Abstract
Objectives
E quisetum arvense preparations have long been used to promote bone healing. The aim of this work was to evaluate osteogenic and antibacterial effects of E . arvense hydromethanolic extracts.
Materials and methods
Dried aerial components of E . arvense were extracted using a mixture of methanol:water (1:1), for 26 days, yielding three extracts that were tested (10–1000 μg/ml) in human osteoblastic cells: E1, E2 and EM (a mixture of E1 and E2, 1:1). Cell cultures, performed on cell culture plates or over hydroxyapatite (HA) substrates, were assessed for osteoblastic markers. In addition, effects of the extracts on S taphylococcus aureus were addressed.
Results
Solution E1 caused increased viability/proliferation and ALP activity at 50–500 μg/ml, and deleterious effects at levels ≥1000 μg/ml. E2 inhibited cell proliferation at levels ≥500 μg/ml. EM presented a profile between those observed with E1 and E2. In addition, E1, E2 and EM, 10–1000 μg/ml, inhibited expansion of S . aureus. Furthermore, E1, tested in HA substrates colonized with osteoblastic cells, causing increase in cell population growth (10–100 μg/ml). E1 also exhibited antibacterial activity against S . aureus cultured over HA.
Conclusions
Results showed that E . arvense extracts elicited inductive effects on human osteoblasts while inhibiting activity of S . aureus, suggesting a potentially interesting profile regarding bone regeneration strategies.
Introduction
Bone repair and regeneration are required in many clinical conditions, including non‐union bone defects in long bones and massive bone loss, caused by trauma or osteoporosis. To compensate for limitation imposed by use of autogenous and allogeneic preparations, a number of biomaterials has been formulated to functionally repair or replace bone tissue 1. Among them, hydroxyapatite (HA), chemical formulation Ca10(PIO4)6(OH)2 and Ca to P ratio of 5:3, exhibits similar chemical and crystallographic structure to bone inorganic apatite, and is the most widely used biomaterial for clinical bone regeneration and repair 1. In addition, a variety of bioactive molecules, either incorporated in the material or applied simultaneously, is being tested to promote migration, proliferation and differentiation of bone cells, to induce regeneration at the bone/material interface 2. Typically, these molecules are expensive growth factors which is a serious limitation to widespread application. The use of phytomedicines in bone regeneration strategies begins to be regarded as a potential alternative. There is good evidence from both in vitro and in vivo studies that a variety of plant products has positive effects on bone metabolism 3, 4, 5, 6, and effectiveness of adding herbal extracts to bone substitutes has already been reported 7, 8, 9.
A further important issue in biomaterial‐mediated bone regeneration is the possibility of bacterial adhesion to the material (which causes material‐related infections), and the lack of successful tissue integration or compatibility with biomaterial surfaces, resulting in significant associated morbidity 10. Treatment of these conditions remains a great challenge due to poor antibiotic distribution at the site of infection, because of limited blood circulation to surrounding skeletal tissue 10. This emphasizes the importance of infection prophylaxis, with preferably, the use of a local antimicrobial approach, a strategy with advantages over the current practice of routine systemic administration 10. Using conventional drugs, there is an increasing problem of microbial resistance and a continuous need for new solutions. Regarding this, many hundreds of plants worldwide are used in traditional medicine as treatments for bacterial infections and some of these have also been subjected to in vivo screening 11.
Equisetum arvense (horsetail) is a well‐known and widespread pteridophyte of the northern hemisphere 12 and its sterile stems are used as medicines in various countries, constituting ‘Equiseti herba’ of European Pharmacopedias 13, 14. Equisetum arvense preparations have been reported to be diuretic, antioxidant, vasorelaxant, antinociceptive, anti‐inflammatory, haemostatic and sedative 13, 14, 15, 16, 17, 18. In the form of tincture (a preparation containing alcohol), E. arvense is used for its antiseptic properties 13, 14, 15 and previous studies have shown a broad antimicrobial spectrum of hydroalcoholic extracts 19 and essential oil 20, 21. In addition, E. arvense preparations are claimed to have beneficial effects on bone disorders 22 and to promote bone healing after bone surgery or fracture 13, 14, 15. Equisetum arvense itself has a highly complex pattern of phenolic compounds, namely various flavonoids, phenolic acids and phytosterols 16, 23. Investigations in animal models and in vitro experiments, as well as studies in humans, provide accumulating evidence of a positive impact of flavonoids on bone metabolism, suggesting that regular intake of these compounds may be important for long‐term bone health 24, 25. In addition, E. arvense has a high concentration of silica 26 and it has been suggested that this pays a significant contribution to its medicinal properties, particularly on bone disorders 13, 14, 15. Since the deprivation studies of Carlise et al. 27, 28, a variety of in vitro and in vivo studies has confirmed the relevance of Si in bone formation events, both in aqueous solution and in the form of calcium phosphate‐based biomaterials 29, 30, 31, 32.
Regarding bone regeneration strategies, association of a biomaterial with an agent that stimulates bone growth, while preventing material‐related infection would contribute to a more predictable and successful outcome. In this context, aim of the present study was to evaluate dose‐dependent profile of E. arvense hydromethanolic extracts on behaviour of human bone marrow osteoblasts, and also to assess their antibacterial activity, both in standard cell culture conditions and over hydroxyapatite‐dense substrates.
Materials and methods
Preparation of E. arvense extracts
Dried aerial components of E. arvense, coarsely cut into pieces, were acquired from a Portuguese herb retail outlet. This plant matter, previously minced into smaller pieces, was transferred into a glass container and compacted. The extracting liquid, methanol–water (1:1), was added to completely cover the plant components, and was set aside for 3 days as the maceration process. Then, the whole biological fluid was percolated and the extracting solution was collected and evaporated in a rotating evaporator, operating at 50 °C and under reduced pressure. The recovered solution was reused as extractor liquid, being added to the glass container again. This process elapsed continuously until exhaustion of the plant material (26 days). During the procedure, two extracts were collected: extract 1 (E1), yielded between days 4 and 10, and extract 2 (E2), yielded between days 11 and 26. Additionally, a mixture (EM, 1:1) of E1 and E2 was prepared. Extracts E1, E2 and EM were concentrated to dryness under reduced pressure, and respective residues were dissolved in dimethylsulphoxide (DMSO; Merck, Darmstadt, Germany) to obtain stock solutions of 100 mg/ml. These were sterilized by filtration through 0.2 μm Millipore filters and maintained in aliquots at −20 °C.
