Abstract
Objectives
Cisplatin is an anti‐neoplastic agent treatment with which causes many side effects including ototoxicity. The aim of this study was to investigate whether acetyl‐l‐carnitine would have protective effects on cisplatin‐induced ototoxicity in vitro, and if present, to reveal roles of apoptotic gene expressions and pro‐inflammatory cytokines.
Materials and methods
House Ear Institute‐Organ of Corti 1 cell line was used for this study. Apoptotic genes were evaluated with an apoptosis PCR array and pro‐inflammatory cytokine levels were measured using ELISA.
Results
Apoptotic cell death reduced by around 22% with acetyl‐l‐carnitine‐cisplatin treatment compared to cisplatin alone. Genes displaying increase in expression of apoptosis, related to cisplatin treatment, were Casp8, Bcl10, Bcl2, Bcl2l1, Bcl2l2, Bid, Naip1, Bnip3l, Card6, Pak7, Cd40, Trp 53inp1, Cideb and Cd70. The acetyl‐l‐carnitine‐cisplatin combination caused reduced expression of genes Casp8, Fas, Casp1, Tnfrsf11b, Tnfrsf10b induced by cisplatin. Acetyl‐l‐carnitine‐cisplatin also caused reduced levels of IL‐6, IL‐1β and TNF‐α, pro‐inflammatory cytokines, induced by cisplatin.
Conclusion
Protective mechanisms of aceytl‐l‐carnitine against cisplatin induced apoptosis, mainly due to activation of anti‐apoptotic Bcl family members' genes, and in an Akt‐related gene expression dependent manner. This is the first study to indicate that acetyl‐L‐carnitine can be an effective agent against cisplatin ototoxicity in auditory cells, with induction of anti‐apoptotic gene expression and attenuating levels of pro‐inflammatory cytokines.
Introduction
Cis‐diamminedicholoroplatinum (CDDP) is a chemotherapeutic agent, widely used, particularly to treat solid tumours of children and adults. Serious side effects, especially neurotoxicity, ototoxicity and nephrotoxicity, however, can limit its therapeutic use and efficacy 1. Ototoxicity is a very serious problem, especially in childhood. CDDP can cause permanent hearing loss due to damage of outer hair cells in the organ of Corti. It provokes cell damage by inducing apoptosis, and in other target tissues, promotes production of pro‐inflammatory cytokines such as TNF (tumour necrosis factor)‐α, IL (interleukin)‐6 and IL‐1β 2. In vivo studies have provided very valuable information on hair cell death and survival; however, due to relatively low accessibility of drugs into the inner ear, cells of these tissues are not suitable to drug screening studies; inner ear cell lines have been found to be more appropriate for this aim. Since 1997, many inner ear cell lines derived from saccular and cochlear tissues have become popular, and Kalinec et al. developed the House Ear Institute‐Organ of Corti 1 (HEI‐OC1) cell line from cochlear cultures of the Immorto mouse 3. These cells express numbers of proteins such as Myosin VIIa and Math 1, suggestive of a hair cell phenotype. The cell line was demonstrated to be susceptible to known ototoxic agents such as gentamycin and cisplatin, hence, it has been suggested to be an excellent model to study mechanisms of cell death and protective drug efficacy, and mechanisms of protection for the organ of Corti of the cochlea.
Acetyl‐l‐carnitine (ALC) is a promising new agent that has protective effects against cisplatin‐induced toxicities. It is a member of the family of carnitines, a group of natural compounds having essential roles in intermediary metabolism – they facilitate essential fatty acid entry and exit from mitochondria 4, 5. Our previous studies on CDDP‐induced toxicities have shown that ALC has protective effects on CDDP‐induced neurotoxicity and nephrotoxicity 6, 7. Moreover, we found that ALC also protected against CDDP ototoxicity, in vivo 8. ALC ameliorates CDDP‐induced oxidative stress by reducing glutathione levels and lipid peroxidation, while apoptotic effects of CDDP are not changed with ALC treatment in neuroblastoma cells 6. The present study was designed to evaluate mechanisms of ALC on CDDP‐induced ototoxicity in vitro in the cochlear cell line by investigating roles of apoptotic gene expressions and levels of pro‐inflammatory cytokines.
Materials and methods
Cell culture and reagents
The House Ear Institute‐Organ of Corti 1 cell line was kindly provided by Professor F.Kalinec. It is an immortalized cell line derived from cochlear cultures from the Immorto mouse 3, 9. Kalinec at al. suggested that it is an excellent in vitro system with 10% foetal bovine serum at 33 °C under 10% CO2 in air. Cells were grown in DMEM (Dulbecco's modified Eagle's medium) (Gibco, Paisley, UK) without antibiotic supplement. Cisplatin (Hexal, Sandoz/Novartis/Bangladesh) and ALC (Sigma‐Aldrich Co, St. Louis, MO, USA) were used in this study; they were prepared freshly before all experiments.
In vitro experimental design of cisplatin ototoxicity
Cells were exposed to CDDP at 10, 20, 40, 100, 200 and 400 μm doses, in the cell viability experiments; ALC was tested at 10, 50, 75, 100 and 200 μm doses in the experiments. With the Acetyl‐l‐carnitine–Cis‐diamminedicholoroplatinum (ALC–CDDP) combination, cells were pre‐treated with ALC for 60 min, then incubated with CDDP for 24 h in other experiments.
