Abstract
Objectives: In this study, we aimed at determining whether human immature dental pulp stem cells (hIDPSC) would be able to contribute to different cell types in mouse blastocysts without damaging them. Also, we analysed whether these blastocysts would progress further into embryogenesis when implanted to the uterus of foster mice, and develop human/mouse chimaera with retention of hIDPSC derivates and their differentiation.
Materials and Methods: hIDPSC and mouse blastocysts were used in this study. Fluorescence staining of hIDPSC and injection into mouse blastocysts, was performed. Histology, immunohistochemistry, fluorescence in situ hybridization and confocal microscopy were carried out.
Results and Conclusion: hIDPSC showed biological compatibility with the mouse host environment and could survive, proliferate and contribute to the inner cell mass as well as to the trophoblast cell layer after introduction into early mouse embryos (n = 28), which achieved the hatching stage following 24 and 48 h in culture. When transferred to foster mice (n = 5), these blastocysts with hIDPSC (n = 57) yielded embryos (n = 3) and foetuses (n = 6); demonstrating presence of human cells in various organs, such as brain, liver, intestine and hearts, of the human/mouse chimaeras. We verified whether hIDPSC would also be able to differentiate into specific cell types in the mouse environment. Contribution of hIDPSC in at least two types of tissues (muscles and epithelial), was confirmed. We showed that hIDPSC survived, proliferated and differentiated in mouse developing blastocysts and were capable of producing human/mouse chimaeras.
Introduction
Embryonic stem cells represent a new prospect in the field of developmental biology by allowing greater understanding of the general mechanisms that occur during embryonic development. Pioneer experiments on human embryonic stem cell transplantation into mice include a model of cell differentiation in vivo that has made the study of both differentiation and therapeutic potential of stem cells possible (1). A characteristic feature of these cells is pluripotency, the capacity to differentiate into cells derived from any of the three germ layers. To characterize pluripotency of mouse embryonic stem cells, various methods have been used: analysis of specific marker expression, formation of embryoid bodies and formation of teratomas. More precisely, embryonic stem cell pluripotency can be evaluated by generation of chimaeras, organisms composed of cells from two or more individuals from the same or different species (2, 3, 4, reviewed in 5, 6).
Production of human/animal chimaeras is a method currently in use to analyse developmental potency of mammalian stem cells in biomedical research (7, 8, 9, 10, 11, 12, 13). For example, Yokoo et al. have demonstrated that human mesenchymal stem cells (MSC) have the ability to differentiate and contribute to the functional complex of a new kidney in rats (8). James et al. showed for the first time biological compatibility between human embryonic stem cells and cells of the mouse inner cell mass (11). Moreover, they demonstrated that a nonhuman embryo surrogate environment could be used to study developmental potential of human embryonic stem cells and their derivates. Since isolation of human embryonic stem cells requires destruction of human embryo, (which can raise a number of ethical objections), adult stem cells (ASC) are now seen as an alternative.
Recently, populations of multipotent ASC have been isolated from different fetal and adult mouse and human tissues that express embryonic stem cell markers, such as Oct‐4, Nanog and Sox2, presenting a differentiation capacity similar to that of embryonic stem cells in vitro (reviewed in 14). Also, we have reported the isolation of a human ASCs, human immature dental pulp stem cells (hIDPSC), which express the aforementioned human embryonic stem cell markers and can differentiate into several cell types in vitro, such as bone, cartilage, skeletal and smooth muscles, and neurones. We found that after their transplantation into adult mice, they engrafted within different mouse organs, such as the liver, heart, spleen, kidney and the brain (15). Although hIDPSC express human embryonic stem cell markers, they also co‐express MSC markers, present fibroblastic phenotype and we isolated them from adult tissues. Recent works have demonstrated that the transfection of somatic cells with pluripotency genes can return these cells to an embryonic‐like state (16, 17). Moreover, direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells has also been suggested (18, 19). In order to demonstrate that hIDPSC are truly multipotent ASC and could undergo further reprogramming to providing cells closer to an embryonic stem cell type, we verified whether these cells were able to generate a chimaera, a prerequisite to characterizing pluripotency similar for embryonic stem cells.
In the present study, we aimed at determining whether hIDPSC, which share some characteristics with human embryonic stem cells, would be able to contribute to different cell types in mouse blastocysts, without damaging them. Besides, we analysed whether these blastocysts, containing hIDPSC, would be able to progress further into embryogenesis, implanted into the uterus of foster mice, and develop a human/mouse chimaera with the retention of hIDPSC derivates.
