Abstract
Objectives
The anti‐cancer potential of melatonin has been examined using a variety of experimental approaches. Melatonin immunomodulatory action was evaluated against the lung cancer cell line SK‐LU‐1, in co‐culture with human peripheral blood mononuclear cells (PBMC).
Materials and methods
Melatonin was tested on the cell line only after 24 h incubation (direct effect), and on the co‐culture system of SK‐LU‐1 and PBMC to investigate any indirect effect. Apoptotic induction of the cancer cells was assessed using annexin V/PI staining with flow cytometric analysis for membrane alteration. Intracellular superoxide anion (O2 •−) and hydrogen peroxide (H2O2) for intracellular oxidative stress and glutathione (GSH) for intracellular anti‐oxidation were measured with specific fluorescence probes. DNA fractions were measured employing propidium iodide (PI) fluorescence staining.
Results
High doses of melatonin were directly toxic to SK‐LU‐1 cells, while PBMC‐mediated indirect effect occurred after moderate doses (1 μm). Under co‐culture conditions, increases in apoptotic cell death, increase in oxidative stress by reduction of GSH and cell cycle arrest in G0/G1 in SK‐LU‐1 cells, were observed as the immunomodulatory effect of melatonin.
Conclusion
Melatonin had indirect effects on lung cancer cells by enhancement of immunomodulatory effects, but further studies of mechanism(s) involved are needed.
Introduction
Non‐small cell lung cancer (NSCLC) accounts for around 60% of all lung malignancies 1 and has a 5‐year relative survival rate of only 16%, for all stages 2. The relapse pattern typically shows that treatment does not completely cause tumour remission, with median time to progression being 4.5–23.7 months 1; thus effective strategies for improving outcome after treatment are needed 3.
Melatonin (N‐acetyl‐5‐methoxytryptamine), an indoleamine molecule produced primarily by the pineal gland. It is secreted into the blood circulation in a rhythmical pattern with nocturnal elevation 4, and commonly recognised to control circadian rhythms 5. As a potent chronobiotic, melatonin is frequently used to treat insomnia, jet lag 6, migraine 7 and headaches 8. In addition, numerous studies have reported its antioxidant 9, 10, anti‐inflammatory 11 and anti‐malignant 12 effects along with its immunomodulatory potential 13, involving various intracellular mechanisms. Previously, direct melatonin activity against breast cancer 14, 15, hepatocellular carcinoma 16, 17 and leukaemia 18 have been demonstrated. These effects are exerted due to its anti‐proliferative action (on breast cancer cells 14) as well as by induction of apoptosis (for example on breast cancer cells 15 and hepatocellular carcinoma cell lines 16); apoptosis is a well known programmed cell death mechanism that avoids an inflammatory response 19. On the other hand, pro‐oxidant effects of melatonin evoked by increasing intracellular reactive oxygen species (ROS) have been reported in leukaemia cancer cell lines, leading to inhibition of cancer cell proliferation 18. Growth inhibition of hepatocellular carcinoma by melatonin through arrest of the cell cycle in the G2/M phase, leading to reduced cell proliferation, has also been reported 16. Likewise, using an animal model has shown that pinealectomized hamsters, thus with endogenous melatonin deficiency, had increased tumour growth compared to controls, which was antagonized by treatment with exogenous melatonin 20.
The goal of efficient cancer treatment is to specifically target tumour cells while avoiding undesirable side effects on normal healthy tissue 21. To achieve this, immunotherapeutical strategies involving activation of the immune system specifically to recognize and eliminate malignant cells, are of interest 22. Immunotherapy aims to augment a weak immune response of the host to its tumour by stimulating cytokines to activate immune cell function and/or proliferation 23, or else by administrating tumour‐specific antibodies or T cells. These lead to limiting growth or destroying the tumour 24. Immunomodulation by melatonin has been reported to stimulate particular cytokine gene expression and to increase numbers of immune cells 13, 25. Melatonin‐induced cytokine production has been shown in human peripheral blood mononuclear cells (PBMC) for IL‐1 26, IL‐2, IL‐6 27, 28, IL‐12 29 and IFN‐γ 28, as well as for TNF‐α in mice 13 and IL‐7 in rat thymic epithelial cells 30. Melatonin administration to pinealectomized mice bearing lung carcinoma also caused significant increases in thymic efficiency by recovery of thymulin activity, increase in IL‐2 production, reduction in tumour volume 31 and increase in granulocyte/macrophage‐colony forming units, in animals receiving chemotherapy 32. On the other hand, melatonin was also reported to suppress a release of IL‐10 from human immune cells 25, 33. IL‐10 is known as an inhibitory cytokine that suppresses synthesis and secretion of other cytokines from T helper 1 (Th1) cells and macrophages, leading to inhibition of the immune cell response 23.
