Abstract
Objectives
Plant lectins, carbohydrate‐binding proteins of non‐immune origin, have recently been reported to induce programmed cell death (including apoptosis and autophagy) in many types of cancer cells. MicroRNAs (miRNAs), small, non‐coding endogenous RNAs, ∼22 nucleotides (nt) in length, have been well characterized to play essential roles in regulation of the autophagy process in cancer; however, how these miRNAs regulate autophagic pathways in plant lectin‐induced cancer cells, still remains an enigma.
Materials and methods
Identification of microRNA‐regulated autophagic pathways was carried out using a series of elegant systems – biology and bioinformatics approaches, such as network construction, hub protein identification, targeted microRNA prediction, microarray analyses and molecular docking.
Results
We computationally constructed the human autophagic protein–protein interaction (PPI) network, and further modified this network into a plant lectin‐induced network. Subsequently, we identified 9 autophagic hub proteins and 13 relevant oncogenic and tumour suppressive miRNAs, that could regulate these aforementioned targeted autophagic hub proteins, in human breast carcinoma MCF‐7 cells. In addition, we confirmed that plant lectins could block the sugar‐containing receptor EGFR‐mediated survival pathways, involved in autophagic hub proteins and relevant miRNAs, thereby ultimately culminating in autophagic cell death.
Conclusions
These results demonstrate that network‐based identification of microRNAs modulate autophagic pathways in plant lectin‐treated cancer cells, which may shed new light on the discovery of plant lectins as potent autophagic inducers, for cancer drug discovery.
Introduction
Plant lectins, a group of highly diverse non‐immune origin proteins, contain at least one non‐catalytic domain, which enables them to selectively recognize and reversibly bind to specific free sugars or glycans, present on glycoproteins and glycolipids, without altering structure of the carbohydrate 1, 2. Hitherto, they have been divided into twelve different families such as the GNA family, proteins with legume lectin domains, and the ricin‐B family that are well characterized, to result in programmed cell death (PCD), as potential anti‐tumour agents for cancer therapy 3, 4.
Apoptosis, or type I PCD, is well known to be a complex, but highly defined, program of cell demolition 5. Distinct from apoptosis, autophagy (type II PCD) refers to an evolutionarily conserved, multistep lysosomal degradation process in which a cell degrades long‐lived proteins and damaged organelles 6, 7. In cancer cells, apoptosis is often executed by caspases and thus contributes to cell death, whereas autophagy plays the Janus role, for regulation of death or survival signalling pathways, by a limited number of autophagy‐related genes (Atgs) 6. Under some circumstances, apoptosis and autophagy may participate in co‐regulation of cancer cell survival or death, thus, ultimately jointly sealing the fate of the cancer cell 5. Recently, plant lectins such as mistletoe lectins (ML‐1, 2 and 3), concanavalin A (ConA) and Polygonatum cyrtonema lectin (PCL) have been widely reported to induce apoptosis in various types of cancer cell 8, 9, 10, 11. Beside this, ConA and PCL have also been found to lead to autophagic cell death in cancers 12, 13.
Of note, microRNAs (miRNAs), small, non‐coding endogenous RNAs ∼22 nucleotides (nt) in length, may play key regulatory roles in apoptotic and autophagic pathways of human cancer cells 14, 15. Accumulating evidence has recently revealed that oncogenic miRNAs such as miRNA‐21, miRNA‐17‐92 cluster, miRNA‐221/222 and miRNA‐272/273 can negatively regulate apoptosis and thus promote cancer initiation and progression 15. On the other hand, tumour suppressive miRNAs including miR‐15a‐miR‐16‐1 cluster, miR‐29 and let‐7 family can positively modulate apoptosis in final decisions of cancer cell death 15. Moreover, miRNAs (except for miR‐101) can negatively regulate autophagy, acting either as oncogenes (miR‐183, miR‐376b, miR‐106a, miR‐221/222, miR‐31 and miR‐34c) or tumour suppressors (miR‐30a, miR‐101 and miR‐9*) 14, 15.
Intriguingly, Chinese mistletoe lectin‐I (CMI) has for the first time, been reported to induce apoptosis in colorectal cancer cells, by downregulating miR‐135a&b expression and upregulating expression of their target gene adenomatous polyposis coli (APC), thereby, reducing activity of its downstream Wnt signalling 16. However, to our knowledge, no report has been produced that any plant lectin induces cancer cell autophagic death, implicated in miRNA regulation.