Materials and methods
Cell culture
Human IDPSC (46, XY) used in the present work have previously been described and characterized (15). Cells were maintained in DMEM/F12 (Dulbecco's modified Eagle's medium/Ham's F12; Gibco, Invitrogen Corporation, Carlsbad, CA, USA) supplemented with 15% fetal bovine serum (FBS, Hyclone, Logan, UT, USA), 100 units/ml penicillin, 100 µg/ml streptomycin, 2 mm l‐glutamine, and 2 mm non‐essential amino acids (all from Invitrogen). The expression of OCT‐4 was checked before each experiment by reverse transcriptase–polymerase chain reaction (RT‐PCR) (15).
Animals
In all experiments, 5‐ to 8‐week‐old CD‐1 mice were obtained from Charles River Laboratories (Wilmington, MA, USA) and used as a source of mouse early embryos and as foster mothers. This project was approved by the Ethical Committee for Animal Experimentation of the Butantan Institute, São Paulo, Brazil (Study of plasticity of nondifferentiated cells: adults stem cells and carcinogenic cells in animal model, protocol no. 250/06, 8 August 2006).
Fluorescent dye staining of hIDPSC
Cell culture was washed twice in calcium and magnesium‐free Dulbecco phosphate solution (DPBS, Invitrogen) and was dissociated with 0.25% trypsin/EDTA solution (Invitrogen). Suspension was centrifuged and the cell pellet was resuspended in DMEM (Invitrogen) with 10% FBS (Invitrogen) containing the fluorescent dye (Vybrant CM‐Dil Cell‐Labelling Solution; Molecular Probes, Invitrogen). Cells were incubated for 15 min at 37 °C, washed twice in DMEM with 10% FBS, and injected into mouse morulae and blastocysts.
Injection of hIDPSC into mouse blastocyst
Injection of one strain of hIDPSC, which expresses OCT‐4 and NANOG (RT‐PCR analysis; data not shown) into mouse morulae and/or blastocysts, was performed using an inverted microscope (Nikon Eclipse T2000, Nikon Instruments Inc., Melville, NY, USA) with ×200 and ×400 magnification and phase contrast observation. During the injection, the morulae and/or blastocysts and the fluorescent‐stained hIDPSC were maintained in Modified HTF medium (HEPES buffered, Irvine Scientific, Santa Ana, CA, USA) supplemented with 15% substitute synthetic serum (Irvine Scientific) in Petri dishes (Falcon 1006, BD Falcon, Bedford, MA, USA) in a hot plate at 37 °C.
Six to eight hIDPSC were introduced into 8 morulae and 20 blastocysts (3.5 days post coitum; d.p.c.). They were cultured for 24/48 h in M16 (Sigma, St. Louis, MO, USA) in vitro and after they were fixed in 4% formaldehyde (Merck, Darmstadt, Germany) and analysed using confocal microscopy. Mouse embryonic fibroblasts stained with Vybrant CM‐Dil Cell‐Labelling Solution (Invitrogen) were used as a control. First, cell homing within the recipient blastocysts was analysed after being cultured for 24/48 h.
In order to analyse hIDPSC contribution in vivo, 57 blastocysts were injected with hIDPSC, with or without staining, and were immediately transferred to the uterus of five foster mothers.
Immunohistochemistry
The mouse anti‐hIDPSC antibody was produced as described previously (15). Whole mouse embryos were embedded in Tissue Freezing Medium (Leica Instruments, Nussloch, Germany) and 10‐µm sections near the body midline were prepared. Additionally, embryo organs were separated and embedded in Tissue Freezing Medium and sections of 10 µm were produced. The sections were washed twice in phosphate‐buffered saline (PBS) pH 7.4 (Gibco, Invitrogen) and fixed in 4% formaldehyde. They were washed twice in PBS and permeabilized with PBS containing 0.25% Triton X‐100 (Sigma) (PBST solution). After blocking with 1% bovine serum albumin (Sigma), sections were incubated with anti‐hIDPSC antibody diluted (1 : 1000) in 1% bovine serum albumin in PBST for 1 h at room temperature. Sections were washed three times in PBS, then FITC‐conjugated anti‐mouse secondary antibody at a 1 : 400 dilution was added; sections were then incubated in the dark for 1 h at room temperature followed by three washes in PBS. Microscope slides were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) with 4′,6‐diamine‐2‐phenylindol (DAPI).
To characterize the ability of hIDPSC to differentiate into specific tissues in the human/mouse chimaeras, we performed immunohistochemistry procedure, as described above. Human‐specific tissue antibodies were used, such as mouse anti‐human cytokeratin peptide 18 antibody (Sigma) at 1 : 100 dilution, mouse anti‐human myosin ventricular heavy chain α/β (Chemicon, Temecula, CA, USA) at 1 : 10 dilution, and mouse anti‐human nuclei (Chemicon) at 1 : 100 dilution. Secondary antibodies used were goat anti‐mouse FITC‐conjugated and rabbit anti‐mouse Cy3‐conjugated, both at 1 : 400 dilution.