Taken together, these findings indicate that melatonin can contribute to inhibition of growth or to induce cancer cell death by modulation of immune cell function. The previously studied co‐culture system of melanoma and monocytes has shown that melatonin increased cytotoxicity (stimulation of cancer cell death) of monocytes against A375 human melanoma cell line 34. Increasing levels of hydrogen peroxide (H2O2), nitric oxide (NO2), superoxide anion (O2 •−) and IL‐1 in monocytes was reported, suggesting involvement of melatonin‐induced enhancement of monocyte cytotoxicity 34; however, the mechanisms by which melatonin affected immune cell activity to induce cancer cell death are still unknown. Moreover, only little published data provide controversial results concerning melatonin effects on lung cancer A549 cell line 35, 36.
This study has aimed, therefore, to study anti‐cancer activity of melatonin against NSCLC cells via immunomodulation. As cisplatin is the first‐line treatment for NSCLC in a majority of countries 3, we used cisplatin‐sensitive NSCLC grade III (SK‐LU‐1) in in vitro co‐culture with human PBMC. Apoptotic induction in the cancer cells was evaluated using annexin V and propidium iodide (PI) staining. Intracellular H2O2, O2 •− and glutathione (GSH) levels were measured by fluorescent probes to evaluate oxidative status of cancer cells. DNA fractions were measured by PI fluorescence staining to define cell populations in each cell cycle phase.
Materials and methods
Cell line and reagents
Human lung adenocarcinoma cisplatin‐sensitive cell line (SK‐LU‐1) was obtained from Cell Line Service – CLS (Eppelheim, Germany). Dulbecco's modified Eagle's medium (DMEM), Roswell Park Memorial Institute (RPMI) 1640 medium and 0.25% trypsin‐EDTA (1X) were from Gibco, Invitrogen Life Technologies, Barcelona, Spain. Melatonin (GMP) was from Huanggang Saikang Pharmaceutical Co. Ltd., Hubei, China (Batch No.: 201111001) with purity confirmed at >99.4% by differential scanning colorimetry (DSC) and high‐performance liquid chromatography (HPLC). Ficoll‐Paque Plus (density 1.077 g/ml) was purchased from Amersham Biosciences (Piscataway, NJ, USA) and 3‐(4, 5‐dimethyl‐2‐thiazolyl)‐2, 5‐diphenyl‐2H‐tetrazolium bromide (MTT) from Amresco LLC (Solon, OH, USA). Mitogens concanavalin A (Con A) and lipopolysaccharide (LPS) from Escherichia coli as well as 2′,7′‐dichlorodihydrofluorescein diacetate (DCFH‐DA) and dihydroethidium (DHE) were from Sigma‐Aldrich (St. Louis, MO, USA), while 5‐chloromethylfluorescein diacetate (CMF‐DA) was from Molecular Probes (Eugene, OR, USA). FITC‐conjugated annexin V and PI were from eBioscience, San Diego, CA, USA (cat no. 88‐8005‐74); PI was purchased from Sigma‐Aldrich Ltd. (Gillingham, Dorset, UK). DMSO was from Lab‐Scan, Analytical Science, Ireland and other reagents were bought from standard commercially available suppliers.
SK‐LU‐1 cell line and PBMC culture
The SK‐LU‐1 cells were cultured in DMEM supplemented with 10% foetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. Human PBMC were isolated from venous blood taken at around 9.00 am throughout this research, and diluted with PBS (1:1). PBMC were isolated by centrifugation at 400 g for 35 min on Ficoll‐Paque Plus. The lymphocyte interface was carefully removed, washed twice in RPMI‐1640 and resuspended in RPMI‐1640 supplemented with 10% foetal calf serum (FCS), 20 mm HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin and mitogens LPS (5 ng/ml) and Con A (1 μg/ml) were used to maintain viable PBMC in culture conditions 37, 38. Cell numbers were counted employing the trypan blue exclusion assay, using a haemocytometer; viable cells exclude the dye, while dead cells take it up and become stained blue.
Melatonin treatment and cell viability measurement: MTT reduction assay
Melatonin solutions were freshly prepared by dissolving in less than 1% dimethyl sulphoxide (DMSO) in culture media, to reach final concentrations of melatonin at 1 nm, 1 μm and 1 mm, further defined as low, medium and high doses respectively. For the cell viability assay, SK‐LU‐1 cells (5 × 103 cells) or PBMC (5 × 104 cells) were plated in triplicate in 96‐well plates and treated with low, medium and high concentrations of melatonin for 24 h after seeding. Metabolic activity of SK‐LU‐1 and PBMC cells was measured using the MTT assay, in which it is converted to formazan crystals by viable cells (as described by Yeap et al., 2007, with minor modification) 39. MTT solution (final concentration 0.5 mg/ml) was added to cells after 21 h treatment and left for a further 3 h. Then reaction plates were centrifuged at 600 g for 10 min before excess MTT solution was removed and formazan crystals dissolved in 200 μl DMSO. Absorbance was measured at 555 nm reference wavelength at 650 nm, by a microplate reader (Sunrise, Tecan Group, Mannedorf, Switzerland).