In this study, we constructed the human autophagic PPI network and modified it to a plant lectin‐induced cancer cell death context. Subsequently, we identified 9 autophagic hub proteins and 13 targeted microRNAs, in MCF‐7 cells. Subsequently, we analysed druggability of two typical plant lectins ConA and PCL, by binding epidermal growth factor receptor (EGFR) on the surface of cancer cells, and blocking its downstream survival signalling pathways involved in core autophagic regulators and relevant targeted miRNAs, thereby finally leading to autophagic cell death.
Materials and methods
Data processing and network construction
To construct the global human PPI network, we collected diverse PPIs from 6 human PPI databases, including the Human Protein Reference Database (HPRD) 17, Biomolecular Object Network Databank (BOND) 18, IntAct 19, HomoMINT 20, BioGRID 21 and Database of Interacting Proteins (DIP) 22. Subsequently, we built the autophagic PPI network according to the condition that there is at least one autophagic protein, by Gene Ontology (GO) consortium, in a pair of proteins. Then, we further extracted known hub autophagic proteins (Table S1) and their related pathways, based on previous reports that these proteins are involved in plant lectin‐induced cancer cell autophagic death 4. Accordingly, these above‐mentioned hub proteins can connect with one another to form the modified autophagic subnetwork, in which at least one protein can interact with the other, in the plant lectin‐treated cancer context.
Multiple analyses of hub proteins
We determined whether or not an autophagic protein is a hub one, and followed the three gold standards, as follows. First, degree of each protein in function‐related networks, was calculated as the number of links that one protein possessed with the other 23. Hub proteins, identified as their levels of high degree were extracted, based on the assumption that high‐degree proteins tended to play a crucial role in the autophagic PPI network. Secondly, the hub proteins should connect known autophagy‐related proteins in lectin‐induced cancer cells 4, thereby making them worth particular focus when developing novel autophagy‐related targets. This method was similar to a previous study that focused on identifying novel cancer‐related genes 24. Thirdly, the network module of the autophagic model is crucial for identification of hub proteins as they often enrich in the ‘dense area′ 25. In this study, we focused on elucidating autophagic hub proteins in plant lectin‐induced cancer cells; thus, combination of known autophagic hub proteins into this context (Table S1) and the aforementioned three gold standards, can together confirm the of autophagic hub proteins.
Targeted microRNA prediction
As available prediction methods have strongly varying degrees of sensitivity and specificity, we developed a combinatory approach that could be predicted by the consensus results from TargetScan 26, MiRanda 27 and Diana‐MicroH 28.
Microarray analyses of autophagic genes and microRNAs
Proteins which can interact with one another often possess similar gene expression patterns, thereby genes that can co‐express should be more likely to interact than those that cannot co‐express. To identify whether certain genes were co‐expressed or not, we used microarray data (No. E‐GEOD‐26459), treated with 2.5 mm DTT, to measure pair‐wise co‐expression genes in autophagy, in tamoxifen treated MCF‐7 cells 29.
Microarray data were carried out three times repeatedly, in control and experimental groups. The E‐GEO database provided preliminary data that were further corrected and standardized. Thus, data pre‐processing would meet standards by significance analysis of microarrays (SAM) 30, and the correspondingly identified probes were by ID from Uniprot database. As the preliminary experimental data did not make log2 processes concerned with the expression data, we set the log2 conduct and T‐test in the statistics model, and took advantage of ‘Two Class (Unpaired)′ model in SAM for analyses of different gene expressions.
Thanks to the above analyses, we used the Delta value favoured by SAM to locate 0.13 and listed all the different expression (upregulation or downregulation) genes. First, we made all data undergo log2 alteration then normalization, and used the correlation and average linkage models to cluster the data (Prior to this analysis, we extracted these gene expression data). When the value of expression data was two, we used the average value, whereas when it was three or more, we took the median value. Finally, we evaluated the microarray data that were delineated into the SAM plot sheet (Figure S1). We also used Cluster 31 and TreeView 32 to gather all the different expression genes for subsequent analyses.
Moreover, we used a similar approach to identify microRNAs that can co‐express. We chose microarray data (No. E‐GEOD‐28267) that were acquired from MCF‐7 cells, treated with 2.5 mm DTT to measure co‐expression miRNAs in cancer, treated with tamoxifen 33.
Molecular docking
Molecular structures of EGFR were downloaded from PDB (untethered EGFR: 1IVO, and tethered EGFR: 1YY9) and molecular structures of plant lectins Concanavalin A (ConA) and Polygonatum cyrtonema lectin (PCL) are also downloaded from PDB (ConA: 3cna and PCL: 3A0E). All PPIs were carried out by ZDOCK and Hex Protein Docking Server, respectively 34, 35.
