Abstract
Abstract. The essential oil extracted from clove (Syzygium aromaticum) is used as a topical application to relieve pain and promote healing in herbal medicine and also finds use in the fragrance and flavouring industries. Clove oil has two major components, eugenol and β‐caryophyllene, which constitute 78% and 13% of the oil, respectively. Clove oil and these components are generally recognized as ‘safe’, but the in‐vitro study here demonstrates cytotoxic properties of both the oil and eugenol, towards human fibroblasts and endothelial cells. Clove oil was found to be highly cytotoxic at concentrations as low as 0.03% (v/v) with up to 73% of this effect attributable to eugenol. β‐caryophyllene did not exhibit any cytotoxic activity, indicating that other cytotoxic components may also exist within the parent oil.
INTRODUCTION
Plant essential oils are widely used in the pharmaceutical, cosmetic and food and beverage industries and fall into the category of the so called ‘natural’ plant products. They are also increasingly being used in the practice of complementary therapies. Even though these oils come from a natural source (plants), their production by distillation before they are added to cosmetics, medicines, food and other products somewhat contradicts their claim as ‘natural’. The final essential oil is a highly concentrated product (1 g of plant material is used to produce 25 mg of lavender oil (Tsuro et al. 2001)) and the addition of essential oils to products is frequently at much higher concentrations than those that occur in the plant of origin. The composition of each sample of essential oil is variable, and depends on a number of biological factors and also on the distillation process employed by the manufacturer. This is further compounded by the fact that the same species of plant grown in different countries, under different soil and altitude conditions, will produce oils that differ in chemical composition and therapeutic properties (Worwood 1996). Evidence of efficacy (and safety) for herbal preparations, including those containing essential oils, should therefore be considered to be extract specific; however, the pharmacovigilance for complementary medicines in general is still very much in its infancy (Barnes 2003).
In the source plant, essential oils are usually found in specialized cells or structures, for example, members of the Labiatae accumulate essential oils in specialized secretory structures such as glandular hairs (trichomes) (Hallahan 2000). The oils are then released in large amounts when these structures are ruptured by herbivore feeding, movement on the plant surface or the growth of pathogens (Wittstock & Gershenzon 2002). This would indicate that these oils may be part of a defence mechanism and their containment in specialized cells/structures prevents them from poisoning the whole plant. Clove oil is extracted from the buds, leaves or stems of the tree Syzygium aromaticum (Family: Myrtaceae) by steam or water distillation. Traditional uses of clove leaf oil include treating burns and cuts, and it has also found use in dental care as a pain reliever, and undiluted clove oil may be rubbed on the gums for treating tooth infections and toothache (Whitehouse, J., personal communication). The oil used in this study was distilled from the leaves.
The major components of the clove oil used in this study were eugenol and β‐caryophyllene. Eugenol (4‐allyl‐1‐hydroxy‐2‐methoxybenzene), a phenolic non‐nutrient compound, is the main component of clove oil with a molecular weight of 164.20 and is a naturally occurring food flavour. Rapidly excreted, eugenol is recognized as ‘safe’, and may be added to foodstuffs in concentrations up to 1500 p.p.m. (Bruneton 1995). Eugenol and clove leaf oil are extensively used in fragrance and flavour formulations as they have a powerful, warm, spicy aroma and have been in widespread use in the United States since before 1900. The soap and detergent industry is a major user of both materials, and eugenol typically finds use in such products at concentrations in the range of 0.05–0.1% (v/v) (Rothenstein et al. 1983). The other major constituent of clove oil is β‐caryophyllene, a sesquiterpenoid with a molecular weight of 204.35.
The purpose of this study was to determine any cytotoxicity of clove oil to human skin cells and to determine any component responsible for its activity. To this end, clove oil and its major components were tested against a range of human skin cells including dermal endothelial cells and fibroblasts.
MATERIALS AND METHODS
All chemicals used in this study were purchased from Sigma‐Aldrich Chemical Co. Ltd (Poole, UK) and VWR International (Poole, UK) and, unless specified otherwise, were of analytical grade or higher. Cell culture materials were purchased from Invitrogen Ltd (Paisley, UK) and clove oil was obtained from Neal's Yard Remedies Ltd (Battersea, London).