Co‐culture conditions and melatonin treatment
For co‐culture observations, human PBMC either non‐pretreated or pre‐treated for 24 h with melatonin at 1 μm, were used as effector cells (E) for SK‐LU‐1 (target cells, T) seeded in triplicate on 24‐well plates at E:T ratio 2:1. Co‐culture was maintained for 24 h with or without additional treatment with melatonin (1 μm). Results obtained for SK‐LU‐1 culture alone (control group) were compared to four groups of different co‐culture conditions (Table 1). After 24 h co‐culture incubation, SK‐LU‐1 cells were tested for apoptotic cell death, intracellular content of O2 •−, H2O2, reduced GSH and cell cycle phase distribution, using the methods described below.
Table 1.
Culture conditions and melatonin treatment in first 24 h of PBMC or SK‐LU‐1 culture and next 24 h of co‐culture period
| Group | Description of group | Individual incubation (First 24 h) | Co‐culture period (Last 24 h) | |
|---|---|---|---|---|
| PBMC (E) | SK‐LU‐1 (T) | Melatonin additional | ||
| Control | SK‐LU‐1 incubated alone | Absent | Non‐treated | Absent |
| I | Co‐culture of SK‐LU‐1 with PBMC | Non‐pretreated with melatonin | Non‐treated | Absent |
| II | Co‐culture of SK‐LU‐1 with PBMC then incubated with additional melatonin | Non‐pretreated with melatonin | Non‐treated | Present |
| III | Co‐culture of SK‐LU‐1 with melatonin pre‐treated PBMC | Pre‐treated with melatonin | Non‐treated | Absent |
| IV | Co‐culture of SK‐LU‐1 with melatonin pre‐treated PBMC then incubated with additional melatonin | Pre‐treated with melatonin | Non‐treated | Present |
Detection of apoptosis by annexin V/PI staining
Annexin V and PI staining were performed to determine the mode of SK‐LU‐1 cell death in the co‐culture system with PBMC. Staining with annexin V allows study of apoptosis, while necrotic cells with internal and external membrane disruption become stained by the vital dye PI 40. At the end of the co‐culture period, both SK‐LU‐1 and PBMC were detached using 0.25% trypsin‐EDTA, washed once in PBS and centrifuged at 700 g for 5 min. Then, supernatant was discarded and pellets were resuspended in 95 μl of binding buffer (HEPES‐buffered saline supplemented with 25 mm CaCl2). Cells were stained with annexin V‐FITC (5 μl) for 15 min, followed by 100 μl of PI solution (5 μl of PI in 95 μl of binding buffer) for further 15 min in the dark at room temperature. Apoptotic and necrotic cell death were assessed by flow cytometry (BD FACSCanto II, BD Biosciences, San Jose, CA, USA) within 1 h after staining. SK‐LU‐1 cells were gated and separated from PBMC by size and cell component (granularity), then percentage of annexin V stained, (that is, apoptotic cells) was calculated using BD FACSDiva software (BD Biosciences, San Jose, CA, USA).
Determination of intracellular superoxide anion level
DHE is converted to ethidium by superoxide anion present in cells 41. After co‐culture incubation, all cells were trypsinized using 0.25% trypsin‐EDTA, washed in PBS and centrifuged at 700 g for 5 min. Subsequently, supernatant was discarded and pellets were stained with 5 μm DHE for 20 min in serum‐free medium, in the dark. Then, cell pellets were washed once in PBS and analysed by flow cytometry. Intracellular O2 •− production was expressed as median fluorescence intensity (MFI), calculated using BD FACSDiva software.
Determination of intracellular hydrogen peroxide level
After penetrating into cells, DCFH‐DA is converted to fluorescent dichlorofluorescein (DCF) by intracellular hydrogen peroxide 42. Following co‐culture incubation, all cells were trypsinized with 0.25% Trypsin‐EDTA, washed in PBS and centrifuged at 700 g for 5 min. Supernatant was discarded and pellets were stained, in the dark, with 10 μm DCFH‐DA in serum‐free medium. After 30 min incubation, pellets were washed once in PBS then analysed by flow cytometry. Intracellular H2O2 production was expressed as MFI, calculated by BD FACSDiva software.
Determination of intracellular glutathione (GSH) level
CMF‐DA conjugates with GSH after crossing cell membranes and subsequently, the acetate group is cleaved from the conjugate by intracellular esterases, to form fluorescent 5‐chloromethylfluorescein 41. In brief, after co‐culture incubation, all cells were trypsinized with 0.25% trypsin‐EDTA, washed in PBS and centrifuged at 700 g for 5 min. Then supernatant was discarded and pellets were incubated with 5 μm CMF‐DA in serum‐free medium for 15 min in the dark. Next, dye was washed out and cells were resuspended in PBS, then assayed using flow cytometry. CMF fluorescence intensity was measured and calculated by BD FACSDiva software. Population of SK‐LU‐1 cells with normal CMF intensity was expressed as percentage of “normal GSH level cells”.
