumonia.
Keywords: intestinal microbiota, lung‐gut axis, mast cells, Staphylococcus aureus pneumonia
1. INTRODUCTION
Staphylococcus aureus is one of the common pathogens in hospital‐ and community‐acquired pneumonia.1 In addition to the symptoms including headache, fever, dyspnoea and cough, patients with pneumonia often present with gastroenteritis‐like discomforts such as vomiting and diarrhoea, especially in young children.2 Increasing evidence indicates that the intestinal microbiome and mucosal tissues broadly affect the progression of multiple diseases.3 The lung‐gut axis, which represents the interaction between lung immunity and intestinal microbiota,4 is thus an area of interest, though the underlying mechanisms remain poorly understood.
Mast cells (MCs) are particularly abundant at host‐environment interfaces, such as the skin and the respiratory and gastrointestinal tracts. Because of their location, MCs have been hypothesized to act as sentinel cells that sense pathogen attacks and initiate a protective immune response.5 However, knowledge of the role of MCs in the host defence against S. aureus is limited. Recent studies have demonstrated that MCs can be activated by S. aureus and exert antimicrobial activity.6, 7 But, anti‐S. aureus mechanisms of MCs have not yet been demonstrated in the lung. The lung is the major target tissue of diverse inhaled microbial pathogens, including S. aureus. To combat them, the respiratory system is armed with diverse mechanisms of innate mucosal immunity, including the secretion of antimicrobial peptides.8, 9 Cathelicidins show anti‐bacterial activity against both Gram‐positive and Gram‐negative bacteria through destruction of bacterial membrane.10, 11, 12 Cathelicidin expression is upregulated in the airways during bacterial infection13 and has been detected in alveolar macrophages, neutrophils and airway epithelial cells.10, 14, 15 There has been increasing interest in the role of cathelicidin‐related antimicrobial peptide (CRAMP) during bacterial pneumonia.
C‐kit receptor plays crucial roles in the differentiation, proliferation and inflammatory reaction of MCs.16, 17 Our prior work has shown that MCs released inflammatory factors (eg, tumour necrosis factor (TNF)‐α) and CRAMP in response to S. aureus, through stimulation of c‐kit receptor and its downstream molecules.18 In this study, using MC‐deficient mice (Kit W‐sh/W‐sh) with defective c‐kit receptor, we evaluated the diversity of the intestinal microbial community in mice with S. aureus pneumonia and determined the role of MCs in regulation of the lung‐gut axis. We showed that MC deficiency impaired lung inflammation and aggravated the imbalance in the gut microbiota upon S. aureus infection. Adoptive transfer of bone marrow mast cells (BMMCs) into the lung largely reconstituted the host defence against S. aureus in both the lung and the gut, thus demonstrating a critical role of MCs in the immune response through regulation of the lung‐gut axis.
2. MATERIALS AND METHODS
2.1. Animals
Kit W‐sh/W‐sh mice on a C57BL/6 background were kindly provided by Harvard Medical School.19 Age‐ and sex‐matched wild‐type (WT) mice were used as controls. All animal experiments were approved by the Animal Care and Use Committee of The Second Affiliated Hospital, Zhejiang University School of Medicine.
2.2. Bacterial culture
A clinical isolate of S. aureus was grown aerobically overnight at 37°C in a shaking incubator.18, 20 S. aureus in the mid‐logarithmic phase was resuspended in phosphate‐buffered saline (PBS). The numbers of bacteria was quantified according to the OD600‐based bacterial growth curve and verified with colony‐forming unit (CFU) assays.20
2.3. BMMCs culture
Bone marrow mast cells were generated from the femurs of C57BL/6 mice and maintained in the presence of 10% pokeweed mitogen‐stimulated spleen‐conditioned medium, as described previously.18, 21 After 4 weeks of culture, about 99% of the cells had developed into MCs.21
2.4. A mouse model of S. aureus lung infection
Eight‐week‐old C57BL/6 and Kit W‐sh/W‐sh mice were used to establish an S. aureus pneumonia model.22 In brief, 40 μL of S. aureus (5 × 107 CFUs) was inoculated intratracheally into anesthetized mice. Non‐infected control mice were administered with an equal volume of PBS intratracheally. Lung specimens were weighed and homogenized in 1 mL PBS to determine bacterial load and production of cytokines and CRAMP.