Preparation and characterization of hydroxyapatite samples
HA powder (Plasma Biotal, Tideswell, UK) was sieved to less than 75 μm and disc samples were prepared by uniaxial pressing at 200 MPa, using steel dies to obtain 8 mm diameter discs. These discs were then sintered at 1300 °C (using a ramp rate of 4 °C/min), with the temperature maintained for 1 h, followed by natural cooling inside the furnace. Phase identification was assessed by X‐ray diffraction (XRD) analysis (Siemens D5000: Munich, Germany, difractometer with CuKα radiation, λ = 1.5418 Å); data were collected between 25° and 40° using a step size of 0.02°, and count time of 2.5 s. For cell culture, discs were mechanically polished to final topology of 1 μm, using silicon carbide paper ultrasonically degreased and cleaned with ethanol, followed by deionized water, then sterilized by autoclaving prior to cell culture.
Cell culture experiments
Effect of E. arvense extracts on osteoblastic cells
Bone marrow was obtained from patients (25–45 years old) undergoing orthopaedic surgery, after informed consent. Bone marrow cells (hBMC) were cultured in λ‐minimum essential medium (λ‐MEM; Gibco, Life Technologies Ltd, Paisly, UK) containing 10% foetal bovine serum (Gibco), 100 IU/ml penicillin (Gibco), 2.5 μg/ml streptomycin (Gibco), 2.5 μg/ml amphotericin B (Gibco) and 50 μg/ml ascorbic acid (Sigma‐Aldrich, Missouri, USA). Cultures were maintained in a 5% CO2 humidified atmosphere at 37 °C, for 10/15 days until near confluence.
Adherent cells were then enzymatically detached using 0.04% trypsin and 0.025% collagenase, and further cultured (104 cell/cm2) in the same medium as described above, but were further supplemented with 10 mm β‐glycerophosphate (Sigma‐Aldrich), as a source of phosphate ions. After overnight incubation, extracts E1, E2 and EM were added at concentrations of 10, 50, 100, 500 and 1000 μg/ml, and cultures were continued for 21 days. In parallel, cell cultures were maintained in presence of the same final concentration of DMSO only (control). The concentration range tested was based on preliminary dose–response assays, which enabled exclusion of levels that caused no effect on cell behaviour, and also, those that caused rapid cell death. Control cultures (absence of extracts) were performed in parallel. Cultures were maintained in a 5% CO2 humidified atmosphere at 37 °C, until near confluence. Culture medium containing the extracts, was renewed over the culture time, twice a week. Complementary experimentation showed that DMSO, at the levels present in the extracts, did not affect cell viability/proliferation of hBMC over the 21‐day culture period. Cultures were characterized for cell viability/proliferation, and were observed using confocal laser scanning microscopy (CLSM). Extract E1, which revealed an inductive effect on cell proliferation, was also characterized for alkaline phosphatase (ALP) activity and ability to form a mineralized matrix.
Effect of Si in osteoblastic cells
Equisetum arvense has high silica content 26, and extract E1 (which resulted in higher osteoblast induction) was evaluated for Si concentration. Standard solutions, 10–75 ppm, were prepared by appropriate dilution of 1000 ppm Si stock solution (Riedel de Haen) with NaCl (20 mg/ml). Absorbance measurements were carried out at λ = 251.6 nm, using an atomic absorption spectrometer (GBC 904 AA) with an acetylene/nitrous oxide flame. Concentration of Si in the 100 mg/ml stock solution was 11.08 ± 0.81 μg/ml.
hBMC (104 cells/cm2) were seeded into 96‐well culture plates. After overnight incubation, Si was added at 0.005–1 μg/ml, levels covering those present in E1 solutions tested over hBMC. Cultures were maintained for 21 days, as described above. As there is little information regarding the exact chemical structure of silica in E. arvense, two different forms of Si were tested, namely the organic form tetraethilorthosilicate (C8H2OO4Si) and silicic acid. Si solutions were prepared from 100 mg/ml standard solutions of tetraethilorthosilicate (Merck) and 100 mg/ml silicic acid (Sigma) diluted in phosphate saline buffer. Cultures were characterized for cell viability/proliferation.
Effect of E. arvense extracts on osteoblastic cells cultured over hydroxyapatite
In a further set of experiments, extract E1 was tested on cultures of hBMC established over HA discs. hBMC (104 cell/cm2) were plated over the HA discs, placed in 48‐well culture plates, and cultured as described above. After overnight incubation to allow for cell adhesion, extract E1 was added, 10–1000 μg/ml, and cultures were handled as described. Colonized HA discs were observed by CLSM and characterized for cell viability/proliferation. In addition, cultures exposed to 50 and 500 μg/ml were evaluated for ALP activity and expression of osteoblastic‐related genes by RT‐PCR.
Characterization of cell behaviour
Cell viability/proliferation
Cell viability/proliferation was evaluated by reduction of tetrazolium salt MTT [3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide] (0.5 mg/ml), by viable cells to form a purple formazan product after 3 h incubation. Absorbance (A) was measured at 600 nm using a microplate reader (WS050 WellScan; Denley Instruments Ltd., Denley, Franklin, USA), after crystal solubilization in DMSO.
Total protein content and alkaline phosphatase activity
Cell cultures were washed twice in PBS, frozen at −20 °C, and evaluated at the end of the culture period. Total protein content and ALP activity were evaluated in cell lysates obtained by treatment of cell layers with 0.1% Triton‐X 100 in water. Protein content was assayed using Lowry's method with bovine serum albumin used as a standard. ALP activity was determined by hydrolysis of p‐nitrophenyl phosphate in alkaline buffer, pH 10.3, and colorimetric determination of the product (p‐nitrophenol) at 405 nm. Hydrolysis was carried out for 30 min at 37 °C. Results were expressed as nanomoles of p‐nitrophenol produced per min per μg of protein (nmolmin−1 μg protein−1).