Histology
To perform the histological analysis, well‐formed embryos were fixed in 4% formaldehyde, embedded in paraffin wax, and 5‐ to 10‐µm sections were cut; de‐paraffination using xylene and alcohols was performed. Sections were stained with haematoxylin and eosin according to routine protocol. Analyses and pictures were obtained using an inverted microscope, Nikon Eclipse TE2000‐S, and a camera, Nikon Coolpix 5400.
Fluorescence in situ hybridization
Presence of hIDPSC in the obtained embryos was assessed by fluorescence in situ hybridization (FISH) with a specific human Y chromosome centromere probe (Sat I, Vysis, Abbott, Downers Grove, IL, USA) according to the manufacturer's protocol. Analysis was performed using a confocal microscope.
Confocal microscopy
An argon ion laser set at 488 nm for FITC and at 536 for rodamine excitation was used. Emitted light was filtered with a 505‐nm (FITC) and 617‐nm (rodamine) long pass filter in a laser scan microscope (LSM 410, Zeiss, Jena, Germany). Sections (5 µm) were prepared at approximately, tissue mid‐height level. Photo‐multiplier gain and laser power were kept constant throughout each experiment.
Results
Human IDPSC contribution into recipient mouse blastocyst
In order to analyse the ability of hIDPSC (46, XY) to contribute to inner cell mass and trophectoderm of mouse early embryos, 6–8 cells stained with vital fluorescent dye (Fig. 1a) were injected into the perivitelline space and/or the blastocell of 8 compacted morulae and 20 early blastocysts (Fig. 1b–d). These cells, while presenting in vitro fibroblast‐like morphology, after their injection were seen to have adopted similar size to those of the recipient mouse embryo (Fig. 1c,d). Fluorescence‐stained hIDPSC proliferated in the recipient mouse embryonic environment and showed a contribution to the inner cell mass and also to the trophoblast cell layer (Fig. 1e,f). The cells injected into the perivitelline space did not seem to be capable of migrating into the blastocele (Fig. 1g). All recipient early mouse blastocysts survived and achieved hatching by 24 or 48 h of culture (Fig. 1h,i). In order to verify whether fluorescence‐stained mouse fibroblast could also be able to contribute to embryonic development, these cells were injected into morulae and/or blastocysts as a control. However, compared to the recipient cells, fibroblast cells displayed different morphology and neither integrated nor proliferated in the mouse embryos after culture (Fig. 1j).
Figure 1.
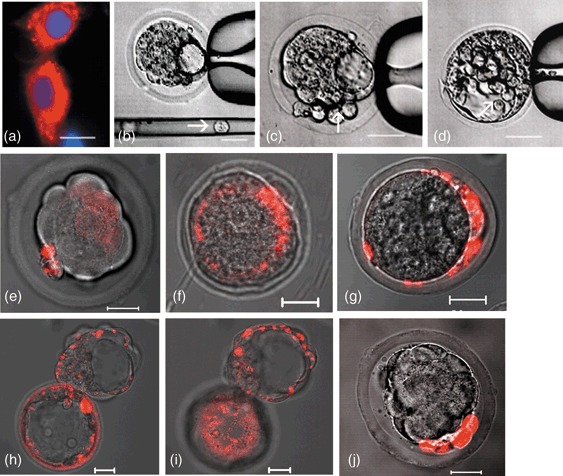
Human immature dental pulp stem cells (hIDPSC) injection into morulae and blastocysts. (a) hIDPSC stained with Vybrant Cell‐Labelling (red) and nuclei stained with DAPI (blue); (b) hIDPSC before injection (arrow) into blastocysts; (c,d) hIPSC injected into perivitelline space and blastocele, respectively; (e) morula with hIDPSC (red) 24 h after injection; (f) contribution of hIDPSC (red) to the inner cell mass and trophoblast cell layer; (g) hIDPSC restricted to the periviteline space, indicating a lack of migration into blastocele; (h,i) hatching stage of mouse blastocyst with hIDPSC (red), 48 h after injection, showing the presence of these cells in throphoblast in different confocal microscope‐captured sections; (j) mouse fibroblast stained with Vybrant Cell‐Labelling (red) injected into morulae, after 48 h, presenting larger cell morphology than recipient morulae cells. These cells did not integrate into compacted morulae but inhibited progress into blastocyst. Scale bars: a–j = 20 µm. a = epifluorescence; b–d = phase contrast; e–j = merged image of fluorescent confocal microscopy and differential interference contrast.
Transfer of mouse early embryos with hIDPSC into recipient mice
To determine the developmental and pluripotent capacity of hIDPSC, six to eight stained cells were injected into the blastocele of 57 early blastocysts (Fig. 1d) and were immediately transferred to the uterus of five foster mothers. Three mice achieved pregnancy and, according to ethical recommendations, human/mouse chimaeras were collected before birth from two mothers: three embryos at the 11 d.p.c. of development (Fig. 2a) and six foetuses at the 18 d.p.c., three of them were well formed (Fig. 2b) and three were malformed (data not shown). One foster mother gave premature birth; however, she killed her offspring.