Results
Construction of the human autophagic network
In this study, we computationally constructed the global human PPI network (about 85,806 protein pairs) covering almost all PPIs, based on IntAct, HPRD, HomoMINT, BOND, BioGRID and DIP (Fig. 1a). To construct the set of true‐positive gene pairs, physical PPIs were derived from these human PPI databases that include 37 710 protein pairs (8982 proteins) from BioGRID, 8044 protein pairs (4073 proteins) from BOND, 14 892 protein pairs (6240 proteins) from HomoMINT, 39 044 protein pairs (9614 proteins) from HPRD, 34 935 protein pairs (8849 proteins) from IntAct and 12 809 protein pairs (4818 proteins) from DIP. These results show that a total number of unique 2633 protein pairs were prepared, as the autophagic PPI network (Fig. 1b).
Figure 1.
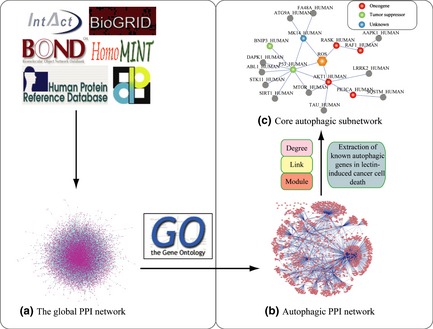
Network‐based identification of hub proteins in plant lectin‐induced autophagy. (a) Global human autophagic PPI network: All PPIs were acquired from Human Protein Reference Database (HPRD), Biomolecular Object Network Databank (BOND), IntAct, HomoMINT, BioGRID and Database of Interacting Proteins (DIP); (b) Autophagic PPI network construction; (c) Core autophagy‐related signalling pathways in plant lectin‐treated cancer cells: Hub autophagic proteins are achieved due to the following four standards: degree, link, module and known autophagic genes, in plant lectin‐induced cancer cells.
Identification of core autophagic pathways in lectin‐treated cancer cells
According to data from Table S1, we extracted all PPIs including oncogenes (PI3KCI, Akt‐1, k‐Ras, Raf‐1), tumour suppressors (TP53, BNIP‐3) and others – including p38 (MAPK14) from the autophagic PPI network. Subsequently, we found that PI3KCI, Akt‐1, TP53, BNIP‐3 and p38 (MAPK14) could interact with other proteins, implicated in autophagic pathways. As reactive oxygen species (ROS) have been known to be involved in plant lectin‐induced autophagic cell death 4, we manually addicted ROS and their relevant autophagic pathways (for example, k‐Ras and Raf‐1) into the autophagic pathways. Thus, combined with three gold standards and the data from Table S1, our result demonstrates that the autophagic pathways are composed of 18 autophagic‐related proteins and ROS (total number of 21 interactions) (Fig. 1c). For instance, the degree of AAPK1 is 37; its link to autophagic proteins from GO annotation is 7 and is located in the dense area (network module area). Moreover, the degree of ATG9A is 8; its link is 2 and located in the dense area (module). Also, the degree of FA48A is 6; its link is 3 and located in the sparse area.
Predicted microRNAs targeting autophagy in MCF‐7 cells
On the basis of above‐mentioned 18 autophagic‐related proteins, we used TargetScan to predict 134 targeted miRNAs, MiRanda to predict 731 targeted miRNAs and Diana‐MicroH to predict 343 targeted miRNAs respectively (Fig. 2a). Subsequently, we integrated these predicted the consensus results that 13 miRNAs, including hsa‐miR‐155, hsa‐miR‐15b, hsa‐miR‐15a, hsa‐miR‐424, hsa‐miR‐16, hsa‐miR‐195, hsa‐miR‐19a, hsa‐miR‐19b, hsa‐miR‐137, hsa‐miR‐96, hsa‐miR‐506, hsa‐miR‐218 and hsa‐miR‐205, were shown to target hub proteins, (k‐Ras, Raf‐1, PI3KCI, AAPK1, Atg9A, DAPK1, FA48A and LRRK2) (Fig. 2b).
Figure 2.
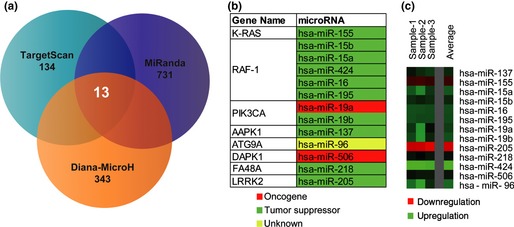
Targeted micro RNA prediction and microarray analyses. (a) Prediction of microRNAs targeting autophagic hub proteins by a combination method including TargetScan, MiRanda and Diana‐MicroH; (b) Novel candidate oncogenic or tumour suppressive microRNAs; (c) Differential expression profiling of microRNAs and their relevance in human breast carcinoma MCF‐7 cells.