Staining F‐actin cytoskeleton filaments and observation using CLSM
Cultures were fixed in 4% methanol free formaldehyde, permeabilized with 0.1% Triton (5 min, RT) and incubated in 10 mg/ml bovine serum albumin with 100 μg/ml RNAse (1 h, RT). F‐actin filaments were stained using Alexa‐Fluor‐conjugated phalloidin (1:100, 1 h, RT) and nuclei were counterstained with 10 μg/ml propidium iodide (10 min, RT). Fluorescence stained cultures were examined using CLSM (Leica TCP SP2 AOBS confocal microscope, Leica, Solms, Germany).
Staining phosphate deposits with von Kossa reagent
Cell cultures were fixed in 1.5% glutaraldehyde in 0.14 m sodium cacodylate buffer (pH 7.3, 10 min). Cell layers were covered with 10 mg/ml silver nitrate solution and were exposed to UV light for 1 h. After rinsing in deionized water, samples were incubated for 2 min in 50 mg/ml sodium thiosulphate solution. After washing once more in deionized water, phosphate deposits (stained black) were visualized using a phase‐contrast optical microscope (Nikon TMS, Nikon, Tokyo, Japan).
Scanning electron microscopy
For scanning electron microscopy (SEM) observation, colonized HA discs were fixed in 1.5% glutaraldehyde in 0.14 m sodium cacodylate buffer (pH 7.3, 10 min), then dehydrated in graded alcohols, critical‐point dried, sputter‐coated with gold and analysed using a Jeol JSM 35C scanning electron microscope (Jeol Ltd., Tokyo, Japan).
Total RNA extraction and RT‐PCR analysis
RT‐PCR was used to access expression of housekeeping gene for GAPDH (glyceraldehyde‐3‐phosphate dehydrogenase) and those for COL1 (collagen type 1), ALP, BMP‐2, M‐CSF, RANKL and OPG, in hBMC grown for 21 days on surfaces of HA substrates. Total RNA was extracted using RiboPure™ Kit (Ambion, Austin, USA), according to manufacturer's instructions. Concentration and purity of total RNA in each sample were determined by UV spectrophotometry at 260 nm and by calculating the A260 nm/A280 nm ratio, respectively. RT‐PCR was performed using the Titan One Tube RT‐PCR System from Roche Applied Science, according to manufacturer's instructions. Briefly, 0.5 μg total RNA from each sample was reverse transcribed into cDNA (30 min at 50 °C), while PCR was performed with an annealing temperature of 55 °C, for 25 cycles; primers used are listed in Table 1. PCR products were then electrophoresed in 1% agarose gel and stained with ethidium bromide. Densitometric analyses were performed with ImageJ 1.41 software and normalized for the corresponding GAPDH value of each experimental condition.
Table 1.
Primers used on RT‐PCR analysis of hBMC cultures established on HA discs
| Gene | 5′ Primer | 3′ Primer |
|---|---|---|
| GAPDH | 5′‐CAGGACCAGGTTCACCAACAAGT‐3′ | 5′‐GTGGCAGTGATGGCATGGACTGT‐3′ |
| Collagen I | 5′‐TCCGGCTCCTGCTCCTCTTA‐3′ | 5′‐ACCAGCAGGACCAGCATCTC‐3′ |
| ALP | 5′‐ACGTGGCTAAGAATGTCATC‐3′ | 5′‐CTGGTAGGCGATGTCCTTA‐3′ |
| BMP‐2 | 5′‐GACGAGGTCCTGAGCGAGTT‐3′ | 5′‐GCAATGGCCTTATCTGTGAC‐3′ |
| M‐CSF | 5′‐CCTGCTGTTGTTGGTCTGTC‐3′ | 5′‐GGTACAGGCAGTTGCAATCA‐3′ |
| RANKL | 5′‐GAGCGCAGATGGATCCTAAT‐3′ | 5′‐TCCTCTCCAGACCGTAACTT‐3′ |
| OPG | 5′‐AAGGAGCTGCAGTACGTCAA‐3′ | 5′‐CTGCTCGAAGGTGAGGTTAG‐3′ |
Antibacterial activity of E. arvense extracts
Equisetum arvense E1, E2 and EM were tested against Staphylococcus aureus (ATCC 29213), Eschericha coli (ATCC 25922), Pseudomonas aeruginosa and Enterococcus (clinical isolated strains). Antibacterial susceptibility testing was performed using a broth microdilution method in accordance with the National Committee for Clinical Laboratory Standards recommendations. Antibacterial testing was performed using 24 h‐old broth cultures (overnight culture). Cell suspensions were adjusted to a 0.5 McFarland turbidity standard, for use inocula (Densimat, Biomérieux). Next, 5 μl of bacterial suspension was inoculated into each well of a 96‐well microplate containing dilutions of extracts, prepared in Muller‐Hinton liquid medium (250 μl) to obtain final concentrations of 10–1000 μg/ml. Cell population growth control and negative control were included in the same microplates. Cultures treated with DMSO, at concentrations present in the extracts, were performed in parallel. Plates were incubated at 37 °C for 24 h and turbidity was measured at 600 nm, using a microplate reader.
In a further experiment, extract E1 was tested for antibacterial activity in bacterial cultures plated over HA discs, using the same experimental protocol.
Statistical analysis
Data were obtained from three separate experiments, each one performed in triplicate, using cell cultures from different donors; cell responses to E. arvense extracts were identical from all. In each experiment, for quantitative assays, six replicates were accomplished. Data are expressed as mean ± standard deviation. Groups of data were evaluated using two‐way analysis of variance (ANOVA) and no significant differences in patterns of cell behaviour were found. Statistical differences between controls and experimental conditions were assessed by Bonferroni's method. Values of P ≤ 0.05 were considered significant.