2.5. Reconstitution of MCs in Kit W‐sh/W‐sh mice
Kit W‐sh/W‐sh mice (5‐6 weeks old) were injected with 1 × 107 BMMCs in 200 μL PBS via the tail vein. The mice treated with PBS were used as controls. After 3 weeks, the reconstituted mice were used to build S. aureus pneumonia model, and tissues were harvested for analysis after 24 hour post‐infection.23
2.6. CRAMP treatment
The effects of CRAMP on S. aureus pneumonia were examined through intratracheal administration of 20 mg/kg CRAMP (GL Biotech, Shanghai, China) in 40 μL PBS to mice for four consecutive days.24
2.7. ELISA
The levels of cytokines including interleukin (IL)‐1β, IL‐6, keratinocyte‐derived chemokine (KC), macrophage inflammatory protein (MIP)‐2, TNF‐α and CRAMP in lung homogenates were detected using enzyme‐linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions.
2.8. Histological analysis
Mouse lung specimens were fixed in 10% paraformaldehyde, embedded in paraffin, cut into 4 μm‐thick sections and stained with haematoxylin‐eosin (H&E). The stained sections were reviewed for morphology under a photomicroscope (Leica, Heidelberg, Germany). The neutrophil infiltration and inflammatory scores of the lung samples were determined as described previously.25, 26 In brief, for evaluation of lung neutrophils, three lung sections from each mouse were analysed and three randomly selected high‐power fields (HPF) were examined for each lung section, the average value of cells/HPF for each mouse was determined by summation of all numbers divided by 9. The inflammatory levels were semi‐quantitatively determined as below: peribronchial/peribronchiolar inflammation 0‐4, perivascular inflammation 0‐4 and alveolar inflammation 0‐2. Each lung section was given a total inflammatory score (maximum score of 10).
2.9. Immunofluorescence staining
Paraffin‐wax sections of mouse lung tissues were placed on glass slides, and non‐specific antibody binding was blocked by incubation with 1% bovine serum albumin for 1 hour. Alexa Fluor 488 anti‐mouse CD117 (c‐kit) antibody (1: 50, BioLegend, San Diego, CA, USA) and phycoerythrin anti‐mouse CRAMP antibody (1: 50, BioLegend) were mixed and used for double staining. The sections were incubated with the above antibodies overnight at 4°C, then incubated at 37°C for 30 minutes. DAPI was used to stain the nuclei before viewing by confocal microscopy (Olympus, Tokyo, Japan).
2.10. Analysis of the faecal microbiota
Faecal samples in ileocecus were collected from WT and Kit W‐sh/W‐sh mice 24 hours after S. aureus infection, then snap‐frozen in liquid nitrogen. Faecal DNA was extracted and used for 16S rRNA sequencing according to manufacturer's instructions (Tianke, Hangzhou, China). Total genomic DNA was extracted using the PowerSoil® DNA Isolation Kit. 16S rRNA/ITS genes of distinct regions were amplified. PCRs were carried out with Phusion® High‐Fidelity PCR Master Mix. PCR products were mixed in equidensity ratios and purified with Qiagen Gel Extraction Kit. Sequencing libraries were generated using TruSeq® DNA PCR‐Free Sample Preparation Kit and examined on an Illumina MiSeq platform.
2.11. Statistical analysis
Data are expressed as mean ± standard error of mean (SEM), unless otherwise stated. Differences between two or multiple groups were analysed with Student's t test or one‐way ANOVA as appropriate. CFUs in lung tissue were compared using the Mann–Whitney U test. A P value <0.05 was considered statistically significant. All calculations were performed in GraphPad software (Version 5.01).
