Abstract
Abstract. The cell cycle is controlled by numerous mechanisms ensuring correct cell division. This review will focus on these mechanisms, i.e. regulation of cyclin‐dependent kinases (CDK) by cyclins, CDK inhibitors and phosphorylating events. The quality checkpoints activated after DNA damage are also discussed. The complexity of the regulation of the cell cycle is also reflected in the different alterations leading to aberrant cell proliferation and development of cancer. Consequently, targeting the cell cycle in general and CDK in particular presents unique opportunities for drug discovery. This review provides an overview of deregulation of the cell cycle in cancer. Different families of known CDK inhibitors acting by ATP competition are also discussed. Currently, at least three compounds with CDK inhibitory activity (flavopiridol, UCN‐01, roscovitine) have entered clinical trials.
GENERAL STRATEGY OF THE CELL CYCLE
Cell division consists of two consecutive processes, mainly characterized by DNA replication and segregation of replicated chromosomes into two separate cells. Originally, cell division was divided into two stages: mitosis (M), i.e. the process of nuclear division; and interphase, the interlude between two M phases (Fig. 1). Stages of mitosis include prophase, metaphase, anaphase and telophase. Under the microscope, interphase cells simply grow in size, but different techniques revealed that the interphase includes G1, S and G2 phases (reviewed in Norbury & Nurse 1992). Replication of DNA occurs in a specific part of the interphase called S phase. S phase is preceded by a gap called G1 during which the cell is preparing for DNA synthesis and is followed by a gap called G2 during which the cell prepares for mitosis. G1, S, G2 and M phases are the traditional subdivisions of the standard cell cycle (Fig. 1). Cells in G1 can, before commitment to DNA replication, enter a resting state called G0. Cells in G0 account for the major part of the non‐growing, non‐proliferating cells in the human body.
Figure 1.
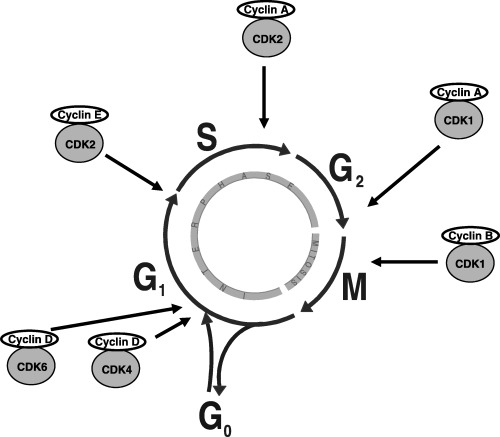
The stages of the cell cycle. The site of activity of regulatory CDK/cyclin complexes is also indicated.
CONTROL OF THE CELL CYCLE
Cyclin‐dependent kinase (CDK) regulation
The transition from one cell cycle phase to another occurs in an orderly fashion and is regulated by different cellular proteins. Key regulatory proteins are the cyclin‐dependent kinases (CDK), a family of serine/threonine protein kinases that are activated at specific points of the cell cycle. Until now, nine CDK have been identified and, of these, five are active during the cell cycle, i.e. during G1 (CDK4, CDK6 and CDK2), S (CDK2), G2 and M (CDK1) (Table 1, Fig. 1). When activated, CDK induce downstream processes by phosphorylating selected proteins (Morgan 1995; Pines 1995). CDK7 acts in combination with cyclin H as CDK activating kinase (CAK, see below) (Fisher & Morgan 1994). The remaining CDK have not yet been shown to have a crucial role in normal cell cycle progression (Rickert et al. 1996). CDK protein levels remain stable during the cell cycle, in contrast to their activating proteins, the cyclins. Cyclin protein levels rise and fall during the cell cycle and in this way they periodically activate CDK (Evans et al. 1983; Pines 1991). Different cyclins are required at different phases of the cell cycle (Table 1). The three D type cyclins (cyclin D1, cyclin D2, cyclin D3) bind to CDK4 and to CDK6 and CDK‐cyclin D complexes are essential for entry in G1 (Fig. 2) (Sherr 1994). Unlike the other cyclins, cyclin D is not expressed periodically, but is synthesized as long as growth factor stimulation persists (Assoian & Zhu 1997). Another G1 cyclin is cyclin E which associates with CDK2 to regulate progression from G1 into S phase (Ohtsubo et al. 1995). Cyclin A binds with CDK2 and this complex is required during S phase (Fig. 2) (Girard et al. 1991; Walker & Maller 1991). In late G2 and early M, cyclin A complexes with CDK1 to promote entry into M. Mitosis is further regulated by cyclin B in complex with CDK1 (Fig. 2) (King et al. 1994; Arellano & Moreno 1997). Sixteen cyclins have been identified so far but, like CDK, not all of them are cell‐cycle related (Peng et al. 1998; Okamoto & Beach 1994; Rickert et al. 1996). Cyclins A and B contain a destruction box and cyclins D and E contain a PEST sequence [segment rich in proline (P), glutamic acid (E), serine (S) and threonine (T) residues]: these are protein sequences required for efficient ubiquitin‐mediated cyclin proteolysis at the end of a cell cycle phase (Glotzer et al. 1991; Rechsteiner & Rogers 1996).
Table 1.
Cyclin‐CDK complexes are activated at specific points of the cell cycle. CAK, CDK activating kinase
| CDK | Cyclin | Cell cycle phase activity | |
|---|---|---|---|
| CDK4 | Cyclin D1, D2, D3 | G1 phase | |
| CDK6 | Cyclin D1, D2, D3 | G1 phase | |
| CDK2 | Cyclin E | G1/S phase transition | |
| CDK2 | Cyclin A | S phase | |
| CDK1 | (cdc2) | Cyclin A | G2/M phase transition |
| CDK1 | (cdc2) | Cyclin B | Mitosis |
| CDK7 | Cyclin H | CAK, all cell cycle phases |
Figure 2.
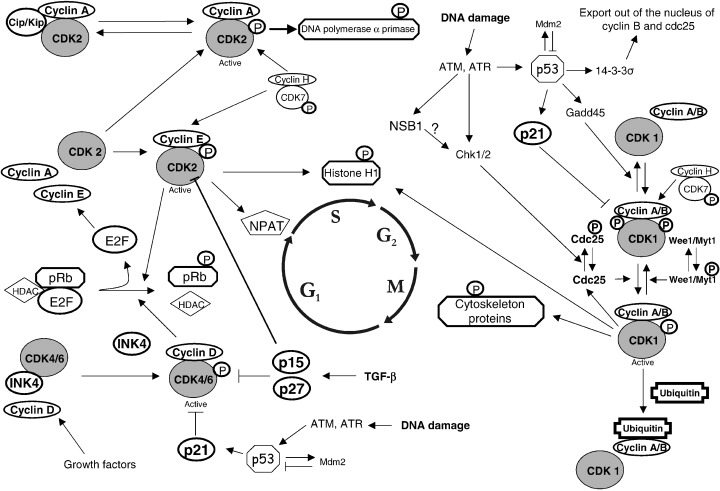
A schematic overwiew of some essential steps in cell cycle regulation.
 , phosphorylated site. (→: activation;
, phosphorylated site. (→: activation;  : inhibition)
: inhibition)
In addition to cyclin binding, CDK activity is also regulated by phosphorylation on conserved threonine and tyrosine residues. Full activation of CDK1 requires phosphorylation of threonine 161 (threonine 172 in CDK4 and threonine 160 in CDK2), brought about by the CDK7‐cyclin H complex, also called CAK ( Fig. 2). These phosphorylations induce conformational changes and enhance the binding of cyclins (Jeffrey et al. 1995; Paulovich & Hartwell 1995). The Wee1 and Myt1 kinases phosphorylate CDK1 at tyrosine‐15 and/or threonine‐14, thereby inactivating the kinase (Fig. 2). Dephosphorylation at these sites by the enzyme Cdc25 is necessary for activation of CDK1 and further progression through the cell cycle (Fig. 2) (Lew & Kornbluth 1996).
CDK activity can be counteracted by cell cycle inhibitory proteins, called CDK inhibitors (CKI) which bind to CDK alone or to the CDK‐cyclin complex and regulate CDK activity. Two distinct families of CDK inhibitors have been discovered, the INK4 family and Cip/Kip family (Table 2) (Sherr & Roberts 1995). The INK4 family includes p15 (INK4b), p16 (INK4a), p18 (INK4c), p19 (INK4d), which specifically inactivate G1 CDK (CDK4 and CDK6). These CKI form stable complexes with the CDK enzyme before cyclin binding, preventing association with cyclin D (Carnero & Hannon 1998). The second family of inhibitors, the Cip/Kip family, includes p21 (Waf1, Cip1), p27 (Cip2), p57 (Kip2). These inhibitors inactivate CDK‐cyclin complexes (Polyak et al. 1994; Harper et al. 1995; Lee et al. 1995). They inhibit the G1 CDK‐cyclin complexes, and to a lesser extent, CDK1‐cyclin B complexes (Hengst & Reed 1998). p21 also inhibits DNA synthesis by binding to and inhibiting the proliferating cell nuclear antigen (PCNA) (Pan et al. 1995; Waga et al. 1997). CKI are regulated both by internal and external signals: the expression of p21 is under transcriptional control of the p53 tumour suppressor gene. The p21 gene promotor contains a p53‐binding site, that allows p53 to transcriptionally activate the p21 gene (el Deiry et al. 1993). The expression and activation of, respectively, p15 and p27, increases in response to transforming growth factor β (TGF‐β), contributing to growth arrest (Hannon & Beach 1994; Reynisdottir et al. 1995).
Table 2.