Results
Effect of E. arvense extracts on behaviour of hBMC
Cell viability/proliferation, ALP activity
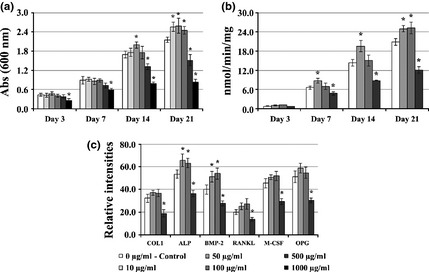
Extract E1 (Fig. 1a) caused stimulatory effects at 50 μg/ml, days 14 and 21 (in the order of 30% and 32%, respectively), and 100 μg/ml, day 21 (~39%). Levels of 500 and 1000 μg/ml caused cumulative inhibitory effects, observed after day 7.
Figure 1.
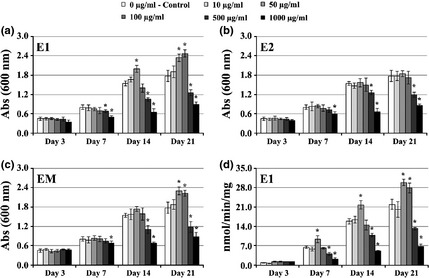
Cell viability/proliferation and ALP activity of hBMC cultures. Effect of E quisetum arvense extracts on viability/proliferation (a–c) and ALP activity (d) of hBMC cultured for 21 days in standard tissue culture plates. *Significantly different from control.
Extract E2 (Fig. 1b) caused dose‐dependent inhibitory effects after 7 days culture (statistically significant at levels >100 μg/ml). Lower concentrations of E2 extract did not affect any cell response significantly.
Regarding extract EM (Fig. 1c), exposure to 50 and 100 μg/ml caused a stimulatory response at day 21, in the order of 27% over control. Presence of 500 and 1000 μg/ml elicited a significant inhibitory effect, which became evident after the first week of culture.
Cell cultures treated with Extract E1 were also assessed for ALP activity (Fig. 1d). Increased ALP activity was found in the presence of 50 μg/ml (at days 7, 14 and 21) and at 100 μg/ml (day 21). The two highest tested concentrations caused dose‐dependent inhibition of ALP activity.
Globally, the inhibitory effect observed for higher concentrations mainly concerned increase in toxicity leading to cell death, as observed by increase in levels of detached cells and/or cell fragments, in cell cultures (data not shown).
F‐actin cytoskeleton organization
CLSM appearance of hBMC cultures exposed to extract E1, 50 and 1000 μg/ml is shown in Fig. 2. At day 7, cultures treated with 50 μg/ml presented well‐spread cells with cytoplasmic extensions, cell‐to‐cell contact and normal morphology and cytoskeleton organization; a continuous cell layer was observed by day 14 and cell multilayers at day 21. High magnification images indicated metabolically active cell population with ongoing processes of cell division. Cultures exposed to 100 and 500 μg/ml E1 presented similar CLSM appearances (not shown). This time‐course behaviour was identical to that seen in control cultures. However, cultures exposed to 1000 μg/ml showed evident signs of toxicity reflected by lower numbers of attached cells at day 7 and significantly disturbed behaviour by days 14 and 21, namely progressively fewer attached cells, lack of cell‐to‐cell contacts and altered cytoskeleton organization, with reduced cytoplasmic volume and dense nuclear material. E2 and EM caused similar dose‐ and time‐dependent behaviour, but deleterious effects on cell morphology were seen at lower levels (data not shown).
Figure 2.
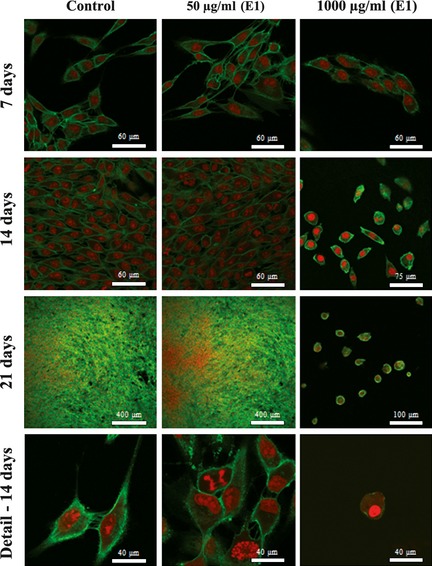
CLSM visualization of hBMC cultures. Cell morphology and proliferation of hBMC cultures treated with extract E1, over culture time. CLSM of cultures stained for F‐actin (green) and nuclei (red).
Presence of phosphate deposits
Cell cultures maintained in absence of any extract revealed production of mineralized deposits at day 14, which had increased by day 21, assessed by von Kossa staining (Fig. 3a). Exposure to 50 μg/ml E1 extract elicited a higher response, specially evident at day 14. Similar behaviour was achieved following supplementation with 100 μg/ml (data not shown). In the presence of higher concentrations, no mineralization was observed and cell death was elicited. This behaviour was confirmed by SEM observation, as shown in representative images of 21‐day cultures (Fig. 3b). Moreover, X‐ray microanalysis of mineralized deposits confirmed that they were mainly composed of calcium phosphate salts.
Figure 3.
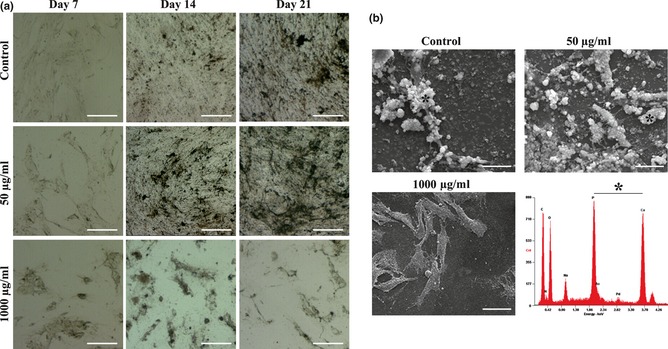
Presence of mineralized deposits in hBMC cultures treated with extract E1. (a) Representative images of cell layers following von Kossa staining (phosphate‐containing deposits); white bars, 100 μm. (b) SEM images after 21 days culture, showing cell layer‐associated mineral deposits; white bars, 15 μm. *Representative X‐ray spectrum of mineralized deposits, displaying Ca and P peaks.