3. RESULTS
3.1. MC deficiency decreases the inflammatory response to S. aureus infection
We evaluated the effect of MCs on the inflammatory response to S. aureus infection. There were no differences in lung neutrophil counts between uninfected Kit W‐sh/W‐sh mice and WT animals; however, the inflammatory levels in Kit W‐sh/W‐sh mice were significantly lower than those in WT mice, based on H&E staining at 24 hours after infection (Figure 1A‐C). We further detected the levels of inflammatory cytokines including IL‐1β, IL‐6, KC, MIP‐2, TNF‐α and CRAMP. The expression levels of all cytokines except IL‐1β were significantly lower in lung specimens from Kit W‐sh/W‐sh mice than those from WT mice 24 hours post‐infection (Figure 1D). Confocal microscopy examinations showed that S. aureus stimulation upregulated the expression of lung MC‐derived CRAMP in WT mice, but weak expression of CRAMP was found in Kit W‐sh/W‐sh mice (Figure 1E). Meanwhile, Kit W‐sh/W‐sh mice had significantly higher numbers of S. aureus in their lungs after infection, compared to WT mice (Figure 1F).
Figure 1.
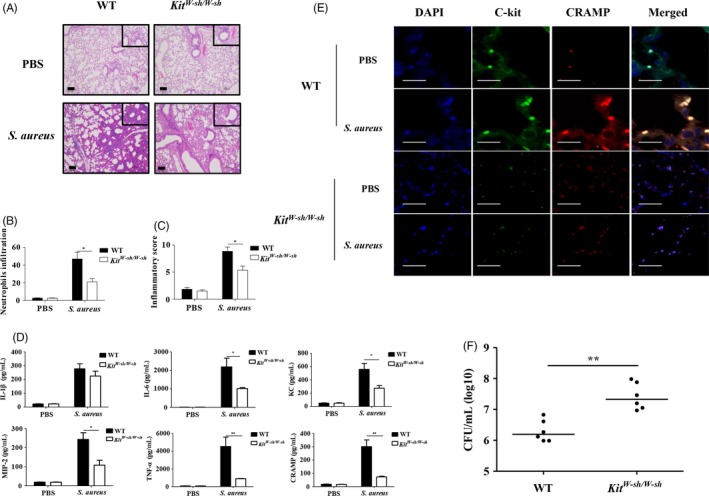
Kit W‐sh/W‐sh mice are susceptible to Staphylococcus aureus infection. WT and Kit W‐sh/W‐sh mice (n = 6 per group) were injected intratracheally with PBS or S. aureus. Lung tissues were harvested at 24 hours after infection. (A) Representative H&E‐stained lung‐tissue sections. Magnification 400× for the inserts, 100× for all others; scale bar, 100 μm. (B) Evaluations for lung neutrophils. (C) Inflammatory scores. (D) Levels of IL‐1β, IL‐6, KC, MIP‐2, TNF‐α and CRAMP. (E) CRAMP expression in c‐kit+ lung MCs by immunofluorescence staining. Magnification 180×; scale bar, 20 μm. (F) Bacterial load in lungs. Individual values and medians are shown for each group. *P < 0.05 and **P < 0.01
3.2. Kit W‐sh/W‐sh mice display impaired intestinal microbiota after S. aureus lung infection
To explore the influence of MCs on the intestinal microbiota, we analysed the intestinal microbiota using 16S rRNA sequencing at 24 hours post‐infection. The mouse intestinal microbiota consisted of three major bacterial phyla: Proteobacteria, Bacteroidetes and Firmicutes. Abundant Proteobacteria were found in the intestinal microbiota in the infected mice. Kit W‐sh/W‐sh mice showed higher burden of Proteobacteria than WT mice after pulmonary S. aureus infection (Figure 2A). E. coli is an important component of the Enterobacteriaceae and a common cause of vomiting and diarrhoea in humans.27 Notably, the most striking change in the faecal microbial community after S. aureus infection was an increase in the abundance of the genus Escherichia (Figure 2B), which accounted for approximately 2% of the total faecal microbiota in uninfected WT and Kit W‐sh/W‐sh mice, but raised to approximately 6% and 15%, respectively, in infected mice.
