Abstract
Abstract. Rhesus monkey embryonic stem cells (ESCs) (R366.4), cultured on a three‐dimensional (3D) collagen matrix with or without human neonatal foreskin fibroblasts (HPI.1) as feeder cells, or embedded in the collagen matrix, formed complex tubular or spherical gland‐like structures and differentiated into phenotypes characteristic of neural, epithelial and endothelial lineages. Here, we analysed the production of endogenous extracellular matrix (ECM) proteins, cell–cell adhesion molecules, cell‐surface receptors, lectins and their glycoligands, by differentiating ESCs, forming a micro‐environment, a niche, able to positively influence cell behaviour. The expression of some of these molecules was modulated by HPI.1 cells while others were unaffected. We hypothesized that both soluble factors and the niche itself were critical in directing growth and/or differentiation of ESCs in this 3D environment. Creating such an appropriate experimental 3D micro‐environment, further modified by ESCs and modulated by exogenous soluble factors, may constitute a template for adequate culture systems in developmental biology studies concerning differentiation of stem cells.
INTRODUCTION
Embryonic stem cells (ESCs), derived from the inner cell mass of mammalian blastocysts, are able to self‐renew and differentiate into cells of the three embryonic germ layers (Thomson & Marshall 1998; Thomson et al. 1998; Itskovitz et al. 2000; Chen et al. 2003; Philp et al. 2005). When cultured in vitro, ESCs differentiate into cells of neuronal, haematopoietic, epithelial, vascular, smooth muscle, cardiac muscle, adipogenic and chondrogenic lineages (Czyz & Wobus 2001). The differentiational fate of ESCs is deeply influenced by their micro‐environment or niche, which plays a fundamental role, not only in cell survival, proliferation, and migration but also in morphogenesis of complex structures and organs (Watt & Hogan 2000; Kleinman et al. 2003). Multicellular structures which are typically formed in normal embryos and in SCID mouse teratomas require cells to be able to migrate in a three‐dimensional extracellular matrix (ECM) in the presence of laminin, collagens, fibronectin and proteoglycans, and to interact with this micro‐environment. We have previously reported that rhesus monkey ESCs (R366.4) cultured in three‐dimensional (3D) collagen gels in the presence of human neonatal fibroblasts (HPI.1) formed dense cell clusters as well as complex tubular or spherical gland‐like structures that were more evident in 3D than in 2D monolayer cultures (Chen et al. 2003). In these structures cells showed signs of differentiation, mainly into neural and epithelial lineages. It is possible that growth factors produced by HPI.1 cells, ECM molecules and cell‐surface receptors expressed by ESCs are critical in setting up the appropriate micro‐environment to drive stem cell differentiation towards particular lineages. When HPI‐1 cells were embedded in collagen gel, ESCs formed multilayered structures and the number of cells expressing neural markers such as nestin, class III β‐tubulin, chromogranin A and N‐CAM was higher relative to that when cultured with HPI.1 cells used as a feeder layer. Epithelial differentiation was enhanced when HPI.1 cells were used as a 2D feeder layer, rather than being embedded in the collagen gel (Chen et al. 2003).
Since the differentiation of ESCs is driven by factors produced in their own niche, the aim of the present study was to analyse the type of ECM molecules, cell‐surface receptors for ECM, cell–cell adhesion molecules and glycoconjugates (that is structural mucopolysaccharides, glycans or highly glycosylated proteins) present in the ESCs’ micro‐environment. ESCs were cultured under three different conditions: (1) control culture with ESCs on a 3D collagen gel in a culture insert; (2) ESCs on a 3D collagen gel with a feeder layer of HPI.1 cells separated by the membrane of the insert; or (3) ESCs in a 3D collagen gel with HPI.1 cells embedded in the collagen gel. We found that ESCs cultured on a collagen gel are able to produce the molecules analysed independently with or without the presence of HPI.1 fibroblasts. Soluble factors released by HPI.1 cells may contribute to modulate the formation of the micro‐environment and the capacity of the ESCs to differentiate.
MATERIALS AND METHODS
ESC cultures
Undifferentiated rhesus monkey ESCs (R366.4) were cultured as previously described (Chen et al. 2003). Briefly, ESCs were cultured with mitomycin C–treated (0.8 mg/ml for 2 h) (Sigma, St. Louis, MO, USA) murine embryonic fibroblasts (MEF) (Cell Essentials Inc., Boston, MA, USA) in gelatin‐coated six‐well plates (Nalge Nunc Inc., Naperville, IL, USA) to prevent spontaneous differentiation. ESCs were maintained in 80% Knock‐Out Dulbecco's modified Eagle's medium (K/O‐DMEM) (Invitrogen, Rockville, MD, USA) supplemented with 20% defined fetal bovine serum (d‐FBS, Hyclone, Logan, UT, USA), 1 mm l‐glutamine, 0.1 mm 2‐mercaptoethanol, and 1% non‐essential amino acids (Invitrogen). In order to induce differentiation, human neonatal foreskin fibroblast HPI.1 cells were used. These cells provide cell–cell contacts and release growth factors, including fibroblast growth factor, epidermal growth factor, interleukin‐6, stem cell factor, granulocyte‐macrophage colony stimulating factor, vascular endothelial growth factor, and insulin‐like growth factor, among others, that favour cell growth and differentiation in human as well as primate cells (Chen et al. 2003; Papini et al. 2003). HPI.1 cells (0.1 × 106 cells/well) were seeded in some wells of six‐well plates as a feeder layer 24 h before ESC seeding. Meanwhile, each of the six‐well inserts was coated with 1 ml of 2.4 mg/ml rat tail type I collagen (Sigma) (Fig. 1A) with or without HPI.1 embedded in the collagen gel. Collagen was neutralized with sterile 1 N NaOH (Chen et al. 2005). ESCs were released from six‐well plates and seeded on top of the collagen gel (with or without HPI.1 cells embedded) (Fig. 1A). After 7 and 19 days of culture at 37 °C in a 5% CO2 in air atmosphere, the inserts were rinsed in PBS, fixed in 4% formaldehyde in PBS at 4 °C for 3 days, washed in PBS and embedded in paraffin wax. Results reported in this paper refer to 19 days of culture since at this time we observed more complex and differentiated structures (Chen et al. 2003). Sections (5 µm) were stained with alcian blue or were subjected to histochemical and immunohistochemical analyses for lectin, ECM molecule production, and integrin expression. In our previous work corresponding sections were positively stained for epithelial and neural markers (Chen et al. 2003).