SAM was performed on data of different expression microarrays from tamoxifen‐treated human breast adenocarcinoma MCF‐7 cells' autophagic stress, to identify divergent expression genes between normal and cancer cells (Fig. 2c). We indicated that these proteins, identified as divergent expression proteins that were extracted as functional hub proteins, dependent on gene co‐expression profiles, thus play their regulatory roles as potential targets (Fig. 3a). Importantly, we confirmed that new oncogenic and tumour suppressive miRNA‐modulated autophagic pathways were involved in MCF‐7 cells. As a result, one autophagic k‐Ras‐Raf‐1‐AAPK‐1 pathway could be predicted to be regulated by hsa‐miR‐155 (targeting k‐Ras), hsa‐miR‐15a/b, hsa‐miR‐16, hsa‐miR‐195 and hsa‐miR‐424 (targeting Raf‐1) and hsa‐miR‐137 (targeting AAPK‐1). The other autophagic p53‐p38‐ATG9A/FA48A pathway could be predicted to be modulated by hsa‐miR‐96 (targeting ATG9A) and hsa‐miR‐218 (targeting FA48A) (Fig. 3b).
Figure 3.
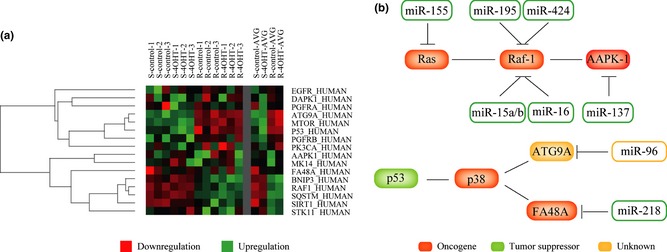
Micro RNA ‐modulated autophagic pathways in cancer. (a) Differential expression profiling of autophagic genes and their relevance in human breast carcinoma MCF‐7 cells; (b) MicroRNA‐modulated autophagic pathways in human breast carcinoma MCF‐7 cells: tumour suppressive microRNA‐modulated Ras‐Raf‐AAPK‐1 signalling pathway; oncogenic microRNA‐regulated p53‐p38‐ATG9A/FA48A signalling pathways.
Plant lectins as autophagy inducers for drug discovery
Within the context of plant lectin‐induced autophagic death, we finally predicted 9 hub proteins and 13 relevant targeted miRNAs in MCF‐7 cells. According to these results, we used bibliometric methods such as Kyoto Encyclopedia of Genes and Genomes (KEGG) search to analyse the key sugar‐containing receptors on the surface of the cancer cells (for example, EGFR), and subsequently analysed EGFR‐mediated survival signalling pathways implicated in autophagic hub proteins and related miRNAs.
On the one hand, we found that plant lectins such as ConA and PCL could bind most (about 95%) of the unliganded EGFR, that may exist in a compact auto‐inhibited or tethered conformation, in which domains II and IV form an intramolecular interaction or tether in cancer cells (Fig. 4A). Also, we demonstrated that plant lectins could possess stronger affinity than the ligands of EGFR (EGF, TGF‐α, HB‐EGF and more), or have similar binding capabilities with them by ZDOCK in Table S2 (higher score means better binding capability) and Hex Protein Docking Server in Table S3 (lower energy value means better binding capability) respectively. Thus, it is suggested that plant lectins may block EGFR‐mediated survival signalling pathways involving BCR‐ABL, PI3K‐Akt‐mTOR and Ras‐Raf signalling, in advance, thereby affecting binding of the inherent ligands of EGFR.
Figure 4.
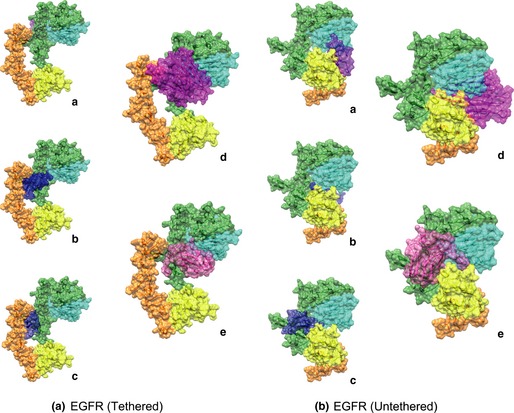
Plant lectins may bind epidermal growth factor receptor ( EGFR ) thus blocking its downstream survival pathways. The typical legume lectin ConA and GNA family lectin PCL may bind the sugar‐containing receptor EGFR on the surface of cancer cells. (A) Tethered monomers: (a) EGF, (b) TGF‐α, (c) HB‐EGF, (d) ConA and (e) PCL; (B) Untethered monomers: (a) EGF, (b) TGF‐α, (c) HB‐EGF, (d) ConA and (e) PCL.