Figure 2.
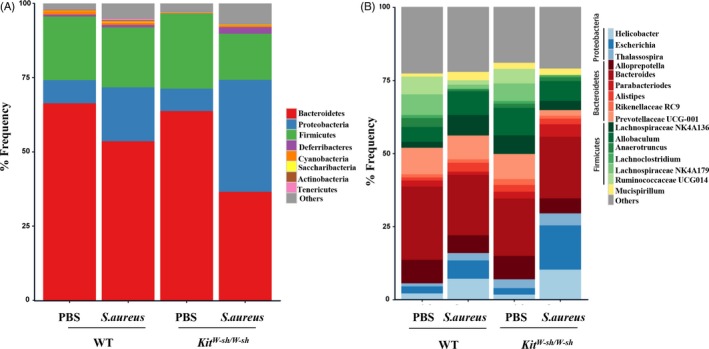
MCs alter the faecal microbiota composition after Staphylococcus aureus infection. Faecal samples were collected from WT and Kit W‐sh/W‐sh mice (n = 5 per group) at 24 h after infection. Analysis of faecal microbiota in WT and Kit W‐sh/W‐sh mice was performed by 16S rRNA sequencing. Composition of the intestinal microbiota was showed at the phylum level (A) and genus level (B), respectively
3.3. Adoptive transfer of BMMCs reconstitutes host defence against S. aureus infection in Kit W‐sh/W‐sh mice
We performed an adoptive MC transfer experiment to determine whether the susceptibility of Kit W‐sh/W‐sh mice to S. aureus was mainly attributed to MC deficiency. Treatment of WT‐derived BMMCs led to significantly elevated levels of IL‐6, TNF‐α, KC, MIP‐2 and CRAMP in KitW-sh/W-sh mice upon infection (Figure 3A). Correspondingly, MC‐reconstituted Kit W‐sh/W‐sh mice exhibited markedly improved bacterial clearance of S. aureus (Figure 3B). Furthermore, sequencing analysis of the microbiota also indicated that the imbalance in the intestinal flora after S. aureus infection was largely reversed in MC‐reconstituted Kit W‐sh/W‐sh mice (Figure 4A‐B).
Figure 3.
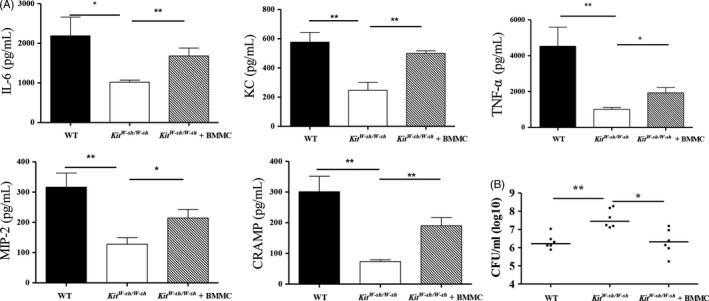
BMMCs transfer reconstitutes host resistance to Staphylococcus aureus infection in Kit W‐sh/W‐sh mice. WT, Kit W‐sh/W‐sh and reconstituted Kit W‐sh/W‐sh mice (n = 6 per group) were injected intratracheally with S. aureus. Lung tissues were collected at 24 h after infection. (A) Levels of IL‐6, KC, MIP‐2, TNF‐α and CRAMP in lung tissues. (B) S. aureus counts in lung tissues. Individual values and medians are shown for each group. *P < 0.05 and **P < 0.01
Figure 4.