Figure 1.
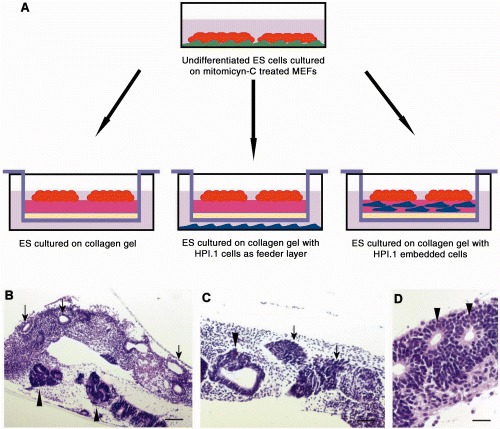
Experimental model. (A) Undifferentiated ESCs cultured on mitomycin‐C‐treated murine embryonic fibroblasts (MEFs) were transferred into a culture insert on top of a collagen gel (left); or on a collagen gel with HPI.1 cells as feeder layer at the bottom of the well (middle); or on a collagen gel with HPI.1 embedded cells (right). Haematoxylin and eosin staining of differentiating ESCs (B–D). Arrows in (B) indicate monolayered structures; arrowheads in (B–D) indicate multilayered structures. Arrow in (C) indicates dense cell clusters. Scale bar in B = 700 µm; C = 400 µm; D = 140 µm.
Histochemical and immunohistochemical analyses
1, 2 list the lectins and antibodies used, their dilutions and specificity. Alcian blue at pH 2.5 (Bio‐Optica staining kit, Milan, Italy) was used to stain structural mucopolysaccharides (sulphated and carboxylated acid) and sulphated and carboxylated sialomucins (glycoproteins) (Prento 2001).
Table 1.
List of lectins used in this study. Carbohydrate binding specificities are from Van Damme et al. (1998)
| Lectin | Carbohydrate specificity | Dilution | Inhibitory sugar (molarity) | Source |
|---|---|---|---|---|
| Glycine max agglutinin (SBA) | N‐acetyl‐D‐galactosamine > α‐D‐galactose | 50 µg/ml | GalNAc (200 mm) | Sigma |
| Bandeirea simplicifolia agglutinin I (BSA‐I) | α‐D‐gal > α‐D‐galNAc | 50 µg/ml | Gal (200 mm) | Sigma |
| Bandeirea simplicifolia agglutinin isolectin B4 (BSA‐I‐B4) | α‐galactose | 50 µg/ml | Gal 200 mm | Sigma |
| Helix pomatia agglutinin (HPA) | α‐N‐acetyl‐D‐galactosamine | 50 µg/ml | GalNAc 200 mm | Sigma |
| Dolichos biflorus agglutinin (DBA) | α‐N‐acetyl‐D‐galactosamine | 50 µg/ml | GalNAc 200 mm | Sigma |
| Sophora japonica agglutinin (SJA) | β‐N‐acetyl‐D‐galactosamine | 50 µg/ml | GalNAc 200 mm | Vector Laboratories |
| Ricinus communis agglutinin I (RCA‐I) | β‐galactose | 50 µg/ml | Gal 200 mm | Sigma |
| Ulex europaeus agglutinin I (UEA‐I) | α‐L‐fucose | 50 µg/ml | L‐fucose 100 mm | Sigma |
Table 2.