Cell cycle phase determination by PI staining
Distribution of cells in different phases of the cell cycle was evaluated by flow cytometry. This determination is based on measurement of DNA content of nuclei stained with PI 43. After co‐culture incubation, all cells were trypsinized with 0.25% trypsin‐EDTA, then washed in PBS and centrifuged at 700 g for 5 min. Supernatant was discarded and pellets were fixed overnight at 4 °C using cold ethanol (70%). Then, cells were incubated in 1 ml PI staining solution (50 μg/ml) for 1 h in the dark. DNA fraction was analysed using flow cytometry. Relative proportions of G0/G1, S and G2/M cells were calculated using BD FACSDiva software.
Statistical analysis
Results are expressed as mean values (±SD) and statistical comparisons were carried out using one‐way ANOVA (SPSS, SPSS Inc., Chicago, IL, USA). Statistical significance was accepted within the 95% confidence limit (P < 0.05).
Results
Direct effect of melatonin on SK‐LU‐1 cells and PBMC in culture
MTT reduction assay of SK‐LU‐1 cells incubated for 24 h with melatonin in concentrations from 1 nm to 1 mm showed significantly reduced viability (92.7 ± 2.7% compared to control), whereas effect of melatonin at lower concentrations was not seen (Fig. 1a). On the contrary, two lower melatonin doses (1 nm and 1 μm) significantly increased PBMC viability by 11.3 ± 2.0% and 13.2 ± 1.3% respectively (Fig. 1b). Thus, for further experiments the medium (1 μm) melatonin dose, being non‐toxic directly to SK‐LU‐1 lung cancer cells and significantly stimulating viability of PBMC, was used.
Figure 1.
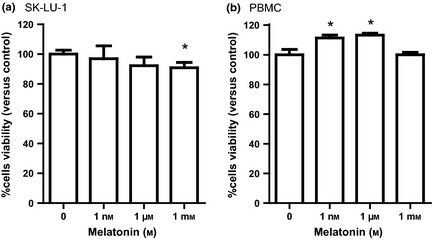
Effect of melatonin on viability of SK ‐ LU ‐1 (a) and human PBMC (b) in culture, evaluated by MTT reduction assay. Viability of melatonin‐treated cells expressed as percent versus control, cultured without melatonin addition. Triplicate samples were averaged for each group. Error bars show SD of triplicate experiments (n = 9). *P < 0.05 versus untreated cells.
Enhancement of SK‐LU‐1 apoptosis by melatonin in PBMC co‐culture
The effect of melatonin on SK‐LU‐1 cell apoptosis was evaluated in co‐culture with PBMC (Fig. 2). Percentage of annexin V‐ and PI‐stained cells in co‐culture (Fig. 2b–e) was compared to that in control SK‐LU‐1 cells incubated alone (Fig. 2a). Additional presence of melatonin in group II and IV increased late apoptotic cells (both annexin V and PI stained) to 9.5% and 11.7%, respectively (Fig. 2c and 2e) compared to 5.0% in group I (Fig. 2b). Ratio of percentage of annexin V/PI‐stained SK‐LU‐1 cells measured in each co‐culture condition versus those from control group I was calculated, Fig. 2f. Co‐culture of SK‐LU‐1 with PBMC significantly increased the ratio of apoptotic SK‐LU‐1 cells by 1.5 ± 0.2 times. Addition of melatonin to co‐culture (group II) significantly increased level of apoptosis 2.1 ± 0.3 times. Co‐culture of SK‐LU‐1 with melatonin pre‐treated PBMC (group III) also increased the level of apoptosis to 2.1 ± 0.3 times, which further increased to 2.3 ± 0.1 times in additional presence of melatonin (group IV).
Figure 2.
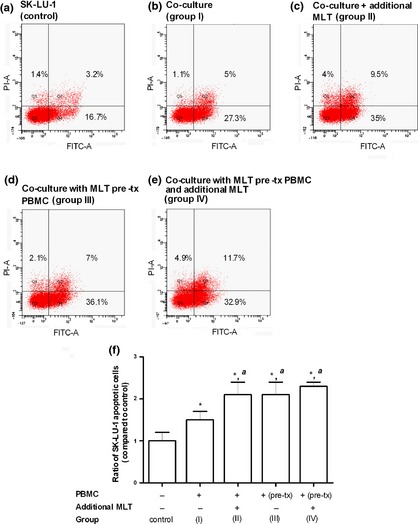
Apoptosis of SK‐LU‐1 cells co‐cultured for 24 h with PBMC in the absence or presence of melatonin, measured by flow cytometry after staining with annexin V/PI. Flow cytometry results of apoptosis are shown in four subpopulations which indicate: early apoptotic cells (lower right), late apoptotic cells (upper right), normal cells (lower left) and necrotic cells (upper left). (a) SK‐LU‐1 cell line alone (control); (b) SK‐LU‐1 co‐cultured with PBMC (group I); (c) SK‐LU‐1 co‐cultured with PBMC in the presence of melatonin (group II); (d) SK‐LU‐1 co‐cultured with PBMC pre‐treated (pre‐tx) with melatonin (group III); (d) SK‐LU‐1 co‐cultured in the presence of melatonin with PBMC pre‐treated with melatonin (group IV); and level of SK‐LU‐1 cell apoptosis expressed as ratio of percentage of apoptotic cells measured in the various co‐culture conditions versus control – bar graph (mean ± SD of at least three experiments). *P < 0.05 versus untreated SK‐LU‐1 cells, a P < 0.05 versus SK‐LU‐1 in co‐culture system.