Yet, in the remaining 5% of unliganded molecules, some of these ligands bind preferentially to untethered molecules (Fig. 4B). Within this context, we found that plant lectins could competitively bind EGFR compared to EGF, TGF‐α, HB‐EGF, and more, afterwards. Therefore, this result indicates that plant lectins bear stronger affinity to EGFR than to its inherent ligands, suggesting that plant lectins (ConA and PCL) may block the EGFR‐mediated survival pathways, thereby, ultimately resulting in autophagic cell death.
Discussion
Hitherto, accumulating evidence has revealed that plant lectins such as ConA and PCL can lead to cancer cell death for regulation of apoptotic and autophagic pathways 3, 4. CM‐I has first been reported to induce cancer cell apoptosis by downregulating miR‐135a&b expression and thus upregulating their target gene APC 16. Interestingly, microRNAs, as autophagic modulators, have not been found to be involved in plant lectin‐induced autophagic death of human cancers.
In our study, we constructed the autophagic PPI network, on the basis of 6 online human PPI databases. A recent study has demonstrated that proteomic analysis of the autophagy interaction network in human cells, under conditions of basal autophagy, revealed a network of 751 interactions among 409 candidate interacting proteins with extensive connectivity among subnetworks. New autophagy interaction network components have their roles in vesicle trafficking, protein or lipid phosphorylation and protein ubiquitination, and affect autophagosome number or flux 36. Different from the above‐mentioned study, we reported herein that our human autophagic PPI network composed of 2633 protein interactions, thus indicating more complicated relationships among these autophagy‐related proteins, in the context of basic autophagic networks.
It is well known that the PPI network is typically complex for its nature, with multiple connections amongst numerous signalling pathways; therefore, it is necessary to represent the networks by further integrating and analyzing these high‐throughput data in a specific biological context, such as in cancer 37, 38. Plant lectins, as potential anti‐tumour agents, have recently been reported to induce autophagic cell death towards various types of cancer cells 3, 4. From the autophagic PPI network, we extracted all the PPIs including PI3KCI, Akt‐1, k‐Ras, Raf‐1, TP53, BNIP‐3 and p38 (MAPK14) in lectin‐induced cancer cells, and further integrated their relevant interactions into the core autophagic subnetwork in cancer.
Recent reports have demonstrated that ConA induces autophagic death in hepatoma cells through a mitochondria‐mediated pathway. After associating with the mannose moiety residing on the cell membrane glycoprotein, ConA was preferentially internalized to mitochondria via clathrin‐mediated endocytosis, and then autophagic cell death was initiated 11. A further study has reported that PCL induces cancer cell autophagy by promoting the ROS‐p38‐p53 signalling pathway, as well as blocking Ras‐Raf‐1 and PI3KCI‐Akt signalling pathways 39, 40. On the basis of these signalling molecules, we constructed the autophagic subnetwork in plant lectin‐induced autophagic death. These predicted autophagic signalling pathways may provide a new clue for further elucidating the complicated autophagic mechanisms in plant lectin‐treated cancer cells.
In the current study, we predicted 13 miRNAs that could target 9 autophagic hub proteins and further refined a few oncogenic and tumour suppressive miRNAs, by microarray analyses, in MCF‐7 cells. According to miRNA differential expressions between tumour tissues and normal ones, miRNAs are aberrantly expressed that are recognized as oncogenic ones, while others are down‐expressed – namely, tumour suppressors 41. Accumulating data have revealed that miRNAs can regulate the autophagy process, and are involved in cancer initiation and progression, which could provide new perspectives for exploring more miRNAs in autophagy regulation 41, 42.
So far, numerous studies have reported that the TP53‐MDMX negative feedback loop constitutes the core module of network of regulatory interactions in cancer cell death 41. The TP53‐MDMX negative feedback loop – the putative cause of oscillations – is embedded in a network involving a positive feedback loop between TP53 and Akt 43, 44, 45. Herein, we found that oncogenic pathways such as k‐Ras‐Raf‐1‐AAPK‐1 could be inhibited by tumour suppressive miRNAs such as hsa‐miR‐155, hsa‐miR‐15a/b, hsa‐miR‐195, hsa‐miR‐424 and hsa‐miR‐137, which may indicate the dynamic oscillations of tumour suppressive miRNA‐oncogene feedback loops. On the other hand, we found that other oncogenic miRNAs (hsa‐miR‐96 and hsa‐miR‐218) could negatively regulate tumour suppressive pathways such as p53‐p38‐ATG9A/FA48A pathway. Thus, the miRNA‐gene oscillation may predict all‐or‐none switching behaviour between pro‐survival and pro‐death states in cancer cells.