List of primary antibodies used in this study
| Immunostaining | Clones | Specificity | Dilution | Source |
|---|---|---|---|---|
| Primary antibodies | ||||
| Laminins | LAM‐89 | Mouse IgG1 | 1 : 300 | Chemicon |
| Fibronectin | 568 | Mouse IgG | 1 : 100 | Novocastra |
| β1‐integrin | 4B7R | Mouse IgG | 1 : 200 | Santa Cruz Biotechnology |
| α5β1‐integrin | JBS5 | Mouse IgG | 1 : 400 | Chemicon |
| αv‐integrin | AV1 | Mouse IgG1 | 1 : 200 | Chemicon |
| N‐cadherin | 3B9 | Mouse IgG1k | 1 : 400 | Zymed |
| E‐cadherin | G‐10 | Mouse IgG | 1 : 100 | Novocastra |
| β‐catenin | E‐5 | Mouse IgG | 1 : 200 | Santa Cruz Biotechnology |
Lectins (Sigma) and immunoperoxidase staining were performed according to the manufacturers’ instructions for each lectin and monoclonal antibody used. Antigen retrieval was performed as necessary with citrate buffer 0.1 m at pH 6.0. Lectins were detected with 3.3 diaminobenzidine (DAB, Sigma). Antigen detection was performed and was visualized using secondary antibodies (EnVision™/HRP detection kit; DakoCytomation, Glostrup, Denmark). All sections were counterstained with haematoxylin. In order to exclude the possibility of antibody staining of serum fibronectin deposited from our culture medium (Hayman & Ruoslhati 1979), our antibody was pre‐incubated with bovine serum before being applied to sections. Lectin controls included competitive inhibition with the appropriate sugar (100–200 mm) for 1 h at room temperature, buffered saline in place of the lectin, and the use of a known positive control tissue. Specificity of immunostaining was verified by incubating sections without primary antibodies. Number of cells stained with the various antibodies was estimated in 10 unconnected fields at ×400 total magnification (Table 3). One thousand cells in total were scored in each observation.
Table 3.
Positive cells for the ECM markers used in the different culture conditions
| Staining | Collagen gel | Collagen gel with HPI.1 feeder layer | Collagen gel with HPI.1 embedded cells |
|---|---|---|---|
| β1‐integrin | 54 ± 1.4 | 121 ± 1.3 | 147 ± 0.9 |
| α5β1‐integrin | 39 ± 0.7 | 68 ± 0.9 | 61 ± 0.5 |
| αv‐integrin | 0 | 0 | 0 |
| N‐Cadherin | 148 ± 1.8 | 213 ± 2.1 | 151 ± 1.5 |
| E‐Cadherin | 0 | 0 | 0 |
| β‐catenin | 474 ± 5.1 | 538 ± 6.2 | 566 ± 5.8 |
| SBA | 244 ± 2.1 | 401 ± 1.6 | 455 ± 6.1 |
| BSA‐I | 149 ± 1.8 | 201 ± 1.4 | 301 ± 2.0 |
| BSA‐I‐B4 | 151 ± 2.1 | 251 ± 2.1 | 302 ± 2.1 |
| HPA | 151 ± 1.7 | 298 ± 2.3 | 352 ± 2.7 |
| DBA | 150 ± 2.2 | 251 ± 2.2 | 302 ± 3.8 |
| SJA | 0 | 0 | 0 |
| RCA‐I | 99 ± 2.4 | 199 ± 2.5 | 249 ± 2.9 |
| UEA‐I | 49 ± 1.8 | 98 ± 2.1 | 148 ± 2.4 |
Number of cells stained with antibodies and lectins on 103 total cells counted (± SD); estimation in 10 unconnected fields at 400X.
RESULTS
Histology of ESC cultures
Embryonic stem cells cultured on 3D‐collagen gels for 19 days formed tubular or spherical gland‐like structures (Fig. 1B, overview) and dense cell clusters (Fig. 1C, arrows). Three different experiments, in triplicate, were performed and results were comparable. Some structures were formed by a single layer of cells (monolayer structures) (Fig. 1B, arrows) while others were formed by multiple layers of cells (multilayered structures) (arrowheads in Fig. 1B–D). On day 19, in all three culture conditions, there were more multilayered than monolayered structures and dense cell clusters. In particular, the number of multilayered structures in the presence of HPI.1 cells (either as a feeder layer or embedded in the collagen gel) was greater than in the controls.
Alcian blue staining
After 19 days of culture, irrespective of the different culture conditions, alcian blue stained the basal lamina of gland‐like structures (Fig. 2A) and the cell layer surrounding cell aggregates (Fig. 2B). In addition, alcian blue staining was observed in the extracellular space (Fig. 2C). Staining was similar in control specimens and in ESCs cultured with HPI.1 cells either as a feeder layer or with them embedded in the collagen gel.
Figure 2.
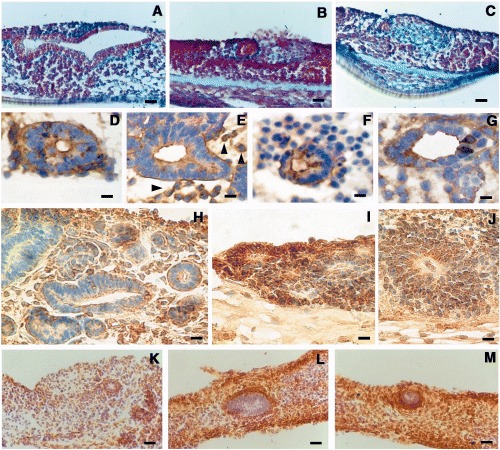
ESCs cultured on collagen gel for 19 days. Alcian blue staining (A, B, C). Basal lamina around multilayered circular structure (A). Basal lamina around a compact structure and defined area in the immediate surrounding of this structure (B); undefined area in the stroma (C). Lectin staining in ES cells cultured for 19 days on a collagen gel with embedded HPI.1, after SBA binding (D), after RCA‐I binding (E), after BSA‐I‐B4 staining (F) and after HPA binding (G). Staining was mainly located, on the basal lamina and on the surface of the external cell layer lining the cavity of the structure. Laminin staining showed a similar pattern in all three different culture conditions: at the level of the basal lamina and in the cytoplasm of scattered cells in the stroma (H); very intense in some monolayered circular structures (I); in the perinuclear area of the cytoplasm of cells forming multilayered circular structures (J). Expression of fibronectin in ESCs cultured on collagen gel (K); on collagen gel with HPI.1 cells as feeder layer in a monolayer separated by a porous membrane (L); on collagen gel with HPI.1 embedded (M). Scale bar in A = 150 µm; B and C = 120 µm; C and D = 30 µm; E = 36 µm; F = 31 µm; G = 36 µm; H and I = 62 µm; J = 70 µm; K = 300 µm; and L and M = 200 µm.