Figure 2.
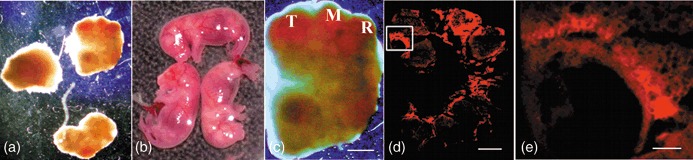
Mouse embryo with human immature dental pulp stem cells (hIDPSC) at 11 and 18 d.p.c. (a,b) 11 d.p.c. embryos and 18 d.p.c. well‐formed mouse foetuses with hIDPSC, respectively. (c) General aspect of the embryo where the neurosystem primary vesicles is evident: telencephalum (T) mesencephalum (M) and rombencephalum (R). (d) Whole embryo frozen section. (e) Selected area from (d) showing the presence of hIDPSC in ocular region. Scale bars: c, d = 1000 µm; e = 200 µm; a–c = estereoscopic microscope. (d,e) Fluorescence confocal microscopy.
Contribution of hIDPSC into tissues of 11 and 18 d.p.c. pretermed human/mouse chimaeras
One of the 11 d.p.c. embryos, which seemed morphologically normal (Fig. 2c), was used to evaluate the hIDPSC incorporation into mouse tissues. Analyses showed the presence of fluorescence‐stained hIDPSC in various parts of the mouse embryo (Fig. 2d,e).
Three of the six 18 d.p.c. mouse foetuses seemed to be well formed, based on their morphological appearance (Fig. 2b) and histological analysis (Fig. 3A). As fluorescence decreases during the process of cell division, the anti‐hIDPSC antibody, (which identifies hIDPSC exclusively (15)), was used to detect their presence in 18 d.p.c. mouse foetuses. Strong fluorescent signals were observed in different organs of the chimaeras, such as the brain, liver, intestine and muscles. Wide individual, organ and tissue variation of hIDPSC engraftment was observed and only representative figures were chosen for demonstration (Fig. 3B1–G3).
Figure 3.
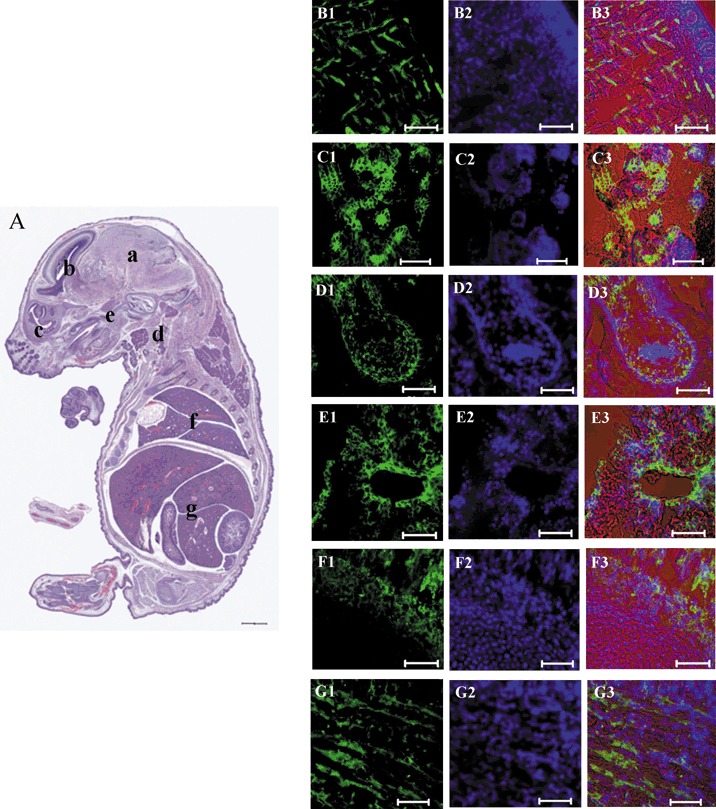
Mouse foetuses with hIDPSC at 18 d.p.c. (A) Histological section of whole embryo, showing apparently normal body organization. At parasagittal or sagittal section where most of the external features of development, which are similar to those seen in newborn mice are evident. The brain (a) shows structures such as lateral ventricles and part of the chorioid plexus. Sub‐arachoid space is also evident (b). Section of parasagittal orbitary cavity is particularly defined (c). Cervical region presents the following structures including mandibular gland, thyroid cartilage and pharynx (d). Palate ossification is a reference to identify nasopharynx and oropharynx (e). Heart is seen only by its lateral wall evident in a trunk region where lung lobes delimitated cranially by the manubrium sterni and costal cartilage (f). Abdominal cavity shows part of the liver lobes, intestine and bladder. Peritoneal cavity is also evident (g). (B1–G3) Confocal microscopy, positive immunostaining with anti‐hIDPSC antibody (Green B1–G1, fluorescent confocal microscopy), nuclei stained with DAPI (Blue B2–G2, epifluorescence) and merged image of fluorescent confocal microscopy, epifluorescence and differential interference contrast (B3–G3). Red is an artificial colour. (B1–B3) brain, (C1–C3) cervical region, (D1–D3) intestine, (E1–E3) liver, (F1–F3) tail, (G1–G3) muscle tissue. Scale bars: A = 1500 µm; B1–G3 = 50 µm.