Of note, EGFR is a receptor tyrosine kinase of the ErbB family that is abnormally activated in many types of human cancers 46, 47. Molecular mechanisms lead to the receptor's aberrant activation that is often observed in cancer, including receptor overexpression, ligand‐dependent receptor dimerization and overexpression of receptor ligands 48. In our study, we found that plant lectins could bind EGFR, which could modulate most of our autophagic hub proteins and relevant microRNAs, and subsequently impeded the sugar‐containing receptor‐mediated survival pathways, thereby, leading to autophagic cell death (Fig. 5). To sum up, plant lectin could be utilized as a potent autophagic inducer via targeting EGFR‐mediated survival pathways for drug discovery.
Figure 5.
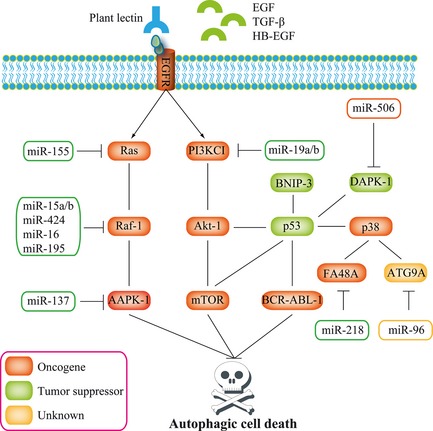
EGFR ‐mediated signalling pathways implicated in micro RNA regulation in plant lectin‐induced autophagic death.
In summary, we constructed the autophagic PPI network, in silico, modified this network into a plant lectin‐induced context, and further identified autophagic hub proteins and relevant microRNAs in MCF‐7 cells. Subsequently, we confirmed that plant lectins could bind the sugar‐containing receptor EGFR, thus modulating the EGFR‐mediated autophagic pathways. Accordingly, these findings may provide a new perspective of microRNA‐regulated autophagic pathways in plant lectin‐induced cancer cell death, which would uncover multifaceted roles of plant lectins as potent autophagic inducers for future cancer therapeutics.
Supporting information
Fig. S1 Evaluation of microarray data from human breast adenocarcinoma MCF‐7 cells.
Table S1 Known autophagic genes involved in plant lectin‐induced cancer cell death.
Table S2 Comparisons between plant lectins and ligands via binding EGFR by ZDOCK
Table S3 Comparisons between plant lectins and ligands via binding EGFR by Hex Protein Docking Server.
Acknowledgements
We are grateful to Qian Liu (National University of Singapore), He‐jiao Bian (Boston University) and Ming‐wei Min (University of Cambridge) for their critical reviews on this manuscript. We also thank Jun‐jie Liu (Tsinghua University) and Qi‐jia Yu (Sichuan University) for their good suggestions. This work was supported in part by the grants from Young teacher's fund of Sichuan University (No. 2010SCU11066), the Science Foundation for Post Doctorate Research of China (No. 20110491725) and the Major State Basic Research Development Program of China (973 Program) (No. 2010cb529900).
L.‐L. Fu, X. Zhao and H.‐L. Xu contributed equally to this work.
References
- 1. Van Damme EJM, Lannoo N, Peumans WJ (2008) Plant lectins. Adv. Bot. Res. 48, 107–209. [Google Scholar]
- 2. Van Damme EJM, Peumans WJ, Barre A, Rouge P (1998) Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 17, 575–692. [Google Scholar]
- 3. Liu B, Bian HJ, Bao JK (2010) Plant lectins: potential antineoplastic drugs from bench to clinic. Cancer Lett. 287, 1–12. [DOI] [PubMed] [Google Scholar]
- 4. Fu LL, Zhou CC, Yao S, Yu JY, Liu B, Bao JK (2011) Plant lectins: targeting programmed cell death pathways as antitumor agents. Int. J. Biochem. Cell. Biol. 43, 1442–1449. [DOI] [PubMed] [Google Scholar]
- 5. Wen X, Lin ZQ, Liu B, Wei YQ (2012) Targeting caspase‐mediated programmed cell death pathways for cancer therapy. Cell. Proliferat. 45, 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu B, Cheng Y, Liu Q, Bao JK, Yang JM (2010) Autophagic pathways as new targets for cancer drug development. Acta Pharmacol. Sin. 31, 1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang SY, Yu QJ, Zhang RD, Liu B (2011) Core signaling pathways of survival/death in autophagy‐related cancer networks. Int. J. Biochem. Cell. Biol. 43, 1263–1266. [DOI] [PubMed] [Google Scholar]
- 8. Hoessli DC, Ahmad I (2008) Mistletoe lectins: carbohydrate‐specific apoptosis inducers and immunomodulators. Curr. Org. Chem. 12, 918–925. [Google Scholar]
- 9. Liu B, Cheng Y, Zhang B, Bian HJ, Bao JK (2009) Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria‐mediated ROS‐p38‐p53 pathway. Cancer Lett. 275, 54–60. [DOI] [PubMed] [Google Scholar]