Cyclin dependent kinases inhibitors (CKI) bind to CDK alone or to the CDK‐cyclin complex and regulate CDK activity. p19 (ARF) is also encoded by the INK4 locus, but has no known CKI activity
| CKI family | Function | Family members | |
|---|---|---|---|
| INK4 family | Inactivationof G1 CDK(CDK4, CDK6) | p15 | (INK4b) |
| p16 | (INK4a) | ||
| p18 | (INK4c) | ||
| p19 | (INK4d) | ||
| Cip/Kip family | Inactivation ofG1 cyclin‐CDKcomplexes andcyclin B‐CDK1 | p21 | (Waf1, Cip1) |
| p27 | (Cip2) | ||
| p57 | (Kip2) |
The intracellular localization of different cell cycle‐regulating proteins also contributes to a correct cell cycle progression. Cyclin B contains a nuclear exclusion signal and is actively exported from the nucleus until the beginning of the prophase. The CDK inactivating kinases Wee1 and Myt1 are located, respectively, in the nucleus and Golgi complex and protect the cell from premature mitosis (Heald et al. 1993; Liu et al. 1997). The 14‐3‐3 group of proteins regulates the intracellular trafficking of different proteins. During interphase, the CDK activating kinase, Cdc25, is kept in the cytoplasm through interaction with 14‐3‐3 proteins. Sequestration of the CDK1‐cyclin B complex in the cytoplasm following DNA damage is also mediated by 14‐3‐3 proteins (Peng et al. 1997; Yang et al. 1999).
CDK substrates
When CDK is active, target proteins become phosphorylated on CDK consensus sites, resulting in changes that are physiologically relevant for cell cycle progression. The most frequently studied target is the substrate of CDK4/6‐cyclin D, i.e. the product of the retinoblastoma tumour suppressor gene (pRb) (Fig. 2). During early G1, pRb becomes phosphorylated and this leads to disruption of the complex with the histone deacetylase protein (HDAC) and release of the transcription factors E2F‐1 and DP‐1, which positively regulate the transcription of genes whose products are required for S phase progression, including cyclin A, cyclin E, Cdc25 (Fig. 2) (Buchkovich et al. 1989; Kato et al. 1993; Brehm et al. 1998). pRb remains hyperphosphorylated for the remainder of the cell cycle and CDK2‐cyclin E participates in maintaining this hyperphosphorylated state. During G1/S the CDK2‐cyclin E complex also phosphorylates its inhibitor p27, inducing its proteasome‐dependent degradation (Hinds et al. 1992; Montagnoli et al. 1999). NPAT (nuclear protein mapped to the ATM locus) associates with, and is also phosphorylated by, CDK2‐cyclin E. The protein level of NPAT peaks at the G1/S boundary and is thought to play a role in S phase entry (Zhao et al. 2000). CDK2‐cyclin E phosphorylates histone H1 and this activity may be important for chromosome condensation required during DNA replication. Histone H1 is also a substrate for CDK1‐cyclin B (Bradbury et al. 1974). Cyclin A‐dependent kinases regulate initiation of DNA replication by phosphorylation of DNA polymerase alpha primase (Voitenleitner et al. 1997). Other CDK substrates include CDK's own regulators Wee1 and Cdc25, and cytoskeletal proteins such as nuclear lamins, microtubules and vimentin, which are required for correct mitosis (Heald & McKeon 1990; Courvalin et al. 1992; Hoffmann et al. 1993; Blangy et al. 1995).
Quality control of the cell cycle: restriction points and checkpoints
The restriction point (R) is defined as a point of no return in G1, following which the cell is committed to enter the cell cycle. Experiments demonstrate that cells starved of serum before the restriction point enter a G0‐like state, while cells starved after R are unaffected and continue through mitosis (Pardee 1974). Additional controls or checkpoints exist further in the cell cycle, ensuring an orderly sequence of events in the cell cycle (Hartwell & Weinert 1989). Up to now, DNA damage checkpoints and spindle checkpoints have (partly) been elucidated. In response to DNA damage, checkpoints arrest the cell cycle in order to provide time for DNA repair. DNA damage checkpoints are positioned before the cell enters S phase (G1‐S checkpoint) or after DNA replication (G2‐M checkpoint) and there appears to be DNA damage checkpoints during S and M phases.
At the G1/S checkpoint, cell cycle arrest induced by DNA damage is p53‐dependent. Usually, the cellular level of p53 is low but DNA damage can lead to rapid induction of p53 activity (Levine 1997). p53 stimulates the transcription of different genes including p21, Mdm2 and Bax (Agarwal et al. 1998). The induction of p21, a CKI, results in CDK inhibition and cell cycle arrest, preventing the replication of damaged DNA (Fig. 2) (Ko & Prives 1996). Mdm2 plays an important role in the regulation of p53: it binds to and inhibits p53 transcriptional activity and contributes to the proteolytic degradation of p53 by facilitating its ubiquitination, hereby providing a negative feedback loop (Oren 1999). Binding of regulatory proteins can also modulate p53 ubiquitination: the p19 (ARF) protein, encoded by the ARF‐INK4 locus (see below), binds to Mdm2 and this prevents the Mdm2‐mediated p53 proteolysis (Zhang et al. 1998). In the case of severely damaged cells, p53 induces cell death by activating genes (e.g. Bax, Fas and genes involved in oxidative stress pathways) that are involved in apoptotic signalling (Owen‐Schaub et al. 1995; Polyak et al. 1997; Gottlieb & Oren 1998).
Different protein kinases ‘recognize’ DNA damage, e.g. ataxia‐telangiectasia‐mutated (ATM), ataxia and rad3 related (ATR). These kinases phosphorylate p53 in response to DNA damage, resulting in p21 blocking the cell cycle, at least at the G1/S checkpoint (Fig. 2) (Siliciano et al. 1997). DNA protein kinase (DNA‐PK), a DNA double‐strand break repair enzyme is related to ATM and ATR, but it is not known yet whether it also plays an important role at the G1/S checkpoint (Burma et al. 1999; Durocher & Jackson 2001).
The mechanisms of the S phase DNA damage checkpoint are poorly understood, but some studies demonstrated suppression of both the initiation and elongation phases of DNA replication (Painter 1986; Paulovich & Hartwell 1995). There is also evidence that ATM‐mediated phosphorylation of (Nijmegen breakage syndrome 1 (NBS1) is required to induce S phase arrest during the S phase checkpoint (Lim et al. 2000).
When DNA damage occurs during G2, cells are able to initiate a cell cycle arrest in the presence or absence of p53. The entry into mitosis is prevented by maintaining CDK1 in its inhibited form through inhibitory phosphorylation or by sequestration of components of the CDK1‐cyclin B complex outside the nucleus. This is achieved by the protein kinases Chk1 and Chk2, which are activated during DNA damage in an ATM‐dependent manner and which phosphorylate Cdc25 (Fig. 2). Phosphorylation of Cdc25 inhibits its activity and promotes its binding to 14‐3‐3 proteins, sequestering it outside the nucleus and preventing it from activating CDK1‐cyclin B and mitotic entry (Sanchez et al. 1997; Zeng et al. 1998). Besides induction of inhibitory phosphorylations on CDK, p53 may also play a role in the regulation of the G2/M checkpoint. DNA damage‐dependent increase of p53 results, as during the G1/S checkpoint, in increased transcription of p21 and of 14‐3‐3 sigma (14‐3‐3 σ). Increased binding of cyclin B to 14‐3‐3 σ actively excludes it from the nucleus. p53 also mediates the dissociation of CDK1‐cyclin B1 complexes by induction of Gadd45 (growth arrest and DNA damage inducible gene) (Hermeking et al. 1997; Taylor & Stark 2001).
The ‘spindle checkpoint’ brings about detection of improper alignment of the chromosomes on the mitotic spindle and stops the cell cycle in metaphase. Initially identified in budding yeast, several mammalian spindle checkpoint‐associated proteins have recently been identified. Mitotic arrest deficient (Mad) and budding uninhibited by benomyl (Bub) proteins are activated when defects in microtubule attachement occur and inhibit the Cdc20 subunit of the anaphase‐promoting complex (APC), resulting in the prevention of metaphase‐anaphase transition (Fang et al. 1998; Amon 1999).
CELL CYCLE AND CANCER
In cancer, there are fundamental alterations in the genetic control of cell division, resulting in an unrestrained cell proliferation. Mutations mainly occur in two classes of genes: proto‐oncogenes and tumour suppressor genes. In normal cells, the products of proto‐oncogenes act at different levels along the pathways that stimulate cell proliferation. Mutated versions of proto‐oncogenes or oncogenes can promote tumour growth. Inactivation of tumour suppressor genes like pRb and p53 results in dysfunction of proteins that normally inhibit cell cycle progression. Cell cycle deregulation associated with cancer occurs through mutation of proteins important at different levels of the cell cycle. In cancer, mutations have been observed in genes encoding CDK, cyclins, CDK‐activating enzymes, CKI, CDK substrates, and checkpoint proteins (reviewed by Sherr 1996; McDonald & el Deiry 2000).
CDK
Alterations of CDK molecules in cancer have been reported, although with low frequency. CDK4 overexpression, that occurs as a result of amplification, has been identified in cell lines, melanoma, sarcoma and glioma (Wolfel et al. 1995). Mutations in CDK4 and CDK6 genes resulting in loss of CKI binding have also been identified (Easton et al. 1998). CDK1 and CDK2 have been reported to be overexpressed in a subset of colon adenomas, a greater overexpresion was seen in focal carcinomas in adenomatous tissue (Yamamoto et al. 1998; Kim et al. 1999).
Cyclins
Cyclin D acts as a growth sensor and provides a link between mitogenic stimuli and the cell cycle. Cyclin D1 binds to CDK4 and CDK6 in early G1. Aberrant cyclin D1 expression has been reported in many human cancers. In the first study that implicated cyclin D1 in human tumours, its gene was linked in parathyroid adenomas to the parathyroid hormone gene (Motokura et al. 1991). It is now clear that cyclin D1 gene translocation is associated with B‐cell malignancies, including mantle cell lymhoma. In the latter case, the characteristic t(11; 14) translocation juxtaposes the cyclin D1 gene (initially described as bcl‐1/PRAD1) to the immunoglobulin heavy chain gene, leading to cyclin D1 overexpression in centrocytic B‐lymphocytes (Weisenburger et al. 1987). Cyclin D1 gene amplification occurs in breast, esophageal, bladder, lung and squamous cell carcinomas (Hall & Peters 1996). Cyclin D2 and cyclin D3 have also been reported to be overexpressed in some tumours and cyclin E has been found to be amplified, overexpressed or both in some cases of breast and colon cancer and in acute lymphoblastic and acute myeloid leukaemias (Leach et al. 1993; Hunter & Pines 1994; Keyomarsi et al. 1995; Scuderi et al. 1996; Iida et al. 1997). Both cyclin A and cyclin E are overexpressed in lung carcinoma and elevated expression of cyclin A but not cyclin E correlated with shorter survival (Dobashi et al. 1998).