Enhancement of SK‐LU‐1 intracellular oxidative stress by melatonin in PBMC co‐culture
Intracellular level of O2 •− in SK‐LU‐1 cells co‐cultured with PBMC (group I) significantly increased (1.5 ± 0.1‐fold) in comparison to the control culture, while melatonin addition under any conditions (group II to IV) did not modify this effect (Fig. 2a). In the same co‐culture conditions, intracellular level of H2O2 did not change significantly (0.7 ± 0.2‐fold) and was not further modified by melatonin addition (Fig. 3b). Contrarily, intracellular content of GSH decreased significantly in SK‐LU‐1 cells co‐cultured with PBMC alone (0.8‐fold) and this effect was significantly reinforced by melatonin addition to the co‐culture (Fig. 3c).
Figure 3.
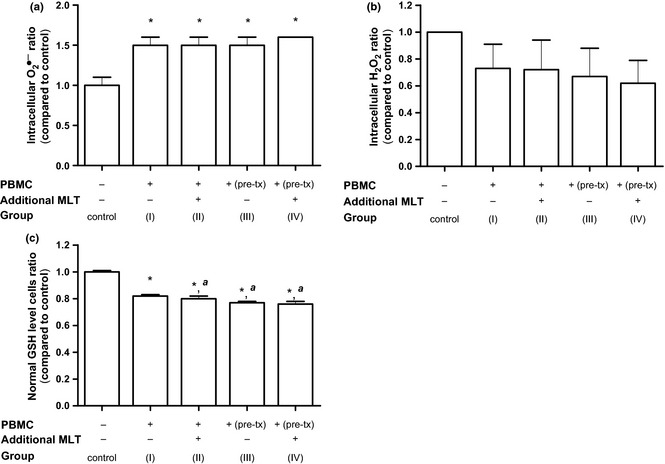
Relative intracellular content of (a) superoxide anion (O 2 •− ), (b) hydrogen peroxide (H 2 O 2 ) and (c) glutathione (GSH) in SK‐LU‐1 cells co‐cultured with PBMC in the presence of melatonin under the different experimental conditions. Ratio of intracellular O2 •− and H2O2 expressed as fold of fluorescence intensity measured in co‐culture conditions versus control. Ratio of normal GSH level cells expressed as fold of normal GSH level cells (%) measured in co‐culture conditions versus control. Error bars – SD of at least two experiments (n = 6). *P < 0.05 versus untreated SK‐LU‐1 cells, a P < 0.05 versus SK‐LU‐1 in co‐culture system.
G0/G1 phase of SK‐LU‐1 cells was arrested by melatonin in PBMC co‐culture
Cell cycle phases under co‐culture conditions (Fig. 4c–f) were compared to the control group of SK‐LU‐1 cells incubated alone (Fig. 4a). Melatonin significantly increased numbers of SK‐LU‐1 cells in G2/M from 20.8 ± 0.0% to 22.4 ± 0.7% (Fig. 4b) compared control SK‐LU‐1 incubated alone (Fig. 4a). Co‐culturing SK‐LU‐1 with PBMC (group I, Fig. 4c) significantly reduced frequency of G2/M phase to 18.8 ± 0.7%, having no effect on G0/G1 and S phases. Under co‐culture conditions supplemented with melatonin (group II and IV, Fig. 4d and 4f), a significant increase in G0/G1 phase from 59.5 ± 2.5% to 63.3 ± 0.7–64.2 ± 0.7% concomitant with significant reduction in frequency of G2/M phase cells (16.5 ± 0.4 – 15.8 ± 0.4%) was observed. However, cell number in G0/G1 phase of SK‐LU‐1 in melatonin pre‐treated PBMC co‐culture (group III, Fig. 4e) was similar to co‐culture alone (group I).
Figure 4.
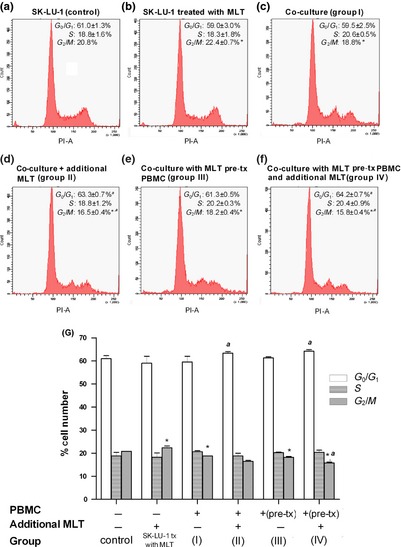
Alterations in cell cycle phase frequency measured in SK‐LU‐1 cell line co‐cultured PBMC and differently supplemented with melatonin; evaluated by flow cytometry. Each experiment was repeated twice, while DNA fraction profile from one representative experiment is shown: (a) SK‐LU‐1 cell alone (control); (b) SK‐LU‐1 treated with melatonin; (c) SK‐LU‐1 co‐cultured with PBMC (group I); (d) SK‐LU‐1 co‐cultured with PBMC in the presence of melatonin (group II); (e) SK‐LU‐1 co‐cultured with PBMC pre‐treated with melatonin (group III); and (f) SK‐LU‐1 co‐cultured in the presence of melatonin with PBMC pre‐treated with melatonin (group IV). (g) Percentage of cell number of each cell cycle phase found in differently treated co‐culture with PBMC compared to those incubated alone, – bar graph (mean ± SD of duplicate experiments). *P < 0.05 versus untreated SK‐LU‐1 cells, a P < 0.05 versus SK‐LU‐1 in co‐culture system.