The cytotoxicity of whole clove oil and its major components, eugenol and β‐caryophyllene, were evaluated against three cell types. 153BR, human fibroblasts were supplied by ECACC (European Collection of Cell Cultures, Porton Down, Salisbury, UK), and used at a cell density of 105 cells/ml for all experiments. Human normal dermal fibroblasts (HNDF), a primary culture obtained from biopsy, were similarly used at 105 cells/ml for all experiments. HMEC‐1 cells, an SV40 transformed human dermal microvascular endothelial cell line were used at 4 × 105 cells/ml for all experiments.
The major components of clove oil have been identified as eugenol (78%) and β‐caryophyllene (13%) by gas chromatography (GC) and gas chromatography linked to Fourier Transform Infra Red spectroscopy (GC‐FTIR) analysis (data not shown). The concentrations of whole clove oil, eugenol and β‐caryophyllene used in this study are summarized in Table 1, the final concentration of a component in the reaction mixture paralleled that which is actually present in the oil. As an example, for comparing cytotoxicity of clove oil and its component eugenol, the concentration of the oil used would be 0.25% (v/v) and the concentration of eugenol would be 0.195% (v/v) (as eugenol constitutes 78% of clove oil). The complete oil and components were diluted in culture medium appropriate to the cells under study. Solubilizers such as dimethyl sulphoxide (DMSO) were not employed.
Table 1.
Concentrations of clove oil, eugenol and β‐caryophyllene used in the study
| Oil/component | Limits of concentrations used (% (v/v)) |
|---|---|
| Clove oil | 0.002–0.25 |
| Eugenol | 0.002–0.195 |
| β‐caryophyllene | 0.0003–0.032 |
Cytotoxicity of clove oil and its major components was determined by the neutral red (NR) assay of Babich et al. (1993) with slight modification. Sterile 96‐well tissue culture microtitre plates were seeded with 100 µl medium containing a defined number of cells which were then allowed to grow to 70% confluence (approximately 48 h). The growth medium was then replaced with medium containing a variety of concentrations of the essential oil or its components, and cells were incubated for 1 h at 37 °C and 5% CO2. After exposure, the medium containing the test agent was removed and 200 µl of medium containing 40 µg/ml NR was added to each well. The plate was then further incubated for 3 h to allow for uptake of the dye by viable, uninjured cells. The NR‐containing medium was then removed and the cells quickly washed with 200 µl fixative (1% CaCl2−0.5% formaldehyde) and then 200 µl of a solution containing 1% v/v acetic acid and 50% v/v ethanol was added to each well to extract the dye. The plate was allowed to stand at room temperature for 10 min followed by rapid agitation on a microtitre plate shaker. The absorbance of the extracted dye was read at 540 nm on a microtitre plate reader (Dynatech MR 5000; Dynex Technologies Ltd, Worthing, UK). All experiments were performed three times or more alongside control (untreated) cells. Total NR uptake constitutes a measure of cell viability (% NR uptake is directly proportional to the number of live, uninjured cells).
Dose‐dependant cytotoxicity graphs were generated from data based on percentage cell viability. Percentage viability was determined as the mean absorbance of treated wells expressed as a function of the mean absorbance of the control wells (untreated). Cytotoxicity of the oil or component was expressed in terms of its NR50 value (the concentration resulting in 50% cell death). NR50 values were calculated by non‐linear regression analysis. All NR50 values were expressed as a percentage (v/v). The baseline of 100% viability corresponded to the absorbance of control (untreated) cells A one‐way analysis of variance (anova) was employed to compare group means. To correlate the activity of the components with the corresponding essential oils, a linear regression was carried out. The r 2 values thus obtained were used to predict such relationships. Statistical analysis was carried out using GraphPad prism software.
RESULTS
The pattern of clove oil cytotoxicity was constant across all cell types (one‐way anova, Bonferroni's test, all P values > 0.05). Viability of all cell types tested dropped by 60–90% when the concentration of the oil was increased from 0.01% to 0.03% (Fig. 1). 153BR fibroblasts exhibited a greater sensitivity to clove oil at all concentrations tested.