- 10. Liu B, Li CY, Bian HJ, Min MW, Chen LF, Bao JK (2009) Antiproliferative activity and apoptosis‐inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch. Biochem. Biophys. 482, 1–6. [DOI] [PubMed] [Google Scholar]
- 11. Li WW, Yu JY, Xu HL, Bao JK (2011) Concanavalin A: a potential anti‐neoplastic agent targeting apoptosis, autophagy and anti‐angiogenesis for cancer therapeutics. Biochem. Biophys. Res. Commun. 414, 282–286. [DOI] [PubMed] [Google Scholar]
- 12. Chang CP, Yang MC, Liu HS, Lin YS, Lei HY (2007) Concanavalin A induces autophagy in hepatoma cells and has a therapeutic effect in a murine in situ hepatoma model. Hepatology 45, 286–296. [DOI] [PubMed] [Google Scholar]
- 13. Liu B, Cheng Y, Bian HJ, Bao JK (2009) Molecular mechanisms of Polygonatum cyrtonema lectin‐induced apoptosis and autophagy in cancer cells. Autophagy 5, 253–255. [DOI] [PubMed] [Google Scholar]
- 14. Fu LL, Wen X, Bao JK, Liu B (2012) MicroRNA‐modulated autophagic signaling networks in cancer. Int. J. Biochem. Cell. Biol. 44, 733–736. [DOI] [PubMed] [Google Scholar]
- 15. Lima RT, Busacca S, Almeida GM, Gaudino G, Fennell DA, Vasconcelos MH (2011) MicroRNA regulation of core apoptosis pathways in cancer. Eur. J. Cancer. 47, 163–174. [DOI] [PubMed] [Google Scholar]
- 16. Li LN, Zhang HD, Zhi R, Yuan SJ (2011) Down‐regulation of some miRNAs by degrading their precursors contributes to anti‐cancer effect of mistletoe lectin‐I. Br. J. Pharmacol. 162, 349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mishra GR, Suresh M, Kumaran K, Kannabiran N, Suresh S, Bala P, et al (2006) Human protein reference database–2006 update. Nucleic Acids Res. 34, D411–D414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alfarano C, Andrade CE, Anthony K, Bahroos N, Bajec M, Bantoft K, et al (2005) The Biomolecular Interaction Network Database and related tools 2005 update. Nucleic Acids Res. 33, D418–D424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kerrien S, Alam‐Faruque Y, Aranda B, Bancarz I, Bridge A, Derow C, et al (2007) IntAct–open source resource for molecular interaction data. Nucleic Acids Res. 35, D561–D565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Persico M, Ceol A, Gavrila C, Hoffmann R, Florio A, Cesareni G (2005) HomoMINT: an inferred human network based on orthology mapping of protein interactions discovered in model organisms. BMC Bioinformatics 6(Suppl. 4), S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winter AG, Wildenhain J (2011) Tyers M. BioGRID REST Service, BiogridPlugin2 and BioGRID WebGraph: new tools for access to interaction data at BioGRID. Bioinformatics 27, 1043–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xenarios I, Rice DW, Salwinski L, Baron MK, Marcotte EM, Eisenberg D (2000) DIP: the database of interacting proteins. Nucleic Acids Res. 28, 289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agarwal S, Deane CM, Porter MA, Jones NS (2010) Revisiting date and party hubs: novel approaches to role assignment in protein interaction networks. PLoS Comput. Biol. 6, e1000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ostlund G, Lindskog M, Sonnhammer EL (2010) Network‐based Identification of novel cancer genes. Mol. Cell. Proteomics 9, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qi Y, Ge H (2009) Modularity and dynamics of cellular networks. PLoS Comput. Biol. 2, e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Betel D, Wilson M, Gabow A, Marks DS, Sander C (2008) MicroRNA target predictions: the microRNA.org resource: targets and expression. Nucleic Acids Res. 36(Database Issue): D149–D153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maragkakis M, Alexiou P, Papadopoulos GL, Reczko M, Dalamagas T, Giannopoulos G, et al (2009) Accurate microRNA target prediction correlates with protein repression levels. BMC Bioinformatics 10, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonzalez‐Malerva L, Park J, Zou L, Hu Y, Moradpour Z., Pearlberg J., et al (2011) High‐throughput ectopic expression screen for tamoxifen resistance identifies an atypical kinase that blocks autophagy. Proc. Natl. Acad. Sci. USA 108, 2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang S (2007) A comprehensive evaluation of SAM, the SAM R‐package and a simple modification to improve its performance. BMC Bioinformatics 8, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamada T, et al (2009) A novel multiple‐walk parallel algorithm for the Barnes–Hut treecode on GPUs – towards cost effective, high performance N‐body simulation. Comput. Sci. Res. Dev. 24, 21–31. [Google Scholar]