FISH analysis of hIDPSC contribution to tissues of mouse foetuses
In order to confirm the presence of hIDPSC, FISH analysis with a human‐specific probe for the centromeric sequence of Y chromosome was performed. This technique was applied to the serial sections, which, in a separate assay (Fig. 3), were also used for immunohistochemistry analysis using the with anti‐hIDPSC antibody. Signals from the Y chromosome probe were detected in a cluster distribution, indicating presence of hIDPSC in defined tissue areas (Fig. 4a–f). As expected, FISH signals were localized around the periphery of the nuclei, which were then stained with DAPI (Fig. 4g,h). Interestingly, signals observed in skeletal muscle of the chimaeras followed the orientation pattern of muscle fibres, in the central position, suggesting an immature state of myotubes present (Fig. 4a,b).
Figure 4.
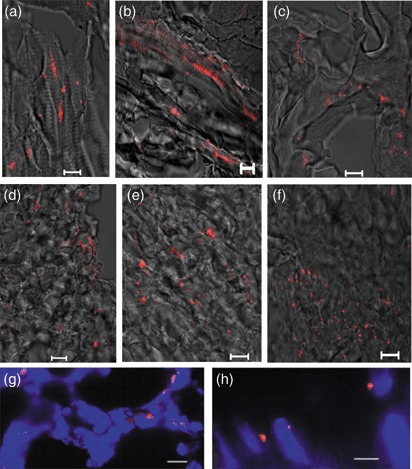
FISH analysis using human Y chromosome probe (red) in human/mouse chimaeras. (a,b) muscle fibres; (c) ventricular cavity; (d) liver; (e) heart; (f) ocular cavity (merged image of fluorescent confocal microscopy and differential interference contrast); (g) chimaera tissue, showing nuclear localization of Y chromosome signal (red), nuclei stained with DAPI (blue, epifluorescence) and (h) higher magnification of nuclei. As expected, FISH signals localized to the periphery of nuclei. Scale bars: a–h = 10 µm.
Differentiation of hIDPSC within mouse embryonic environment
Using a variety of methods we demonstrated hIDPSC contribution to mouse embryos, which did not present any type of morphological deficiency (3, 4). These data strongly indicate that hIDPSC underwent the differentiation process into functional cell types during the mouse embryonic development. In accordance with this observation we were able to produce evidence (by immunohistochemistry), that these cells accomplished differentiation within local tissues, by the presence of human‐specific tissue proteins, such as myosin and cytokeratin. Moreover, we used a specific antibody against human nuclei (HumN) to confirm, once more, that the cells were indeed of human origin. All human/mouse tissues analysed, with specific human antibodies, showed positive immunostaining, confirming that hIDPSC had differentiate into muscles and epithelial cells within the mouse tissues (Fig. 5).
Figure 5.
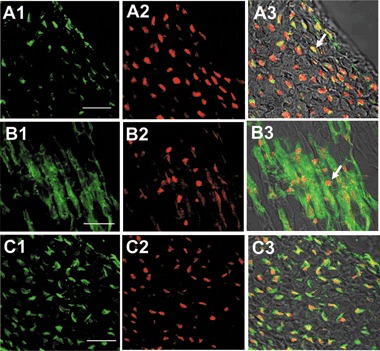
In vivo differentiation of hIDPSC into muscle and epithelial cells. (A1) Positive immunostaining with anti‐HumN antibody (green), (A2) nuclei stained with PI (red), A3) merged image of A1 and A2, arrows indicate human nuclei in yellow as a result of superposition of green and red. (B1) Human myosin‐positive muscle fibres (green), B2) anti‐HumN antibody (red), (B3) merged image of B1 and B2, arrows indicate human nuclei. C1) Anti‐human cytokeratin positive immunostaining (green), C2) positive reaction with anti‐HumN antibody (red), (C3) merged image of C1 and C2. Confocal microscopy: A1–G1, B1–G1 = fluorescent confocal microscopy, C1–G1 = fluorescent confocal microscopy + differential interference contrast. Scale bars: A1–C3 = 20 µm.