CDK activating enzymes
Activation of CDK is regulated through dephosphorylation by members of the Cdc25 phosphatase family. Cdc25A plays an important role at the G1/S‐phase transition, Cdc25B undergoes activation during S‐phase and Cdc25C activates CDK1‐cyclin B during entry into mitosis. Deregulation or overexpression of Cdc25 allows for unscheduled activation of CDK‐cyclins and can be associated with tumour formation. Cdc25A and Cdc25B are potential human oncogenes (Nilsson & Hoffmann 2000). Cdc25B is overexpressed in 32% of primary breast cancers. Transcription of Cdc25A and Cdc25B genes is activated by c‐myc, an oncogene found to be frequently mutated in human cancers (Galaktionov et al. 1996). Raf, a kinase downstream of the frequently mutated ras oncogene, is able to bind, activate and deregulate Cdc25 protein (Galaktionov et al. 1995).
CKI
The inhibitory activity of CKI results in growth suppression through activation of pRb, reflecting the tumour suppressor function of CKI. The p16 gene is altered in a high percentage of human tumours and can be inactivated by a variety of mechanisms including deletion, point mutations and hypermethylation (Kamb 1998). Cells with altered p16 will be unrestrained to proceed through G1. The p16 protein is a specific inhibitor of CDK‐cyclin D, preventing phosphorylation of the pRb protein and arresting cells in G1 phase (Table 2). As pRb, p16 and CDK/cyclin D are functionally interconnected, perturbations in any of these cell cycle regulators are likely to have similar consequences. Moreover, alterations of at least one of these regulators are found in nearly all human cancers (Jiang et al. 1993; Bates et al. 1994; Okamoto et al. 1994; Parry et al. 1995; Gronbaek et al. 1998). Deletions of p16 have been reported in approximately 50% of gliomas and mesotheliomas, 40–60% of nasopharyngeal, pancreatic and bilary tract tumours and 20–30% of acute lymphoblastic leukaemias (reviewed by Hall & Peters 1996). The p16 gene product is encoded by the ARF‐INK4 locus. Besides the p16 gene product, this locus also encodes p19 (ARF), following an alternative reading frame. The p19 transcript acts independently of p16 in regulating p53 stability. Large deletions of the ARF‐INK4 locus can also affect the p19 gene, resulting in a mutated p19 and in deregulation of p53. The gene encoding p15, another CKI, is located close to the p16 gene on chromosome 9 and is also often simultaneously deleted (Harper & Elledge 1996). Loss of p27 expression has been reported for a number of human tumour types (lung, breast, bladder) and has been correlated with poor prognosis and tumour aggressiveness. It has been shown in colorectal carcinomas that increased proteasome‐dependent proteolysis, rather than gene deletion, is responsible for p27 down‐regulation (King et al. 1996; Pagano 1997). A few alterations have been found in p18 and p21 in breast tumour and leukaemia, respectively (Lapointe et al. 1996; Shi et al. 1996; Esposito et al. 1997; Loda et al. 1997; Tan et al. 1997). p21 has been implicated in tumorigenesis through its regulation by the p53 tumour supressor protein. The p53 gene is the most commonly mutated gene in human cancer and regulation of p21 in response to DNA damage is lost when p53 is inactivated (Deng et al. 1995).
Substrate
pRb, the most important CDK substrate during G1, is frequently mutated in human retinoblastoma and lung cancer (Knudson 1971; Hall & Peters 1996). Deletion and mis‐sense mutations result in truncated, non‐functional pRb or in complete absence of pRb, while binding of certain tumour virus proteins (e.g. human papillomaviruses (HPV) E7, adenovirus E1A and simian virus 40 (SV40) large (T) inactivate pRb (Gage et al. 1990; Hu et al. 1990). Absence or loss of function of pRb is associated with unrestrained cell cycle progression and is common in acute lymphoblastic leukaemia (Horsthemke 1992; Tsai et al. 1996). The implications for human cancer of the interconnection of pRb with CDK4/6‐cyclin D and p16 have been mentioned above. Approximately 90% of human cancers have abnormalities in some component of the pRb pathway (Hall & Peters 1996). The other pRb family members, p107 and p130, have not yet been linked with tumour‐promoting mutations, neither have tumour‐associated mutations of the E2F family of transcription factors been described (Bartek et al. 1996).
Checkpoint proteins
Mutations of checkpoint proteins are frequent in all types of cancer. The tumour suppressor protein p53 is a sequence‐specific DNA‐binding protein, that is able to induce either cell cycle arrest or apoptosis at the cell cycle checkpoints. The p53 tumour suppressor gene was first discovered in SV40 transformed cells by the finding that its protein product p53 was tightly bound to the SV40 large T oncogene product. Now, the p53 gene is known to be the most frequently mutated gene in human cancer (Miller & Koeffler 1993; Greenblatt et al. 1994). Point and mis‐sense mutations lead to conformational changes and inactivation of the protein (Nataraj et al. 1995). Other mechanisms, like binding of viral oncoproteins including SV40 T antigen, HPV E6 and adenovirus E1B‐55K, can alter or block p53 function (van den Heuvel et al. 1990; Crook & Vousden 1994). In general, tumours that retain wild type p53 have a better prognosis and have a better response to therapy (Lowe et al. 1994). Overexpression by gene amplification or other mechanisms of Mdm2, the negative regulator of p53, has been reported in leukaemia and lymphoma, breast carcinoma, sarcoma and glioma and may represent an alternative mechanism to p53 mutation for escaping p53‐mediated growth control (Bueso‐Ramos et al. 1995; Bueso‐Ramos et al. 1996; Moller et al. 1999).
CDK INHIBITORS IN ANTI‐CANCER DRUG DEVELOPMENT
The process of searching for new cancer drugs has undergone a major change: it has moved from a strategy identifying drugs that kill tumour cells towards a more mechanistic strategy acting on molecular targets that underly cell transformation. The evidence that CDK, their regulators and substrates are targets of genetic alteration in different types of human cancer has stimulated the search for chemical CDK inhibitors. Different strategies for therapeutic intervention can modulate CDK activity: targeting the major regulators of CDK activity (indirect strategy) or inhibiting the catalytic activity of the CDK kinases (direct strategy). Approaches for the indirect strategy include overexpression of CKI, synthesis of peptides mimicking the effects of CKI, decrease of cyclin levels, modulation of the proteasomal machinery, modulation of the phosphorylated state of CDK and of the enzymes regulating it. Details of these indirect strategies are beyond the scope of this review; for further information, readers are referred to other reviews (McDonald & el Deiry 2000; Senderowicz & Sausville 2000). Until now, direct inhibition of CDK kinase activity has been the most successful strategy for the development of potent cell cycle inhibitors. All inhibitors identified so far act by competitive inhibition of ATP binding to CDK. The potential for disruption of ATP binding in the small, defined ATP pocket of CDK is much higher than disrupting a large protein–protein interface, such as a CDK‐cyclin binding surface. More than 50 inhibitors have been described and most studied families will be described below and in Table 3.
Table 3.