Discussion
To investigate the widely accepted direct anti‐tumour activity of melatonin 12, 14, 15, 16, 18 on NSCLC treatment, we elaborated an experimental protocol in which the SK‐LU‐1 cisplatin‐sensitive lung cancer cell line was co‐cultured with human PBMC pre‐treated or/and supplemented with melatonin. Our preliminary experiments demonstrated that melatonin added at high dose (1 mm) inhibited SK‐LU‐1 cell growth after 24 h incubation. This supports the result of Sanchez‐Sanchez et al. (2011) obtained with a different type of lung cancer cell, cisplatin non‐sensitive A549, where 1 mm melatonin inhibited their proliferation after 72 h incubation, without influence on cell antioxidant capacity 35. However, such results are still controversial, as other research groups 36 have not found any change in number of A549 cells in culture after exposure to 0.1 and 1 mm melatonin for 24 h 36. These results suggest that the SK‐LU‐1 cell line might be more sensitive to melatonin than A549 cells, as the inhibitory effect occurred earlier, at 24 h co‐culture, which seems to be more applicable to treatment in cisplatin‐sensitive conditions. These studies also indicate that the response of cancer cell lines varies widely, even under identical treatment conditions 35, 36.
Immunomodulation‐enhancing efficacy of the immune system receives considerable interest as part of a search for new effective anti‐cancer therapies 44. In our experimental protocol, melatonin used at low and medium concentrations (1 nm and 1 μm respectively) and had a mitogenic effect on human PBMCs, in culture. This is in line with the previously reported ability of melatonin to lower production of inhibitory cytokine IL‐10, by Th2 lymphocytes, leading thereby to increase in their proliferation 25. Induction of immune cell proliferation may be mediated via MT2 melatonin membrane receptors as reported in melatonin‐treated mouse splenocytes 45.
A previous study of human melanoma cell line A375 and monocytes in co‐culture demonstrated that melatonin activated monocytes and induced their cytotoxic effects against A375 cells evaluated by MTT assay 34. As preferable melatonin binding to human Th lymphocytes and monocytes has been demonstrated, these subpopulations seem to be the primary melatonin target within human PBMC 46. Thus, we used PBMC co‐culture with SK‐LU‐1 as a model to examine immunomodulatory effects of melatonin on his lung cancer cell line. Flow cytometry performed on the annexin V/PI‐stained cells resulted in increase in frequency of number of apoptotic SK‐LU‐1 cells in co‐culture with melatonin‐activated PBMC. Moreover, pro‐apoptotic effects of co‐culture on SK‐LU‐1 cells was similar, regardless of mode of melatonin addition, melatonin added to the co‐culture (group II) versus pre‐treatment of PBMC with melatonin (group III) versus both (group IV). Results obtained in SK‐LU‐1 co‐culture with melatonin pre‐treated PBMC (group III) particularly suggest immune‐enhancing effects of melatonin exerted before contact with the target cells. More intense late apoptosis observed after additional presence of melatonin in co‐culture (groups II and IV) suggests increase in SK‐LU‐1 cell line sensitivity to enter into the end state. On the other hand, it has been demonstrated that activated PBMC are able to synthesize and secrete endogenous melatonin, exerting intra‐, auto‐ and paracrine immunomodulatory effects 47, 48. Thus, enhanced SK‐LU‐1 cancer cell apoptosis in co‐culture with PBMC might result from immunomodulatory effects of melatonin, of both exo‐ and endogenous origin.
In the experimental protocol applied here, a significant increase in intracellular O2 •− level accompanied by reduction in GSH and no change in H2O2 were observed in SK‐LU‐1 cells co‐cultured with PBMC for 24 h, suggesting involvement of oxidative imbalance of target SK‐LU‐1 cells. Melatonin introduced to the co‐culture caused further depletion of intracellular GSH in the SK‐LU‐1 cells with no effect on O2 •− and H2O2, which can be interpreted as higher oxidative stress of target cells, due to action of melatonin. Reactive oxygen species (ROS) play an important role in normal physiological signalling pathways 49. High levels of ROS, however, increase DNA damage and ATP depletion via inhibition of Akt phosphorylation leading to induction of apoptosis 43, 50, 51. Morrey et al. have shown that induction of H2O2, NO2 and O2 •− in monocytes can be involved in the mechanism of cytotoxic activity of melatonin‐activated monocytes at 0.5–12 h incubation 34. Depletion of GSH in SK‐LU‐1 cells observed in our experimental protocol in vitro might result from scavenging of H2O2. It is also possible that 24 h incubation applied in our protocol (much longer than that described by Morrey et al. (1994)), may be the reason for observed differences in intracellular ROS production.