Lectin staining
After 19 days of culture on collagen gel matrix, the incubation with SBA (Fig. 2D), RCA‐I (Fig. 2E), BSA‐I, BSA‐I‐B4 (Fig. 2F), DBA and HPA (Fig. 2G) caused intense staining of the cells of the gland‐like circular structures. Expression was moderate after UEA‐I binding, and negative after SJA binding. Lectin staining was positive for all three culture conditions but was detected in a larger cell fraction when HPI.1 cells were embedded in the collagen gel (Table 3). Positive staining was located on apical surfaces of cells lining cavities of structures as well as on the basal lamina surrounding the gland‐like structures (Fig. 2D, E). In addition, small clusters of lectin‐positive cells were found in the stroma (arrowheads in Fig. 2E). Some cells in the monolayered circular structures displayed an intense lectin binding either in the cytoplasm or on both apical and basal surfaces of the cells (Fig. 2F, G). In the three different experiments run in triplicate, the pattern of staining was similar.
ECM molecules
Laminins
After 19 days of culture in all three different culture conditions, the pattern of laminin expression was similar. Laminins were present around the monolayered structures at the basal lamina and in the cytoplasm of many scattered cells located in the stroma (Fig. 2H). In some monolayered structures, laminin staining was intense (Fig. 2I). Virtually all cells of the multilayered circular structures contained laminins in the perinuclear cytoplasm (Fig. 2J).
Fibronectin
After 19 days of culture on collagen gel, fibronectin formed a ring‐like matrix surrounding the gland‐like structures. It was also demonstrated scattered in the stroma (Fig. 2K–M) and was present in the cytoplasm of cell aggregates within the stroma (not shown). Fibronectin was more intensely and broadly expressed in ESCs cultured with HPI.1 as a feeder layer or with HPI.1 cells embedded in collagen gel than it was in controls. The pattern of fibronectin staining was similar in samples cultured with HPI.1 as a feeder layer or embedded in collagen gel. More importantly, fibronectins formed a dense network of fibrils (Fig. 2L, M). Counting both laminin‐positive and fibronectin‐positive cells was difficult due to the abundant secretion of this protein.
Cell‐surface receptors to the ECM (integrins)
β‐1‐integrin
In control cultures in a small group of cells, β1‐integrin staining was more intense in cells of monolayered circular structures than on cells of multilayered structures after 19 days (Fig. 3A). This was also observed in ESCs co‐cultured with HPI.1 as a feeder layer (not shown); however, β1‐integrin positive cells were apparently more abundant (Table 3). In ESCs cultured in collagen gel with HPI.1 cells embedded, β1‐integrin positive cells were more abundant than in control cultures (Fig. 3B and Table 3). In addition, small tight aggregates of β‐1‐integrin positive‐cells were found within the stroma not associated to any particular gland‐like or tubular structure (Fig. 3A).
Figure 3.
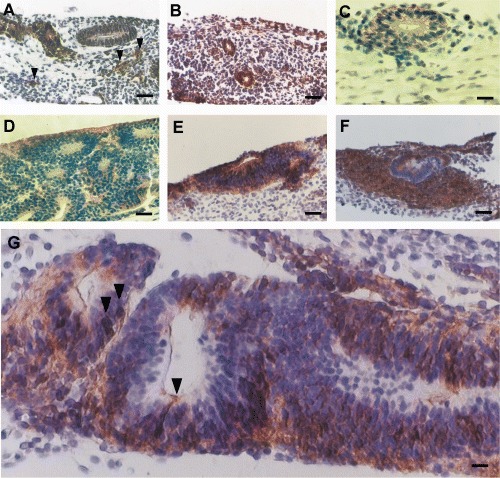
ESCs cultured for 19 days. Distribution of β‐1‐integrin in ESCs cultivated on collagen gel (A); on collagen gel with HPI.1 embedded (B). Note β‐1‐integrin positive cells in small cell clusters in A (arrows). Expression of α5β1 integin in ESCs cultured on a collagen gel (C). The expressin of N‐cadherin in differentiating ESCs on a collagen gel with HPI.1 embedded (D). Distribution of β‐Catenin in ESCs cultivated on collagen gel (E); or on a collagen gel with HPI.1 embedded (F) and (G). Arrows in G show some nuclear β‐catenin staining in neuroepithelial‐like cells. Scale bar in A and B = 200 µm; C and D = 90 µm; E and F = 200 µm; G = 75 µm.
α5β1‐integrin and αv‐integrin
In all the culture conditions, α5β1‐integrin expression was similarily low. Positive staining was detected at the plasma membrane of cells, but confined to the basolateral side (opposite to the luminal side; Fig. 3C) of some monolayered circular structures in ESCs cultured in collagen gel with HPI.1 cells embedded. Common to all the culture conditions, αv‐integrin expression was not found.