Cell viability assay
Briefly, cells were seeded at approximately 1 × 104/well in a final volume of 200 μl, in 96‐well microtiter culture plates, with at least 6 replicates for each group. After 24 h plating, incubation was continued for a further 24 h in the absence (control) or presence of the test reagents. At the end of the incubation period, colorimetric WST‐1 assay was performed using Cell Proliferation Reagent WST‐1 assay kit (Roche Applied Science, Mannheim, Germany), according to manufacturer's instructions. The reaction was allowed to proceed for 4 h at 33 °C. Intensity of the resulting colour developed (a reflection of number of live cells) was measured 450 nm wavelength against 630 nm reference wavelength, using an ELISA reader (Thermo Instruments Inc, Shanghai, China). All values were compared to the corresponding controls. Mean of triplicate experiments for each dose was used to calculate 50% cell proliferation inhibitor doses of chemicals and combinations.
TdT‐mediated dUTP nick end labelling
Cells were exposed to CDDP, ALC and ALC‐CDDP combinations, after treatment of ALC for 60 min and then incubation with CDDP for 24 h. At 24 h incubation, apoptotic cell death was monitored using the TUNEL assay to detect fragmented DNA in apoptotic nuclei (GenScript TUNEL Apoptosis Detection Kit Cat. No. L00299, for adherent cells, FITC‐labelled POD). The kit was applied to slides according to the manufacturer's instructions, at the end of experiments. After fixation and washing, TUNEL reaction mix containing equilibration buffer, FITC‐12‐dUTP and TdT was applied for 60 min at 37 °C. Results of the assay were observed using an Olympus fluorescence microscope, excitation wave 450–500 nm, and emission wave 515–565 nm (green). A total of 5000 cells per condition were evaluated and scored as per cent of apoptosis per total cells.
Pro‐Inflammatory cytokine analyses
Cisplatin has the capability of inducing of pro‐inflammatory cytokine secretion, related to ototoxicity, both in vitro and in vivo 10, 11. Thus, cisplatin‐induced pro‐inflammatory cytokines IL‐6, IL‐1β and TNF‐α were selected to evaluate this in vitro ototoxicity study. Cells were seeded with at least 6 replicates for each group and exposed to CDDP, ALC and ALC‐CDDP combinations, after treatment of ALC for 60 min and then incubated with CDDP for 24 h. At 24 h incubation results of all groups were compared to control groups. Cell culture supernatants were preserved at −80 °C until analyses were to be performed, when they were thawed and analysed immediately. Levels of pro‐inflammatory cytokines IL‐6, IL‐1β and TNF‐α were measured using mouse‐specific ELISA kits, according to the manufacturers' instructions. IL‐6 (Boster Biological Tehcnologies Ltd, Wuhan, China), IL‐1β (eBioscience, benderMedSystems, GmbH Vienna, Austria) and TNF‐α (Boster Biological Tehcnologies) concentrations were provided as a pg/ml.
RNA isolation and apoptosis gene expression analysis by real‐time PCR
Most important apoptotic and anti‐apoptotic genes have been designed as a standard apoptotic gene array at SABiosciences, and 84 apoptosis‐related gene expressions were evaluated in this study. After the HEI‐OC1 cell line was cultured and CDDP, ALC and ALC‐CDDP combinations, after treatment of ALC for 60 min and then incubated with CDDP for 24 h, were applied for 24 h, RNA isolation and complementary DNA (cDNA)‐converting expression of the 84 Standard Array Genes of Mouse Apoptosis (PAMM‐012A; SABiosciences, Hilden, Germany) were determined by real‐time PCR, for each condition. The PCR array (84 genes, 5 housekeeping genes, 1 genomic DNA control, 3 reverse transcriptase control, 3 positive PCR control) was studied on 96‐well PCR plates. Total RNA extraction was performed according to the manufacturer's instructions (Macharey Nagel RNA Isolation Kit, Düren, Germany). cDNA synthesis was performed using RT2 First Strand Kit (QIAGEN Cat No. 330401, Hilden, Germany). For gene expression analysis, real‐time PCR on ABI PRISM 7000 Sequence Detection System (Singapore) was applied on standard arrayed plates. SABiosciences's PAMM‐012A array included Master Mix primary for each gene or housekeeping genes, matched by array code. Only cDNA and SYBR Green was loaded on PCR 96‐well plates. The protocol was loaded as one cycle of 10 min at 95 °C, followed by 45 cycles of 15 s 95 °C and 1 min 65 °C each. SYBR Green Fluorescence was the detection method. Cp values were uploaded on to an Excel file listed according to A1‐H12 array codes on http://www.sabiosciences.com/pcr/arrayanalysis.php, online free array analysis system. Fold changes of each condition compared to control HEI‐OC1 cells were calculated. Genes that showed increase or decrease of more than 5‐fold were taken into consideration for expression changes. The list of genes in this PCR array is shown in Table 1.
Table 1.