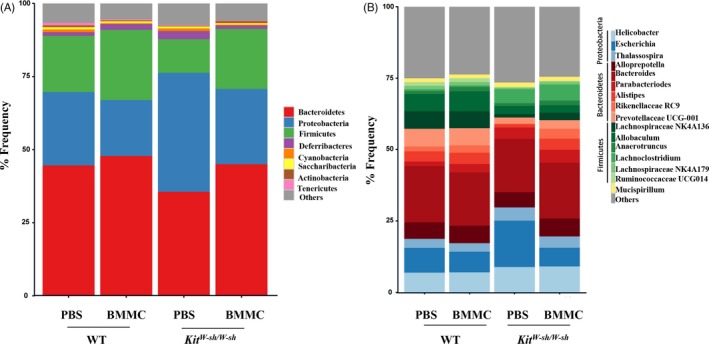
Intestinal microbiota is reversed in MC‐reconstituted Kit W‐sh/W‐sh mice. Faecal samples were collected from WT, reconstituted WT, Kit W‐sh/W‐sh and reconstituted Kit W‐sh/W‐sh mice (n = 5 per group) at 24 h after infection. Analysis of faecal microbiota was performed by 16S rRNA sequencing. Composition of the intestinal microbiota was shown at the phylum level (A) and genus level (B), respectively
3.4. Exogenous CRAMP treatment significantly ameliorates S. aureus pneumonia
Exogenous CRAMP was intratracheally administrated into the mice for 4 consecutive days. Although the lung‐tissue levels of the pro‐inflammatory cytokines IL‐6, TNF‐α, KC and MIP‐2 were not significantly affected by CRAMP treatment (Figure 5A), the bacterial load was obviously decreased by CRAMP treatment in both WT and Kit W‐sh/W‐sh mice (Figure 5B).
Figure 5.
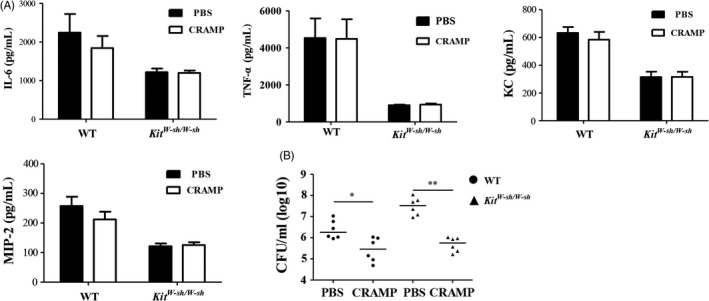
Treatment with CRAMP restores the host defence against Staphylococcus aureus infection in Kit W‐sh/W‐sh mice. WT and Kit W‐sh/W‐sh mice (n = 6 per group) were intratracheally injected with mouse CRAMP (20 mg/kg) or PBS for four consecutive days prior to S. aureus infection (5 × 107 CFUs). Lung tissues were harvested at 24 h after infection. (A) Levels of IL‐6, KC, MIP‐2 and TNF‐α in lung tissues. (B) S. aureus counts in lung tissues. Individual values and medians are shown for each group.*P < 0.05 and **P < 0.01
4. DISCUSSION
Staphylococcus aureus is one of the most common causative pathogens of pneumonia.28 Although pneumonia is known to be a lung infection, many studies have shown that pneumonia is also associated with substantial extrapulmonary effects, including intestinal inflammation, apoptosis and injury.29, 30 Mucosal tissues, including those in the respiratory and gastrointestinal tracts, act as the first line of host defence against invading pathogens. Gill et al31 have proposed that the mucosal immune system, which is associated with the commensal microbial community, is not isolated. Agwu et al32 observed that respiratory S. aureus infection in mice caused immune injury not only in the lung but also in the gut, and resembled the symptoms exhibited by humans infected with S. aureus. Staphylococcus aureus‐infected mice provide a good model for exploring the mechanisms of lung‐gut axis during respiratory S. aureus infection. Furthermore, these observations give more evidence supporting the existence of a broad mucosal immune system.