Cell–cell adhesion molecules
N‐cadherin and E‐cadherin
After 19 days of culture, N‐cadherin was expressed in the cytoplasm and on the plasma membrane of cells predominantly in multilayered gland‐like structures (Fig. 3D). The number of N‐cadherin positive cells was similar among the three different culture conditions (Table 3). However, E‐cadherin was not expressed in any samples.
β‐catenin
In day 19 control cultures, β‐catenin was present in the cytoplasm and plasma membrane of cells in multilayered gland‐like structures (Fig. 3E). A similar staining pattern for β‐catenin, though less intense, was observed in ESCs cultured on collagen gel with HPI.1 as a feeder layer (not shown). On the other hand, when HPI.1 cells were embedded in collagen gel, β‐catenin was expressed widely and intensely. β‐Catenin was expressed not only in the cells forming the gland‐like structures but also in dense cell aggregates surrounding these structures (Fig. 3F). In addition, in some gland‐like structures, we found cells with strong β‐catenin expression both in the nucleus and in the cytoplasm (Fig. 3G). Interestingly, the shape of these cells, an oval cell body extending a long and thin neurite‐like process reaching the lumen of the multilayered structure, resembled neuro‐epithelial cells. Furthermore, the number of β‐catenin positive cells was slightly higher in cultures with HPI.1 embedded in the collagen gels than that in the other culture conditions (Table 3).
DISCUSSION
For years, the ECM has been traditionally viewed as a substrate for cell attachment and a scaffold for tissue formation. More recently, the ECM has received increasing attention since it has been shown to play a fundamental biological role in cell adhesion, migration, and differentiation – through integrins and other cell‐surface molecules, and also to serve as a reservoir for growth factors (Aumailley & Gayraud 1998; Green & Watt 2004; Zaidel‐Bar et al. 2004; Li et al. 2005). This complex role of the ECM has to be studied under controlled experimental conditions. Our present study has demonstrated that primate ESCs cultured on 3D collagen gels in different controlled experimental environments differentiated, produced ECM, and expressed cell‐surface receptors and cell–cell adhesion molecules. We have shown that the factors released by HPI.1 cells may modulate the amount of these molecules.
Below we discuss the possible role of ECM components in the observed differentiation pattern.
In our current study, we found structural mucopolysaccharides in the basal lamina of the gland‐like structures and in scattered cells in the stroma. However, the deposition of polysaccharides was not modulated by the presence of human neonatal HPI.1 fibroblasts either as feeder cells nor when embedded in the collagen gels. Yet polysaccharides probably participated in the ECM organization by providing a general and default structural support.
Cell‐surface glycoconjugates play a key role in many cell processes, including cell–cell recognition, adhesion, regulation of cell growth and differentiation (Fukuda et al. 2000; Kudo et al. 2004). Cell‐surface molecules containing carbohydrates are thought to play a crucial role in morphogenesis and differentiation in many systems by means of selective adhesion to adjacent cell surfaces and extracellular structures (Fenderson et al. 1990; Jessel et al. 1990; Zalik 1991; Fukuda et al. 2000). The spatial and temporal distribution of glycoconjugates in many developing systems also suggests a close relationship between their expression and maturation of the organs (Khan et al. 1999). It is possible that glycoconjugates, selectively bound by lectins, found on differentiated ESCs of gland‐like structures might play a role in determining cell connections and pattern formation as it occurs during in vivo development (Kresse & Schonherr 2001). In addition, as summarized in Table 3, the expression of glycoconjugates in these gland‐like structures was influenced by the presence of HPI.1 cells. This indicates that the expression of these molecules was possibly modulated by soluble factors released by HPI.1 cells.
The ECM produced by stromal cells and basement membrane plays an important role in directing polarization and differentiation of cells (Salamonsen 1994). Laminins are required for the assembly of the earliest basement membranes. Loss‐of‐function mutation in laminins causes missing or distrupted ECM (Huang et al. 2003). Genetic data available so far argue that laminins alone are indispensable for early embryonic basement membrane assembly and that no other basement membrane component can accumulate into a cell‐associated ECM within a tissue in the absence of laminin deposition (reviewed in Yurchenco & Wadsworth 2004). Thus laminins are a prerequisite for the integration of all basement membrane components. Interestingly, the pattern of laminin staining in our cultures was not influenced by the presence or absence of HPI.1 cells. This has further indicated that laminins together with polysaccharides may be part of a default requirement for initial ECM deposition. In addition, laminins are highly glycosylated proteins, and glycans on laminins may modulate their binding to other cell‐surface receptors. To date the biological function of the glycans on laminin remains unclear; however, current evidence suggests that laminins are important in cell spreading and neurite outgrowth (Tanzer et al. 1993). Interestingly, this correlates with the presence of neural differentiation in our ESCs culture (Chen et al. 2003).