Effect of Si on behaviour of hBMC
hBMC cultures exposed to 0.005–1 μg/ml Si (concentration range representative of that present in the tested E1 solutions), had increased viability/proliferation by days 14 and 21, in the order of 16% or 24% for tetraethilorthosilicate or silicic acid respectively (Fig. 4a). Also, increase in ALP activity in the order of 13% or 31% (tetraethilorthosilicate or silicic acid respectively) was observed (Fig. 4b).
Figure 4.
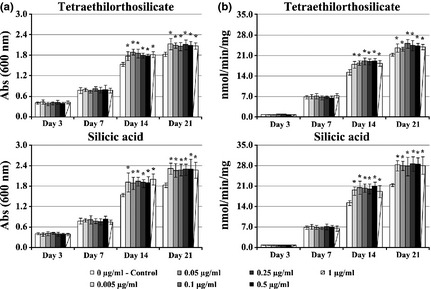
Effects of Si on the behaviour of hBMC cultures. Effect of Si on viability/proliferation (a) and ALP activity (b) of hBMC cultures. *Significantly different from control.
Effect of E. arvense extracts on behaviour of hBMC cultured on hydroxyapatite
XRD pattern of prepared HA (Fig. 5a) revealed it to be pure phase HA, as no secondary phases were formed. On CLSM observation, colonized HA substrates treated with low levels of E1 showed well‐spread cells distributed over the HA surface on day 7, which proliferated throughout the culture period forming a thick cell layer by day 21, as seen in Fig. 5c–e for cultures treated with 50 μg/ml E1. In addition, E1 induced a statistically significant increase in cell proliferation at 50 μg/ml (in the order of 17% and 20%, at days 14 and 21 respectively) and 10 and 100 μg/ml (~16%, at day 21), and inhibitory effects at levels >500 μg/ml, Fig. 6a. Compared to controls, ALP activity following supplementation with E1 50 and 100 μg/ml increased from day 14 (36%) and 21 (21%) respectively, Fig. 6b. Concentration of 500 μg/ml elicited a significant reduction in ALP activity (~42% at day 21). In addition, presence of 50 and 100 μg/ml E1 extract appeared to induce a slight increase in gene expressions of COL1, ALP, BMP‐2, M‐CSF, RANKL and OPG, evaluated at day 21, which reached statistical significance for ALP (20%) and BMP‐2 (31%) genes (Fig. 6c). On the other hand, treatment with 500 μg/ml significantly reduced expression of osteoblast‐related genes.
Figure 5.
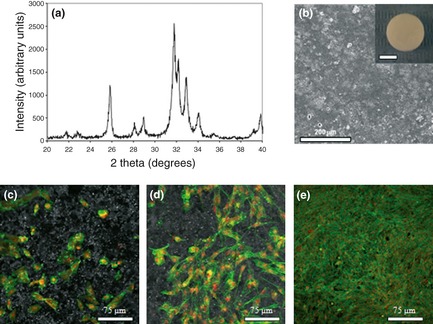
X‐ray diffraction pattern of hydroxyapatite (a) SEM appearance of surface of HA‐dense discs used in the cell culture experiments (b) inset: macroscopic appearance, bar = 5 mm). HA colonized with hBMC grown in the presence of 50 μg/ml extract E1, at days 7 (c) 14 (d) and 21 (e) observed by CLSM.
Figure 6.
Effect of Equisetum arvense E1 extract on behaviour of hBMC cultured on hydroxyapatite discs. (a) viability/proliferation; (b) ALP activity; (c) RT‐PCR analysis for expression of COL1, ALP, BMP‐2, RANKL, M‐CSF and OPG. *Significantly different from control.
Antibacterial activity of E. arvense extracts
Extracts, 10–1000 μg/ml, caused dose‐dependent inhibitory activity of S. aureus, with all three extracts presenting similar profiles (Fig. 7a), but did not cause any apparent effect against tested Gram‐negative bacteria (not shown). Extract E1, tested on S. aureus culture plated over HA discs, presented identical antibacterial activity, that is 20–95% inhibition of bacterial cell growth over the tested concentration range, Fig. 7b.
Figure 7.
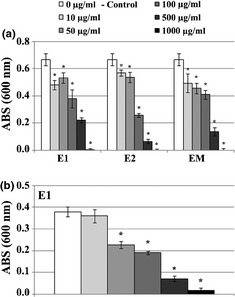
Effect of Equisetum arvense extracts on proliferation of Staphylococcus aureus, after 24 h. (a) Antibacterial activity of E1, E2 and EM on cultures performed in standard tissue culture plates. (b) Antibacterial activity of extract E1 in cultures performed on hydroxyapatite discs. *Significantly different from control.
Discussion
In contrast to synthetic pharmaceuticals based on single chemicals, many phytomedicines exert their beneficial effects due to additive or synergistic action of several chemical compounds acting at single or multiple target sites, associated with a physiological process 33. Also, because of the presence of a high number of potential bioactive compounds, plant preparations are anticipated to exhibit a wide range of effects 33, with the possibility of interesting combinations regarding a specific therapeutic aim. The present study describes the effects of hydromethanolic extracts of E. arvense on behaviour of hBMC, and also, any antimicrobial activity they may have against Gram+ and Gram− bacteria, relevant in bone‐associated infections 10. Additionally, activity of the extracts was evaluated on colonized HA‐dense substrates, exploring a potential utility for bone regeneration strategies.
Dried aerial components of E. arvense were extracted using a mixture of methanol:water (1:1) continuously until exhaustion of the plant material sample. As alcohol has a lower boiling point than water, the extracting solution became progressively richer. This procedure resulted in decreased capacity to extract apolar compounds and increased extraction of polar compounds. Two extracts were collected and tested, E1 (yielded between days 4 and 10) and E2 (yielded between days 11 and 26), with different compositions regarding hydrophilicity and lipophilicity characters of the compounds present. Thus, E1 was more concentrated in apolar compounds and E2 was expected to be richer in polar compounds. A mixture of the two extracts, EM, was also evaluated.