Chemical CDK inhibitors and their IC50 (concentration at which 50% inhibition occured) on CDK1
| Inhibitor | IC50 (µM) | Reference no. |
|---|---|---|
| Purine analogues | ||
| Dimethylaminopurine | 120 | Meijer & Pondaven 1988; Neant & Guerrier 1988 |
| N6‐isopentenyladenine | 55 | Rialet & Meijer 1991 |
| Olomoucine | 7 | Vesely et al. 1994 |
| Roscovitine | 0.2–0.8 | De Azevedo et al. 1997; Meijer et al. 1997 |
| CVT‐313 | 4.2 | Brooks et al. 1997 |
| Purvalanol A | 0.004 | Gray et al. 1998 |
| Purvalanol B | 0.006 | Gray et al. 1998 |
| New cytokinin analogues | 0.1–3.8 | Vermeulen et al. 2002a; Vermeulen et al. 2002b |
| Olomoucine II | 0.02 | Krystof et al. 2002 |
| NU2058 | 5 | Arris et al. 2000 |
| Pyrimidine analogues | ||
| NU6027 | 2.5 | Arris et al. 2000 |
| Butyrolactone | 0.6 | Kitagawa et al. 1993; Kitagewa et al. 1994 |
| Flavonoïds | ||
| Flavopiridol | 0.4 | Losiewicz et al. 1994 |
| Oxoflavopiridol | 0.130 | Kim et al. 2000 |
| Thioflavopiridol | 0.110 | Kim et al. 2000 |
| Paullones | ||
| Kenpaullone | 0.4 | Zaharevitz et al. 1999 |
| Alsterpaullone | 0.035 | Schultz et al. 1999 |
| Indolinones | ||
| Indirubin | 10˙ | Hoessel et al. 1999 |
| Indirubin‐3′‐monoxime | 0.18 | Hoessel et al. 1999 |
| 5‐chloro‐indirubin | 0.4 | Hoessel et al. 1999 |
| Indirubin‐5‐sulphonic acid | 0.055 | Hoessel et al. 1999 |
| SU9516 | 0.04 | Lane et al. 2001 |
| Staurosporine and derivatives | ||
| Staurosporine | 0.003–0.009 | Gadbois et al. 1992 |
| UCN‐01 | 0.031–1 | Wang et al. 1995; Kawakami et al. 1996 |
| 9‐hydroxyellipticine | 1 | Ohasi et al. 1995 |
| Suramin | 4 | Larsen 1993 |
| Hymenialdisine | 0.022 | Meijer et al. 2000 |
| Toyocamycin | 0.88 | Park et al. 1996 |
Purine analogues, plant cytokinin analogues and pyrimidine analogues
The natural phytohormones or cytokinins dimethylaminopurine and N6‐isopentenyladenine were first identified as CDK1‐cyclin B kinase inhibitors, but they were found to be non‐specific kinase inhibitors (Meijer & Pondaven 1988; Neant & Guerrier 1988; Rialet & Meijer 1991). Screening of chemically synthesized aromatic cytokinin analogues for inhibition of CDK1‐cyclin B kinase led to the discovery of the highly CDK‐specific inhibitor olomoucine (Vesely et al. 1994; Schulze‐Gahmen et al. 1995). Olomoucine has been found to inhibit cell proliferation and to induce apoptosis in tumour cells (Abraham et al. 1995; Schutte et al. 1997). Olomoucine also potentiated mitoxantrone‐induced apoptosis (Ongkeko et al. 1995) and initiated apoptosis in a case of dog malignant melanoma (Hajduch et al. 1997). Among 35 highly purified kinases, only the cell cycle regulating CDK1‐cyclin B, CDK2‐cyclin A, CDK2‐cyclin E, brain CDK5‐p35 and ERK1/MAP kinase were substantially inhibited by olomoucine. Among 81 cytokinin analogues tested, only C2‐, C6‐, N9‐substituted purines showed strong inhibitory effect on CDK1 (Vesely et al. 1994). Roscovitine, a more recently developed C2‐, C6‐, N9‐substituted purine has a 10‐fold more potent inhibitory activity on CDK1 than olomoucine. Roscovitine showed strong anti‐proliferative effects and it has currently entered clinical trials (De Azevedo et al. 1997; Meijer et al. 1997; Clough 2002). CVT‐313 is another CDK inhibitor, identified from a purine analogue library; this compound blocks proliferation of human lung fibroblasts (Brooks et al. 1997). Another group of purine‐based structures was identified in a screening of trisubstituted purine combinatorial libraries designed for CDK inhibition. This screening led to the development of new CDK inhibitors, e.g. purvalanol A and B (Gray et al. 1998). Recently, different studies on synthesis and in vitro evaluation of C2‐, C6‐, N9‐substituted purines have been published and research on the mechanistic basis of anti‐proliferative effect is currently ongoing (Chang et al. 1999; Legraverend et al. 1999; Legraverend et al. 2000; Davis et al. 2001; Dreyer et al. 2001; Davies et al. 2002; Gibson et al. 2002). Our own studies show that cytokinin analogues with anti‐CDK activity have marked anti‐proliferative effects on leukaemic cell lines, primary myeloid cells and on primary lymphocytes, that this anti‐proliferative effect is correlated with anti‐CDK activity and that it is due, at least in part, to induction of apoptosis (Vermeulen et al. 2002a). Apoptosis seems to be induced through the mitochondrial pathway (Vermeulen et al. 2002b). Another newly synthesized compound, olomoucine II, is a potent and specific CDK1 inhibitor with 10 times higher inhibitory activity than roscovitine and with a cytotoxic activity that exceeds that of purvalanol A (Krystof et al. 2002). NU2058 and NU6027, respectively, a guanine and pyrimidine analogue, show potent CDK1 and CDK2 inhibition, and both have a growth inhibitory pattern distinct from flavopiridol and olomoucine (Arris et al. 2000; Davies et al. 2002; Gibson et al. 2002). Also, pyridopyrimidines have been identified as CDK inhibitors and the study of the structure–activity relationship of more than 60 analogues showed clear trends that might be exploited in the design of more potent inhibitors (Barvian et al. 2000).
Butyrolactone
Butyrolactone, isolated from Aspergillus strain F‐25799, was found to be a CDK inhibitor in a screen of micro‐organism culture media. Butyrolactone inhibits CDK1 and CDK2 and shows good specificity against other kinases, including CDK4 (Kitagawa et al. 1993). Butyrolactone inhibits phosphorylation of pRb and of histone H1, inhibits G1/S and G2/M transition and DNA synthesis in human fibroblasts and inhibits proliferation of lung, colon and pancreatic cancer cell lines (Kitagawa et al. 1994; Nishio et al. 1996; Wada et al. 1998; Yamamoto et al. 1998).
Flavonoids
Flavonoids, like quercitin and genistein, are known to exhibit biological activity through inhibition of various kinases, of which protein kinase C (PKC) is the most prominent example. However, flavopiridol, another flavonoid and a synthetic analogue of a natural alkaloid extracted from an Indian plant, Dysoxylum binectariferum, specifically inhibits CDK1 and CDK2 (Maik et al. 1988; Losiewicz et al. 1994). In contrast to olomoucine, roscovitine and butyrolactone, it is also active against CDK4 (Carlson et al. 1996). Flavopiridol was first discovered as a potent growth inhibitor of several breast and lung cancer cell lines (Kaur et al. 1992). Different studies have shown that flavopiridol has the potential to inhibit the proliferation of a broad range of different types of cell lines, human tumours, leukaemias and lymphomas (Drees et al. 1997; Parker et al. 1998). Several phase I and phase II clinical trials with different regimens have been completed. Activity has been shown in some patients with non‐Hodgkin's lymphoma and in renal, prostate, colon and gastric carcinoma (reviewed by Senderowicz & Sausville 2000; Senderowicz 2001). However, many questions remain: What is the best treatment schedule? Is there a ‘best’ combination with standard chemotherapeutic agents (Zhai et al. 2002)?
Recently, flavopiridol analogues thioflavopiridol and oxoflavopiridol were shown to have selective anti‐CDK activity and to inhibit the colony forming ability of multiple human tumour cell lines (Kim et al. 2000).
Paullones
Paullones were discovered following analysis of the in vitro anti‐proliferative profile in the National Cancer Institute (NCI) anti‐cancer drug screen panel, that was performed in order to detect compounds that show a similar pattern of activity as flavopiridol. Kenpaullone was identified as an inhibitor of CDK1‐cyclin B, CDK2‐cyclin A, CDK2‐cyclin E and CDK5‐p35; it arrests breast epithelial cells at the G1/S boundary (Zaharevitz et al. 1999). Derivatives of the lead compound kenpaullone were synthesized and alsterpaullone was developed. This compound showed a high CDK1‐cyclin B inhibitory activity and exceeded the in vitro anti‐tumour potency of the other paullones by one order of magnitude (Schultz et al. 1999).
Indolinones
Indirubin was isolated from a Chinese herbal mixture, that was used to treat chronic myeloid leukaemia (CML). Recenly, indirubin and its analogues 5‐chloro‐indirubin, indirubin‐3′‐monoxime and indirubin‐5‐sulphonic acid were identified as potent and selective CDK inhibitors. They show inhibitory activity against CDK1, CDK2, CDK4 and CDK5 (Hoessel et al. 1999). Indirubin‐3′‐monoxime has activity in several tumour models and blocks the cell cycle at G1/S and G2/M (Marko et al. 2001). SU9516, a novel 3‐substituted indolinone inhibits the activity of CDK1, CDK2 and CDK4 and induces apoptosis in colon carcinoma cells (Lane et al. 2001). Oxindole (2‐indolinone)‐based inhibitors with effect on CDK have been developed and are described by (Andreani et al. 2001; Bramson et al. 2001; Davis et al. 2001).
Other (non‐specific) CDK inhibitors
Staurosporine is a microbial alkaloid, isolated from Streptomyces sp. cultures. Staurosporine is a general non‐specific inhibitor of PKC and of CDK1 and it induces G2 cell cycle arrest of normal and transformed cells (Tamaoki 1991; Gadbois et al. 1992). UCN‐01 or 7‐hydroxystaurosporine, a staurosporine analogue that is also a non‐specific CDK inhibitor, shows an anti‐proliferative effect on human tumour cell lines and has now entered clinical trials (Takahashi et al. 1989; Wang et al. 1995; Kawakami et al. 1996). It is not yet clear whether the effect is the result of inhibiton of CDK or of other kinases (reviewed by Senderowicz 2001).
Other CDK inhibitors include suramin, hymenialdisine, 9‐hydroxyellipticine, toyocamycin, quinazolines and aminothiazoles (Bojanowski et al. 1994; Ohashi et al. 1995; Park et al. 1996; Meijer et al. 2000; Shewchuk et al. 2000; Sielecki et al. 2001; Kim et al. 2002). Suramin is a naturally occurring glycosaminoglycan currently used as an anti‐helminthic, anti‐protozoal and anti‐tumour agent (reviewed by Larsen 1993). Suramin inhibits various enzymes including CDK1 (Bojanowski et al. 1994). Hymenialdisine is a compound isolated from a marine sponge and is a very potent inhibitor of CDK1, CDK2 and CDK5, of glycogen synthase kinase‐3β (GSK‐3β) and of casein kinase 1 (CK1) (Meijer et al. 2000). The aminothiazole 2‐acetamido‐thiozolylthio acetic ester 1 showed CDK2 inhibition but was inactive in cells. Synthesis and structure‐activity relationship studies of more than 100 analogues revealed many analogues with CDK2 inhibitory activity and with potent and broad spectrum anti‐proliferative activity across a panel of tumour cell lines in vitro (Kim et al. 2002).