Correlation between signals position produced by FISH analysis and those observed by imunofluorescence, for human‐specific antibodies, confirmed the cluster distribution of human cells in defined positions within the mouse tissues and organs (3, 4, 5).
Discussion
Production of human/mouse chimaeras using human embryonic stem cells has shown that human and mouse embryonic stem cells are biologically compatible. Moreover, human embryonic stem cells have been shown to be able to respond to the spatial and temporal program of the mouse embryonic environment (11). According to our knowledge, ASC obtained from dental pulp are a promising source of cell that might be used in cell therapies (20, 21). We have previously described a population of hIDPSC, that had characteristics of both MSC and human embryonic stem cells (15). The results described here extend previous findings concerning the capacity of ASC (22) and human embryonic stem cells (11) to contribute to formation of chimaeras. We showed that hIDPSC are also biologically compatible with the mouse embryonic environment and were capable to contribute functionally into a variety of mouse tissues.
Improbable doubts could be raised concerning the possibility of fluorescent dye (Vybrant CM‐Dil) being transferred between human and mouse adjacent cells after injection into early mouse embryo. To overcome such a hypothesis, two different methods immunohistochemistry with anti‐hIDPSC antibody and FISH analysis with human‐specific probe for the centromeric sequence of Y chromosome were used. Furthermore, we performed an in vitro assay using hIDPSC stained with two different colours (red and green) of fluorescent dye. We did not observe any cells presenting double staining, which could suggest a possible transfer between cells (data not shown).
Although the human embryonic stem cells that were introduced into mouse blastocysts did not affect embryonic development in the early stages, only one (approximately 8.5 d.p.c.) of 28 embryos presented normal morphology and retained human embryonic stem cell derivatives. Another three chimaeras with human embryonic stem cell contribution had abnormal development (11). In our experiment, hIDPSC were capable of engrafting and proliferating inside mouse morulae and blastocysts and forming pretermed chimaeras. These cells contributed not only to inner cell mass, as do human embryonic stem cells, but also to the trophoblast cell layer – without any embryo damage. Furthermore, hIDPSC integrated into host embryos and developed foetuses, undergoing the process of differentiation.
Little is known about the initial reprogramming events that occur after transference of ASC into mouse blastocysts (8, 22). For the first time, Grinnell et al. de‐differentiated mouse interfollicular keratinocytes with Oct‐4 transfection, improving their developmental potential and, subsequently, their capacity to differentiate into neuronal cells (23). This finding suggests that somatic cells, express a single pluripotent marker, Oct‐4, could be reverted to less differentiated cells. Several recent publications have demonstrated reprogramming and the complete reversion into a pluripotent state of mouse and human somatic cells by retroviral transfection of four transcription factors, Oct4, Sox2, c‐Myc and Klf4 (16, 17, 18, 19, 24). In accordance with this finding, our data demonstrate that adult stem cells, positive for OCT‐4, NANOG and other human embryonic stem markers, even presenting a fibroblast phenotype, can be ‘reprogrammed’ to a cell type closer to the embryonic stem cells by the host environment without any genetic modification and/or drug selection.
Several explanations for stem cell plasticity mechanisms have been proposed, including differentiation and cell fusion (25, 26, reviewed in 27). It has been shown that mouse euploid multipotent adult progenitor cells isolated from bone marrow were able to produce chimaeras and to differentiate into cells of all three germ layers. Based on the multipotent adult progenitor cell differentiation capacity observed in vitro and in vivo, when cell fusion was not observed, the authors concluded that plasticity could be attributed to mechanism of stem cell differentiation (22). Cell plasticity as a result of cell fusion has been demonstrated between donor haematopoietic stem and differentiated cells in recipient tissues (after inoculation), resulting in tetraploid daughter cells, which expressed markers of both donor–recipient cells (28). However, it has been shown that polyploid cells have a slower rate of proliferation (29), which would seem to diminish the stem cell contribution to different tissues. We observed a significant contribution of hIDPSC to various mouse tissues that confirmed the high proliferation rate of these cells during recipient embryo development; thus, this suggests the absence of cell fusion in several tissues. However here, signals for the human Y chromosome were observed along muscle fibres, indicating their fusion with hIDPSC. Nevertheless, this fusion is a process that is likely to occur anyway since skeletal muscle ontogeny requires fusion of mononucleated myoblasts into myotubes. Thus, muscle fibres originated from mouse and hIDPSC have yielded chimaeric muscles. We believe that the mechanisms, proposed to explain stem cell plasticity, differentiation and cell fusion, are indeed feasible depending on cell characteristics and/or cell environment. Plasticity of hIDPSC was demonstrated by these two mechanisms since they were able to differentiate into several cell types and to fuse, thus generating muscle fibres.
