Abstract
Objectives
Stromal cell‐derived factor‐1α (SDF‐1α) plays an important role in tissue regeneration in various tissues including the periodontium. A potential limitation for its use derives from its sensitivity to cleavage by dipeptidyl peptidase‐IV (DPP‐IV). Parathyroid hormone (PTH) reduces enzymatic activity of DPP‐IV and is suggested to be a promising agent for periodontal tissue repair. The purpose of this study was to provide insight into how SDF‐1α and intermittent PTH treatment might affect proliferation, migration and osteogenic differentiation of human periodontal ligament stem cells (PDLSCs) in vitro.
Materials and methods
PDLSCs were isolated by the limiting dilution method. Surface markers were quantified by flow cytometry. Cell‐counting kit‐8 (CCK8), cell migration assay, alkaline phosphatase (ALP) activity assay, alizarin red staining and RT‐PCR were used to determine viability, migration and osteogenic differentiation of PDLSCs.
Results
PDLSCs were positive for CD44, CD73, CD90, CD105, CD166 and STRO‐1 and negative for CD14, CD34 and CD45. PTH/SDF‐1α cotherapy significantly promoted cell proliferation, chemotactic capability, ALP activity and mineral deposition (P<.05). Gene expression level of bone sialoprotein (BSP), runt‐related transcription factor 2 (Runx2) and osteocalcin (OCN) were all up‐regulated (P<.05).
Conclusions
PTH/SDF‐1α cotherapy promoted proliferation, migration and osteogenic differentiation of PDLSCs in vitro. Cotherapy seemed to have potential to promote periodontal tissue regeneration by facilitating chemotaxis of PDLSCs to the injured site, followed by promoting proliferation and osteogenic differentiation of these cells.
1. Introduction
Periodontitis are infectious diseases characterized by destruction of periodontium including gingiva, periodontal ligament (PDL), cementum and alveolar bone. Effective reconstruction of periodontium destroyed by periodontal diseases is a major goal of periodontal therapy.1 PDL is a fibrous connective tissue embedded between the cementum and the inner wall of the alveolar bone socket, which contributes to tooth homoeostasis, nutrition and repair of damaged tissue.2, 3 Seo et al.4 first isolated a population of multipotent stem cells within PDL, termed periodontal ligament stem cells (PDLSCs). PDLSCs exhibit osteogenic, adipogenic and chondrogenic characteristics under inductive culture conditions in vitro.5, 6 Furthermore, PDLSCs transplantation therapies have the potential to promote the formation of new bone, new cementum and functional PDL in damaged periodontium in animal models.7, 8, 9, 10 PDLSC may be an ideal cellular source for periodontal tissue repair and regeneration. However, all the aforementioned PDLSCs‐based therapies required tissue biopsies and extensive manipulation of PDLSCs. Apart from its limited tissue source, additional time, cost and potential morbidity are associated with harvesting procedures, obtaining enough PDLSCs from human autologous PDL tissues for clinical use is a formidable challenge. Thus, application of chemokines or cytokines to recruit autologous stem cells to defects may provide a potential to increase the number of local stem cells and improve periodontal tissue regeneration.11, 12 Cytokines and chemokines are important factors that regulate mobilization, trafficking and homing of stem/progenitor cells.13, 14 Among the various cytokines and chemokines, stromal cell‐derived factor‐1α (SDF‐1α/CXCL12) is a promising candidate for tissue engineering.15, 16, 17, 18, 19, 20, 21, 22, 23
SDF‐1α, a member of CXC chemokine subfamily, plays important roles in survival, growth and migration of different cell types by activation of a G protein‐coupled receptor, CXCR4.15, 16, 17, 18, 19, 20, 21, 22, 23 The interaction of SDF‐1α/CXCR4 may play an essential role in promoting migration of CXCR4‐expressing MSCs into injured tissues and mediate tissue regeneration.17, 18, 21, 22 Previously, we reported that human PDLSCs expressed SDF‐1α receptor CXCR4, and SDF‐1α promoted the proliferation and chemotaxis of human PDLSCs in a dose‐dependent manner.24 Our previous work suggested that local application of exogenous SDF‐1α may be a simple and safe technique for periodontal tissue regeneration.25 However, a potential limitation for the therapeutic use of SDF‐1α derives from its sensitivity to cleavage by CD26/dipeptidyl peptidase‐IV (DPP‐IV).26
DPP‐IV is a membrane‐bound extracellular peptidase that is ubiquitously expressed.27, 28 SDF‐1α is N‐terminally cleaved at position‐2 proline by DPP‐IV, and the truncated form of SDF‐1α is both inactive as a chemotactic molecule, and also blocks chemotaxis of full‐length SDF‐1α.26, 29 Therefore, stabilization of the active form of SDF‐1α by the inhibition of DPP‐IV may maintain the therapeutic activity of SDF‐1α and enhance the recruitment of stem/progenitor cells to injured tissues. Huber et al.30 reported that parathyroid hormone (PTH) significantly reduced the enzymatic activity of DPP‐IV in a dose‐dependent manner in vitro. PTH was also shown to lead to an increased cardiac SDF‐1α protein level by inhibiting DPP‐IV, thus enhancing stem cell migration into the ischaemic heart and improving cardiac function in vivo.
Approved by the U.S. Food and Drug Administration (FDA), PTH is the unique bone anabolic drug for the treatment of osteoporosis.31, 32 Reports have demonstrated that intermittent administration of PTH increases bone strength and prevents bone fractures, while continuous use of PTH may lead to bone loss.32, 33, 34 Intermittent administration of PTH can play a significant role in periodontal repair via stimulation of bone mineral content especially in the alveolar region.35 PTH has been suggested as a promising agent for periodontal tissue repair.35, 36 Addition of PTH to SDF‐1α may improve the efficiency of SDF‐1α on PDLSCs, making their application for periodontal regeneration more feasible and effective. To date, the effect of the combination of PTH and SDF‐1α on the biological behaviour of PDLSCs has not been elucidated.
Therefore, the aim of this study was to investigate the effects of the combination of PTH and SDF‐1α on proliferation, migration and osteogenic differentiation of human PDLSCs and provide the experimental basis for their cotherapy in periodontal tissue regeneration.