The low molecular weight intracellular thiol compound, GSH, is central in cell redox buffering capacity and primarily maintains appropriate cell oxidative status 52. Our study showed that SK‐LU‐1 cell depletion of GSH was concomitant with G0/G1 phase arrest induction in co‐culture with melatonin‐supplemented PBMC. These results support published data that alteration in redox state causes a delay in progression from G0/G1 to S phase, in mouse embryo fibroblasts 53. Elevated intracellular ROS content generated in the CaCo‐2 cell line by lipid hydroperoxide, leading to reduction in GSH/GSSG ratio is known to be a factor responsible for cell arrest in G0/G1 phase and diminished proliferation 54. Therefore, SK‐LU‐1 cell cycle arrest observed here, may result from change in intracellular oxidative balance caused by melatonin under co‐culture conditions applied. Increased SK‐LU‐1 cell arrest in G0/G1 observed after additional presence of melatonin in co‐culture (groups II and IV) suggests increase in SK‐LU‐1 cell line sensitivity to enter into G0/G1 phase. A possible mechanism of blocking G1 phase is disruption of the actin cytoskeleton 55 by melatonin as a cytoskeletal modulator 56
In summary, direct cytotoxic activity of melatonin at high doses, on lung cancer cell line SK‐LU‐1 was observed in culture. In contrast, an indirect effect was exhibited at lower doses, enhancing human PBMC to counteract proliferation of cancer cells. Increased apoptotic cell death, arrest of cell cycle phase and imbalance of oxidative status in the cancer cell lie were observed, as the immunomodulatory effect of melatonin in co‐culture. These results imply that melatonin had an indirect effect on these lung cancer cells by enhancement of immunomodulatory activity. Further study is needed to identify mechanism(s) involved in melatonin immunomodulatory action towards malignant cells in co‐culture with immune cells. However, a set of melatonin and cisplatin combination treatment in clinical trials must be performed before application of melatonin as the supplementary (supporting) agent for therapeutic use for NSCLC treatment.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The financial support is acknowledged from Khon Kaen University (KKU) through the Melatonin Research Group (MRG), the Incubation Researcher Project, the Integrated Multidisciplinary Research Cluster under the National Research University Project of Thailand (NRU‐KKU) and KKU Research Funding (552401). We are grateful to Prof. Krystyna Skwarlo‐Sonta and Dr.Jeffrey Johns for their assistance in paper proofing.
References
- 1. Ashworth A, Rodrigues G, Boldt G, Palma D (2013) Is there an oligometastatic state in non‐small cell lung cancer? A systematic review of the literature. Lung Cancer 20, 376–379. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J. Clin. 61, 69–90. [DOI] [PubMed] [Google Scholar]
- 3. Stahel R, Peters S, Baas P, Brambilla E, Cappuzzo F, De Ruysscher D et al (2013) Strategies for improving outcomes in NSCLC: a look to the future. Lung Cancer 8, 390–395. [DOI] [PubMed] [Google Scholar]
- 4. Stehle JH, Saade A, Rawashdeh O, Ackermann K, Jilg A, Sebesteny T et al (2011) A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J. Pineal Res. 51, 17–43. [DOI] [PubMed] [Google Scholar]
- 5. Reiter RJ, Rosales‐Corral S, Coto‐Montes A, Boga JA, Tan DX, Davis JM et al (2011) The photoperiod, circadian regulation and chronodisruption: the requisite interplay between the suprachiasmatic nuclei and the pineal and gut melatonin. J. Physiol. Pharmacol. 62, 269–274. [PubMed] [Google Scholar]
- 6. Hardeland R, Madrid JA, Tan DX, Reiter RJ (2012) Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 52, 139–166. [DOI] [PubMed] [Google Scholar]
- 7. Peres MF (2011) Melatonin for migraine prevention. Curr. Pain Headache Rep. 15, 334–335. [DOI] [PubMed] [Google Scholar]
- 8. Miano S, Parisi P, Pelliccia A, Luchetti A, Paolino MC, Villa MP (2008) Melatonin to prevent migraine or tension‐type headache in children. Neurol. Sci. 29, 285–287. [DOI] [PubMed] [Google Scholar]
- 9. Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX (2009) Melatonin: an established antioxidant worthy of use in clinical trials. Mol. Med. 15, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El‐Sokkary GH (2008) Melatonin and vitamin C administration ameliorate diazepam‐induced oxidative stress and cell proliferation in the liver of rats. Cell Prolif. 41, 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, Gonzalez‐Gallego J (2012) A review of the molecular aspects of melatonin's anti‐inflammatory actions: recent insights and new perspectives. J. Pineal Res. 54, 1–14. [DOI] [PubMed] [Google Scholar]