Epigenetic mechanisms seem to allow an organism to respond to its local environment through changes in gene expression (30). During embryonic development, cells express different set of genes that are characteristic of their temporal stage and organ locations. As hIDPSC express pluripotent markers, epigenetic changes could occur during their differentiation in the mouse blastocyst. Although three well‐formed chimaeras were obtained in the present work, suggesting that normal epigenetic modifications could occur during hIDPSC differentiation within the embryonic mouse environment, it still needs further investigation.
As previously described, hIDPSC were well tolerated by adult mouse organism (15). However, it is important to highlight that hIDPSC rejection was prevented due to the immune system of the recipient early mouse embryos and foetuses not yet being fully developed. This finding is also supported by other authors, who have demonstrated that human stem cells isolated from dental pulp present MSC characteristics when there is immunosuppressive activity (31). Moreover, it also has been demonstrated that MSC, isolated from human bone marrow, showed long‐term engraftment after their in utero transplantation in sheep, while present unique immunological characteristics that allow their persistence in a xenogenetic environment (32). Yokoo et al. described that human MSC could be reprogrammed to other cell types and organs depending on the embryonic environment in which they find themselves (8). Despite their mesenchymal origin, MSC are able to differentiate into cell types that are derived from ectoderm and endoderm. Once hIDPSC present all the basic characteristics of MSC, they are not rejected by mouse embryos and foetuses and contribute to their embryonic development (33). Moreover, another work that also used hIDPSC demonstrated that they were capable of reconstructing large cranial defects in non‐immunosuppressed rats (34).
Additional work is required to verify whether the pluripotent capacity of the hIDPSC strain used in the present work could be extended to other hIDPSC isolated from different patients.
Conclusions
We have shown that hIDPSC can survive and proliferate within the mouse developing blastocyst and are capable of producing human/mouse chimaeras, as well as exhibiting differentiation into human‐specific tissues. Our data demonstrate that hIDPSC could respond to signals emanating from mouse blastocyst and produce chimaeras similar to embryonic stem cells. This model suggests the contribution of hIDPSC to embryogenesis in which their homing was observed to different organs and tissues without rejection by the recipient embryos or foetuses. Production of human/animal chimaeras using ASC opens new opportunities for understanding of human genetic diseases and embryogenesis.
It is of great biomedical importance to obtain patient‐specific ASC with the potential of human embryonic stem cells but without any ethical implications. We believe that ASC, which express embryonic stem cell markers and are obtained without any genetic modification, are an alternative route to advancement of clinical deliberations regarding stem cell therapy.
Acknowledgements
This study was made possible thanks to the financial support of the Roger Abdelmassih Human Reproduction Clinic and Research Center. We are also grateful to Alexsander S. Souza for his excellent technical assistance with confocal microscopy, Laboratory of Parasitology, Butantan Institute and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo, Brazil. We are also indebted to Danielle Martins, PhD, for her help on histological photographs.
References
- 1. Doss MX, Koehler CI, Gissel C, Hescheler J, Sachinidis A (2004) Embryonic stem cells: a promising tool for cell replacement therapy. J. Cell. Mol. Med. 8, 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. [DOI] [PubMed] [Google Scholar]
- 3. Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 78, 7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jeffrey M (1998) Jones Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- 5. Smith A (2001) Embryonic stem cells In: Marshak DR, Gardner RL, Gottlieb D, eds. Stem Cell Biology, pp. 205–230, Cold Spring Harbor, NY: Cold Spring; Harbor Laboratory Press. [Google Scholar]
- 6. Morris MB, Rathjen J, Keough RA, Rathjen PD (2005) Biology of embryonic stem cells In: Odorico J, Zhang S, Pedersen R, eds. Human Embryonic Stem Cells, pp. 1–15. Oxford, UK: BIOS Scientific Publishers, Taylor & Francis Group. [Google Scholar]
- 7. Lensch MW, Schlaeger TM, Zon LI, Daley GQ (2007) Teratoma formation assays with human embryonic stem cells: a rationale for one type of human‐animal chimaera. Cell Stem Cell 1, 253–258. [DOI] [PubMed] [Google Scholar]
- 8. Yokoo T, Ohashi T, Shen JS, Sakurai K, Miyazaki Y, Utsunomiya Y, Takahashi M, Terada Y, Eto Y, Kawamura T, Osumi N, Hosoya T (2005) Human mesenchymal stem cells in rodent whole‐embryo culture are reprogrammed to contribute to kidney tissues. Proc. Natl. Acad. Sci. USA 102, 3296–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldstein RS (2006) Transplantation of human embryonic stem cells to the chick embryo. Methods Mol. Biol. 331, 137–151. [DOI] [PubMed] [Google Scholar]