List of genes of mouse apoptosis Real‐Time PCR array
| Pro‐apoptotic genes | Anti‐apoptotic genes |
|---|---|
| Apaf1, Bad, Bak1, Bax, Bcl10, Bid, Bnip3l, Bok, Nod1, Card6, Casp1, Casp12, Casp14, Casp2, Casp3, Casp4, Casp6, Casp7, Casp8, Casp9, Cidea, Cideb, Cradd, Dapk1, Dffa, Dffb, Fadd, Fasl, Ltbr, Nol3, Pycard, Ripk1, Tnfrsf10b, Tnfrsf11B, Tnfrsf1A, Cd40, Tnfsf10, Tnfsf12, Cd40lg, Cd70, Traf2, Traf3, TRP53, Tp53bp2, Trp53inp1, Trp63, Trp73 | Akt1, Api5, Atf5, Bag1, Bag3, Bcl2, Bcl2l1, Bcl2l10, Bcl2l2, Naip1, Naip2, Birc2, Birc3, Birc4 (Xiap), Birc5, Bnip2, Bnip3, Card10, Cflar, Dad1, Tsc22d3, Fas (Tnfrsf6), Hells, Il10, Lhx4, Mcl1, Nfkb1, Nme5, Pak7, Pim2, Polb, Prdx2, Rn7, Sphk2, Tnf, Traf1, Zc3hc1 |
A list of the genes analysed in the profile is also available online (<http://www. sabiosciences.com/rt_pcr_product/HTML/PAMM‐012A.html>).
Statistical analysis
Statistical analyses were performed using spss 15.0 software program (Chicago, IL, USA). All results are expressed as means ± SEM. Continuous variables were compared using the Mann–Whitney rank sum test. All treatment experiments were repeated at least three times to generate statistically relevant data. P < 0.05 was considered statistically significant.
Results
Acetyl‐l‐carnitine protection from CDDP‐induced cell death of HEI‐OC1 cells
Acetyl‐l‐carnitine did not change HEI‐OC1 cell viability up to 75 μm concentrations as shown in Fig. 1. More than 100 μm doses of ALC inhibited viability in a time‐ and dose‐dependent manner. In this study, CDDP inhibited HEI‐OC1 cell population growth in a time‐ and dose‐dependent manner as shown in Fig. 2. CDDP inhibited 50% cell population growth at 20 μm dose by 24 h incubation. Non‐proliferative ALC dose at 50 μm concentration at 24 h incubation was determined from cell viability experiments. In the ALC‐CDDP combination group, after 60 min pre‐treatment with 50 μm ALC, the auditory cells were protected from 20 μm CDDP‐induced cytotoxicity to the level of 22% (Fig. 3). Apoptotic cell death was reduced with combination of ALC‐cisplatin compared to CDDP alone at 24 h incubation as shown in Fig. 4 (P < 0.05). Cell viability and apoptotic analyses indicated that protective effects of ALC on CDDP‐induced ototoxicity occurred in the same ratios.
Figure 1.
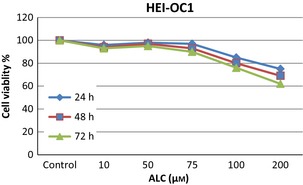
Aceytl‐l‐carnitine did not affect cell viability up to 75 μm doses by time in HEI‐OC1 cells. More than 100 μm doses of ALC inhibited cell viability in a time and dose‐dependent manner.
Figure 2.
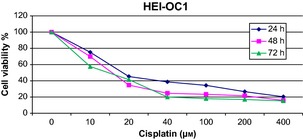
Cisplatin inhibited cell growth by time and dose‐dependent manner in HEI‐OC1 cells.
Figure 3.
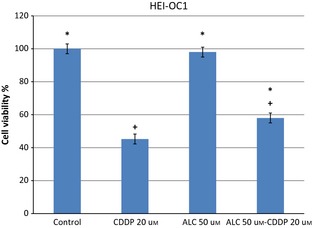
After 60 min pretreatment with 50 μm acetyl‐l‐carnitine, auditory cells were protected from 20 μm cisplatin induced cytotoxicity at 24 h incubation. Values represent the mean ± SEM of six observations. Asterisk indicates significance relative to cisplatin alone (Mann–Whitney U, P < 0.05). Asterisk indicates significance relative to cisplatin alone; plus sign indicates statistically significant values compared to ALC.
Figure 4.
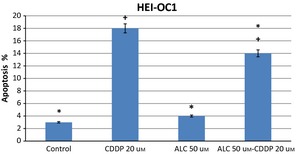
Apoptotic cell death was decreased about 33% with combination of acetyl‐l‐carnitine‐cisplatin at 24 h incubation. Asterisk indicates significance relative to cisplatin alone (Mann–Whitney U, P < 0.05). Values represent the mean ± SEM of six observations. Asterisk indicates significance relative to cisplatin alone; plus sign indicates statistically significant values compared to ALC.
Acetyl‐l‐carnitine protected cells from CDDP‐induced apoptosis mainly by expression of apoptotic genes
Apoptotic genes highly expressed with CDDP treatment were Casp 8, Bid, Bcl10, Trp 53inp1, Card6, Cideb, Bnip3l and Cd70 when gene expressions were evaluated relative to control groups at 24 h incubation (Fig. 5). Caspase 3 gene expression was also induced by CDDP treatment (2.6‐fold) and ALC supplementation slightly increased (4.6‐fold); expression of this gene was more than CDDP alone. Expressions of anti‐apoptotic genes Atf5, Akt1, Bcl2 and Bcl2l1 were also induced 5‐fold or more by CDDP treatment.
Figure 5.