Figure 1.
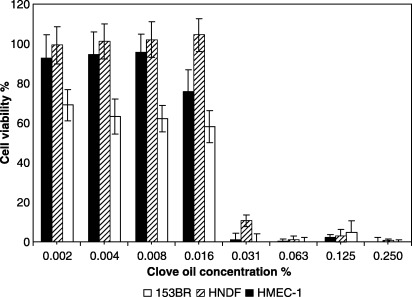
Dose‐dependent cytotoxicity of clove oil (1 h exposure) to HMEC‐1 endothelial cells, HNDF fibroblasts and 153BR fibroblasts as determined by the NR assay. Following exposure to the oil, the medium was replaced with NR‐containing medium for 3 h, which was then removed and the cells were fixed with 1% CaCl2–0.5% formaldehyde. Cells were then lysed with 1% v/v acetic acid – 50% v/v ethanol to extract the dye, incubated for 10 min at room temperature, and read at 540 nm. Error bars indicate the standard deviation (n > 16).
The r 2 values (linear regression) suggested that the 54–73% variation in the cytotoxicity of clove oil could be explained by variation in the cytotoxicity of eugenol (HMEC‐1, r 2 = 0.54, P = 0.03; HNDF, r 2 = 0.73, P = 0.006; 153BR, r 2 = 0.64, P = 0.01). On increasing the concentration of eugenol from 0.03% to 0.06% (Fig. 2), viability dropped from 60% to less than 20% in all cells tested. With endothelial cells, eugenol was highly cytotoxic (Fig. 2), with about 50% viability at a concentration as low as 0.002%.
Figure 2.
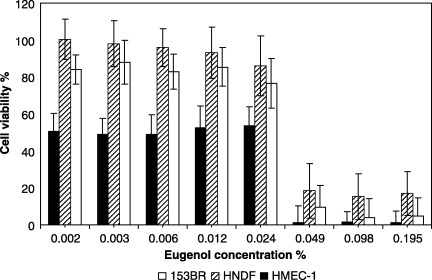
Dose‐dependent cytotoxicity of eugenol (1 h exposure) to HMEC‐1 endothelial cells, HNDF fibroblasts and 153BR fibroblasts as determined by the NR assay. Following exposure to eugenol, the medium was replaced with NR‐containing medium for 3 h, which was then removed and the cells fixed with 1% CaCl2−0.5% formaldehyde. Cells were then lysed with 1% v/v acetic acid – 50% v/v ethanol to extract the dye, incubated for 10 min at room temperature, and read at 540 nm. Error bars indicate the standard deviation (n > 16). (The concentrations of eugenol used were proportionate to that actually present in the oil).
NR50 values for all components and cell types are summarized in Table 2. The NR50 values for cells treated with eugenol were higher in relation to clove oil as more eugenol was required to affect the viability of 50% cells. β‐caryophyllene (which constitutes 13% of the total oil) was not cytotoxic at all concentrations tested. Cytotoxicity of clove oil was significantly related to that of eugenol (one‐way anova, Dunnett's test, eugenol P > 0.05, all cell types).
Table 2.
NR50 values of clove oil and its major components expressed as percentage of the oil/component diluted in the medium (v/v). NR50 values could not be determined in some cases where the component was either highly cytotoxic or non‐cytotoxic
| Oil/component | NR50 value (%) | ||
|---|---|---|---|
| HMEC‐1 | HNDF | 153BR | |
| Clove oil | 0.018 | 0.025 | 0.017 |
| Eugenol | HC | 0.030 | 0.032 |
| β‐caryophyllene | NC | NC | NC |
HC, highly cytotoxic at all concentrations tested; NC, non‐cytotoxic.
DISCUSSION
Reports of essential oil cytotoxicity are in their infancy and very few attempts have been made to determine active cytotoxic components of essential oils. In vitro toxicological studies have shown that lemon myrtle oil and citral exhibit high toxicity against primary cell cultures of dermal fibroblasts and a fibroblast cell line with cytotoxicity IC50 values ranging from 0.008% to 0.014% (w/v) (Hayes & Markovic 2002). Lavender oil is also cytotoxic at 0.25% (v/v) to endothelial cells and fibroblasts (Prashar et al. 2004).