REFERENCES
- Abraham RT, Acquarone M, Andersen A, Asensi A, Belle R, Berger F, Bergounioux C, Brunn G, Buquet FC, Fagot D, Glab N, Goudeau H, Goudeau M, Guerrier P, Houghton P, Hendriks H, Kloareg B, Lippai M, Marie D, Maro B, Meijer L, Mester J, Mulner‐Lorillon O, Poulet S, Schierenberg E, Schutte B, Vaulot D, Verlhac M (1995) Cellular effects of olomoucine, an inhibitor of cyclin‐dependent kinases. Biol. Cell 83, 105. [DOI] [PubMed] [Google Scholar]
- Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR (1998) The p53 network. J. Biol. Chem. 273, 1. [DOI] [PubMed] [Google Scholar]
- Amon A (1999) The spindle checkpoint. Curr. Opin. Genet. Dev. 9, 69. [DOI] [PubMed] [Google Scholar]
- Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Giorgi G, Salvini L, Garaliene V (2001) Synthesis and antitumor activity of substituted 3‐(5‐imidazo[2,1‐b]thiazolylmethylene)‐2‐indolinones. Anticancer Drug Des. 16, 167. [PubMed] [Google Scholar]
- Arellano M, Moreno S (1997) Regulation of CDK/cyclin complexes during the cell cycle. Int. J. Biochem. Cell Biol. 29, 559. [DOI] [PubMed] [Google Scholar]
- Arris CE, Boyle FT, Calvert AH, Curtin NJ, Endicott JA, Garman EF, Gibson AE, Golding BT, Grant S, Griffin RJ, Jewsbury P, Johnson LN, Lawrie AM, Newell DR, Noble MEM, Sausville EA, Schultz R, Yu W (2000) Identification of novel purine and pyrimidine cyclin‐dependent kinase inhibitors with distinct molecular interactions and tumor cell growth inhibition profiles. J. Med. Chem. 43, 2797. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Zhu X (1997) Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr. Opin. Cell Biol. 9, 93. [DOI] [PubMed] [Google Scholar]
- Bartek J, Bartkova J, Lukas J (1996) The retinoblastoma protein pathway and the restriction point. Curr. Opin. Cell Biol. 8, 805. [DOI] [PubMed] [Google Scholar]
- Barvian M, Boschelli DH, Cossrow J, Dobrusin E, Fattaey A, Fritsch A, Fry D, Harvey P, Keller P, Garrett M, La F, Leopold W, McNamara D, Quin M, Trumpp‐Kallmeyer S, Toogood P, Wu Z, Zhang E (2000) Pyrido[2,3‐d]pyrimidin‐7‐one inhibitors of cyclin‐dependent kinases. J. Med. Chem. 43, 4606. [DOI] [PubMed] [Google Scholar]
- Bates S, Parry D, Bonetta L, Vousden K, Dickson C, Peters G (1994) Absence of cyclin D/cdk complexes in cells lacking functional retinoblastoma protein. Oncogene 9, 1633. [PubMed] [Google Scholar]
- Blangy A, Lane HA, D’Herin P, Harper M, Kress M, Nigg EA (1995) Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin‐related motor essential for bipolar spindle formation in vivo . Cell 83, 1159. [DOI] [PubMed] [Google Scholar]
- Bojanowski K, Nishio K, Fukuda M, Larsen AK, Saijo N (1994) Effect of suramin on p34cdc2 kinase in vitro and in extracts from human H69 cells: evidence for a double mechanism of action. Biochem. Biophys. Res. Commun. 203, 1574. [DOI] [PubMed] [Google Scholar]
- Bradbury EM, Inglis RJ, Matthews HR (1974) Control of cell division by very lysine rich histone (F1) phosphorylation. Nature 247, 257. [DOI] [PubMed] [Google Scholar]
- Bramson HN, Corona J, Davis ST, Dickerson SH, Edelstein M, Frye SV, Gampe R‐TJ, Harris PA, Hassell A, Holmes WD, Hunter RN, Lackey KE, Lovejoy B, Luzzio MJ, Montana V, Rocque WJ, Rusnak D, Shewchuk L, Veal JM, Walker DH, Kuyper LF (2001) Oxindole‐based inhibitors of cyclin‐dependent kinase 2 (CDK2): design, synthesis, enzymatic activities, and X‐ray crystallographic analysis. J. Med. Chem. 44, 4339. [DOI] [PubMed] [Google Scholar]
- Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T (1998) Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 391, 597. [DOI] [PubMed] [Google Scholar]
- Brooks EE, Gray NS, Joly A, Kerwar SS, Lum R, Mackman RL, Norman TC, Rosete J, Rowe M, Schow SR, Schultz PG, Wang X, Wick MM, Shiffman D (1997) CVT‐313, a specific and potent inhibitor of CDK2 that prevents neointimal proliferation. J. Biol. Chem. 272, 29207. [DOI] [PubMed] [Google Scholar]
- Buchkovich K, Duffy LA, Harlow E (1989) The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell 58, 1097. [DOI] [PubMed] [Google Scholar]
- Bueso‐Ramos CE, Manshouri T, Haidar MA, Huh YO, Keating MJ, Albitar M (1995) Multiple patterns of MDM‐2 deregulation in human leukemias: implications in leukemogenesis and prognosis. Leuk. Lymphoma 17, 13. [DOI] [PubMed] [Google Scholar]
- Bueso‐Ramos CE, Manshouri T, Haidar MA, Yang Y, McCown P, Ordonez N, Glassman A, Sneige N, Albitar M (1996) Abnormal expression of MDM‐2 in breast carcinomas. Breast Cancer Res. Treat. 37, 179. [DOI] [PubMed] [Google Scholar]
- Burma S, Kurimasa A, Xie G, Taya Y, Araki R, Abe M, Crissman HA, Ouyang H, Li GC, Chen DJ (1999) DNA‐dependent protein kinase‐independent activation of p53 in response to DNA damage. J. Biol. Chem. 274, 17139. [DOI] [PubMed] [Google Scholar]
- Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ (1996) Flavopiridol induces G1 arrest with inhibition of cyclin‐dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 56, 2973. [PubMed] [Google Scholar]
- Carnero A, Hannon GJ (1998) The INK4 family of CDK inhibitors. Curr. Top. Microbiol. Immunol. 227, 43. [DOI] [PubMed] [Google Scholar]
- Chang YT, Gray NS, Rosania GR, Sutherlin DP, Kwon S, Norman TC, Sarohia R, Leost M, Meijer L, Schultz PG (1999) Synthesis and application of functionally diverse 2,6,9‐trisubstituted purine libraries as CDK inhibitors. Chem. Biol. 6, 361. [DOI] [PubMed] [Google Scholar]
- Clough J (2002) CDK inhibitor shows promise for inflammatory kidney disease. Drug Discovery Today 7, 789. [DOI] [PubMed] [Google Scholar]
- Courvalin JC, Segil N, Blobel G, Worman HJ (1992) The lamin B receptor of the inner nuclear membrane undergoes mitosis‐specific phosphorylation and is a substrate for p34cdc2‐type protein kinase. J. Biol. Chem. 267, 19035. [PubMed] [Google Scholar]
- Crook T, Vousden KH (1994) Interaction of HPV E6 with p53 and associated proteins. Biochem. Soc. Trans. 22, 52. [DOI] [PubMed] [Google Scholar]
- Davis ST, Benson BG, Bramson HN, Chapman DE, Dickerson SH, Dold KM, Eberwein DJ, Edelstein M, Frye SV, Gampe RT, Griffin Jr RJ, Harris PA, Hassell AM, Holmes WD, Hunter RN, Knick VB, Lackey K, Lovejoy B, Luzzio MJ, Murray D, Parker P, Rocque WJ, Shewchuk L, Veal JM, Walker DH, Kuyper LF (2001) Prevention of chemotherapy‐induced alopecia in rats by CDK inhibitors. Science 291, 134. [DOI] [PubMed] [Google Scholar]
- Davies TG, Bentley J, Arris CE, Boyle FT, Curtin NJ, Endicott JA, Gibson AE, Golding BT, Griffin RJ, Hardcastle IR, Jewsbury P, Johnson LN, Mesguiche V, Newell DR, Noble MEM, Tucker JA, Wang L, Whitfield HJ (2002) Structure‐based design of a potent purine‐based cyclin‐dependent kinase inhibitor. Nat. Struct. Biol. 9, 745. [DOI] [PubMed] [Google Scholar]
- De Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH (1997) Inhibition of cyclin‐dependent kinases by purine analogues: crystal structure of human CDK2 complexed with roscovitine. Eur. J. Biochem. 243, 518. [DOI] [PubMed] [Google Scholar]
- El Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817. [DOI] [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P (1995) Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675. [DOI] [PubMed] [Google Scholar]
- Dobashi Y, Shoji M, Jiang SX, Kobayashi M, Kawakubo Y, Kameya T (1998) Active cyclin A‐CDK2 complex, a possible critical factor for cell proliferation in human primary lung carcinomas. Am. J. Pathol. 153, 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees M, Dengler WA, Roth T, Labonte H, Mayo J, Malspeis L, Grever M, Sausville EA, Fiebig HH (1997) Flavopiridol (L86–8275): selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clin. Cancer Res. 3, 273. [PubMed] [Google Scholar]
- Dreyer MK, Borcherding DR, Dumont JA, Peet NP, Tsay JT, Wright PS, Bitonti AJ, Shen J, Kim SH (2001) Crystal structure of human cyclin‐dependent kinase 2 in complex with the adenine‐derived inhibitor H717. J. Med. Chem. 44, 524. [DOI] [PubMed] [Google Scholar]
- Durocher D, Jackson SP (2001) DNA‐PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr. Opin. Cell Biol. 13, 225. [DOI] [PubMed] [Google Scholar]
- Easton J, Wei T, Lahti JM, Kidd VJ (1998) Disruption of the cyclin D/cyclin‐dependent kinase/INK4/retinoblastoma protein regulatory pathway in human neuroblastoma. Cancer Res. 58, 2624–2632. [PubMed] [Google Scholar]
- Esposito V, Baldi A, De Luca A, Groger AM, Loda M, Giordano GG, Caputi M, Baldi F, Pagano M, Giordano A (1997) Prognostic role of the cyclin‐dependent kinase inhibitor p27 in non‐small cell lung cancer. Cancer Res. 57, 3381. [PubMed] [Google Scholar]
- Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T (1983) Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33, 389. [DOI] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschner MW (1998) The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase‐promoting complex to control anaphase initiation. Genes Dev. 12, 1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RP, Morgan DO (1994) A novel cyclin associates with MO15/CDK7 to form the CDK‐activating kinase. Cell 78, 713. [DOI] [PubMed] [Google Scholar]