2. Materials and methods
2.1. Collection and culture of human PDLSCs
The study protocol was approved by the Medical Ethical Committee of School of Stomatology, Shandong University (Protocol Number: GR201603) and written informed consent was obtained from each individual participant. All the protocols were carried out in accordance with the approved guidelines. Six human premolars, which were extracted for orthodontic reasons from six systemically healthy patients, were used for tissue biopsy and PDL cell isolation. The age of the participants ranged from 12 to 16 years (three males, three females). The teeth were stored in Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 100 U/mL penicillin G and 100 μg/mL streptomycin (Sigma‐Aldrich, St Louis, MO, USA). Human PDL tissue was scraped from the middle third of the root surface as previously described.37 PDL tissue was cut into small pieces and digested with 3 mg/mL collagenase I (Invitrogen, Carlsbad, CA, USA) and 4 mg/mL dispase II (Invitrogen) for 2 hours at 37°C. The dissociated cell suspension was filtered through a 70 μm cell strainer (BD Falcon, BD Biosciences, Bedford, MA, USA). After cell counting, single cell suspension was plated at a concentration of 60 cells/cm2 on non‐treated 10 cm Petri dishes for single cell‐derived colony selection, and was cultured in DMEM with 10% foetal calf serum (FCS; Hyclone), 2 mm l‐glutamine (Sigma‐Aldrich), 100 mm l‐ascorbate‐2‐phosphate (Wako Pure Chemical Industries, Richmond, VA, USA), 1 mm sodium pyruvate (Sigma‐Aldrich), 50 U/mL penicillin G and 50 mg/mL streptomycin for 10–14 days. Individual colonies were isolated with colony rings and expanded into individual vessels for further cultivation, as previously described.38 All cell‐based experiments were repeated at least three times.
2.2. Flow cytometric analysis
Surface markers for human PDLSCs were quantified by flow cytometry. After reaching confluence, cells were detached by 0.05% trypsin/ethylenediaminetetraacetic acid (EDTA) and resuspended in blocking buffer containing Hanks’ balanced salt solution (Sigma‐Aldrich) supplemented with 5% FCS. Approximately, 1 × 105 cells were incubated with fluorescein isothiocyanate (FITC)‐conjugated mouse monoclonal antibodies (10 μg/mL) specific for CD44, CD73, CD90, CD105, CD166 (Becton Dickinson Biosciences, San Jose, CA, USA) and STRO‐1 (R&D Systems, Inc., Minneapolis, MN, USA), CD14, CD34, CD45 (Beckman Coulter, Brea, CA, USA) or isotype‐matched control IgGs for 1 hour on ice. Isotype‐matched controls were then incubated with FITC‐conjugated goat anti‐mouse IgG (Southern Biotech, Birmingham, AL, USA) for 45 minutes on ice. After washing, cells were fixed in fluorescence‐activated cell sorting fix solution. Then the samples were subjected to flow cytometric analyses using an Epics‐XL/MCL flow cytometer (Beckman Coulter, Fullerton, CA, USA).
2.3. PTH and SDF‐1α administration
In this study, cells were cultured in the presence of 50 ng/mL PTH (Sigma‐Aldrich) and/or 200 ng/mL SDF‐1α (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as experiment groups according to a previous study.24, 39 Cells were maintained in PTH for 6 hours within a 48‐hour incubation cycle. For the remaining time, experimental media were replaced with basic culture media without PTH. Cells maintained without PTH and SDF‐1α were referred as control group. The duration of PTH application and the four groups (a‐d) were shown in Fig. 1.
Figure 1.
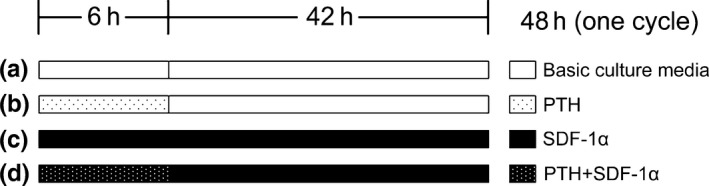
Schematic diagram of SDF‐1α and PTH application duration and four groups
2.4. Cell proliferation assay
Once 80% confluence had been achieved, cells were seeded in 96‐well plates at a density of 7.5 × 104 cells/mL and then treated with maintenance medium (DMEM supplemented with 0.5% FCS) or maintenance medium with PTH and/or SDF‐1α for 2 days. The proliferation of the cells was determined using the cell‐counting kit‐8 (CCK8) according to the manufacturer's instructions (Dojindo Laboratories, Kumamoto, Japan). Briefly, the culture medium was replaced with 100 μL DMEM containing 10 μL CCK8 and the plates were incubated for 3 hours at 37°C. Absorbance at 450 nm was measured in a multiwell spectrophotometer.
2.5. Cell migration assay
The migratory effects of SDF‐1α and/or PTH on human PDLSCs were evaluated in 24‐well plates with an 8 μm pore size polycarbonate membrane. In brief, 1×105 cells in 200 μL DMEM containing 0.1% FCS were cultured in the upper chamber. The lower wells were supplemented with PTH and/or SDF‐1α in 500 μL DMEM containing 0.1% FCS. Medium without PTH and SDF‐1α but 0.1% FCS served as a negative control and medium containing 10% FCS served as a positive control. The chambers were incubated for 20 hours at 37°C. After removal of non‐migrated cells on top of the filter, cells that had migrated through the membrane were fixed in 4% paraformaldehyde (Sigma‐Aldrich), stained with 0.1% crystal violet (Sigma‐Aldrich). The number of cells that had migrated into the lower chamber was counted in six randomly selected high‐power microscopic fields (×200) per filter by blind evaluation.
2.6. ALP activity assay
Human PDLSCs were seeded in six‐well plates at a density of 5 × l04 cells/mL in osteogenic inductive medium (DMEM supplemented with 10% FCS, 10−8 mol/L dexamethasone, 50 mg/L ascorbic acid, and 10 mmol/L β‐glycerophosphate) or osteogenic inductive medium with the presence of 50 ng/mL PTH and/or 200 ng/mL SDF‐1α. After 7 and 14 days of induction, cells were washed with 0.01 m PBS and scraped into 150 μL 1% TritonX‐100 (Sigma‐Aldrich). Then cells were sonicated and cell lysates were centrifuged at 12 000 g for 5–10 minutes at 4°C. ALP activity in the supernatant was assayed according to the instruction of the manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In brief, 30 μL supernatant, 50 μL buffer solutions and 50 μL matrix liquid were added to a 96‐well plate and mixed. The plate was incubated for 15 minutes at 37°C. Next, 150 μL colouration solution was added to each well and the absorbance of the samples was measured at 520 nm wavelength with a spectrophotometer. For standard wells and negative control wells, the supernatant was replaced with standard phenol solution and double distilled water. ALP activity was calculated according to the concentration of the phenol in a standard well. Results were adjusted according to the protein content detected by bicinchoninic acid standard (Jiangsu KeyGEN BioTECH Corp., Ltd, Nanjing, China).