Equisetum arvense extracts affected viability/proliferation of hBMC over the range of 50–1000 μg/ml and presented distinct dose‐ and time‐dependent profiles. E1 exhibited the most favourable response with significant dose‐dependent increase in the cell population expansion at 50 and 100 μg/ml. E2 had a less favourable profile and EM, a 1.1 mixture of E1 and E2, presented activity between those observed with the isolated extracts. Furthermore, presence of 50 and 100 μg/ml of E1 also stimulated activity of osteoblastic enzyme ALP on hBMC 34 and formation of mineralized deposits. The results obtained for cell viability and ALP activity, combined with absence of significant effects on actin filaments (at lower extract concentrations), suggest that the observed extract‐inducing effects might not involve significant changes in cytoskeleton reorganization. That is, other processes, rather than alterations in F‐actin filaments, could be involved in stimulation observed in the osteoblastic behaviour, induced by low extract concentrations. On the other hand, actin staining suggested that at high extract concentrations, cells had aberrant morphology, which is in line with the proposed toxicity and, consequently, cell death. In cells, F‐actin is highly concentrated just beneath the plasma membrane and it controls cell shape and surface movements modulating intracellular mechanics proximal to proliferation and differentiation events 35, 36, in addition to providing the molecular basis for many mechanical properties of cytoplasm 37. Distinct profiles of E1 and E2 are most probably related to differences in composition of the two extracts and/or in concentrations of the active compounds, as expected considering procedures of the extract preparations. Also, E1 might contain metabolites, present only in smaller quantities in the plant and totally extracted in the first few days, that may not be present in E2. E2 itself, also, also might contain different compounds. Results suggest that E1 had active constituents that stimulated cell proliferation and additionally that E2 appeared to be poorer in such molecules or be richer in proliferation inhibitory compounds.
Equisetum arvense is known to have a high content of silica, being a natural organic source of its water‐soluble colloidal 13, 14, 15. Considering the role of this ion on bone metabolism 27, 28, 29, 30, 31, 32, extract E1, which caused increased cell viability/proliferation, was analysed for Si content. Levels in the range of those present in E1‐tested solutions caused slightly higher proliferation of hBMC after a few days exposure. This observation is in line with previous studies reporting stimulatory effects in human osteoblast cultures treated with levels <1.4 μg/ml 38 and 0.1–100 μg/ml 39. Also, presence of Si concentrations up to 1 μg/ml caused increase in ALP activity of hBMC, particularly when the source of Si was silicic acid, one of the forms of Si found in E. arvense 40. Taken together, results reported here suggest that in addition to an eventual modest contribution of Si, other bioactive compounds played a role in induced cell proliferation caused by extract E1. Regarding this, it is known that E. arvense contains a variety of phenolic compounds 13, 14, 15, 16, 23, which might contribute to the observed inductive effect. Positive association between phenolic phytochemicals and bone metabolism, based on cell culture experiments, animal models and clinical studies, has been addressed in two reviews 24, 25. Equisetum arvense accumulates phenolics, mainly flavonols quercetin and kaempferol and flavone apigenin, among others 23. Studies on osteoblast cultures showed higher alkaline phosphatase activity and matrix mineralization in the presence of these two molecules 41, 42, 43, 44, with some authors suggesting involvement of ERK and oestrogen receptor activation 44. Apigenin has also been reported to influence bone cell behaviour 45, but its effect on bone health remains unclear 24.
The extracts were also evaluated with regard to antibacterial activity, at 10–1000 μg/ml, concentration range that elicited dose‐dependent effects of proliferation of hBMC. E1, E2 and EM caused dose‐dependent population growth inhibition of S. aureus, but were not active against the tested Gram‐negative bacteria. Antibacterial activity of E. arvense extracts might be related to high content of its phenolic compounds 19, 23 and these results are in agreement with studies on E. arvense essential oil 20 and are in line with previous screenings of medicinal plants that show that most active plant extracts have activity against Gram‐positive bacteria, while Gram‐negative microorganisms are more resistant 11, 34, 46, 47.
To address suitability of E. arvense extracts in biomaterial‐mediated bone regeneration, extract E1, which yielded a promising profile from the fore‐going experiments, was tested on HA‐dense substrates colonized with hBMC. HA is widely used in bone regeneration/repair applications, and our cultured material samples exposed to E1 exhibited an increase in cell proliferation (at 50 and 100 μg/ml), without any deleterious effects on phenotype expression of hBMC. Accordingly, hBMC cultures displayed expression of osteoblastic‐related markers COL1, ALP and BMP‐2 48, and increased expression was observed of ALP and BMP‐2. In addition, no significant changes were found in expression of genes or their products essential in cross‐talk between osteoblasts and osteoclasts during bone modelling and remodelling, namely osteoclastogenic modulators such as inducers M‐CSF and RANKL, and the inhibitor OPG 49, 50. Extract E1 also exhibited antibacterial activity against S. aureus in cultures performed on HA. This is a relevant aspect considering that bone‐related infections are caused particularly by Gram‐positive bacteria 10 and S. aureus is a common pathogen in biomaterial‐related infections 10, 51. Following implantation, biomaterials rapidly acquire a layer of host proteins such as collagen, fibrinogen and fibronectin, providing a suitable adhesive substratum for bacterial colonization that, in the case of S. aureus, is mediated by specific adhesion molecules (Microbial surface components recognizing adhesive matrix molecules, MSCRAMMs) 51. Bacterial colonization prevents attachment of bone precursor cells to the material surface, thus reducing host response and impairing the repair process 10. Presence, in the bone environment, of an agent with both osteoblast‐inducing ability. and antibacterial properties would favour bone formation and prevent bacterial colonization, useful profiles in bone regeneration strategies.
In conclusion, the present study has shown that the hydromethanolic extracts of E. arvense exhibit a dose‐dependent stimulatory effect on proliferation of hBMC, at concentrations up to 100 μg/ml. In addition, activity against S. aureus was observed over the same concentration range. Experiments performed on colonized HA substrates also showed increase in proliferation of osteoblasts expressing normal phenotype features, along with antibacterial activity. These results provide information regarding for eventual utility of E. arvense extracts in promoting osteoblastic response, while preventing risk of infection at the biomaterial/bone interface, by local delivery strategy. However, aspects related to establishment of basic levels of chemical and pharmacological standardization, together with safety issues, need to be further addressed.