In this study, clove oil has also been shown to be highly toxic at a concentration of 0.03%. Depending on the target cell type, 54–73% of the cytotoxic activity of clove oil was ascribed to a phenolic terpene, eugenol, which constitutes 78% of the oil and was shown to be highly cytotoxic to the skin cells when tested at concentrations as low as 0.06%. The second major component, β‐caryophyllene (which constitutes 13% of the oil), did not contribute towards the cytotoxicity. The data from this study also indicate that the cytotoxic properties of clove oil may be a function of more than one component. A possible mediator for the ‘unaccounted for’ cytotoxicity of the whole oil is eugenyl acetate, which constitutes up to 10% of clove oil (Lis‐Balchin 1995) and is likely to show similar effects to eugenol. Further studies are, however, required to determine whether this component of clove oil makes any contribution towards the activity of the whole oil. All experiments performed in this study utilized a 1 h incubation following preliminary experiments using a range of times, which indicated no notable further increases in cell death with prolonged incubation. This timing is in agreement with previous work (Friedman et al. 2002), which demonstrated that the bactericidal activity of 96 essential oils and 23 components was near maximum at approximately 1 h.
The midpoint cytotoxicity values of eugenol, as reported by other laboratories using different assays, are in the region of 100–300 µm (Hume 1984; Babich et al. 1993; Atsumi et al. 2000). The NR50 value of eugenol obtained in this piece of research was 0.03%, which is equivalent to 1.8 µm. The lower value obtained in our investigation may be the result of our use of unsolubilized and therefore more active eugenol. The studies cited above involved the use of solubilizers, which can cause changes in the physicochemical properties of the test system. Lipophilic molecules such as essential oil components are likely to become solubilized within micelles formed by non‐ionic surfactants (e.g., Tween 20 and Tween 80), and are thus partitioned out of the aqueous phase of the suspension (Schmolka 1973). The activity of cinnamon oil, for example, has been shown to decrease by 50‐fold when the oil was solubilized in DMSO compared to when it was used without solubilizers. Partitioning between the aqueous phase and DMSO is likely to have resulted in distancing the oil from the cells, potentially accounting for the reduced activity (Hili et al. 1997).
An NR50 value of 0.03%, as determined for fibroblasts in this study, represents a concentration of eugenol far lower than those used commercially, which is a potential cause for concern. While we recognize that cytotoxicity studies in isolated cells are not fully analogous to the in vivo situation, studies have also identified other potentially harmful effects of eugenol in vivo at similar low concentrations. Eugenol, present in a household product at a concentration of 0.05%, has been shown to induce hypersensitivity, while a fragrance blend containing 0.09% eugenol has been shown to elicit a reaction in a patch test (Rothenstein et al. 1983).
The NR assay used for these cytotoxicity studies quantifies membrane integrity (Cornelis et al. 1992) and it has been suggested that tissue damage by essential oils may be related to membrane lysis and surface activity. A previous report of eugenol cytotoxicity to mouse fibroblasts also implicates direct cell damage, in particular to the cell membrane phospholipids, as a mechanism (Hume 1984). Studies with eugenol, thymol and menthol on rat erythrocytes and hepatocytes suggest that their penetration into tissue may be dependant on membrane affinity and lipid solubility (Manabe et al. 1987).
The cytotoxicity of clove oil (or eugenol) may therefore be a result of cell membrane attack leading to necrotic or apoptotic cell death, depending on the nature of the injury. While membrane damage and subsequent cell lysis seem to be a likely mechanism given the nature of the materials in question, apoptosis has also previously been suggested as a mechanism in the observed cytotoxicity of the essential oil components, carvacrol, carvone and cinnamaldehyde (Stammati et al. 1999). Other support for a pro‐apoptotic action of essential oils and their components comes from observations of mitochondrial damage (Park et al. 2005; Yoo et al. 2005) and membrane ion flux following treatment with essential oils and their components. Apoptosis requires K+ and Cl− efflux (Segal & Beem 2001) and eugenol‐mediated leakage of intracellular potassium has been reported from studies on rat liver slices (Thompson et al. 1995). Ca2+ conversely, accumulates in dead and dying cells and appears to be directly involved in the regulation of cell injury and apoptosis (Orrenius et al. 1989). Previous studies on yeast (Saccharomyces cerevisiae) cells show release of K+ and Mg2+ but no release of Ca2+ following treatment with oils and components (Bard et al. 1988; Prashar et al. 2003). A possible mechanism for these ion fluxes may be a result of an inhibitory action of clove oil on membrane Na+‐K+‐ATPase, which has been attributed to eugenol (Kreydiyyeh et al. 2000). Further experiments are, however, required before these data can be interpreted unequivocally.