2.7. Alizarin red staining
Human PDLSCs cells were seeded in six‐well plates at a density of 5 × l04 cells/mL and were cultured with osteogenic inductive medium or osteogenic inductive medium with the presence of 50 ng/mL PTH and/or 200 ng/mL SDF‐1α. After 28 days of induction, extracellular matrix calcification was estimated by using 2% Alizarin Red S with a pH value of 4.3 (Sigma‐Aldrich) for 15 minutes. For quantifying the relative amount of calcium, 300 μL of 10% (w/v) cetylpyridinium chloride (Sigma‐Aldrich) and 10 mm sodium phosphate (pH 7.0) solution were added to the stained dishes and the absorbance of extracted dye was determined at 562 nm.
2.8. RNA isolation and real‐time PCR analysis of osteogenesis‐related gene expression
Human PDLSCs were inoculated in six‐well plates at a density of 5 × l04 cells/mL and cultured with osteogenic inductive medium or osteogenic inductive medium with the presence of 50 ng/mL PTH and/or 200 ng/mL SDF‐1α. After 7, 14, 21 and 28 days of induction, total RNA of PDLSCs with different treatments was extracted with Trizol® reagent (TaKaRa Bio Inc, Tokyo, Japan), and 1 μg RNA was transcribed with reverse transcriptase (TaKaRa Bio Inc) according to the manufacturer's recommendation. Real‐time PCR (RT‐PCR) was performed using 1 μL of cDNA in a 20 μL reaction volume with Roche 480 in triplicate. The relative expression level of the housekeeping gene, GAPDH, was used to normalize gene expression in each sample in different groups. PCR conditions were as follows: hot‐start enzyme activation at 95°C for 30 seconds; 55 cycles of denaturation at 95°C for 5 seconds, annealing at 60°C for 35 seconds and extension at 72°C for 1 minute; finally at 40°C for 30 seconds. The sequences of the primers for amplification of human GAPDH, Runx2, BSP and OCN were as follows: GAPDH: 5′‐GCACCGTCAAGGCTGAGAAC‐3′ and 5′‐TGGTGAAGACGCCAGTGGA‐3′; Runx2: 5′‐TCCACACCATTAGGGACCATC‐3′ and 5′‐TGCTAATGCTTCGTGTTTCCA‐3′; BSP: 5′‐CCCCACCTTTTGGGAAAACCA‐3′ and 5′‐TCCCCGTTCTCACTTTCATAGAT‐3′; OCN: 5′‐TCACACTCCTCGCCCTATT‐3′ and 5′‐GATGTGGTCAGCCAACTCG‐3′. The amount of mRNA was calculated for each sample based on the standard curve using the lightcycler ® Software 4.0 (Roche, Basel, Switzerland).
2.9. Statistical analysis
Data were expressed as the mean ± standard error of the mean and were analysed using SPSS software (SPSS Inc., Chicago, IL, USA) with differences between groups assessed by one‐way ANOVA. Statistical probability of P<.05 was considered significant.
3. Results
3.1. Isolation and characteristics of human PDLSCs
Primary cultures of single cell suspensions from human periodontal ligament tissues exhibited a spindle‐shaped fibroblast‐like morphology (Fig. 2a), PDLSCs were identified as single cell colonies generated from human PDL‐derived cells, which exhibited typical spindle shape and oval nuclei containing two or three nucleoli (Fig. 2b). All trials of single cell‐derived colony‐forming efficiency were successfully performed. Cell clusters were derived from a single colony of PDLSCs (Fig. 2c,d). By flow cytometric analysis, PDLSCs were uniformly positive (>95%) for MSCs markers CD44, CD73, CD90, CD105, CD166 and the level of STRO‐1 expression was 20.40%, while negative for hematopoietic stem cells markers CD14, CD34 and CD45 (<5%) (Fig. 2e).
Figure 2.
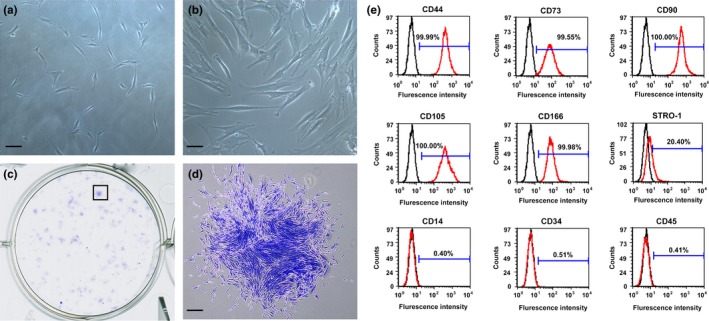
Characterization of PDLSCs. (a) Cultured primary cells derived from human periodontal ligament tissue exhibited typical fibroblast‐like morphology. Scale bar: 100 μm. (b) Cultured PDLSCs exhibited typical fibroblast‐like morphology. Scale bar: 50 μm. (c) Single colonies formed after PDLSCs were plated at low density and cultured for 10–14 days. (d) Cell clusters derived from the PDLSCs formed a single colony and were stained with 0.1% toluidine blue. Scale bar: 250 μm. (e) Flow cytometric analyses of PDLSCs. They were positive for MSCs markers CD44, CD73, CD90, CD105, CD166 and the level of STRO‐1 expression was 20.4%, while negative for hematopoietic markers CD14, CD34 and CD45 (red line). Isotype controls 1B5 (IgG1) and 1D4.5 (IgG2a) were black line
3.2. The combination of PTH and SDF‐1α enhanced the proliferation of PDLSCs
CCK8 results showed that the proliferation of PDLSCs treated with 50 ng/mL PTH was higher than that in control group, but there was no significant difference between the two groups (P>.05) (Fig. 3). However, 200 ng/mL SDF‐1α significantly promoted cell proliferation compared with control group (P<.05). Moreover, cell proliferation was synergistically promoted by the combination of PTH and SDF‐1α, which was significantly higher than other three groups (P<.05).
Figure 3.