Mast cells act as effector cells during infection and are increasingly recognized to have a major role in modulating the course of infection.33 Previous studies demonstrated that S. aureus infection causes a series of inflammatory responses in the lung, in line with infiltration of various inflammatory cells including neutrophils, macrophages and natural killer cells.34, 35 Nevertheless, the role of MCs in host defence against S. aureus infection is largely unknown. Kit W‐sh/W‐sh mice with the Kit W‐sh mutation were reported to have a significant deficiency of MCs in all tissues but normal levels in other differentiated hematopoietic and lymphoid cells, showing the dampen immune responses.36, 37 In this study, using Kit W‐sh/W‐sh mice, we revealed that MC‐deficient mice were significantly more susceptible to S. aureus lung infection than WT animals, accompanied by a greater imbalance of the intestinal flora. We further carried out adoptive transfer of BMMCs into Kit W‐sh/W‐sh mice prior to S. aureus infection, to obtain additional supportive evidence for the role of MCs in keeping intestinal microflora homeostasis. Interestingly, the suppressed inflammatory response was largely reconstituted in Kit W‐sh/W‐sh mice after adoptive BMMCs administration. Moreover, the KitW-sh/W-sh mice received WT‐derived BMMCs had significantly fewer harmful bacteria in the gut than did controls. The present study thus indicates the critical role of MCs in keeping the host defence and maintaining gut microbial homeostasis against S. aureus infection.
Inflammation is an integral part of the reactions of the innate immune systems against micro‐organisms.38 We recently showed that the activated MCs contributed to a series of inflammatory actions to clear the pathogen in a mouse model of skin S. aureus infection.18 The host inflammatory and antimicrobial responses against S. aureus were impaired in Kit W‐sh/W‐sh mice, which suggested that MCs may act as an immune booster in the lung. Consistently, our previous data showed that MCs released multiple inflammatory cytokines directly upon S. aureus infection by means of the c‐kit‐activated phosphoinositide 3‐kinase (PI3K)/AKT/P65‐nuclear factor (NF‐κB) signal pathway.18
A couple of studies have revealed that MCs can inhibit bacterial growth by secreting the murine cathelicidin CRAMP.39, 40 CRAMP expression in the lung was significantly upregulated in response to different pathogen invasion.9, 12, 41 Here, we reported that CRAMP expression in the lung increased after infection, directly proportional to the amounts of MCs. Moreover, exogenous CRAMP treatment effectively decreased bacterial burden in a model of S. aureus pneumonia.
In summary, our results demonstrated that lung MCs are involved in regulation of the lung‐gut axis during S. aureus pneumonia. This study thus provides a potential clinical strategy focused on MCs for patients with S. aureus pneumonia accompanied by severe gut symptoms.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (81770008 and 81570004).