Another proposed mechanism for eugenol cytotoxicity is the intracellular formation of a quinone methide, resulting in the depletion of intracellular glutathione, and its covalent binding to cellular proteins in hepatocytes (Thompson et al. 1989). This is supported by the work of Jeng et al. (1994) who observed the depletion of intracellular glutathione in oral fibroblasts treated with eugenol. These studies would imply that metabolic activation of eugenol to a quinone methide may be necessary for its cellular toxicity but, although a number of peroxidase enzymes capable of catalysing this are present in mammalian cells, it is not yet known if this occurs in vivo (Thompson et al. 1989).
Further research is clearly required to establish the mode of action of essential oils and their components, but the cytotoxicity data from this study suggests that essential oils or their components should be used with care and in highly diluted forms, especially when directly applied to the skin.
REFERENCES
- Atsumi T, Fujisawa S, Satoh K, Sakagami H, Iwakura I, Ueha T, Sugita Y, Yokoe I (2000) Cytotoxicity and radical intensity of eugenol, isoeugenol or related dimers. Anticancer Res. 20, 2519–2524. [PubMed] [Google Scholar]
- Babich H, Stern A, Borenfreund E (1993) Eugenol cytotoxicity evaluated with continuous cell lines. Toxicol. In Vitro 7, 105–109. [DOI] [PubMed] [Google Scholar]
- Bard M, Albrecht MR, Gupta N, Guynn CJ, Stillwell W (1988) Geraniol interferes with membrane functions in strains of Candida and Saccharomyces . Lipids 23, 534–538. [DOI] [PubMed] [Google Scholar]
- Barnes J (2003) Quality, efficacy and safety of complementary medicines: fashions, facts and the future. Part II: Efficacy and safety. Br. J. Clin. Pharmacol. 55, 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneton J (1995) Pharmacognosy, Phytochemistry, Medicinal Plants. Hampshire: Intercept Limited. [Google Scholar]
- Cornelis M, Dupont C, Wepierre J (1992) Prediction of eye irritancy potential of surfactants by cytotoxicity tests in vitro on cultures of human skin fibroblasts and keratinocytes. Toxicol. In Vitro 6, 119–128. [DOI] [PubMed] [Google Scholar]
- Friedman M, Henika PR, Mandrell RE (2002) Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica . J. Food Prot. 65, 1545–1560. [DOI] [PubMed] [Google Scholar]
- Hallahan DL (2000) Monoterpenoid biosynthesis in glandular trichomes of Labiate family. Adv. Bot. Res. 31, 77–119. [Google Scholar]
- Hayes AJ, Markovic B (2002) Toxicity of Australian essential oil Backhousia citriodora (lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem. Toxicol. 40, 535–543. [DOI] [PubMed] [Google Scholar]
- Hili P, Evans CS, Veness RG (1997) Antimicrobial action of essential oils: the effect of dimethylsulphoxide on the activity of cinnamon oil. Lett. Appl. Microbiol. 24, 269–275. [DOI] [PubMed] [Google Scholar]
- Hume WR (1984) Effect of eugenol on respiration and division in human pulp, mouse fibroblasts, and liver cells in vitro . J. Dent. Res. 63, 1262–1265. [DOI] [PubMed] [Google Scholar]
- Jeng JH, Hahn LJ, Lu FJ, Wang YJ, Kuo MYP (1994) Eugenol triggers different pathobiological effects on human oral mucosal fibroblasts. J. Dent. Res. 73, 1050–1055. [DOI] [PubMed] [Google Scholar]