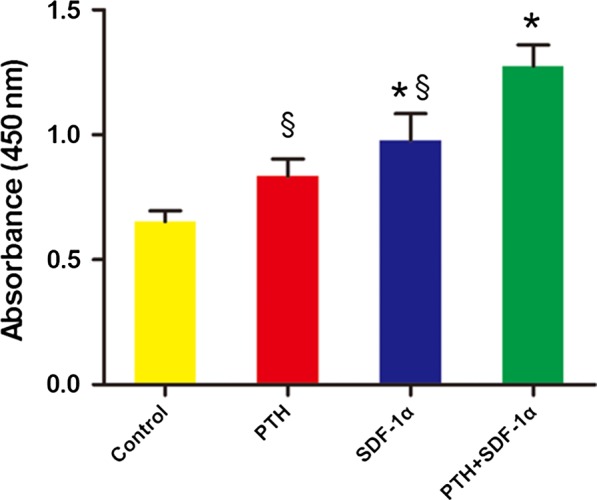
PTH combined with SDF‐1α promoted the proliferation of human PDLSCs. Cell proliferation was measured using a CCK‐8 kit. Each value was the mean ± SEM of four independent experiments. *P<.05 compared with control group; §P<.05 PTH+SDF‐1 group compared with other experimental groups
3.3. The combination of PTH and SDF‐1α enhanced the migration of PDLSCs
We tested the chemotactic capability of PTH and SDF‐1α on PDLSCs and the numbers of migrated cells were quantified (Fig. 4a,b). SDF‐1α significantly enhanced the migration capacity of PDLSCs compared with negative control group (62.92 ± 7.87 vs 6.28 ± 1.28 cells/field, P<.05) and positive control group (62.92 ± 7.87 vs 45.5 ± 2.24 cells/field, P<.05). About 50 ng/mL PTH also significantly induced cell migration compared with negative control group (54.53 ± 5.11 vs 6.28 ± 1.28 cells/field, P<.05). More importantly, when combined with PTH, the chemotactic capability of SDF‐1α was further enhanced. The migration capacity of PDLSCs cultured in 200 ng/mL SDF‐1α plus 50 ng/mL PTH was significantly higher than that in negative, positive controls and PTH group (77.36 ± 7.71 vs 6.28 ± 1.28, 45.5 ± 2.24, 54.53 ± 5.11 cells/field, P<.05).
Figure 4.
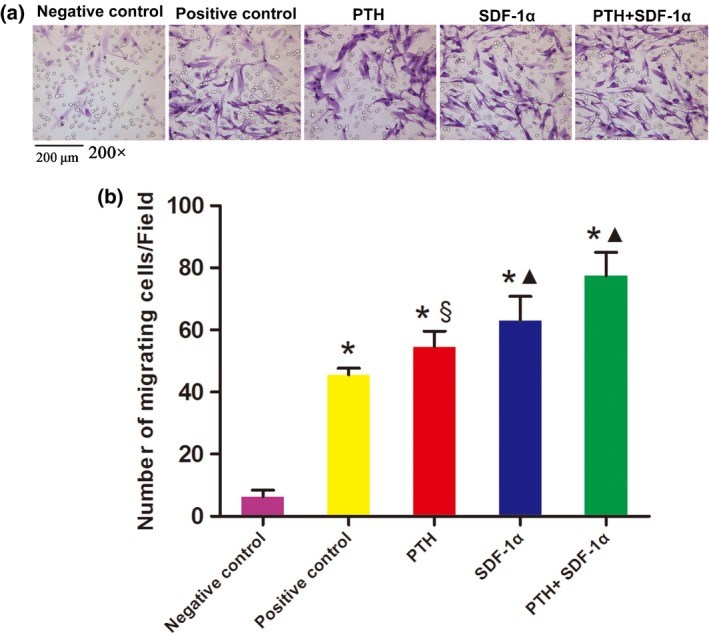
PTH combined with SDF‐1α promoted the migration of human PDLSCs. (a) Crystal violet staining showed the cells that migrated to the undersurface of the membrane in different groups. (b) All three experiment groups had positive effects on the migration capacity of PDLSCs in transwell assays. The data were presented as mean ± SEM. *P<.05 compared with negative control; ▲P<.05 experimental groups compared with positive control; §P<.05 PTH+SDF‐1α group compared with other experimental groups
3.4. The combination of PTH and SDF‐1α significantly increased ALP activity of PDLSCs
ALP activity has been widely used as a marker of the early osteogenic differentiation of stem cells or progenitor cells. In our study, ALP activity was measured at day 7 and 14 (Fig. 5). At day 7, 200 ng/mL SDF‐1α significantly promoted ALP activity compared with PTH and control groups (P<.05). Moreover, the combination of PTH and SDF‐1α stimulated significantly higher level of ALP activity compared with other three groups (P<.05). At day 14, ALP activity in both SDF‐1α and PTH+SDF‐1α groups dramatically decreased to the level of PTH and control groups.
Figure 5.
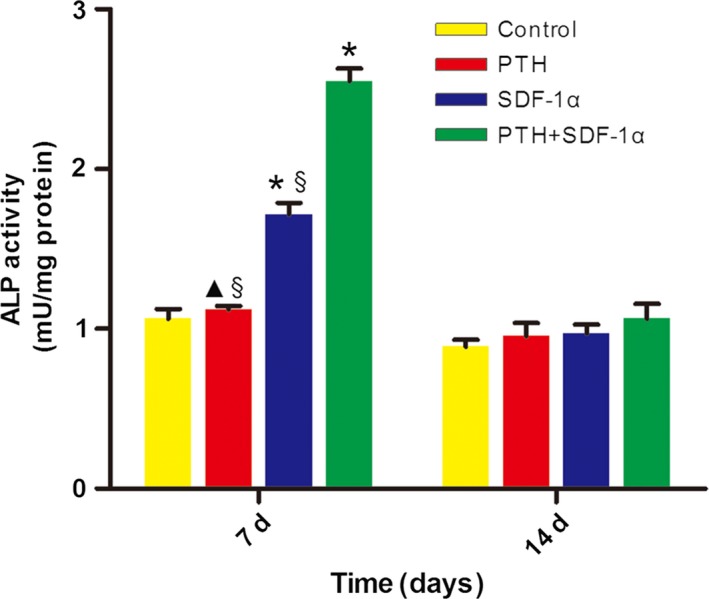
The combination of PTH and SDF‐1α enhanced ALP activity of human PDLSCs. ALP activity was significantly promoted at day 7 in SDF‐1α and PTH+SDF‐1α groups, while decreased at day 14 and there was no significant difference between experiment groups and control group. The data were presented as mean ± SEM. *P<.05 compared with control group; ▲P<.05 PTH group compared with SDF‐1α group; §P<.05 PTH+SDF‐1α group compared with other experimental groups
3.5. The combination of PTH and SDF‐1α significantly promoted the formation of mineral deposition of PDLSCs
In our research, extracellular matrix calcification was determined by Alizarin Red S staining (Fig. 6a) and the relative amount of calcium was quantified (Fig. 6b). The application of both PTH and SDF‐1α significantly enhanced the mineralization in comparison with control group (P<.05) and SDF‐1α significantly increased the concentration of calcium deposition in comparison with PTH (P<.05). More importantly, data indicated cells treated with PTH+SDF‐1α significantly increased the calcium deposition compared with the other three groups (P<.05).