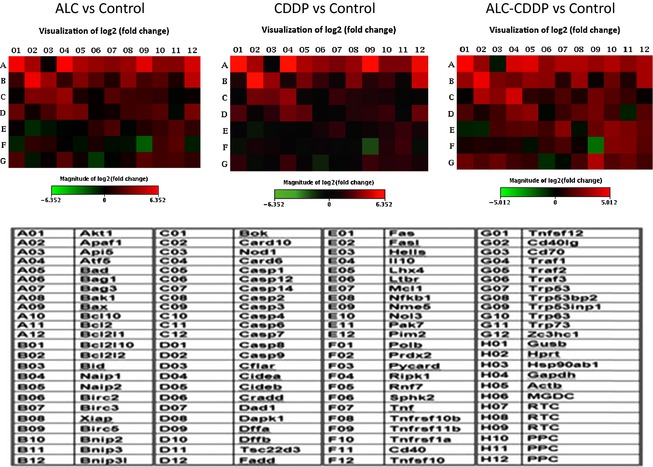
The heatmap presentation of apoptotic gene expressions induced by ALC, CDDP and ALC‐CDDP combination versus Control group at 24 h incubation. Also, the list of apoptosis gene array was given as a table. H lines were control of the array (housekeeping genes in H1‐H5, one genomic DNA control in H6, three reverse transcriptase controls in H7‐9; three positive PCR control in H10‐H12 lines), so H1‐H12 lines not represented in the table. Green color indicates lower expression of genes and red color indicates higher expressions of genes.
Expressions of apoptotic genes Apaf1, Bad, Bak1, Bax, Nod1, Casp1, Casp3, Casp14, Dffb, Ltbr, Mcl1, Nol3, Tnfrsf10b, Tnfrsf1a and Traf2 also increased by around 2‐fold or more compared to controls. Expressions of anti‐apoptotic genes Bag1, Bag3, Birc2, Xiap, Bnip2, Card10, Cflar, Fas, Nme5, Tnf, Tnfsf12 and Traf1 increased 2‐fold or more with CDDP. CDDP mostly caused increases in apoptotic gene expressions over anti‐apoptotic gene expressions.
Acetyl‐l‐carnitine alone induced both apoptotic and anti‐apoptotic gene expressions. Apoptotic genes Apaf1, Bad, Bak1, Bax, Bcl10, Bid, Nod1, Bnip3l, Casp8 and Cideb were induced by ALC alone compared to controls (Fig. 5). Anti‐apoptotic genes Akt1, Atf5, Bag1, Bag3, Bcl2l1, Card10 and Xiap expressions were induced by ALC alone compared to controls (Fig. 5). Only Tnfrsf11b apoptotic gene expression had in the order of 5‐fold reduction and Traf3 had around a 2‐fold reduction. Moreover, Casp2, casp3, Ltbr, Prdx2, Tnfrsf1a and Trp53inp1 increased in the region of 2‐fold or more with ALC treatment over control. Anti‐apoptotic genes Birc2, Birc5, Bnip2, Cflar, Mcl1, Pim2 and Zc3hc1 were also induced 2‐fold or more with ALC treatment.
In comparison to control groups, apoptotic genes Apaf1, Bad, Bak1, Bax, Bcl10, Bid, Bnip3l, Nod1, Cideb, Dapk, Nol3, Tnfrsf1a, Trp53inp1 and anti‐apoptotic genes Akt1, Atf5, Bag1, Bag3, Bcl2l1, Birc2, Xiap, Card10 and Mcl1 were highly expressed with ALC‐CDDP treatment (Fig. 5). Also, Casp3, Casp4, Casp6, Casp8, Ltbr, Tnfsf12 and Trp53bp2 apoptotic gene expressions increased around 2‐fold or more with ALC‐CDDP over controls. Anti‐apoptotic genes Bnip2, Cflar, Hells, Pims and Zc3hc1 were highly expressed 2‐fold or more with ALC‐CDDP treatment. Tnfrsf11b apoptotic gene expression had around a 2‐fold reduction.
The ALC‐CDDP combination caused reduction in expressions of genes mainly involved with the extrinsic apoptotic pathway, such as Casp8, Fasl, and also Casp1, Tnfrsf10b and Tnfrsf11b like other groups. Furthermore, anti‐apoptotic genes Atf5, Fas and Naip1 were reduced by the ALC‐CDDP combination compared to CDDP alone. ALC‐CDDP treatment increased expressions of apoptotic genes Bad and Bak1 (4.99‐fold) and anti‐apoptotic gene Akt1 compared to CDDP alone.
Acetyl‐l‐carnitine reduced CDDP‐induced pro‐inflammatory cytokine levels
Cis‐diamminedicholoroplatinum treatment increased production of IL‐6, TNF‐α and IL‐1β pro‐inflammatory cytokines in HEI‐OC1 cells by 24 h incubation. ALC supplementation significantly reduced CDDP‐induced pro‐inflammatory cytokine levels in these auditory cells (Figs 6, 7, 8) (P < 0.05).
Figure 6.
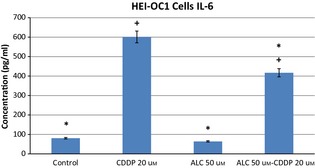
Acetyl‐l‐carnitine‐cisplatin decreased pro‐inflammatory cytokine levels of IL‐6 induced by cisplatin at 24 h incubation. Asterisk indicates significance relative to cisplatin alone (Mann–Whitney U, P < 0.05). Values represent the mean ± SEM of six observations. Asterisk indicates significance relative to cisplatin alone; plus sign indicates statistically significant values compared to ALC.
Figure 7.