Liu C, Yang L, Han Y, Ouyang W, Yin W, Xu F. Mast cells participate in regulation of lung‐gut axis during Staphylococcus aureus pneumonia. Cell Prolif. 2019;52:e12565 10.1111/cpr.12565
REFERENCES
- 1. Amin AN, Cerceo EA, Deitelzweig SB, Pile JC, Rosenberg DJ, Sherman BM. The hospitalist perspective on treatment of community‐acquired bacterial pneumonia. Postgrad Med. 2014;126:18‐29. [DOI] [PubMed] [Google Scholar]
- 2. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costa MC, Weese JS. Understanding the intestinal microbiome in health and disease. Vet Clin North Am Equine Pract. 2018;34:1‐12. [DOI] [PubMed] [Google Scholar]
- 4. Bradley CP, Teng F, Felix KM, et al. Segmented filamentous bacteria provoke lung autoimmunity by inducing gut‐lung axis Th17 cells expressing dual TCRs. Cell Host Microbe. 2017;22:697‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piliponsky AM, Romani L. The contribution of mast cells to bacterial and fungal infection immunity. Immunol Rev. 2018;282:188‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rönnberg E, Johnzon CF, Calounova G, et al. Mast cells are activated by Staphylococcus aureus in vitro but do not influence the outcome of intraperitoneal S. aureus infection in vivo. Immunology. 2014;143:155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abel J, Goldmann O, Ziegler C, et al. Staphylococcus aureus evades the extracellular antimicrobial activity of mast cells by promoting its own uptake. J Innate Immun. 2011;3:495‐507. [DOI] [PubMed] [Google Scholar]
- 8. Chung DR, Huh K. Novel pandemic influenza A (H1N1) and community‐associated methicillin‐resistant Staphylococcus aureus pneumonia. Expert Rev Anti Infect Ther. 2015;13:197‐207. [DOI] [PubMed] [Google Scholar]
- 9. Kovach MA, Ballinger MN, Newstead MW, et al. Cathelicidin‐related antimicrobial peptide is required for effective lung mucosal immunity in Gram‐negative bacterial pneumonia. J Immunol. 2012;189:304‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bals R, Wang X, Zasloff M, et al. The peptide antibiotic LL‐37/hCAP‐18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A. 1998;95:9541‐9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byfield FJ, Kowalski M, Cruz K, et al. Cathelicidin LL‐37 increases lung epithelial cell stiffness, decreases transepithelial permeability, and prevents epithelial invasion by Pseudomonas aeruginosa . J Immunol. 2011;187:6402‐6409. [DOI] [PubMed] [Google Scholar]
- 12. Yu FS, Cornicelli MD, Kovach MA, et al. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin‐related antimicrobial peptide. J Immunol. 2010;185:1142‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schaller‐Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med. 2002;165:992‐995. [DOI] [PubMed] [Google Scholar]
- 14. Agerberth B, Grunewald J, Castaños‐Velez E, et al. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med. 1999;160:283‐290. [DOI] [PubMed] [Google Scholar]
- 15. Beisswenger C, Bals R. Antimicrobial peptides in lung inflammation. Chem Immunol Allergy. 2005;86:55‐71. [DOI] [PubMed] [Google Scholar]
- 16. da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014;62:698‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ainsua‐Enrich E, Serrano‐Candelas E, Álvarez‐Errico D, et al. The adaptor 3BP2 is required for KIT receptor expression and human mast cell survival. J Immunol. 2015;194:4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu C, Ouyang W, Xia J, Sun X, Zhao L, Xu F. Tumor necrosis factor‐α is required for mast cell‐mediated host immunity against cutaneous Staphylococcus aureus infection. J Infect Dis. 2018;218:64‐74. [DOI] [PubMed] [Google Scholar]
- 19. Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719‐724. [DOI] [PubMed] [Google Scholar]
- 20. Xu F, Kang Y, Zhang H, et al. Akt1‐mediated regulation of macrophage polarization in a murine model of Staphylococcus aureus pulmonary infection. J Infect Dis. 2013;208:528‐538. [DOI] [PubMed] [Google Scholar]
- 21. Supajatura V, Ushio H, Nakao A, et al. Differential responses of mast cell Toll‐like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qian J, Hu Y, Zhao L, et al. Protective role of adipose‐derived stem cells in Staphylococcus aureus‐induced lung injury is mediated by RegIIIγ secretion. Stem Cells. 2016;34:1947‐1956. [DOI] [PubMed] [Google Scholar]