Figure 6.
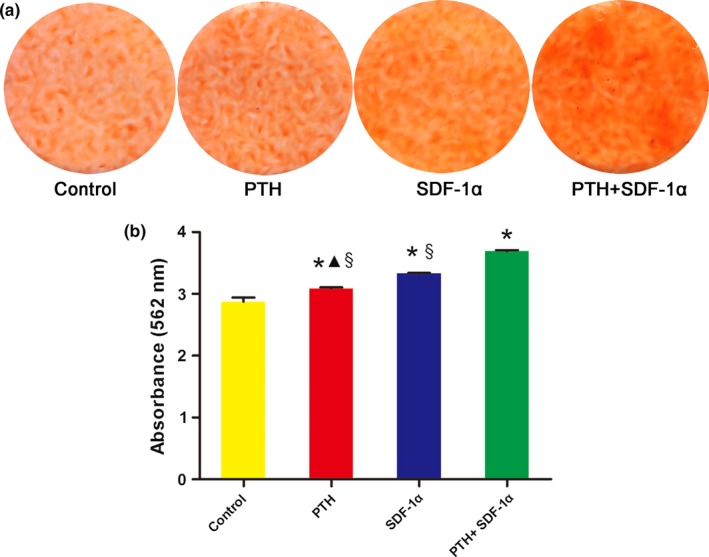
The combination of PTH and SDF‐1α enhanced mineral deposition of PDLSCs. (a) Representative photographs of Alizarin Red staining in different groups. (b) The concentration of calcium deposition was quantified by absorbance at 562 nm. The data were presented as mean ± SEM. *P<.05 compared with control group; ▲P<.05 PTH group compared with SDF‐1α group; §P<.05 PTH+SDF‐1α group compared with other experimental groups
3.6. PTH and SDF‐1α enhanced the gene expression of osteogenic markers
The effect of PTH and SDF‐1α on osteogenic differentiation of PDLSCs was determined by evaluating the gene expression of BSP, OCN and Runx2. At day 7, the expression level of BSP was up‐regulated significantly by PTH+SDF‐1α in comparison with other three groups (P<.05) (Fig. 7a). SDF‐1α promoted significantly higher level of BSP mRNA than PTH and the expression level of BSP mRNA in PTH group was higher than that in control group (P<.05). Not surprisingly, BSP expression level decreased dramatically at day 14 and the difference among the four groups kept the same trend as day 7, with the exception that there was no significant difference between PTH and SDF‐1α groups (P>.05). At day 21, BSP expression level continued to decline and PTH+SDF‐1α up‐regulated higher BSP gene level than PTH and control groups (P<.05). Finally, there was no significant difference among four groups at day 28 (P>.05). As a relatively late marker of osteoblastic differentiation related to mineralization, OCN expression increased with time in all four groups and PTH+SDF‐1α promoted significantly higher OCN expression than other three groups at all four time points (P<.05) (Fig. 7b). At day 14, 21 and 28, though OCN expression level was significantly lower in PTH group in comparison with SDF‐1α, both SDF‐1α and PTH up‐regulated higher OCN expression than control group (P<.05). As for Runx2, the highest expression level appeared at day 14 and both PTH and SDF‐1α groups significantly up‐regulated Runx2 expression in comparison with control group at four time points (P<.05) (Fig. 7c). Furthermore, PTH+SDF‐1α induced significantly higher Runx2 expression than the other three groups at day 7, 21 and 28 (P<.05).
Figure 7.
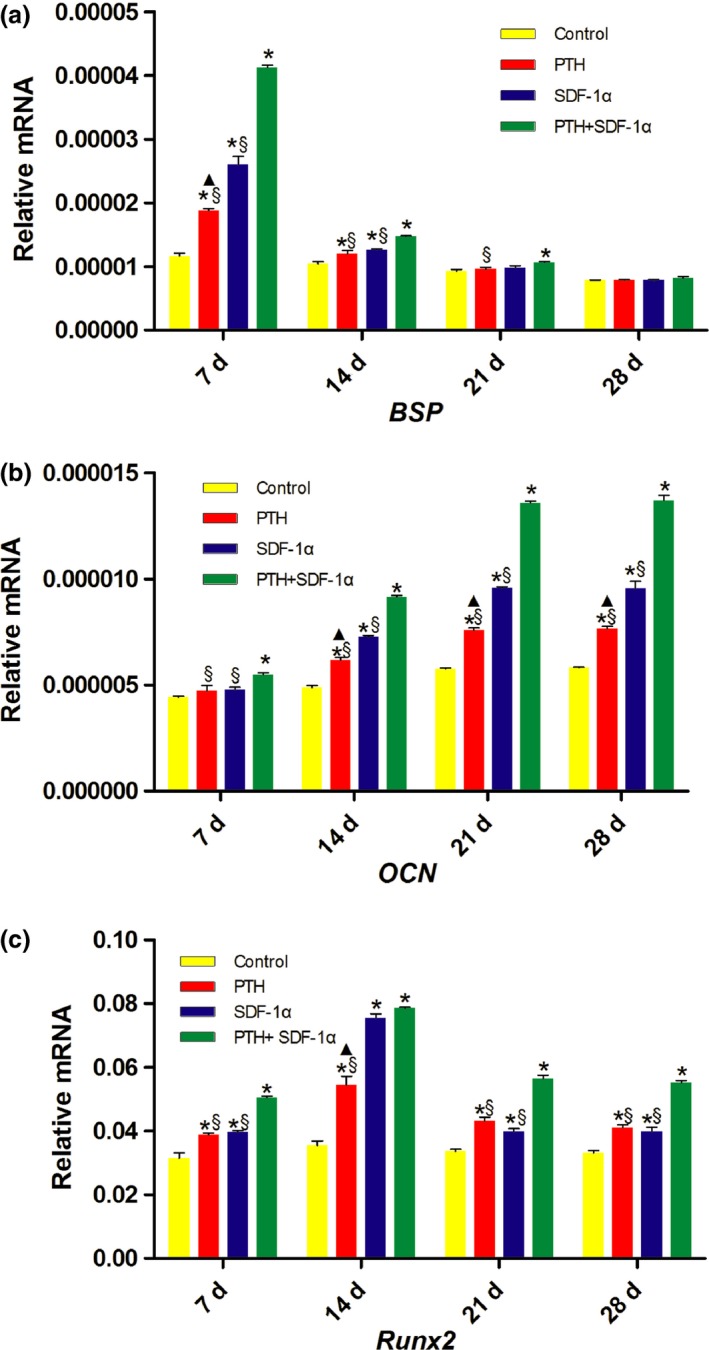
PTH and SDF‐1α up‐regulated the gene expression levels of BSP,OCN and Runx2. (a) Gene expression levels of BSP after 7, 14, 21, 28 days of induction. (b) Gene expression levels of OCN after 7, 14, 21, 28 days of induction. (c) Gene expression levels of Runx2 after 7, 14, 21, 28 days of induction. Each bar represents the mean ± SEM. *P<.05 compared with control group; ▲P<.05 PTH group compared with SDF‐1α group; §P<.05 PTH+SDF‐1α group compared with other experimental groups
4. Discussion
In this study, human PDLSCs were successfully isolated and identified. For the first time, we demonstrated that intermittent PTH treatment enhanced the proliferative and chemotactic effects of SDF‐1α on PDLSCs in vitro. Moreover, the combination of intermittent PTH and SDF‐1α significantly increased ALP activity, promoted the formation of mineral deposition and increased osteogenesis‐related gene expression levels of human PDLSCs.