- 10. Muotri AR, Nakashima K, Toni N, Sandçer VM, Gage FH (2005) Development of functional human embryonic stem cell‐derived neurons in mouse brain. Proc. Natl. Acad. Sci. USA 102, 18644–18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. James D, Noggle SA, Swigut T, Brivanlou AH (2006) Contribution of human embryonic stem cells to mouse blastocysts. Dev. Biol. 295, 90–102. [DOI] [PubMed] [Google Scholar]
- 12. McLaren A (2007) A scientist's view of the ethics of human embryonic stem cell research. Cell Stem Cell 1, 23–26. [DOI] [PubMed] [Google Scholar]
- 13. Behringer RR (2007) Human‐animal chimaeras in biomedical research. Cell Stem Cell 1, 259–262. [DOI] [PubMed] [Google Scholar]
- 14. Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M (2007) A hypothesis for an embryonic origin of pluripotent Oct‐4+ stem cells in adult bone marrow and other tissues. Leukemia 21, 1–8. [DOI] [PubMed] [Google Scholar]
- 15. Kerkis I, Kerkis A, Dozortsev D, Stutkart‐Parson GC, Massironi AMG, Pereira LV, Caplan AI, Cerruti HF (2006) Isolation and characterization of a population of immature dental pulp stem cells expressing OCT‐4 and other embryonic stem cells markers. Cells Tissues Organs 184, 105–116. [DOI] [PubMed] [Google Scholar]
- 16. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- 17. Yu J, Vodyanik MA, Smuga‐Otto K, Antosiewicz‐Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920. [DOI] [PubMed] [Google Scholar]
- 18. Meissner A, Wernig M, Jaenisch R (2007) Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat. Biotechnol. 25, 1177–1181. [DOI] [PubMed] [Google Scholar]
- 19. Qin D, Li W, Zhang J, Pei D (2007) Direct generation of ES‐like cells from unmodified mouse embryonic fibroblasts by Oct4/Sox2/Myc/Klf4. Cell Res. 17, 958–962. [DOI] [PubMed] [Google Scholar]
- 20. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2000) SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl Acad. Sci. USA 97, 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grothos S, Mankani M, Brahim J, Gehron Robey P, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo . Proc. Natl. Acad. Sci. USA 97, 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz‐Gonzalez XR, Reyes M, Lenvik T, Lund T, Du Blackstad MJ, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49. [DOI] [PubMed] [Google Scholar]
- 23. Grinnell KL, Yang B, Eckert RL, Bickenbach JR (2007) De‐differentiation of mouse interfollicular keratinocytes by the embryonic transcription factor Oct‐4. J. Invest. Dermatol. 127, 260–262. [DOI] [PubMed] [Google Scholar]
- 24. Maheralli N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K (2007) Directly reprogrammed fibrablasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55–70. [DOI] [PubMed] [Google Scholar]
- 25. Alison MR, Poulsom R, Otto WR, Vig P, Brittan M, Direkze NC, Lovell M, Fang TC, Preston SL, Wright NA (2004) Recipes for adult stem cell plasticity: fusion cuisine or readymade? J. Clin. Pathol. 57, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wagers A, Weissman I (2004) Plasticity of adult stem cells. Cell 116, 639–648. [DOI] [PubMed] [Google Scholar]
- 27. Schwartz RE, Verfaillie CM (2005) Adult stem cell plasticity In: Odorico J, Zhang S, Pedersen R, eds. Human Embryonic Stem Cells, pp. 45–60. Oxford, UK: BIOS Scientific Publishers, Taylor & Francis Group. [Google Scholar]
- 28. Kucia M, Campbell FR, Zuba‐Surma E, Majka M, Ratajczak J, Ratajczak MZ (2006) A population of very small embryonic‐like (VSEL) CXCR4+SSEA‐1+Oct‐4+ stem cells identified in adult bone marrow. Leukemia 20, 857–869. [DOI] [PubMed] [Google Scholar]
- 29. Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, Amon A (2007) Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 317, 916–924. [DOI] [PubMed] [Google Scholar]
- 30. Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33, 245–254. [DOI] [PubMed] [Google Scholar]
- 31. Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V, Staffolani N, Franchina M, Grossi A, Bagnara GP (2005) Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 80, 836–842. [DOI] [PubMed] [Google Scholar]
- 32. Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AB, Deans R, Marshak DR, Flake AW (2000) Human mesenchymal stem cells engraft and demonstrate site‐specific differentiation after in utero transplantation in sheep. Nat. Med. 6, 1282–1286. [DOI] [PubMed] [Google Scholar]
- 33. Dominici M, Le Blanc K, Mueller I, Slaper‐Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop, Dj. Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. [DOI] [PubMed] [Google Scholar]
- 34. De Mendonça Costa A, Bueno DF, Martins MT, Kerkis I, Kerkis A, Fanganiello RD, Cerruti H, Alonso N, Passos‐Bueno MR (2008) Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. Craniofac. Surg. 19, 204–210. [DOI] [PubMed] [Google Scholar]