- Gadbois DM, Hamaguchi JR, Swank RA, Bradbury EM (1992) Staurosporine is a potent inhibitor of p34cdc2 and p34cdc2‐like kinases. Biochem. Biophys. Res. Commun. 184, 80. [DOI] [PubMed] [Google Scholar]
- Gage JR, Meyers C, Wettstein FO (1990) The E7 proteins of the nononcogenic human papillomavirus type 6b (HPV‐6b) and of the oncogenic HPV‐16 differ in retinoblastoma protein binding and other properties. J. Virol. 64, 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaktionov K, Jessus C, Beach D (1995) Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev. 9, 1046. [DOI] [PubMed] [Google Scholar]
- Galaktionov K, Chen X, Beach D (1996) Cdc25 cell‐cycle phosphatase as a target of c‐myc. Nature 382, 511. [DOI] [PubMed] [Google Scholar]
- Gibson AE, Arris CE, Bentley J, Boyle FT, Curtin NJ, Davies TG, Endicott JA, Golding BT, Grant S, Griffin RJ, Jewsbury P, Johnson LN, Mesguiche V, Newell DR, Noble MEM, Tucker JA, Whitfield HJ (2002) Probing the ATP ribose‐binding domain of cyclin‐dependent kinases 1 and 2 with O(6)‐substituted guanine derivatives. J. Med. Chem. 45, 3381. [DOI] [PubMed] [Google Scholar]
- Girard F, Strausfeld U, Fernandez A, Lamb NJ (1991) Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell 67, 1169. [DOI] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW (1991) Cyclin is degraded by the ubiquitin pathway [see comments]. Nature 349, 132. [DOI] [PubMed] [Google Scholar]
- Gottlieb TM, Oren M (1998) p53 and apoptosis. Semin. Cancer Biol. 8, 359. [DOI] [PubMed] [Google Scholar]
- Gray NS, Wodicka L, Thunnissen AM, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, Leclerc S, Meijer L, Kim SH, Lockhart DJ, Schultz PG (1998) Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281, 533. [DOI] [PubMed] [Google Scholar]
- Greenblatt MS, Bennett WP, Hollstein M, Harris CC (1994) Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 54, 4855. [PubMed] [Google Scholar]
- Gronbaek K, Nedergaard T, Andersen MK, Thor SP, Guldberg P, Moller P, Zeuthen J, Ebbe HN, Hou JK, Ralfkiaer E (1998) Concurrent disruption of cell cycle associated genes in mantle cell lymphoma: a genotypic and phenotypic study of cyclin D1, 16, 15, 53 and pRb. Leukemia 12, 1266. [DOI] [PubMed] [Google Scholar]
- Hajduch M, Kolar Z, Novotny R, Hanus J, Mihal V, Hlobilkova A, Noskova V, Strnad M (1997) Induction of apoptosis and regression of spontaneous dog melanoma following in vivo application of synthetic cyclin‐dependent kinase inhibitor olomoucine. Anticancer Drugs 8, 1007. [DOI] [PubMed] [Google Scholar]
- Hall M, Peters G (1996) Genetic alterations of cyclins, cyclin‐dependent kinases, and CDK inhibitors in human cancer. Adv. Cancer Res. 68, 67. [DOI] [PubMed] [Google Scholar]
- Hannon GJ, Beach D (1994) p15INK4B is a potential effector of TGF‐β‐induced cell cycle arrest. Nature 371, 257. [DOI] [PubMed] [Google Scholar]
- Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell CL, Swindell E, et al. (1995) Inhibition of cyclin‐dependent kinases by p21. Mol. Biol. Cell 6, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Elledge SJ (1996) Cdk inhibitors in development and cancer. Curr. Opin. Genet. Dev. 6, 56. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA (1989) Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629. [DOI] [PubMed] [Google Scholar]
- Heald R, McKeon F (1990) Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 61, 579. [DOI] [PubMed] [Google Scholar]
- Heald R, McLoughlin M, McKeon F (1993) Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell 74, 463. [DOI] [PubMed] [Google Scholar]
- Hengst L, Reed SI (1998) Inhibitors of the Cip/Kip family. Curr. Top. Microbiol. Immunol. 227, 25. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B (1997) 14–3−3 sigma is a p53‐regulated inhibitor of G2/M progression. Mol. Cell 1, 3. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel SJ, Van Laar T, Kast WM, Melief CJ, Zantema A, Van Der Eb AJ (1990) Association between the cellular p53 and the adenovirus 5 E1B‐55kd proteins reduces the oncogenicity of Ad‐transformed cells. EMBO J. 9, 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA (1992) Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell 70, 993. [DOI] [PubMed] [Google Scholar]
- Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, Niederberger E, Tang W, Eisenbrand G, Meijer L (1999) Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin‐dependent kinases. Nat. Cell Biol. 1, 60. [DOI] [PubMed] [Google Scholar]
- Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G (1993) Phosphorylation and activation of human cdc25‐C by cdc2 – cyclin B and its involvement in the self‐amplification of MPF at mitosis. EMBO J. 12, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsthemke B (1992) Genetics and cytogenetics of retinoblastoma. Cancer Genet. Cytogenet. 63, 1. [DOI] [PubMed] [Google Scholar]
- Hu QJ, Dyson N, Harlow E (1990) The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 9, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Pines J (1994) Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell 79, 573. [DOI] [PubMed] [Google Scholar]
- Iida H, Towatari M, Tanimoto M, Morishita Y, Kodera Y, Saito H (1997) Overexpression of cyclin E in acute myelogenous leukemia. Blood 90, 3707. [PubMed] [Google Scholar]
- Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich NP (1995) Mechanism of CDK activation revealed by the structure of a cyclinA‐CDK2 complex. Nature 376, 313. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang YJ, Kahn SM, Hollstein MC, Santella RM, Lu SH, Harris CC, Montesano R, Weinstein IB (1993) Altered expression of the cyclin D1 and retinoblastoma genes in human esophageal cancer. Proc. Natl Acad. Sci. USA 90, 9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamb A (1998) Cyclin‐dependent kinase inhibitors and human cancer. Curr. Top. Microbiol. Immunol. 227, 139. [DOI] [PubMed] [Google Scholar]
- Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ (1993) Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin d‐dependent kinase CDK4. Genes Dev. 7, 331. [DOI] [PubMed] [Google Scholar]
- Kaur G, Stetler SM, Sebers S, Worland P, Sedlacek H, Myers C, Czech J, Naik R, Sausville E (1992) Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86–8275. J. Natl Cancer Inst. 84, 1736. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Futami H, Takahara J, Yamaguchi K (1996) UCN‐1,7‐hydroxyl‐staurosporine, inhibits kinase activity of cyclin‐dependent kinases and reduces the phosphorylation of the retinoblastoma susceptibility gene product in A549 human lung cancer cell line. Biochem. Biophys. Res. Commun. 219, 778. [DOI] [PubMed] [Google Scholar]
- Keyomarsi K, Conte D, Toyofuku W, Fox MP (1995) Deregulation of cyclin E in breast cancer. Oncogene 11, 941. [PubMed] [Google Scholar]
- Kim JH, Kang MJ, Park CU, Kwak HJ, Hwang Y, Koh GY (1999) Amplified CDK2 and cdc2 activities in primary colorectal carcinoma. Cancer 85, 546. [PubMed] [Google Scholar]
- Kim KS, Sack JS, Tokarski JS, Qian L, Chao ST, Leith L, Kelly YF, Misra RN, Hunt JT, Kimball SD, Humphreys WG, Wautlet BS, Mulheron JG, Webster KR (2000) Thio‐ and oxoflavopiridols, cyclin‐dependent kinase 1‐selective inhibitors: synthesis and biological effects. J. Med. Chem. 43, 4126. [DOI] [PubMed] [Google Scholar]
- Kim KS, Kimball SD, Misra RN, Rawlins DB, Hunt JT, Xiao HY, Lu S, Qian L, Han WC, Shan W, Mitt T, Cai ZW, Poss MA, Zhu H, Sack JS, Tokarski JS, Chang CY, Pavletich N, Kamath A, Humphreys WG, Marathe P, Bursuker I, Kellar KA, Roongta U, Batorsky R., Mulheron JG, Bol D, Fairchild CR, Lee FY, Webster KR (2002) Discovery of aminothiazole inhibitors of cyclin‐dependent kinase 2: synthesis, X‐ray crystallographic analysis, and biological activities. J. Med. Chem. 45, 3905. [DOI] [PubMed] [Google Scholar]
- King RW, Jackson PK, Kirschner MW (1994) Mitosis in transition. Cell 79, 563. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters JM, Kirschner MW (1996) How proteolysis drives the cell cycle. Science 274, 1652. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Okabe T, Ogino H, Matsumoto H, Suzuki TI, Kokubo T, Higashi H, Saitoh S, Taya Y, Yasuda H et al. (1993) Butyrolactone I, a selective inhibitor of cdk2 and cdc2 kinase. Oncogene 8, 2425–2432. [PubMed] [Google Scholar]
- Kitagawa M, Higashi H, Takahashi IS, Okabe T, Ogino H, Taya Y, Hishimura S, Okuyama A (1994) A cyclin‐dependent kinase inhibitor, butyrolactone I, inhibits phosphorylation of RB protein and cell cycle progression. Oncogene 9, 2549. [PubMed] [Google Scholar]
- Knudson AGJ (1971) Mutation and cancer: statistical study of retinoblastoma. Proc. Natl Acad. Sci. USA 68, 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko LJ, Prives C (1996) p53: puzzle and paradigm. Genes Dev. 10, 1054. [DOI] [PubMed] [Google Scholar]
- Krystof V, Lenobel R, Havlicek L, Kuzma M, Strnad M (2002) Synthesis and biological activity of olomoucine II. Bioorg. Med. Chem. Lett. 12, 3283. [DOI] [PubMed] [Google Scholar]
- Lane MEYuB, Rice A, Lipson KE, Liang C, Sun L, Tang C, McMahon G, Pestell RG, Wadler S (2001) A novel cdk2‐selective inhibitor, SU9516, induces apoptosis in colon carcinoma cells. Cancer Res. 61, 6170. [PubMed] [Google Scholar]