PDLSCs, isolated from periodontal ligament tissues, have the capacity of multipotent differentiation in vitro and regeneration potential in vivo.4, 5, 6, 7, 8, 9 Consequently, they are regarded as candidates for periodontal tissue regeneration. In the present study, human PDLSCs were successfully isolated from periodontal ligament tissues, displayed typical fibroblastic appearance and demonstrated remarkable colony‐forming capacity. We detected the expression of CD44, CD73, CD90, CD105, CD166, STRO‐1, CD14, CD34 and CD45 in PDLSCs by flow cytometry. The results showed that PDLSCs were positive for MSCs markers CD44, CD73, CD90, CD105, CD166 and negative for hematopoietic markers CD14, CD34, and CD45, in accord with the marker expression profile defined for multipotent stromal cells in the International Society for Cellular Therapy position statement.40 STRO‐1 antigen is one of the important early markers of MSCs, and it can also be used to isolate a homogeneous stem cell population from periodontal ligament.4, 6, 24, 37 As for STRO‐1, the expression level in our study was 20.4%, which was consistent with previous studies.24, 37
In our previous studies, we demonstrated that human PDLSCs expressed SDF‐1α receptor CXCR4 at mRNA and protein level. Moreover, PDLSCs showed a dose‐dependent chemotactic response to SDF‐1α at concentrations of 100–400 ng/mL in vitro and the optimal effect appeared at the concentration of 200 ng/mL.24 In this study, we first investigated in vitro chemotactic potency of PTH, and confirmed that PTH promoted the migration of PDLSCs and slightly promoted the proliferation of PDLSCs at the concentration of 50 ng/mL. About 200 ng/mL SDF‐1α and 50 ng/mL PTH were determined to be the optimal concentrations for PDLSCs in this study.
To explore the effects of PTH and SDF‐1α on the proliferation and migration of PDLSCs, we compared in vitro cell proliferation and migration capacity among different treatment conditions of SDF‐1α and PTH. Consistent with the results of our previous studies, SDF‐1α promoted the proliferation and migration of human PDLSCs. Blocking experiment demonstrated a crucial participation of the SDF‐1α/CXCR4 axis in these effects.24 Despite extensive in vivo and in vitro studies conducted to investigate the effects of anabolic PTH treatment on bone, other biological effects on MSCs remain unclear. We demonstrated that PTH also directly regulated the proliferation and migration of human PDLSCs. Not surprisingly, PTH/SDF‐1α cotherapy synergistically promoted the proliferation and migration of PDLSCs, which suggested that PTH enhanced the proliferative and chemotactic effects of SDF‐1α on PDLSCs in vitro. The administration of PTH resulted in an up‐regulation of SDF‐1α and improved the homing capacity via SDF‐1α/CXCR4 axis by analyses of osteoblasts in bone marrow.41 A recent report demonstrated that PTH significantly reduced the enzymatic activity of DPP‐IV in a dose‐dependent manner in vitro.30 PTH enhanced the proliferation and migration of PDLSCs might through stabilizing the active form of SDF‐1α by inhibition of DPP‐IV. Activation of chemokine receptor CXCR4 by SDF‐1α is associated with cell proliferation and migration in many cell types.16, 17, 18, 19, 20, 21, 22, 23 The ability of SDF‐1α/CXCR4 axis to promote cell proliferation may be regulated by different members of the MAP kinase family.42 SDF‐1α/CXCR4 pathway is a key stimulus for the homing of stem cells to sites of damaged tissue, including bone injury.22, 23, 25 SDF‐1α/CXCR4 axis results in increased phosphorylation of focal adhesion components, including the related adhesion focal tyrosine kinase (RAFTK/Pyk2), Crk, and paxillin, which are important in the adhesion process, leading to cell migration.42 Cell migration induced by SDF‐1α/CXCR4 axis involved activation of multiple downstream pathways, including activation of phosphoinositide‐3 kinase (PI3K), phospholipase C, and inactivation of adenylyl cyclase.42, 43
While MSCs have been scrutinized and studied for years, attention was focused on SDF‐1α originally only for its function as a chemotaxis regulator in MSCs engraftment and in tissue engineering.18, 20, 22, 25 In this study, we demonstrated that SDF‐1α also directly played a role in regulating osteogenic differentiation of PDLSCs in addition to its chemotactic effects. These results were in agreement with previous studies of SDF‐1α in osteogenic differentiation of mesenchymal C2C12 cells.44 Hosogane et al.45 reported that SDF‐1α increased BMP‐induced ALP activity and OCN expression in human and mouse BMSCs. It has also been documented that SDF‐1α improves ALP activity and mineralized nodule formation in human dental pulp cells.46 To explore the effects of SDF‐1α on the osteogenic differentiation of PDLSCs in vitro, we performed differentiation assays as we have previously reported.47 The addition of SDF‐1α in the culture media promoted the mRNA expression of osteogenic markers of BSP, OCN and Runx2, and resulted in enhanced relative amount of calcium deposition, indicating that SDF‐1α enhanced osteoblastic differentiation of PDLSCs in vitro. PTH, as a major regulator of bone remodelling and calcium homoeostasis, stimulated osteoblastic bone formation not only in vitro but also in vivo.35, 36, 39 Tokunaga et al.35 demonstrated that intermittent treatment with PTH stimulated osteoblastic differentiation in foetal rat calvaria cell cultures, and topical intermittent administration of PTH recovered alveolar bone loss in rat experimental periodontitis. The anabolic effect of intermittent PTH application on bone varies with different cell types as well as with the concentrations and duration of PTH.34 Our results suggested that PTH alone directly enhanced osteogenic differentiation of PDLSCs. When combined PTH with SDF‐1α, osteogenic capability of PDLSCs was markedly enhanced compared with PTH or SDF‐1α alone. The process of osteogenic differentiation consists of three phases: proliferation with matrix formation, maturation and mineralization. During this process, orchestrated expression of osteogenic genes takes