Acknowledgements
This work was supported by Faculdade de Medicina Dentária da Universidade do Porto (FMDUP). CLSM observation was performed at Advanced Light Microscopy, IBMC, University of Porto (IBMC.INEB).
References
- 1. Betz RR (2002) Limitations of autograft and allograft: new synthetic solutions. Orthopedics 5, 561–570. [DOI] [PubMed] [Google Scholar]
- 2. Varkey M, Gittens SA, Vludag H (2004) Growth factor delivery for bone tissue repair: an update. Expert Opin. Drug Deliv. 1, 19–36. [DOI] [PubMed] [Google Scholar]
- 3. Putnam SE, Scutt AM, Bicknell K, Priestley CM, Williamson EM (2007) Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health. Phytother. Res. 21, 99–112. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Leung P‐C, Che C‐T, Chow H‐K, Wu C‐F, Wong M‐S (2008) Improvement of bone properties and enhancement of mineralization by ethanol extracts of Fructus Ligustri Lucidi . Br. J. Nutr. 99, 494–502. [DOI] [PubMed] [Google Scholar]
- 5. Jeong J‐C, Lee J‐W, Yoon C‐H, Lee Y‐C, Chung K‐H, Kim M‐G et al (2005) Stimulative effects of Drynariae rhizome extracts on the proliferation of osteoblastic MC3T3‐E1 cells. J. Ethnopharmacol. 96, 489–495. [DOI] [PubMed] [Google Scholar]
- 6. Sun J‐S, Lin C‐Y, Dong GC, Shen S‐Y, Lin F‐H, Chen L‐T et al (2002) The effect of Gu‐Sui‐Bu (Drynaria fortunei J. Sm) on bone cells activities. Biomaterials 23, 3377–3385. [DOI] [PubMed] [Google Scholar]
- 7. Dong G‐C, Chen HM, Yao C‐H (2008) A novel bone substitute composite composed of tricalcium phosphate, gelatine and drynaria fortunei herbal extract. J. Biomed. Mater. Res. 84A, 167–177. [DOI] [PubMed] [Google Scholar]
- 8. Yao C‐H, Tsai H‐M, Chen Y‐S, Liu B‐S (2005) Fabrication and evaluation of a new composite composed of tricalcium phosphate, gelatin and Chinese medicine as a bone substitute. J. Biomed. Mater. Res. 75B, 277–288. [DOI] [PubMed] [Google Scholar]
- 9. Sun J‐S, Dong G‐C, Lin C‐Y, Sheu S‐Y, Lin F‐H, Chen L‐T et al (2003) The effect of Gu‐Sui‐Bu (Drynaria fortunei J. Sm) immobilized modified calcium hydrogenphosphate on bone cell activities. Biomaterials 24, 873–882. [DOI] [PubMed] [Google Scholar]
- 10. Brause B (2000) Infections with prostheses in bones and joints In: Mandell G, Bennet J, Dolin R, eds. Principles and Practice of Infectious Diseases, 5th edn, pp. 1196–1200. Philadelphia: Churchill Livingstone. [Google Scholar]
- 11. Martin KW, Ernst E (2003) Herbal medicines for treatment of bacterial infections: a review of controlled clinical trials. J. Antimicrob. Chemother. 51, 241–246. [DOI] [PubMed] [Google Scholar]
- 12. Pryer KM, Schneider H, Smith AR (2001) Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature 409, 618–622. [DOI] [PubMed] [Google Scholar]
- 13. Van Wyk B, Wink M (2004) Medicinal Plants of the World, pp. 136 Portland, OR: Timber Press. [Google Scholar]
- 14. Duke J, Bogenschutz J, du Cellier J, Duke P (2002) Handbook of Medicinal Herbs, 2nd edn pp. 391–392. Boca Raton, FL: CRC Press. [Google Scholar]
- 15. Wichtl M (2004) Herbal Drugs and Phytopharmaceuticals, 3rd edn, pp. 195–199. Boca Raton, FL: CRC Press. [Google Scholar]
- 16. Graefe EV, Veit M (1999) Urinary metabolites of flavonoids and hydroxycinnamic acids in humans after application of a crude extract from Equisetum arvense . Phytomedicine 6, 239–246. [DOI] [PubMed] [Google Scholar]
- 17. Trouillas P, Calliste CA, Allais DP (2003) Antioxidant, anti‐inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin country side as herbal teas. Food Chem. 80, 399–407. [Google Scholar]
- 18. Blumenthal M, Goldberg A, Brinckmann J (2000) Herbal Medicine: Expanded Commission E Monographs, pp. 208–211. Newton, MA: Lippincott Williams & Wilkins. [Google Scholar]
- 19. Milovanović V, Radulović N, Todorović Z, Stanković M, Stojanović G (2007) Antioxidant, antimicrobial and genotoxicity screening of hydro‐alcoholic extracts of five Serbian equisetum species. Plant Foods Hum. Nutr. 62, 113–119. [DOI] [PubMed] [Google Scholar]
- 20. Radulović N, Stojanović G, Palić R (2006) Composition and antimicrobial activity of Equisetum arvense L. essential oil. Phytother. Res. 20, 85–88. [DOI] [PubMed] [Google Scholar]
- 21. Sommer L, Mintzer L, Rindasu G (1962) Antimicrobial activity of the volatile oil extracted from Equisetum arvense . Farmacia 10, 535–541. [Google Scholar]
- 22. Corletto F (1999) Female climacteric osteoporosis therapy with titrated horsetail (Equisetum arvense) extracts plus calcium (osteosil calcium): randomized double bind study. Miner. Ortoped. Traumatol. 50, 201–206. [Google Scholar]
- 23. Veit M, Bauer K, Beckert C, Kast B, Geiger H, Czygan F‐C (1995) Phenolic characters of British hybrid taxa in Equisetum subgenus Equisetum . Biochem. Syst. Ecol. 23, 79–87. [Google Scholar]
- 24. Habauzit V, Horcajada M‐N (2008) Phenolic phytochemicals and bone. Phytochem. Rev. 7, 313–344. [Google Scholar]
- 25. Dew TP, Day AJ, Morgan MRA (2007) Bone mineral density, polyphenols and caffeine: a reassessment. Nutr. Res. Rev. 20, 89–105. [DOI] [PubMed] [Google Scholar]
- 26. Holzhiter G, Narayanan K, Gerber T (2003) Structure of silica in Equisetum arvense . Anal. Bioanal. Chem. 376, 512–517. [DOI] [PubMed] [Google Scholar]