Fibronectins are amongst the most ubiquitous and the best characterized ECM macromolecules (Hynes 1981; Romberger 1997). They are large glycoproteins consisting of repeating units of amino acids which form domains that enable the molecules to interact with a variety of cells through both integrin and non‐integrin receptors. Fibronectin is most abundant during embryonic development and tissue remodeling and acts as an insoluble protein that is extensively cross‐linked to a variety of macromolecules, including components of the cytoskeleton and the extracellular matrix, and to several types of cell‐surface receptor (Hynes 1990). Fibronectin also interacts with itself, forming a fibrillary network, and also with other cell‐surface receptors including cell‐surface proteoglycans (Wierzbicka‐Patynowski & Schwarzbauer 2003). Here, we found that differentiating ESCs, cultured for 19 days on collagen gels, synthesized abundant fibronectin. The addition of HPI.1 cells, either as a feeder layer or embedded in the collagen gel matrix, enhanced not only fibronectin synthesis but also the formation of a well‐organized fibrillary network, particularly around the gland‐like circular structures. It is possible that soluble factors secreted by HPI.1 cells facilitate fibronectin syntheis and fibrillary network formation. It is also possible that HPI.1 cell fibres directly participated in the fibrillary network.
The most common receptors for ECM molecules are integrins. They act as powerful regulators of morphogenetic processes. This is in part due to their ability to modulate cytoskeletal organization, and selectively activate important signalling components (Damsky & Ilic 2002). For example, the assembly of a fibronectin network by fibroblasts is a multistep process initiated by the binding of α5β1 integrin (the most common integrin receptor for fibronectin together with αvβ1 integrin) to the arginine‐glycine‐aspartate domain of fibronectin, followed by inside–outside signals that enhance integrin–fibronectin affinity and promote cytoskeletal re‐organization. This results in fibronectin matrix deposition in the extracellular micro‐environment (Wierzbicka‐Patynowski & Schwarzbauer 2003). In our present study, we observed a slightly increased proportion of gland‐like structures expressing β‐1‐integrin in ESCs grown in the presence of HPI.1 cells, particularly when embedded in the collagen gel, where we more clearly observed the presence of a fibronectin fibrillary network. Although fibronectin was abundantly expressed around cells in all the culture conditions, the major receptors for fibronectin α5β1‐integrins were barely found and αv integrin expression was not detected. It is possible that ESCs, in our experimental conditions, interact with fibronectin through other types of β1‐integrin receptors (Johansson et al. 1997) or not through the RGD domain, using non‐integrin receptors (Singleton & Menino 2005).
In these complex interactions between ECM molecules and gland‐like structures formed by differentiating ESCs, cell adhesion is crucial in maintaining cell shape and allowing differentiation and appropriate interaction with the micro‐environment. Cadherins, a family of homophilic adhesion receptors, play a crucial role in cell–cell adhesion (Takeichi 1995), as well as in cell migration, tumour growth and cell patterning (Takeichi et al. 2000). Cadherin activity can be regulated by catenins associated to the cadherin‐cytoplasmic domain (Barth et al. 1997). N‐cadherin is a key molecule in embryogenesis during gastrulation and neural crest development and it is involved in neural as well as skeletal muscle differentiation (Derycke & Bracke 2004). Since we observed that multilayered structures differentiated predominantly into neuronal phenotypes (Chen et al. 2003), we hypothesize that N‐cadherin expression could be predictive of neuronal differentiation. We found that N‐cadherin was expressed by differentiating ESCs within multilayered circular structures under all three culture conditions without any significant variation (Table 3). This suggests that N‐cadherin may be part of a default requirement for the formation of these structures without being apparently affected by HPI.1 cells.
Despite the presence of epithelial‐like structures in our cultures (Chen et al. 2003), we never observed the expression of E‐chaderin in any samples analysed after 19 days of culture. It is possible that E‐cadherin is expressed in the gland‐like structures at very early stages and later is replaced by N‐cadherin. This is consistent with the in vivo situation where E‐cadherin is expressed in the early stages of embryonic development and replaced later on by other cadherins (Derycke & Bracke 2004).
β‐Catenin is a cytoplasmic protein with a dual function in cell–cell adhesion and signal transduction (Nelson & Nusse 2004). At the plasma membrane, β‐catenin is associated with cadherins, providing a link between cadherins and the actin cytoskeleton, thus ensuring strong intercellular adhesion. However, β‐catenin is also a downstream component of the Wnt signal transduction pathway which is involved in many aspects of embryonic patterning (Kikuchi 2000) and its signalling function is distinct from its role in adhesion. Activation of the Wnt pathway allows β‐catenin to enter the nucleus and interact with several target genes involved in cell survival, proliferation and/or differentiation. Recently, β‐catenin has been shown to be important for the survival and differentiation of haematopoietic stem cells (Reya et al. 2003). Wnt signalling together with cadherin‐mediated cell adhesion viaβ‐catenin may contribute to regulating haematopoietic stem cell growth and differentiation within their specific niches (Zhang et al. 2003). In our present study, we found that in differentiating ESCs, β‐catenin was highly expressed (about 40–50%) in all culture conditions, particularly in cells forming multilayered structures. β‐Catenin was detected on plasma membrane and/or cytoplasmically. In addition, β‐catenin was found in the nucleus of cells which morphologically resembled neuroepithelial cells, although the proportion of this type of cells was less than 5% cells. β‐Catenin signalling, via nuclear translocation of β‐catenin, has recently been shown to be required for neural differentiation of embryonic stem cells (Otero et al. 2004). This finding is in agreement with our previous observation of ESC‐multilayered structures strongly expressing neural markers such as β‐III‐tubulin, nestin, and N‐CAM (Chen et al. 2003). In addition, the translocation of β‐catenin into the nucleus implies activation of the Wnt signalling pathway. As nuclear β‐catenin was mainly detected in ESCs cultured on collagen gel with HPI.1 cells embedded within, we hypothesize that HPI.1 cells embedded in collagen might provide the signals that activate the Wnt pathway inducing β‐catenin translocation to the nucleus and subsequently enhancing neural differentiation.