place. ALP, as an important marker for osteogenic differentiation, is expressed in the very early stages of differentiation of stem cells or progenitors.48 Our results demonstrated that ALP activity increased as early as day 7 when cells were treated with PTH and SDF‐1α, which suggested that combination of PTH and SDF‐1α promoted osteoblast differentiation at an early stage. Runx2 is an osteoblast‐specific transcription factor, which is implicated as a major regulator of osteoblast differentiation and gene expression.45 SDF‐1α and/or PTH increased expression level of Runx2 compared with control group. BSP is a component of mineralized tissues, including bone, dentin, cementum, and calcified cartilage.47 Our results showed that the expression level of BSP was up‐regulated significantly by PTH+SDF‐1α in comparison with other three groups as early as day 7. OCN, the most abundant non‐collagenous protein of the bone matrix, serves as a late marker of osteoblast differentiation in several osteogenic lines.45 SDF‐1α/PTH cotherapy significantly increased OCN mRNA as early as day 7. The results indicated that combination of PTH and SDF‐1α synergistically promoted ALP activity, gene expression of osteogenic markers including Runx2, BSP and OCN, and consequently leading to accelerated calcium deposition in PDLSCs.
In summary, our results suggested that the combination of PTH and SDF‐1α enhance the proliferation, chemotaxis and osteogenic differentiation of PDLSCs in vitro. Therefore, PTH/SDF‐1α cotherapy could not only be applied as chemotactic factors to promote stem cell recruitment but also as osteogenic inducers in regenerative dentistry. The cooperative effects of PTH and SDF‐1α had new implications for periodontal tissue regeneration. To confirm the synergistic effect of PTH and SDF‐1α and explore the underlying mechanisms, we will investigate in vivo effects of PTH/SDF‐1α cotherapy on periodontal regeneration by a cell‐free tissue engineering system in further studies.
5. Conclusion
The combination of PTH and SDF‐1α promotes the proliferation, migration and osteogenic differentiation of PDLSCs in vitro. All these findings indicate that application of SDF‐1α and PTH holds promise for in situ therapies of periodontal tissue regeneration based on guiding PDLSCs to periodontal defects, and encourage further in vivo studies.
Competing interests
The authors declare that no financial or other potential competing interests exist with regard to this study.
Author contributions
SG conceived the project and designed the experiments; LD performed PDLSCs culture and identification (colony selection and flow cytometric analysis), proliferation and migration assay; RF performed ALP activity assay, osteogenic differentiation and RT‐PCR evaluation, and analysed the data; LD and SG wrote the manuscript; SG financially and administratively supported this project. All authors made critical revisions of the manuscript and all approved the final version.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Nos. 81371157 and 81100756), Science and Technology Program of Shandong Province (No. 2014GSF118075), The Construction Engineering Special Fund of “Taishan Scholars” (No.ts201511106). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31:7892–7927. [DOI] [PubMed] [Google Scholar]
- 2. Shimono M, Ishikawa T, Ishikawa H, et al. Regulatory mechanisms of periodontal regeneration. Microsc Res Tech. 2003;60:491–502. [DOI] [PubMed] [Google Scholar]
- 3. Beertsen W, McCulloch CA, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000. 1997;13:20–40. [DOI] [PubMed] [Google Scholar]
- 4. Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. [DOI] [PubMed] [Google Scholar]
- 5. Gay I, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10:149–160. [DOI] [PubMed] [Google Scholar]
- 6. Xu J, Wang W, Kapila Y, Lotz J, Kapila S. Multiple differentiation capacity of STRO‐1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009;18:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park JY, Jeon SH, Choung PH. Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant. 2011;20:271–285. [DOI] [PubMed] [Google Scholar]
- 8. Liu Y, Zheng Y, Ding G, et al. Periodontal ligament stem cell‐mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng F, Akiyama K, Liu Y, et al. Utility of PDL progenitor cells for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 2010;16:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsumanuma Y, Iwata T, Washio K, et al. Comparison of different tissue‐derived stem cell sheets for periodontal regeneration in a canine 1‐wall defect model. Biomaterials. 2011;32:5819–5825. [DOI] [PubMed] [Google Scholar]
- 11. Murakami S, Takayama S, Kitamura M, et al. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J Periodontal Res. 2003;38:97–103. [DOI] [PubMed] [Google Scholar]
- 12. Chen FM, Shelton RM, Jin Y, Chapple IL. Localized delivery of growth factors for periodontal tissue regeneration: Role, strategies, and perspectives. Med Res Rev. 2009;29:472–513. [DOI] [PubMed] [Google Scholar]
- 13. Cui Y, Madeddu P. The role of chemokines, cytokines and adhesion molecules in stem cell trafficking and homing. Curr Pharm Des. 2011;17:3271–3279. [DOI] [PubMed] [Google Scholar]
- 14. Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. [DOI] [PubMed] [Google Scholar]
- 15. Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal‐derived factor‐1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–3801. [DOI] [PubMed] [Google Scholar]
- 16. Stich S, Loch A, Leinhase I, et al. Human periosteum‐derived progenitor cells express distinct chemokine receptors and migrate upon stimulation with CCL2, CCL25, CXCL8, CXCL12, and CXCL13. Eur J Cell Biol. 2008;87:365–376. [DOI] [PubMed] [Google Scholar]
- 17. Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–427. [DOI] [PubMed] [Google Scholar]
- 18. Abbott JD, Huang Y, Liu D, et al. Stromal cell derived factor‐1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. [DOI] [PubMed] [Google Scholar]