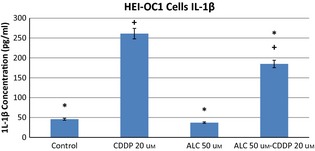
Acetyl‐l‐carnitine‐cisplatin decreased pro‐inflammatory cytokine levels of IL‐1beta induced by cisplatin at 24 h incubation. Asterisk indicates significance relative to cisplatin alone (Mann–Whitney U, P < 0.05). Values represent the mean ± SEM of six observations. Asterisk indicates significance relative to cisplatin alone; plus sign indicates statistically significant values compared to ALC.
Figure 8.
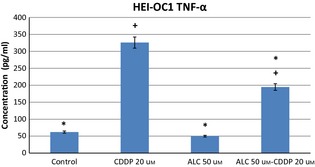
Acetyl‐l‐carnitine‐cisplatin decreased pro‐inflammatory cytokine levels of TNF‐α induced by cisplatin at 24 h incubation. Asterisk indicates significance relative to cisplatin alone (Mann–Whitney U, P < 0.05). Values represent the mean ± SEM of six observations. Asterisk indicates significance relative to cisplatin alone; plus sign indicates statistically significant values compared to ALC.
Discussion
Hearing loss is a serious side effect of anti‐cancer chemotherapy with platinum drugs and can have a negative impact on a patient's quality of life. CDDP is a platinum‐based chemotherapeutic agent widely used in treatment of different kinds of cancers such as ovarian, head and neck and lung cancer, as well as solid tumours of children mainly, neuroblastoma 12, 13. Ototoxicity is a common side effect of CDDP, especially in growing children 26–68% of treated patients being affected 14. CDDP causes cytotoxicities by distinct mechanisms. One of these is formation of DNA adducts 15, 16, 17, 18, and another involves reactive oxygen species generation 19, 20, 21, 22, 23. Pro‐inflammatory cytokine secretion is also linked to ototoxic effects of CDDP 10, 24, 25, and all these cytotoxic mechanisms are associated with apoptotic or necrotic cell death 17, 18, 26. Thus, it is very important to analyse the apoptotic mechanism of CDDP activation and possible protective agents against it. It is very difficult to study and investigate mechanisms of drug ototoxicity in experimental animal models; there are limitations in vivo. However, in vitro studies are very suitable for this aim, and Kalinec et al. developed the House Ear Institute‐organ of Corti 1 cell line from cochlear cultures of the Immorto mouse for in vitro ototoxicity studies 3, 9.
In the literature, there are different kinds of study investigating mechanisms of carnitine as a protective agent against cytotoxic agents 27, 28; it is well known that carnitine has a neuroprotective effect. Kalinec et al. have reported that l‐carnitine supplementation prevents gentamicin‐induced outer hair cell damage through mitochondrial pathways 25. ALC has a cell stress modulator function in different cell process, and might have protective functions against chemotherapy‐induced neurotoxicity 6, 8, 29, 30. Acetylation of cell proteins is a critical step in epigenetic changes. Furthermore, the acetyl ester group of L‐carnitine may perhaps modulate critical cell processes such as gene transcription and apoptosis. ALC plays a major role in different biological activities related to energy metabolism 30, 31. Moreover, previous studies have also shown that ALC has anti‐apoptotic effects 32, 33. One of these has demonstrated that ALC reduced neuronal apoptotic cell death in primary cultures at the concentration of 100 um 32. There, 50 um concentrations of ALC did not change viability of cells and reduced the apoptotic effect of cisplatin.
The main problem with using preventative or protective agents against chemotherapeutical drugs is changing or reducing anti‐tumour effects of the drugs. Ideal preventative agents must not disturb the anti‐tumoural effects of chemotherapeutics 6. Our previous study on neuroblastoma cells indicated that ALC inhibits lipid peroxidation; it did not alter the cytotoxic effect of cisplatin treatment. ALC has the capacity to reduce adverse effects such as peripheral neuropathy, neuronal toxicity or neuropathic pains due to cisplatin and similar chemotherapeutic drugs, in experimental models in vivo 8, 33, 34.
Our study of expressions of 84 apoptotic genes in HEI‐OC1 cells has revealed that apoptotic genes highly expressed with CDDP treatment were Casp 8, Bid, Bcl10, Trp 53inp1, Card6, Cideb, Bnip3l and Cd70 when evaluated relative to controls. Caspase 3 gene expression was also induced by CDDP treatment (2.6‐fold) and ALC supplementation slightly increased this gene's expression more than CDDP alone. Expressions of anti‐apoptotic genes Atf5, Akt1, Bcl2 and Bcl2l1 were also induced with CDDP treatment. CDDP mostly caused increases in expressions of apoptotic genes more than of anti‐apoptotic genes. This study has indicated that the apoptotic effect of CDDP was associated with increases in expressions of Apaf1, Bad, Bak1, Bax, Nod1, Casp1, Casp3, Casp14, Dffb, Ltbr, Mcl1, Nol3, Tnfrsf10b, Tnfrsf1a and Traf2 apoptotic genes in these auditory cells. One previous study has revealed that some CDDP‐induced apoptotic gene expressions in HEI‐OC1 cells were similar to those in lung cancer cells 35. Similar to our study, Devarajan et al. demonstrated that 36 CDDP induces both extrinsic (death receptor‐related) and intrinsic (mitochondrial cell death) apoptotic pathways. However, mainly p53, Casp8, Bid and Bax gene expressions were elevated.