- 23. Wolters PJ, Mallen‐St Clair J, Lewis CC, et al. Tissue‐selective mast cell reconstitution and differential lung gene expression in mast cell‐deficient Kit (W‐sh) /Kit (W‐sh) sash mice. Clin Exp Allergy. 2005;35:82‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Chu X, Liu C, et al. Exogenous murine antimicrobial peptide CRAMP significantly exacerbates Ovalbumin‐induced airway inflammation but ameliorates oxazolone‐induced intestinal colitis in BALB/c mice. Hum Vaccin Immunother. 2018;14:146‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun CK, Lee FY, Sheu JJ, et al. Early combined treatment with cilostazol and bone marrow‐derived endothelial progenitor cells markedly attenuates pulmonary arterial hypertension in rats. J Pharmacol Exp Ther. 2009;330:718‐726. [DOI] [PubMed] [Google Scholar]
- 26. Zhou Y, Wang GF, Yang L, et al. Treatment with 1,25(OH)2D3 induced HDAC2 expression and reduced NF‐κB p65 expression in a rat model of OVA‐induced asthma. Braz J Med Biol Res. 2015;48:654‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ochoa TJ, Contreras CA. Enteropathogenic escherichia coli infection in children. Curr Opin Infect Dis. 2011;24:478‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial‐resistant pathogens associated with healthcare‐associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006‐2007. Infect Control Hosp Epidemiol. 2008;29:996‐1011. [DOI] [PubMed] [Google Scholar]
- 29. Lobo SM, Orrico SR, Queiroz MM, et al. Pneumonia‐induced sepsis and gut injury: effects of a poly‐(ADP‐ribose) polymerase inhibitor. J Surg Res. 2005;129:292‐297. [DOI] [PubMed] [Google Scholar]
- 30. Perrone EE, Jung E, Breed E, et al. Mechanisms of methicillin‐resistant Staphylococcus aureus pneumonia‐induced intestinal epithelial apoptosis. Shock. 2012;38:68‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gill N, Wlodarska M, Finlay BB. The future of mucosal immunology: studying an integrated system‐wide organ. Nat Immunol. 2010;11:558‐560. [DOI] [PubMed] [Google Scholar]
- 32. Agwu A, Brady KM, Ross T, Carroll KC, Halsey NA. Cholera‐like diarrhea and shock associated with community‐acquired methicillin‐resistant Staphylococcus aureus (USA400 clone) pneumonia. Pediatr Infect Dis J. 2007;26:271‐273. [DOI] [PubMed] [Google Scholar]
- 33. Johnson‐Weaver B, Choi HW, Abraham SN, Staats HF. Mast cell activators as novel immune regulators. Curr Opin Pharmacol. 2018;41:89‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen TS, Jones‐Nelson O, Hotz M, et al. S. aureus blocks efferocytosis of neutrophils by macrophages through the activity of its virulence factor alpha toxin. Sci Rep. 2016;6:35466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Small CL, McCormick S, Gill N, et al. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J Immunol. 2008;180:5558‐5568. [DOI] [PubMed] [Google Scholar]
- 36. Grimbaldeston MA, Chen CC, Piliponsky AM, et al. Mast cell‐deficient W‐sash c‐kit mutant Kit W‐sh/W‐sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Michel A, Schüler A, Friedrich P, et al. Mast cell‐deficient Kit W‐sh ‘‘Sash’’ mutant mice display aberrant myelopoiesis leading to the accumulation of splenocytes that act as myeloid‐derived suppressor cells. J Immunol. 2013;190:5534‐5544. [DOI] [PubMed] [Google Scholar]
- 38. Alalwani SM, Sierigk J, Herr C, et al. The antimicrobial peptide LL‐37 modulates the inflammatory and host defense response of human neutrophils. Eur J Immunol. 2010;40:1118‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Di Nardo A, Yamasaki K, Dorschner RA, Lai Y, Gallo RL. Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin. J Immunol. 2008;180:7565‐7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li G, Domenico J, Jia Y, Lucas JJ, Gelfand EW. NF‐kappaB‐dependent induction of cathelicidin‐related antimicrobial peptide in murine mast cells by lipopolysaccharide. Int Arch Allergy Immunol. 2009;150:122‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Braff MH, Jones AL, Skerrett SJ, Rubens CE. Staphylococcus aureus exploits cathelicidin antimicrobial peptides produced during early pneumonia to promote staphylokinase‐dependent fibrinolysis. J Infect Dis. 2007;195:1365‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]