- Lapointe J, Lachance Y, Labrie Y, Labrie C (1996) A p18 mutant defective in CDK6 binding in human breast cancer cells. Cancer Res. 56, 4586–4589. [PubMed] [Google Scholar]
- Larsen AK (1993) Suramin: an anticancer drug with unique biological effects. Cancer Chemother. Pharmacol. 32, 96. [DOI] [PubMed] [Google Scholar]
- Leach FS, Elledge SJ, Sherr CJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B (1993) Amplification of cyclin genes in colorectal carcinomas. Cancer Res. 53, 1986. [PubMed] [Google Scholar]
- Lee MH, Reynisdottir I, Massague J (1995) Cloning of p57KIP2, a cyclin‐dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 9, 639. [DOI] [PubMed] [Google Scholar]
- Legraverend M, Ludwig O, Bisagni E, Leclerc S, Meijer L, Giocanti N, Sadri R, Favaudon V (1999) Synthesis and in vitro evaluation of novel 2,6,9‐trisubstituted purines acting as cyclin‐dependent kinase inhibitors. Bioorg. Med. Chem. 7, 1281. [DOI] [PubMed] [Google Scholar]
- Legraverend M, Tunnah P, Noble M, Ducrot P, Ludwig O, Grierson DS, Leost M, Meijer L, Endicott J (2000) Cyclin‐dependent kinase inhibition by new C‐2 alkynylated purine derivatives and molecular structure of a CDK2‐inhibitor complex. J. Med. Chem. 43, 1282. [DOI] [PubMed] [Google Scholar]
- Levine AJ (1997) p53, the cellular gatekeeper for growth and division. Cell 88, 323. [DOI] [PubMed] [Google Scholar]
- Lew DJ, Kornbluth S (1996) Regulatory roles of cyclin‐dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol. 8, 795. [DOI] [PubMed] [Google Scholar]
- Lim DS, Kim ST, Xu B, Maser RS, Lin J, Petrini JH, Kastan MB (2000) ATM phosphorylates p95/nbs1 in an S‐phase checkpoint pathway. Nature 404, 613. [DOI] [PubMed] [Google Scholar]
- Liu F, Stanton JJ, Wu Z, Piwnica WH (1997) The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol. Cell Biol. 17, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M (1997) Increased proteasome‐dependent degradation of the cyclin‐dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 3, 231. [DOI] [PubMed] [Google Scholar]
- Losiewicz MD, Carlson BA, Kaur G, Sausville EA, Worland PJ (1994) Potent inhibition of CDC2 kinase activity by the flavonoid L86–8275. Biochem. Biophys. Res. Commun. 201, 589. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, Housman DE, Jacks T (1994) p53 status and the efficacy of cancer therapy in vivo . Science 266, 807. [DOI] [PubMed] [Google Scholar]
- Maik RG, Kattice SL, Bhat SV (1988) An anti‐inflammatory cum immodulatory piperidinylbenzopyranone from Dysoxylum binectariferum: isolation, structure, and total synthesis. Tetrahedron 44, 2081. [Google Scholar]
- Marko D, Schatzle S, Friedel A, Genzlinger A, Zankl H, Meijer L, Eisenbrand G (2001) Inhibition of cyclin‐dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br. J. Cancer 84, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald ER III, El Deiry WS (2000) Cell cycle control as a basis for cancer drug development. Int. J. Oncol. 16, 871. [PubMed] [Google Scholar]
- Meijer L, Pondaven P (1988) Cyclic activation of histone H1 kinase during sea urchin egg mitotic divisions. Exp. Cell Res. 174, 116–129. [DOI] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP (1997) Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin‐dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243, 527. [DOI] [PubMed] [Google Scholar]
- Meijer L, Thunnissen AM, White AW, Garnier M, Nikolic M, Tsai LH, Walter J, Cleverley KE, Salinas PC, Wu YZ, Biernat J, Mandelkow EM, Kim SH, Pettit GR (2000) Inhibition of cyclin‐dependent kinases, GSK‐3beta and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 7, 51. [DOI] [PubMed] [Google Scholar]
- Miller C, Koeffler HP (1993) p53 mutations in human cancer. Leukemia 7, S18. [PubMed] [Google Scholar]
- Moller MB, Ino Y, Gerdes A, Skjodt K, Louis D, Pedersen N (1999) Aberration of the p53 pathway components p53, MDM2 and CDKN2A appear independent in diffuse large B cell lymphoma. Leukemia 13, 453. [DOI] [PubMed] [Google Scholar]
- Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, Pagano M (1999) Ubiquitination of p27 is regulated by CDK‐dependent phosphorylation and trimeric complex formation. Genes Dev. 13, 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO (1995) Principles of CDK regulation. Nature 374, 131. [DOI] [PubMed] [Google Scholar]
- Motokura T, Bloom T, Kim HG, Juppner H, Ruderman JV, Kronenberg HM, Arnold A (1991) A novel cyclin encoded by a bcl1‐linked candidate oncogene. Nature 350, 512. [DOI] [PubMed] [Google Scholar]
- Nataraj AJ, Trent JC, Ananthaswamy HN (1995) p53 gene mutations and photocarcinogenesis. Photochem. Photobiol. 62, 218. [DOI] [PubMed] [Google Scholar]
- Neant I, Guerrier P (1988) 6‐Dimethylaminopurine blocks starfish oocyte maturation by inhibiting a relevant protein kinase activity. Exp. Cell Res. 176, 68. [DOI] [PubMed] [Google Scholar]
- Nilsson I, Hoffmann I (2000) Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res. 4, 107. [DOI] [PubMed] [Google Scholar]
- Nishio K, Ishida T, Arioka H, Kurokawa H, Fukuoka K, Nomoto T, Fukumoto H, Yokote H, Saijo N (1996) Antitumor effects of butyrolactone I, a selective cdc2 kinase inhibitor, on human lung cancer cell lines. Anticancer Res. 16, 3387. [PubMed] [Google Scholar]
- Norbury C, Nurse P (1992) Animal cell cycles and their control. Annu. Rev. Biochem. 61, 441. [DOI] [PubMed] [Google Scholar]
- Ohashi M, Sugikawa E, Nakanishi N (1995) Inhibition of p53 protein phosphorylation by 9‐hydroxyellipticine: a possible anticancer mechanism. Jpn. J. Cancer Res. 86, 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M (1995) Human cyclin E, a nuclear protein essential for the G1‐to‐S phase transition. Mol. Cell Biol. 15, 2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Beach D (1994) Cyclin G is a transcriptional target of the p53 tumor suppressor protein. EMBO J. 13, 4816–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto A, Demetrick DJ, Spillare EA, Hagiwara K, Hussain SP, Bennett WP, Forrester K, Gerwin B, Serrano M, Beach DH (1994) Mutations and altered expression of p16INK4 in human cancer. Proc. Natl Acad. Sci. USA 91, 11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongkeko W, Ferguson DJ, Harris AL, Norbury C (1995) Inactivation of Cdc2 increases the level of apoptosis induced by DNA damage. J. Cell Sci. 108, 2897. [DOI] [PubMed] [Google Scholar]
- Oren M (1999) Regulation of the p53 tumor suppressor protein. J. Biol. Chem. 274, 36031. [DOI] [PubMed] [Google Scholar]
- Owen‐Schaub LB, Zhang W, Cusack JC, Angelo LS, Santee SM, Fujiwara T, Roth JA, Deisseroth AB, Zhang WW, Kruzel EA (1995) Wild‐type human p53 and a temperature‐sensitive mutant induce Fas/APO‐1 expression. Mol. Cell Biol. 15, 3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M (1997) Cell cycle regulation by the ubiquitin pathway. FASEB J. 11, 1067. [DOI] [PubMed] [Google Scholar]
- Painter RB (1986) Inhibition of mammalian cell DNA synthesis by ionizing radiation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 49, 771. [DOI] [PubMed] [Google Scholar]
- Pan ZQ, Reardon JT, Li L, Flores RH, Legerski R, Sancar A, Hurwitz J (1995) Inhibition of nucleotide excision repair by the cyclin‐dependent kinase inhibitor p21. J. Biol. Chem. 270, 22008. [DOI] [PubMed] [Google Scholar]
- Pardee AB (1974) A restriction point for control of normal animal cell proliferation. Proc. Natl Acad. Sci. USA 71, 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Cheon JY, Lee YH, Park JS, Lee KY, Lee CH, Lee SK (1996) A specific inhibitor of cyclin‐dependent protein kinases, CDC2 and CDK2. Mol. Cell 6, 679. [Google Scholar]
- Parker BW, Kaur G, Nieves NW, Taimi M, Kohlhagen G, Shimizu T, Losiewicz MD, Pommier Y, Sausville EA, Senderowicz AM (1998) Early induction of apoptosis in hematopoietic cell lines after exposure to flavopiridol. Blood 91, 458. [PubMed] [Google Scholar]
- Parry D, Bates S, Mann DJ, Peters G (1995) Lack of cyclin D‐Cdk complexes in Rb‐negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. 14, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovich AG, Hartwell LH (1995) A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell 82, 841. [DOI] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica WH (1997) Mitotic and G2 checkpoint control: regulation of 14–3−3 protein binding by phosphorylation of Cdc25C on serine‐216 [see comments]. Science 277, 1501. [DOI] [PubMed] [Google Scholar]
- Peng J, Marshall NF, Price DH (1998) Identification of a cyclin subunit required for the function of Drosophila P‐TEFb. J. Biol. Chem. 273, 13855. [DOI] [PubMed] [Google Scholar]
- Pines J (1991) Cyclins: wheels within wheels. Cell Growth Differ. 2, 305. [PubMed] [Google Scholar]
- Pines J (1995) Cyclins and cyclin‐dependent kinases: theme and variations. Adv. Cancer Res. 66, 181. [DOI] [PubMed] [Google Scholar]
- Polyak K, Lee MH, Erdjument BH, Koff A, Roberts JM, Tempst P, Massague J (1994) Cloning of p27Kip1, a cyclin‐dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78, 59. [DOI] [PubMed] [Google Scholar]