- 27. Carlise E (1970) Si: a possible factor in bone calcification. Science 167, 279–280. [DOI] [PubMed] [Google Scholar]
- 28. Carlise E (1979) Biochemical and morphological changes associated with long bone abnormalities in Si deficiency. J. Nutr. 110, 1046–1055. [DOI] [PubMed] [Google Scholar]
- 29. Pietack AM, Reid JW, Stott MY, Sayer M (2007) Silicon substitution in the calcium phosphate bioceramics: a review. Biomaterials 28, 4023–4032. [DOI] [PubMed] [Google Scholar]
- 30. Thian ES, Huang J, Best SM, Barber ZH, Bonfield W (2007) Silicon‐substituted hydroxyapatite: the next generation of bioactive compounds. Mater. Sci. Eng. C 27, 251–256. [Google Scholar]
- 31. Thian ES, Huang J, Best SM, Barber ZH, Brooks RA, Rushton N et al (2006) The response of osteoblasts to nanocrystalline silicon‐substituted hydroxyapatite thin films. Biomaterials 27, 2692–2698. [DOI] [PubMed] [Google Scholar]
- 32. Patel N, Best S, Bonfield W, Gibson I, Hing K, Damien E et al (2002) A comparative study on the in vivo behaviour of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci.: Mater. Med. 13, 1199–1206. [DOI] [PubMed] [Google Scholar]
- 33. Briskin DP (2000) Medicinal plants and phytomedicines linking plant biochemistry and physiology to human health. Plant Physiol. 24, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ali NAA, Jülich WD, Kusnick C, Lindequist U (2001) Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J. Ethnopharmacol. 74, 173–179. [DOI] [PubMed] [Google Scholar]
- 35. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) The cytoskeleton In: Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, eds. Molecular Biology of the Cell, 4th edn, pp. 907–982. New York: Garland Publishing. [Google Scholar]
- 36. Stamenovic D (2005) Effects of cytoskeletal prestress on cell rheological behaviour. Acta Biomater. 1, 255–262. [DOI] [PubMed] [Google Scholar]
- 37. Titushkin I, Cho M (2007) Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophysical J. 93, 3693–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reffit D, Ogston N, Jugdaohsingh R, Cheung H, Evans B, Thompson R et al (2003) Orthosilicic acid stimulates collagen type I synthesis and osteoblastic differentiation in human osteoblast‐like cells in vitro. Bone 32, 127–135. [DOI] [PubMed] [Google Scholar]
- 39. Keeting P, Oursler M, Wiegand K, Bonde S, Spelsberg T, Riggs B (1992) Zeolite A increases proliferation, differentiation and TGF‐beta production in normal adult human osteoblastic‐like cells in vitro. J. Biomed. Mater. Res. 7, 1281–1289. [DOI] [PubMed] [Google Scholar]
- 40. Mimica‐Dukic N, Simin N, Cvejic J, Jovin E, Orcic D, Bozin B (2008) Phenolic compounds in field horsetail (Equisetum arvense L.) as natural antioxidants. Molecules 13, 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim D‐S, Takai H, Arai M, Araki S, Mezawa M, Kawai Y et al (2007) Effects of quercetin and quercetin 3‐glucuronide on the expression of bone sialoprotein gene. J. Cell. Biochem. 101, 790–800. [DOI] [PubMed] [Google Scholar]
- 42. Kim YJ, Bae YC, Suh KT, Suh KT, Jung JS (2006) Quercetin, a flavonoid inhibits proliferation and increases osteogenic differentiation in human adipose stromal cells. Biochem. Pharmacol. 72, 1268–1278. [DOI] [PubMed] [Google Scholar]
- 43. Miyake M, Arai N, Ushio S, Iwaki K, Ikeda M, Kurimoto M (2003) Promoting effect of kaempferol on the differentiation and mineralization of murine pre‐osteoblastic cell line MC3T3‐E1. Biosci. Biotechnol. Biochem. 67, 1199–1205. [DOI] [PubMed] [Google Scholar]
- 44. Prouillet C, Maziere JC et al (2004) Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatise activity in MG‐63 human osteoblasts through ERK and estrogen receptor pathway. Biochem. Pharmacol. 67, 1307–1313. [DOI] [PubMed] [Google Scholar]
- 45. Bandyopadhyay S, Lion JM, Mentaverri R, Ricupero DA, Kamel S, Romero JR et al (2006) Attenuation of osteoclastogenesis and osteoclast function by apigenin. Biochem. Pharmacol. 72, 184–197. [DOI] [PubMed] [Google Scholar]
- 46. Herrera RM, Perez M, Martin‐Herrera DA, Lopez‐Garcia R, Rabanal RM (1996) Antimicrobial activity of extracts from plants endemic to the Canary Islands. Phytother. Res. 10, 364–366. [Google Scholar]
- 47. Kelmanson JE, Jäger AK, Van Staden J (2000) Zulu medicinal plants with antibacterial activity. J. Ethnopharmacol. 69, 241–246. [DOI] [PubMed] [Google Scholar]
- 48. Chau JF, Leong WF, Li B (2009) Signaling pathways governing osteoblast proliferation, differentiation and function. Histol. Histopathol. 24, 1593–1606. [DOI] [PubMed] [Google Scholar]
- 49. Matsuo K, Irie N (2008) Osteoclast‐osteoblast communication. Arch. Biochem. Biophys. 473, 201–209. [DOI] [PubMed] [Google Scholar]
- 50. Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodelling. Arch. Biochem. Biophys. 473, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hudson MC, Ramp WK, Frankenburg KP (1999) Staphylococcus aureus adhesion to bone matrix and bone‐associated biomaterials. FEMS Microbiol. Lett. 173, 279–284. [DOI] [PubMed] [Google Scholar]