- 19. Ratajczak MZ, Majka M, Kucia M, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF‐1 by muscle‐derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. [DOI] [PubMed] [Google Scholar]
- 20. Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF‐1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. [DOI] [PubMed] [Google Scholar]
- 21. Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF‐1 signals mobilization and homing of CXCR4‐positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. [DOI] [PubMed] [Google Scholar]
- 22. Kitaori T, Ito H, Schwarz EM, et al. Stromal cell‐derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. [DOI] [PubMed] [Google Scholar]
- 23. Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Circulating bone marrow‐derived osteoblast progenitor cells are recruited to the bone‐forming site by the CXCR4/stromal cell‐derived factor‐1 pathway. Stem Cells. 2008;26:223–234. [DOI] [PubMed] [Google Scholar]
- 24. Du L, Yang P, Ge S. Stromal cell‐derived factor‐1 significantly induces proliferation, migration and collagen type I expression in human periodontal ligament stem cell subpopulation. J Periodontal. 2012;83:379–388. [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Li M, Du L, Yang P, Ge S. Local administration of stromal cell‐derived factor‐1 promotes stem cell recruitment and bone regeneration in a rat periodontal bone defect model. Mater Sci Eng C Mater Biol Appl. 2015;53:83–94. [DOI] [PubMed] [Google Scholar]
- 26. Christopherson KW, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell‐derived factor‐1 alpha‐mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. [DOI] [PubMed] [Google Scholar]
- 27. Matheeussen V, Baerts L, De Meyer G, et al. Expression and spatial heterogeneity of dipeptidyl peptidases in endothelial cells of conduct vessels and capillaries. Biol Chem. 2011;392:189–198. [DOI] [PubMed] [Google Scholar]
- 28. Huhn J, Ehrlich S, Fleischer B, von Bonin A. Molecular analysis of CD26‐mediated signal transduction in T cells. Immunol Lett. 2000;72:127–132. [DOI] [PubMed] [Google Scholar]
- 29. Jungraithmayr W, De Meester I, Matheeussen V, Baerts L, Arni S, Weder W. CD26/DPP‐4 inhibition recruits regenerative stem cells via stromal cell‐derived factor‐1 and beneficially influences ischaemia‐reperfusion injury in mouse lung transplantation. Eur J Cardiothorac Surg. 2012;41:1166–1173. [DOI] [PubMed] [Google Scholar]
- 30. Huber BC, Brunner S, Segeth A, et al. Parathyroid hormone is a DPP‐IV inhibitor and increases SDF‐1‐driven homing of CXCR4(+) stem cells into the ischaemic heart. Cardiovasc Res. 2011;90:529–537. [DOI] [PubMed] [Google Scholar]
- 31. Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42:806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barnes LG, Kakar S, Vora S, Morgan FE, Gerstenfeld CL, Einhorn AT. Stimulation of fracture‐healing with systemic intermittent parathyroid hormone treatment. J Bone Joint Surg Am. 2008;90:120–127. [DOI] [PubMed] [Google Scholar]
- 33. De Paula FJ, Rosen CJ. Back to future: revisiting parathyroid hormone and calcitonin control of bone remodelling. Horm Metab Res. 2010;42:299–306. [DOI] [PubMed] [Google Scholar]
- 34. Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tokunaga K, Seto H, Ohba H, et al. Topical and intermittent application of parathyroid hormone recovers alveolar bone loss in rat experimental periodontitis. J Periodont Res. 2011;46:655–662. [DOI] [PubMed] [Google Scholar]
- 36. Lossdörfer S, Götz W, Jäger A. Parathyroid hormone modifies human periodontal ligament cell proliferation and survival in vitro. J Periodontal Res. 2006;41:519–526. [DOI] [PubMed] [Google Scholar]
- 37. Ge S, Zhao N, Wang L, et al. Bone repair by periodontal ligament stem cell‐seeded nanohydroxyapatite–chitosan scaffold. Int J Nanomed. 2012;7:5405–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ge S, Zhao N, Wang L, Liu H, Yang P. Effects of hydroxyapatite nanostructure on channel surface of porcine acellular dermal matrix scaffold on cell viability and osteogenic differentiation of human periodontal ligament stem cells. Int J Nanomeds. 2013;8:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guimarães GN, Stipp RN, Rodrigues TL, de Souza AP, Line SR, Marques MR. Evaluation of the effects of transient or continuous PTH administration to odontoblast‐like cells. Arch Oral Biol. 2013;58:638–645. [DOI] [PubMed] [Google Scholar]
- 40. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 41. Jung Y, Wang J, Schneider A, et al. Regulation of SDF‐1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. [DOI] [PubMed] [Google Scholar]
- 42. Ganju RK, Brubaker SA, Meyer J, et al. The alpha‐chemokine, stromal cell‐derived factor‐1alpha, binds to the transmembrane G‐protein‐coupled CXCR‐4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–23175. [DOI] [PubMed] [Google Scholar]
- 43. Zhao M, Discipio RG, Wimmer AG, Schraufstatter IU. Regulation of CXCR4‐mediated nuclear translocation of extracellular signal‐related kinases 1 and 2. Mol Pharmacol. 2006;69:66–75. [DOI] [PubMed] [Google Scholar]
- 44. Zhu W, Boachie‐Adjei O, Rawlins BA, et al. A novel regulatory role for stromal‐derived factor‐1 signaling in bone morphogenic protein‐2 osteogenic differentiation of mesenchymal C2C12 cells. J Biol Chem. 2007;282:18676–18685. [DOI] [PubMed] [Google Scholar]
- 45. Hosogane N, Huang Z, Rawlins BA, et al. Stromal derived factor‐1 regulates bone morphogenetic protein 2‐induced osteogenic differentiation of primary mesenchymal stem cells. Int J Biochem Cell Biol. 2010;42:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim DS, Kim YS, Bae WJ, et al. The role of SDF‐1 and CXCR4 on odontoblastic differentiation in human dental pulp cells. Int Endod J. 2014;47:534–541. [DOI] [PubMed] [Google Scholar]
- 47. Ge S, Mrozik KM, Menicanin D, Gronthos S, Bartold PM. Isolation and characterization of mesenchymal stem cell‐like cells from healthy and inflamed gingival tissue: potential use for clinical therapy. Regen Med. 2012;7:819–832. [DOI] [PubMed] [Google Scholar]
- 48. Wlodarski KH, Reddi AH. Alkaline phosphatase as a marker of osteoinductive cells. Calcif Tissue Int. 1986;39:382–385. [DOI] [PubMed] [Google Scholar]