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B (1997) A model for p53‐induced apoptosis. Nature 389, 300. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M, Rogers SW (1996) PEST sequences and regulation by proteolysis. Trends Biochem. Sci. 21, 267. [PubMed] [Google Scholar]
- Reynisdottir I, Polyak K, Iavarone Massague J (1995) Kip/cip and INK4 CDK inhibitors co‐operate to induce cell cycle arrest in respons to TGF‐β. Genes Dev. 9, 1831. [DOI] [PubMed] [Google Scholar]
- Rialet V, Meijer L (1991) A new screening test for antimitotic compounds using the universal M phase‐specific protein kinase, p34cdc2/cyclin Bcdc13, affinity‐immobilized on p13suc1‐coated microtitration plates. Anticancer Res. 11, 1581. [PubMed] [Google Scholar]
- Rickert P, Seghezzi W, Shanahan F, Cho H, Lees E (1996) Cyclin C/CDK8 is a novel CTD kinase associated with RNA polymerase II. Oncogene 12, 2631. [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica WH, Elledge SJ (1997) Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497. [DOI] [PubMed] [Google Scholar]
- Schultz C, Link A, Leost M, Zaharevitz DW, Gussio R, Sausville EA, Meijer L, Kunick C (1999) Paullones, a series of cyclin‐dependent kinase inhibitors: synthesis, evaluation of CDK1/cyclin B inhibition, and in vitro antitumor activity. J. Med. Chem. 42, 2909. [DOI] [PubMed] [Google Scholar]
- Schulze‐Gahmen U, Brandsen J, Jones HD, Morgan DO, Meijer L, Vesely J, Kim SH (1995) Multiple modes of ligand recognition: crystal structures of cyclin‐dependent protein kinase 2 in complex with ATP and two inhibitors, olomoucine and isopentenyladenine. Proteins 22, 378. [DOI] [PubMed] [Google Scholar]
- Schutte B, Nieland L, Van Engeland M, Henfling ME, Meijer L, Ramaekers FC (1997) The effect of the cyclin‐dependent kinase inhibitor olomoucine on cell cycle kinetics. Exp. Cell Res. 236, 4. [DOI] [PubMed] [Google Scholar]
- Scuderi R, Palucka KA, Pokrovskaja K, Bjorkholm M, Wiman KG, Pisa P (1996) Cyclin E overexpression in relapsed adult acute lymphoblastic leukemias of B‐cell lineage. Blood 87, 3360. [PubMed] [Google Scholar]
- Senderowicz A (2001) Development of cyclin‐dependent kinase modulators as novel therapeutic approaches for hematological malignancies. Leukemia 15, 1. [DOI] [PubMed] [Google Scholar]
- Senderowicz AM, Sausville EA (2000) Preclinical and clinical development of cyclin‐dependent kinase modulators. J. Natl Cancer Inst 92, 376. [DOI] [PubMed] [Google Scholar]
- Sherr CJ (1994) G1 phase progression: cycling on cue. Cell 79, 551. [DOI] [PubMed] [Google Scholar]
- Sherr CJ (1996) Cancer cell cycles. Science 274, 1672. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM (1995) Inhibitors of mammalian G1 cyclin‐dependent kinases. Genes Dev. 9, 1149. [DOI] [PubMed] [Google Scholar]
- Shewchuk L, Hassell A, Wisely B, Rocque W, Holmes W, Veal J, Kuyper LF (2000) Binding mode of the 4‐anilinoquinazoline class of protein kinase inhibitor: X‐ray crystallographic studies of 4‐anilinoquinazolines bound to cyclin‐dependent kinase 2 and p38 kinase. J. Med. Chem. 43, 133. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zou M, Farid NR, Al Sedairy ST (1996) Evidence of gene deletion of p21 (WAF1/CIP1), a cyclin‐dependent protein kinase inhibitor, in thyroid carcinomas. Br. J. Cancer 74, 1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sielecki TM, Johnson TL, Liu J, Muckelbauer JK, Grafstrom RH, Cox S, Boylan J, Burton CR, Chen H, Smallwood A, Chang CH, Boisclair M, Benfield PA, Trainor GL, Seitz SP (2001) Quinazolines as cyclin dependent kinase inhibitors. Bioorg. Med. Chem. Lett. 11, 1157. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Canman CE, Taya Y, Sakaguchi K, Appella E, Kastan MB (1997) DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 11, 3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I, Saitoh Y, Yoshida M, Sano H, Nakano H, Morimoto M, Tamaoki T (1989) UCN‐01 and UCN‐02, new selective inhibitors of protein kinase C. II. Purification, physico‐chemical properties, structural determination and biological activities. J. Antibiot. Tokyo 42, 571. [DOI] [PubMed] [Google Scholar]
- Tamaoki T (1991) Use and specificity of staurosporine, UCN‐01, and calphostin C as protein kinase inhibitors. Meth. Enzymol. 201, 340. [DOI] [PubMed] [Google Scholar]
- Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi GC, Lavin P, Draetta G, Pagano M, Loda M (1997) The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res. 57, 1259. [PubMed] [Google Scholar]
- Taylor WR, Stark GR (2001) Regulation of the G2/M transition by p53. Oncogene 20, 1803. [DOI] [PubMed] [Google Scholar]
- Tsai T, Davalath S, Rankin C, Radich JP, Head D, Appelbaum FR, Boldt DH (1996) Tumor suppressor gene alteration in adult acute lymphoblastic leukemia (ALL). Analysis of retinoblastoma (Rb) and p53 gene expression in lymphoblasts of patients with de novo, relapsed, or refractory ALL treated in Southwest Oncology Group studies. Leukemia 10, 1901. [PubMed] [Google Scholar]
- Vermeulen K, Strnad M, Krystof V, Havlicek L, Van der Aa A, Lenjou M, Nijs G, Rodrigus I, Stockman B, Van Onckelen H, Van Bockstaele D, Berneman ZN (2002a) Antiproliferative effect on plant cytokinin analogues with an inhibitory activity on cyclin‐dependent kinases. Leukemia 16, 299. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Strnad M, Havlicek L, Van Onckelen H, Lenjou M, Nijs G, Van Bockstaele D, Berneman ZN (2002b) Plant cytokinin analogues with inhibitory activity on cyclin dependent kinases (CDK) exert their antiproliferative effect through induction of apoptosis initiated by the mitochondrial pathway: determination by a multiparametric flow cytometric analysis. Exp. Hematol. 30, 1107. [DOI] [PubMed] [Google Scholar]
- Vesely J, Havlicek L, Strnad M, Blow JJ, Donella DA, Pinna L, Letham DS, Kato J, Detivaud L, Leclerc S, Meijer L (1994) Inhibition of cyclin‐dependent kinases by purine analogues. Eur. J. Biochem. 224, 771. [DOI] [PubMed] [Google Scholar]
- Voitenleitner C, Fanning E, Nasheuer HP (1997) Phosphorylation of DNA polymerase alpha‐primase by cyclin A‐dependent kinases regulates initiation of DNA replication in vitro . Oncogene 14, 1611. [DOI] [PubMed] [Google Scholar]
- Wada M, Hosotani R, Lee JU, Doi R, Koshiba T, Fujimoto K, Miyamoto Y, Tsuji S, Nakajima S, Okuyama A, Imamura M (1998) An exogenous cdk inhibitor, butyrolactone‐I, induces apoptosis with increased Bax/Bcl‐2 ratio in p53‐mutated pancreatic cancer cells. Anticancer Res. 18, 2559. [PubMed] [Google Scholar]
- Waga S, Li R, Stillman B (1997) p53‐induced p21 controls DNA replication. Leukemia 11, 321. [PubMed] [Google Scholar]
- Walker DH, Maller JL (1991) Role for cyclin A in the dependence of mitosis on completion of DNA replication. Nature 354, 314. [DOI] [PubMed] [Google Scholar]
- Wang Q, Worland PJ, Clark JL, Carlson BA, Sausville EA (1995) Apoptosis in 7‐hydroxystaurosporine‐treated T lymphoblasts correlates with activation of cyclin dependent kianses 1 and 2. Cell Growth Differ. 6, 927. [PubMed] [Google Scholar]
- Weisenburger DD, Sanger WG, Armitage JO, Purtilo DT (1987) Intermediate lymphocytic lymphoma: immunophenotypic and cytogenetic findings. Blood 69, 1617. [PubMed] [Google Scholar]
- Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann HE, De Plaen E, Hankeln T, Meyer‐zum‐Buschenfelde KH, Beach D (1995) A p16INK4a‐insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 269, 1281. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Monden T, Miyoshi H, Izawa H, Ikeda K, Tsujie M, Ohnishi T, Sekimoto M, Tomita N, Monden M (1998) Cdk2/cdc2 expression in colon carcinogenesis and effects of cdk2/cdc2 inhibitor in colon cancer cells. Int. J. Oncol. 13, 233. [DOI] [PubMed] [Google Scholar]
- Yang J, Winkler K, Yoshida M, Kornbluth S (1999) Maintenance of G2 arrest in the Xenopus oocyte: a role for 14–3−3 mediated inhibition of Cdc25 nuclear import. EMBO J. 18, 2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharevitz DW, Gussio R, Leost M, Senderowicz AM, Lahusen T, Kunick C, Meijer L, Sausville EA (1999) Discovery and initial characterization of the paullones, a novel class of small‐molecule inhibitors of cyclin‐dependent kinases. Cancer Res. 59, 2566. [PubMed] [Google Scholar]
- Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica WH, Enoch T (1998) Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature 395, 507. [DOI] [PubMed] [Google Scholar]
- Zhai S, Senderowicz AM, Sausville EA, Figg WD (2002) Flavopiridol, a novel cyclin‐dependent kinase inhibitor, in clinical development. Ann. Pharmacother. 36, 905. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y, Yarbrough WG (1998) ARF promotes MDM2 degradation and stabilizes p53: ARF‐INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 92, 725. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kennedy BK, Lawrence BD, Barbie DA, Matera AG, Fletcher JA, Harlow E (2000) NPAT links cyclin E‐Cdk2 to the regulation of replication‐dependent histone gene transcription. Genes Dev. 14, 2283. [PMC free article] [PubMed] [Google Scholar]