Acetyl‐l‐carnitine treatment alone induced both apoptotic and anti‐apoptotic gene expressions. Apoptotic genes Apaf1, Bad, Bak1, Bax, Bcl10, Bid, Nod1, Bnip3l, Casp8, and Cideb were induced by ALC alone compared to controls. Expressions of anti‐apoptotic genes Akt1, Atf5, Bag1, Bag3Bcl2l1, Card10 and Xiap were induced by ALC alone compared to controls. These results suggest that ALC has the capacity to induce both apoptotic and anti‐apoptotic‐related genes of auditory cells.
In comparison to control groups, apoptotic genes Apaf1, Bad, Bak1, Bax, Bcl10, Bid, Bnip3l, Nod1, Cideb, Dapk1, Nol3, Tnfrsf1a, Trp53inp1, and anti‐apoptotic genes Akt1, Atf5, Bag1, Bag3, Bcl2l1, Birc2, Xiap, Card10, Mcl1 were highly expressed with ALC‐CDDP treatment. The ALC‐CDDP combination caused reduction in expressions of genes involved mainly in the extrinsic apoptotic pathway; genes such as Casp8, Fasl, and also Casp1, Tnfrsf10b, and Tnfrsf11b with other groups. Furthermore, anti‐apoptotic genes Atf5, Fas and Naip1 were reduced by the ALC‐CDDP combination compared to CDDP alone. ALC‐CDDP treatment increased expressions of apoptotic genes Bad and Bak1 and expression of anti‐apoptotic gene Akt1, compared to CDDP alone. ALC‐CDDP treatment increased pro‐apoptotic and anti‐apoptotic gene expressions compared to CDDP treatment. These results suggest that the ALC–CDDP combination upregulates apoptotic and downregulates anti‐apoptotic gene expressions in these auditory cells. The ALC–CDDP combination did not disturb apoptotic effects of CDDP. Furthermore, the ALC–CDDP combination induced further gene expressions compared to CDDP alone via both apoptotic and anti‐apoptotic features. Protective agents must maintain anti‐cancer effects of the chemotherapeutics while preventing their toxic effects on normal cells. Our previous study relating to this issue demonstrated that ALC was a very suitable micronutrient, without compromising anti‐tumour effects of CDDP on cancer cells 6. Moreover, a previous study has shown that ALC might also potentiate anti‐tumour effects of platinum compounds in higher doses, for non‐small cell lung cancer 37.
CDDP, ALC or ALC–CP combination changed the expressions of the other apoptotic/anti‐apoptotic gene expressions. Until now, there have been no studies on possible anti‐inflammatory effects of ALC on CDDP stimulation. l‐carnitine and its acyl esters have reactive oxygen scavenger function 38, 39 and immunomodulatory features in mammalian and avian species 40. Here, CDDP has been shown to induce production of pro‐inflammatory cytokines IL‐6, TNF‐α and IL‐1β in HEI‐OC1 cells, similar to previous studies 24, 25. Pro‐inflammatory cytokine secretion is one cell death mechanism of cisplatin‐induced ototoxicity, demonstrated both in vitro and in vivo (10, 11 ). In previous studies, it has been shown that l‐carnitine can be effective in male infertility associated with infection, and septic conditions, through anti‐inflammatory mechanisms 41. A further recent study has shown that carnitine reduced inflammatory cytokine levels in chronic diseases, with cancer being an example 42. The present study here has demonstrated that ALC supplementation significantly reduced CDDP‐induced pro‐inflammatory cytokine levels in the tested auditory cells. These results suggest that one of the protective mechanisms of ALC against CDDP ototoxicity is related to reduction of pro‐inflammatory cytokine levels. Our previous in vitro and in vivo research has shown that ALC has protective effects against CDDP neurotoxicity, ototoxicity and nephrotoxicity, and it has good in vivo tolerability 6, 7, 8. Moreover, ALC does not impair apoptotic effects of CDDP, besides having protective effects via apoptosis‐related genes and anti‐inflammatory proteins, when used in combination therapy. All these results taken together indicate that ALC can be protective against CDDP‐induced auditory hair cell damage.
Acetyl‐l‐carnitine has modulator functions in critical cell processes including gene transcription and apoptosis. The present study demonstrates that ALC has protective effects on CDDP‐induced ototoxicity by modulating apoptotic gene expressions and inflammatory cytokine levels. ALC can be considered to be an innovative therapeutic intervention against iatrogenic hearing loss due to CDDP and CDDP‐like ototoxic agents. Translation of these findings to clinical practice requires further support by in vivo studies.
Acknowledgements
We specially thank Professor F. Kalinec for providing HEI‐OC1 cells. This study was approved by DEU ethics committee and the research was carried out at Dokuz Eylul University, Institute of Oncology and Basic Oncology Laboratory, Turkey. This study was financially supported by a grant from the Dokuz Eylul University Research Foundation (project 2011.KB.SAG.009).
A part of the study was presented orally at the 17th National Paediatric Cancer Congress, Turkish Society of Paediatric Oncology, Abant‐BOLU, Turkey, 1–5 May 2012, and presented as a poster at the 44th Congress of the International Society of Paediatric Oncology (SIOP) London, United Kingdom, 5–8 October 2012.