In conclusion, our present findings demonstrate that ESCs, cultured in a three‐dimensional system, synthesize their own endogenous repertoire of molecules and contribute to form a micro‐environment vital to cell survival and differentiation. Moreover, small variations in the micro‐environment have affected the type and the amount of proteins that might direct lineage differentiation. External factors, such as those released by HPI.1 cells, changed the proportion of those molecules in the micro‐environment in particular of glycoconjugates, fibronectin, and β‐catenin. These molecules are particularly important in collagen‐mediated formation of epithelial tubules in vitro (Ojakian et al. 2001). This correlates with the increased number of tubular structures we observed in ESCs cultured with HPI.1 cells either as a 2D feeder layer or embedded in collagen gel.
Creating an appropriate experimental 3D micro‐environment, further modified by ESCs and modulated by exogenous soluble factors, may become an adequate culture system particularly suited to ESCs development.
ACKNOWLEDGEMENTS
This work has been supported in part by grants from the Italian Ministry of Education, University and Research. MIUR:PNR 2001/3 (FIRB art 8) DM199; Italian Ministry of Health, RC 2004; IZS: Tuscany and Lazio; Joint project Italy Czech Republic (Project Kontakt) awarded to R.P.R.; MIUR‐University of Bologna: ‘Molecular signal and mechanisms in cellular survival’ awarded to V.F. The authors gratefully thank Prof Marie A. DiBerardino for critically reading the manuscript.
M. Michelini, V. Franceschini, S. Sihui Chen and R. P. Revoltella contributed equally to this paper.
REFERENCES
- Aumailley M, Gayraud B (1998) Structure and biological activity of the extracellular matrix. J. Mol. Med. 76, 253–265. [DOI] [PubMed] [Google Scholar]
- Barth AIM, Nathke IS, Nelson WJ (1997) Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr. Opin. Cell Biol. 9, 683–690. [DOI] [PubMed] [Google Scholar]
- Chen SS, Revoltella RP, Papini S, Michelini M, Fitzgerald W, Zimmerberg J, Margolis L (2003) Multilineage differentiation of rhesus monkey embryonic stem cells in three‐dimensional culture systems. Stem Cells 21, 281–295. [DOI] [PubMed] [Google Scholar]
- Chen Sihui S, Revoltella RP, Zimmerberg J, Margolis L (2005) Differentiation of rhesus monkey embryonics stem cell in three‐dimensional collagen matrix In: Turksen K. ed. Methods in Molecular Biology, vol. 330: Nonhuman Embryonic Stem Cell Protocols. Humana Press, Totowa, NJ Vol. 2, pp. 431–444. [DOI] [PubMed] [Google Scholar]
- Czyz J, Wobus AM (2001) Embryonic stem cell differentiation: the role of extracellular factors. Differentiation 68, 167–174. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Ilic D (2002) Integrin signalling: it's were the action is. Curr. Opin. Cell Biol. 5, 594–608. [DOI] [PubMed] [Google Scholar]
- Derycke LMD, Bracke ME (2004) N‐cadherin in the spot light of cell‐cell adhesion, differentiation, embryogenesis, invasion and signalling. Int. J. Dev. Biol. 48, 463–476. [DOI] [PubMed] [Google Scholar]
- Fenderson BA, Eddy EM, Hakomori S (1990) Glycoconjugate expression during embryogenesis and its biological significance. Bioessays 12, 173–179. [DOI] [PubMed] [Google Scholar]
- Fukuda M. (2000) Cell surface carbohydrates: cell type‐specific expression In: Fukuda M, Hindsgaul O, eds. Molecular and Cell Glycobiology. Oxford University Press, New York, pp. 1–61. [Google Scholar]
- Green K, Watt F (2004) Cell‐to cell contact and extracellular matrix. Curr. Opin. Cell Biol. 16, 465–469. [Google Scholar]
- Hayman EG, Ruoslhati E (1979) Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J. Cell Biol. 83, 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Hall DH, Hedgecock EM, Kao G, Karantza V, Vogel BE, Hutter H, Chisholm AD, Yurchenco PD, Wadsworth WG (2003) Laminin α subunit and their role in C. elegans development. Development 130, 3343–3358. [DOI] [PubMed] [Google Scholar]
- Hynes RO (1981) Cell biology of the extracellular matrix. In: Hay ED, ed. Plenum Press, New York, pp. 295–333. [Google Scholar]
- Hynes RO (1990) Fibronectins. Springer‐Verlag, New‐York. [Google Scholar]
- Itskovitz‐Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N (2000) Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2, 88–95. [PMC free article] [PubMed] [Google Scholar]
- Jessell TM, Hymes MA, Dodd J (1990) Carbohydrates and carbohydrate‐binding proteins in the nervous system. Annu. Rev. Neurosci. 13, 227–255. [DOI] [PubMed] [Google Scholar]
- Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L (1997) Fibronectin‐integrin interactions. Front. Biosci. 2, 126–146. [DOI] [PubMed] [Google Scholar]