- Kreydiyyeh SI, Usta J, Copti R (2000) Effect of cinnamon, clove and some of their constituents on the Na+‐K+‐ATPase activity and alanine absorption in the rat jejunum. Food Chem. Toxicol. 38, 755–762. [DOI] [PubMed] [Google Scholar]
- Lis‐Balchin M (1995) Aroma Science, the Chemistry and Bioactivity of Essential Oils. England: Amberwood Publishing, Ltd. [Google Scholar]
- Manabe A, Nakayama S, Sakamoto K (1987) Effects of essential oils on erythrocytes and hepatocytes from rats and dipalmitoyl phosphatidylcholine‐liposomes. Jpn. J. Pharmacol. 44, 77–84. [DOI] [PubMed] [Google Scholar]
- Orrenius S, McConkey DL, Bellomo G, Nicotera P (1989) Role of Ca2+ in toxic cell killing. Trends Pharmacol. Sci. 10, 281–285. [DOI] [PubMed] [Google Scholar]
- Park BS, Song YS, Yee SB, Lee BG, Seo SY, Park YC, Kim JM, Kim HM, Yoo YH (2005) Phospho‐ser 15‐p53 translocates into mitochondria and interacts with Bcl‐2 and Bcl‐xL in eugenol‐induced apoptosis. Apoptosis 10, 193–200. [DOI] [PubMed] [Google Scholar]
- Prashar A, Hili P, Veness RG, Evans CS (2003) Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae . Phytochemistry 63, 569–575. [DOI] [PubMed] [Google Scholar]
- Prashar A, Locke IC, Evans CS (2004) Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolif. 37, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenstein AS, Booman KA, Dorsky J, Kohrman KA, Schwoeppe EA, Sedlak RI, Steltenkamp RJ, Thompson GR (1983) Eugenol and clove leaf oil: a survey of consumer patch‐test sensitisation. Food Chem. Toxicol. 21, 727–733. [DOI] [PubMed] [Google Scholar]
- Schmolka IR (1973) The synergistic effects of nonionic surfactants upon cationic germicidal agents. J. Soc. Cosmet. Chem. 24, 577–592. [Google Scholar]
- Segal MS, Beem E (2001) Effect of pH, ionic charge, and osmolality on cytochrome c‐mediated caspase‐3 activity. Am. J. Physiol. 281, C1196–C1204. [DOI] [PubMed] [Google Scholar]
- Stammati A, Bonsi P, Zucco F, Moezelaar R, Alakomi HL, Von Wright A (1999) Toxicity of selected plant volatiles in microbial and mammalian short‐term assays. Food Chem. Toxicol. 37, 813–823. [DOI] [PubMed] [Google Scholar]
- Thompson D, Norbeck K, Olsson L, Constantin‐Teodosiu D, Van der Zee J, Moldeus P (1989) Peroxidase‐catalyzed oxidation of eugenol: formation of a cytotoxic metabolite(s). J. Biol. Chem. 264, 1016–1021. [PubMed] [Google Scholar]
- Thompson DC, Perera K, Krol ES, Bolton JL (1995) o‐Methoxy‐4‐alkylphenols that form quinone methides of intermediate reactivity are the most toxic in rat liver slices. Chem. Res. Toxicol. 8, 323–327. [DOI] [PubMed] [Google Scholar]
- Tsuro M, Inoue M, Kameoka H (2001) Variation in essential oil components in regenerated lavender (Lavandula vera DC) plants. Sci. Hort. 88, 309–317. [Google Scholar]
- Wittstock U, Gershenzon J (2002) Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 5, 1–8. [DOI] [PubMed] [Google Scholar]
- Worwood VA (1996) The Fragrant Pharmacy. London: Transworld Publishers, Ltd. [Google Scholar]
- Yoo CB, Han KT, Cho KS, Ha J, Park HJ, Nam JH, Kil UH, Lee KT (2005) Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species‐mediated apoptosis in HL‐60 human promyelocytic leukemia cells. Cancer Lett. 225, 41–52. [DOI] [PubMed] [Google Scholar]