References
- 1. Rybak LP, Mukherjea D, Jajoo S, Ramkumar V (2009) Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J. Exp. Med. 219, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park HJ, Kim HJ, Bae GS, Seo SW, Kim DY, Jung WS et al (2009) Selective GSK‐3beta inhibitors attenuate the cisplatin‐induced cytotoxicity of auditory cells. Hear. Res. 257, 53–62. [DOI] [PubMed] [Google Scholar]
- 3. Kalinec F, Kalinec G, Boukhvalova M, Kachar B (1999) Establishment and characterization of conditionally immortalized organ of corti cell lines. Cell Biol. Int. 23, 175–184. [DOI] [PubMed] [Google Scholar]
- 4. Fritz IB (1963) Carnitine and its role in fatty acid metabolism. Adv. Lipid Res. 1, 285–3348. [PubMed] [Google Scholar]
- 5. Stanley CA (1987) New genetic defects in mitochondrial fatty acid oxidation and carnitine deficiency. Adv. Pediatr. 34, 59–88. [PubMed] [Google Scholar]
- 6. Altun ZS, Gunes D, Aktaş S, Erbayraktar Z, Olgun N (2010) Protective effects of acetyl‐L‐carnitine on cisplatin cytotoxicity and oxidative stress in neuroblastoma. Neurochem. Res. 35, 437–443. [DOI] [PubMed] [Google Scholar]
- 7. Tufekci O, Gunes D, Ozoğul C, Kolatan E, Altun Z, Yilmaz O et al (2009) Evaluation of the effect of acetyl L‐carnitine on experimental cisplatin nephrotoxicity. Chemotherapy 55, 451–459. [DOI] [PubMed] [Google Scholar]
- 8. Gunes D, Kirkim G, Kolatan E, Guneri EA, Ozogul C, Altun Z et al (2011) Evaluation of the effect of acetyl L‐carnitine on experimental cisplatin ototoxicity and neurotoxicity. Chemotherapy 57, 186–194. [DOI] [PubMed] [Google Scholar]
- 9. Kalinec GM, Webster P, Lim DJ, Kalinec F (2003) A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol. Neurootol. 8, 177–189. [DOI] [PubMed] [Google Scholar]
- 10. Kim HJ, So HS, Lee JH, Park C, Lee JB, Youn MJ et al (2008) Role of proinflammatory cytokines in cisplatin‐induced vestibular hair cell damage. Head Neck 30, 1445–1456. [DOI] [PubMed] [Google Scholar]
- 11. Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, Ramkumar V (2011) Short interfering RNA against STAT1 attenuates cisplatin‐induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2, e180. doi: 10.1038/cddis.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rybak LP, Whitworth CA (2005) Ototoxicity: therapeutic opportunities. Drug Discov. Today 10, 1313–1321. [DOI] [PubMed] [Google Scholar]
- 13. Olgun N, Kansoy S, Aksoylar S, Cetingul N, Vergin C, Oniz H et al (2003) Experience of the Izmir Pediatric Oncology Group on Neuroblastoma: IPOG‐NBL‐92 Protocol. Pediatr. Hematol. Oncol. 20, 211–218. [PubMed] [Google Scholar]
- 14. Musial‐Bright L, Fengler R, Henze G, Hernáiz Driever P (2011) Carboplatin and ototoxicity: hearing loss rates among survivors of childhood medulloblastoma. Childs Nerv. Syst. 27, 407–413. [DOI] [PubMed] [Google Scholar]
- 15. Huang H, Zhu L, Reid BR, Drobny GP, Hopkins PB (1995) Solution structure of a cisplatin‐induced DNA interstrand crosslink. Science 270, 1842–1845. [DOI] [PubMed] [Google Scholar]
- 16. Kharbanda S, Ren R, Pandey P, Shafman TD, Feller SM, Weichselbaum RR et al (1995) Activation of the c‐Abl tyrosine kinase in the stress response to DNA‐damaging agents. Nature Lond. 376, 785–788. [DOI] [PubMed] [Google Scholar]
- 17. Jordan P, Carmo‐Fonseca M (2000) Molecular mechanisms involved in cisplatin cytotoxicity. Cell. Mol. Life Sci. 57, 1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kartalou M, Essigmann JM (2001) Mechanisms of resistance to cisplatin. Mutat. Res. 478, 23–43. [DOI] [PubMed] [Google Scholar]
- 19. Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D et al (1997) Use of organotypic cultures of Corti's organ to study the protective effects of antioxidant molecules on cisplatin‐induced damage of auditory hair cells. Am. J. Otol. 18, 559–571. [PubMed] [Google Scholar]
- 20. Rybak LP, Whitworth C, Somani S (1999) Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope 109, 1740–1744. [DOI] [PubMed] [Google Scholar]
- 21. Evans P, Halliwell B (1999) Free radicals and hearing. Cause, consequence, and criteria. Ann. N. Y. Acad. Sci. 884, 19–40. [DOI] [PubMed] [Google Scholar]
- 22. Teranishi M, Nakashima T, Wakabayashi T (2001) Effects of alpha‐tocopherol on cisplatin‐induced ototoxicity in guinea pigs. Hear. Res. 151, 61–70. [DOI] [PubMed] [Google Scholar]
- 23. Feghali JG, Liu W, Van de Water TR (2001) L‐n‐acetyl‐cysteine protection against cisplatin‐induced auditory neuronal and hair cell toxicity. Laryngoscope 111, 1147–1155. [DOI] [PubMed] [Google Scholar]