- 32. Page T (2002) Visualizing phylogenetic trees using TreeView. Curr. Protoc. Bioinformatics 6, Unit 6.2. [DOI] [PubMed] [Google Scholar]
- 33. Manavalan TT, Teng Y, Appana SN, Datta S, Kalbfleisch TS, Li Y, et al (2011) Differential expression of microRNA expression in tamoxifen‐sensitive MCF‐7 versus tamoxifen‐resistant LY2 human breast cancer cells. Cancer Lett. 313, 26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen R, Li L, Weng Z (2003) ZDOCK: an initial‐stage protein‐docking algorithm. Proteins 52, 80–87. [DOI] [PubMed] [Google Scholar]
- 35. Macindoe G, Mavridis L, Venkatraman V, Devignes MD, Ritchie DW (2010) HexServer: an FFT‐based protein docking server powered by graphics processors. Nucleic Acids Res. 38(Web Server issue), W445–W449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Behrends C, Sowa ME, Gygi SP, Harper JW (2010) Network organization of the human autophagy system. Nature 466, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ng AC (2010) Integrative systems biology and networks in autophagy. Semin. Immunopathol. 32, 355–361. [DOI] [PubMed] [Google Scholar]
- 38. Huett A, Goel G, Xavier RJ (2010) A systems biology viewpoint on autophagy in health and disease. Curr. Opin. Gastroenterol. 26, 302–309. [DOI] [PubMed] [Google Scholar]
- 39. Wang SY, Yu QJ, Bao JK, Liu B (2011) Polygonatum cyrtonema lectin, a potential antineoplastic drug targeting programmed cell death pathways. Biochem. Biophys. Res. Commun. 406, 497–500. [DOI] [PubMed] [Google Scholar]
- 40. Liu B, Wu JM, Li J, Liu JJ, Li WW, Li CY, et al (2010) Polygonatum cyrtonema lectin induces murine fibrosarcoma L929 cell apoptosis and autophagy via blocking Ras‐Raf and PI3K‐Akt signaling pathways. Biochimie 92, 1934–1938. [DOI] [PubMed] [Google Scholar]
- 41. Gandellini P, Profumo V, Folini M, Zaffaroni N (2011) MicroRNAs as new therapeutic targets and tools in cancer. Expert Opin. Ther. Targets 15, 265–279. [DOI] [PubMed] [Google Scholar]
- 42. Wang N, Xu HL, Zhao X, Wen X, Wang FT, Wang SY, Liu B, Bao JK (2012) Network‐based identification of novel microRNA‐regulated apoptotic pathways in cancer. Appl. Biochem. Biotechnol. 15, 265–279. [DOI] [PubMed] [Google Scholar]
- 43. Wee KB, Surana U, Aguda BD (2009) Oscillations of the p53‐Akt network: implications on cell survival and death. PLoS One 4, e4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knight ZA, Lin H, Shokat KM (2010) Targeting the cancer kinome through polypharmacology. Nat. Rev. Cancer 10, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu JJ, Lin M, Yu JY, Liu B, Bao JK (2011) Targeting apoptotic and autophagic pathways for cancer therapeutics. Cancer Lett. 300, 105–114. [DOI] [PubMed] [Google Scholar]
- 46. Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 7, 301–311. [DOI] [PubMed] [Google Scholar]
- 47. Han W, Lo HW (2012) Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 318, 124–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu QJ, Li ZY, Yao S, Ming M, Wang SY, Liu B, Bao JK (2011) In silico analysis of molecular mechanisms of GNA‐related lectin‐induced cancer cell death from carbohydrate‐binding motif evolution hypothesis. Appl. Biochem. Biotechnol. 165, 1037–1046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Evaluation of microarray data from human breast adenocarcinoma MCF‐7 cells.
Table S1 Known autophagic genes involved in plant lectin‐induced cancer cell death.
Table S2 Comparisons between plant lectins and ligands via binding EGFR by ZDOCK
Table S3 Comparisons between plant lectins and ligands via binding EGFR by Hex Protein Docking Server.