- Khan KM, Sarfaraz N, Salim Z (1999) Lectin binding patterns in nonsensory regions of rat cochlea during postnatal development. J. Anat. 194, 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A (2000) Breakthroughs and views. Regulations of β‐catenin signaling in the Wnt pathway. Bioch. Biophys. Res. Commun. 286, 243–248. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Philip D, Hoffman MP (2003) Role of the extracellular matrix in morphogenesis. Curr. Opin. Biotechnol 14, 526–532. [DOI] [PubMed] [Google Scholar]
- Kresse H, Schonherr E (2001) Proteoglycans of the extraellular matrix and growth control. J. Cell. Physiol. 189, 266–274. [DOI] [PubMed] [Google Scholar]
- Kudo T, Kaneko M, Iwasaki H, Togayachi A, Nishihara S, Abe K, Narimatsu H (2004) Normal embryonic and germ cell development in mice lacking alpha 1,3‐fucosyltransferse IX (Fut9) which show disappearance of stage‐specific embryonic antigen 1. Mol. Cell. Biol. 24, 4221–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Guan JL, Chien S (2005) Biochemistry and biomechanics of cell motility. Annu. Rev. Biomed. Eng. 7, 105–150. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R (2004) Convergence of Wnt, B‐catenin and Cadherin pathways. Science 303, 1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojakian GK, Ratcliffe DR, Schwimmer R (2001) Integrin regulation of cell–cell adhesion during epithelial tubule formation. J. Cell Sci. 114, 941–952. [DOI] [PubMed] [Google Scholar]
- Otero JJ, Fu W, Kan L, Quadre AE, Kessler JA (2004) β‐catenin signaling is required for neural differentiation of embryonic stem cells. Development 131, 3545–3557. [DOI] [PubMed] [Google Scholar]
- Papini S, Cecchetti D, Campani D, Fitzgerald W, Grivel JC, Chen S, Margolis L, Revoltella RP (2003) Isolation and clonal analysis of human epidermal keratinocyte stem cells in long‐term culture. Stem Cells 4, 481–494. [DOI] [PubMed] [Google Scholar]
- Philp D, Chen SS, Fitzgerald W, Orenstein J, Margolis L, Kleinman HK (2005) Complex extracellular matrices promote tissue‐specific stem cell differentiation. Stem Cells 23, 288–296. [DOI] [PubMed] [Google Scholar]
- Prento P (2001) A contribution to the theory of biological staining based on the principles for structural organization of biological macromolecules. Biotech. Histochem. 3, 137–161. [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL (2003) A role for Wnt signalling in self‐renewal of hematopoietic stem cells. Nature 423, 409–414. [DOI] [PubMed] [Google Scholar]
- Romberger DJ (1997) Molecules in focus:fibronectin. Int. J. Biochem. Cell Biol. 29, 939–943. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA (1994) Matrix metalloproteinases and endometrial remodelling. Cell Biol. Int. 18, 1139–1144. [DOI] [PubMed] [Google Scholar]
- Singleton C, Menino AR Jr (2005) Effects of inhibitors of integrin binding on cellular outgrowth from bovine inner cell masses in vitro. In Vitro Cell. Dev. Biol. Anim. 41, 29–37. [DOI] [PubMed] [Google Scholar]
- Takeichi M (1995) Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 7, 619–627. [DOI] [PubMed] [Google Scholar]
- Takeichi M, Nakagawa S, Aono S, Usui T, Uemura T (2000) Patterning of cell assemblies regulated by adhesion receptors of the cadherin superfamily. Philos. Trans. R. Soc. Lond., B., Biol. Sci. 355, 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer ML, Giniger MS, Chandrasekaran S (1993) Laminin oligosaccarides play a pivotal role in cell spreading. Symp. Soc. Exp. Biol. 47, 147–154. [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 5391, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Marshall VS (1998) Primate embryonic stem cells. Curr. Top. Dev. Biol. 38, 133–165. [DOI] [PubMed] [Google Scholar]
- Van Damme EJM, Peumans WJ, Pusztai A, Bardocz S (1998) Handbook of Plant Lectins: Properties and Biomedical Applications. J. Wiley & Sons, New York, pp. 31–50. [Google Scholar]
- Watt FM, Hogan BLM (2000) Out of eden: stem cells and their niches. Science 287, 1427–1430. [DOI] [PubMed] [Google Scholar]
- Wierzbicka‐Patynowski I, Schwarzbauer JE (2003) The ins and outs of fibronectin matrix assembly. J. Cell. Sci. 116, 3269–3276. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Wadsworth WG (2004) Assembly and tissue functions of early embryonic laminins and netrins. Curr. Opin. Cell Biol. 16, 572–579. [DOI] [PubMed] [Google Scholar]
- Zaidel‐Bar R, Cohen M, Addadi L, Geiger B (2004) Hierarchical assembly of cell‐matrix adhesion complexes. Biochem. Soc. Trans. 32, 416–420. [DOI] [PubMed] [Google Scholar]
- Zalik SE (1991) On the possible role of endogenous lectins in early animal development. Anat. Embryol. 183, 521–536. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L (2003) Identification of the hematopoietic stem cell niche and control of the niche size. Nature 425, 836–841. [DOI] [PubMed] [Google Scholar]