- 24. So H, Kim H, Lee JH, Park C, Kim Y, Kim E et al (2007) Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF‐kappaB. J. Assoc. Res. Otolaryngol. 8, 338–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myung NY, Choi IH, Jeong HJ, Kim HM (2011) Ameliorative effect of purple bamboo salt‐pharmaceutical acupuncture on cisplatin‐induced ototoxicity. Acta Otolaryngol. 131, 14–21. [DOI] [PubMed] [Google Scholar]
- 26. Davies KJA (2000) Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 50, 279–289. [DOI] [PubMed] [Google Scholar]
- 27. Kalinec GM, Fernandez‐Zapico ME, Urrutia R, Esteban‐Cruciani N, Chen S, Kalinec F (2005) Pivotal role of Harakiri in the induction and prevention of gentamicin‐induced hearing loss. Proc. Natl. Acad. Sci. USA 102, 16019–16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Grandis D (2007) Acetyl‐L‐carnitine for the treatment of chemotherapy induced peripheral neuropathy: a short review. C.N.S. Drugs 21(Suppl 1), 39–43; discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 29. Pisano C, Pratesi G, Laccabue D, Zunino F, Lo Giudice P, Belluci A et al (2003) Paclitaxel and cisplatin induced neurotoxicity: a protective role of acetyl‐L‐carnitine. Clin. Cancer Res. 9, 5756–5767. [PubMed] [Google Scholar]
- 30. Calabrese V, Giuffrida Stella AM, Calvani M, Butterfield DA (2006) Acetylcarnitine and cellular stress response: roles in nutritional redox homeostasis and regulation of longevity genes. J. Nutr. Biochem. 17, 73–88. [DOI] [PubMed] [Google Scholar]
- 31. Steiber A, Kerner J, Hoppel CL (2004) Carnitine: a nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 25, 455–473. [DOI] [PubMed] [Google Scholar]
- 32. Ishii T, Shimpo Y, Matsuoka Y, Kinoshita K (2000) Anti‐apoptotic effect of acetyl‐l‐carnitine and I‐carnitine in primary cultured neurons. Jpn. J. Pharmacol. 83, 119–124. [DOI] [PubMed] [Google Scholar]
- 33. Di Cesare Mannelli L, Ghelardini C, Calvani M, Nicolai R, Mosconi L, Vivoli E et al (2007) Protective effect of acetyl‐L‐carnitine on the apoptotic pathway of peripheral neuropathy. Eur. J. Neurosci. 26, 820–827. [DOI] [PubMed] [Google Scholar]
- 34. Ghirardi O, Lo Giudice P, Pisano C, Vertechy M, Bellucci A, Vesci L et al (2005) Acetyl‐L‐Carnitine prevents and reverts experimental chronic neurotoxicity induced by oxaliplatin, without altering its antitumor properties. Anticancer Res. 25, 2681–2687. [PubMed] [Google Scholar]
- 35. Gupta S, Koru‐Sengul T, Arnold SM, Devi GR, Mohiuddin M, Ahmed MM (2011) Low‐dose fractionated radiation potentiates the effects of cisplatin independent of the hyper‐radiation sensitivity in human lung cancer cells. Mol. Cancer Ther. 10, 292–302. [DOI] [PubMed] [Google Scholar]
- 36. Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban‐Cruciani N, Kalinec G et al (2002) Cisplatin‐induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hear. Res. 174, 45–54. [DOI] [PubMed] [Google Scholar]
- 37. Pisano C, Vesci L, Milazzo FM, Guglielmi MB, Foderà R, Barbarino M et al (2010) Metabolic approach to the enhancement of antitumor effect of chemotherapy: a key role of acetyl‐L‐carnitine. Clin. Cancer Res. 16, 3944–3953. [DOI] [PubMed] [Google Scholar]
- 38. Abd‐Allah AR, Al‐Majed AA, Al‐Yahya AA, Fouda SI, Al‐Shabana OA (2005) L‐Carnitine halts apoptosis and myelosuppression induced by carboplatin in rat bone marrow cell cultures (BMC). Arch. Toxicol. 79, 406–413. [DOI] [PubMed] [Google Scholar]
- 39. Liu J, Head E, Kuratsune H, Cotman CW, Ames BN (2004) Comparison of the effects of l‐carnitine and acetyl‐l‐carnitine on carnitine levels, ambulatory activity and oxidative stress biomarkers in the brain of old rats. Ann. NY. Acad. Sci. 1033, 117–131. [DOI] [PubMed] [Google Scholar]
- 40. Buyse J, Swennen Q, Niewold TA, Klasing KC, Janssens GPJ, Baumgartner M et al (2007) Dietary l‐carnitine supplementation enhances the lipopolysaccharide‐induced acutephase protein response in broiler chickens. Veterinary Immunol. Immunopathol. 118, 154–159. [DOI] [PubMed] [Google Scholar]
- 41. Abd‐Allah AR, Helal GK, Al‐Yahya AA, Aleisa AM, Al‐Rejaie SS, Al‐Bakheet SA (2009) Pro‐inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L‐carnitine. Oxid. Med. Cell. Longev. 2, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laviano A, Molfino A, Seelaender M, Frascaria T, Bertini G, Ramaccini C et al (2011) Carnitine administration reduces cytokine levels, improves food intake, and ameliorates body composition in tumour‐bearing rats. Cancer Invest. 29, 696–700. [DOI] [PubMed] [Google Scholar]