- 12. Cutando A, Lopez‐Valverde A, Arias‐Santiago S, De Vicente J, De Diego RG (2012) Role of melatonin in cancer treatment. Anticancer Res. 32, 2747–2753. [PubMed] [Google Scholar]
- 13. Liu F, Ng TB, Fung MC (2001) Pineal indoles stimulate the gene expression of immunomodulating cytokines. J. Neural. Transm. 108, 397–405. [DOI] [PubMed] [Google Scholar]
- 14. Ram PT, Dai J, Yuan L, Dong C, Kiefer TL, Lai L et al (2002) Involvement of the mt1 melatonin receptor in human breast cancer. Cancer Lett. 179, 141–150. [DOI] [PubMed] [Google Scholar]
- 15. Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu L et al (2012) Simultaneous modulation of COX‐2, p300, Akt, and Apaf‐1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J. Pineal Res. 53, 77–90. [DOI] [PubMed] [Google Scholar]
- 16. Martin‐Renedo J, Mauriz JL, Jorquera F, Ruiz‐Andres O, Gonzalez P, Gonzalez‐Gallego J (2008) Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J. Pineal Res. 45, 532–540. [DOI] [PubMed] [Google Scholar]
- 17. Ordonez R, Carbajo‐Pescador S, Prieto‐Dominguez N, Garcia‐Palomo A, Gonzalez‐Gallego J, Mauriz JL (2014) Inhibition of matrix metalloproteinase‐9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. J. Pineal Res. 56, 20–30. [DOI] [PubMed] [Google Scholar]
- 18. Buyukavci M, Ozdemir O, Buck S, Stout M, Ravindranath Y, Savasan S (2006) Melatonin cytotoxicity in human leukemia cells: relation with its pro‐oxidant effect. Fundam. Clin. Pharmacol. 20, 73–79. [DOI] [PubMed] [Google Scholar]
- 19. Lowe SW, Lin AW (2000) Apoptosis in cancer. Carcinogenesis 21, 485–495. [DOI] [PubMed] [Google Scholar]
- 20. el‐Domeiri AA, Das Gupta TK (1973) Reversal by melatonin of the effect of pinealectomy on tumor growth. Cancer Res. 33, 2830–2833. [PubMed] [Google Scholar]
- 21. Biemar F, Foti M (2013) Global progress against cancer‐challenges and opportunities. Cancer Biol. Med. 10, 183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swann JB, Smyth MJ (2007) Immune surveillance of tumors. J. Clin. Invest. 117, 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y (2004) Cytokines in cancer immunity and immunotherapy. Immunol. Rev. 202, 275–293. [DOI] [PubMed] [Google Scholar]
- 24. Mellman I, Coukos G, Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480, 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuhlwein E, Irwin M (2001) Melatonin modulation of lymphocyte proliferation and Th1/Th2 cytokine expression. J. Neuroimmunol. 117, 51–57. [DOI] [PubMed] [Google Scholar]
- 26. Arias J, Melean E, Valero N, Pons H, Chacin‐Bonilla L, Larreal Y et al (2003) Effect of melatonin on lymphocyte proliferation and production of interleukin‐2 (IL‐2) and interleukin‐1 beta (IL‐1 beta) in mice splenocytes. Invest. Clin. 44, 41–50. [PubMed] [Google Scholar]
- 27. Garcia‐Maurino S, Gonzalez‐Haba MG, Calvo JR, Goberna R, Guerrero JM (1998) Involvement of nuclear binding sites for melatonin in the regulation of IL‐2 and IL‐6 production by human blood mononuclear cells. J. Neuroimmunol. 92, 76–84. [DOI] [PubMed] [Google Scholar]
- 28. Garcia‐Maurino S, Gonzalez‐Haba MG, Calvo JR, Rafii‐El‐Idrissi M, Sanchez‐Margalet V, Goberna R et al (1997) Melatonin enhances IL‐2, IL‐6, and IFN‐gamma production by human circulating CD4+ cells: a possible nuclear receptor‐mediated mechanism involving T helper type 1 lymphocytes and monocytes. J. Immunol. 159, 574–581. [PubMed] [Google Scholar]
- 29. Garcia‐Maurino S, Pozo D, Carrillo‐Vico A, Calvo JR, Guerrero JM (1999) Melatonin activates Th1 lymphocytes by increasing IL‐12 production. Life Sci. 65, 2143–2150. [DOI] [PubMed] [Google Scholar]
- 30. Huang Y‐M LH, Wei J‐E, Lin J, Zhou R‐X (2008) Effect of pinealectomy and melatonin on IL‐7 expression of rat thymic epithelial cells. Acta Anat. Sin. 39, 901–905. [Google Scholar]
- 31. Mocchegiani E, Perissin L, Santarelli L, Tibaldi A, Zorzet S, Rapozzi V et al (1999) Melatonin administration in tumor‐bearing mice (intact and pinealectomized) in relation to stress, zinc, thymulin and IL‐2. Int. J. Immunopharmacol. 21, 27–46. [DOI] [PubMed] [Google Scholar]
- 32. Maestroni GJ, Covacci V, Conti A (1994) Hematopoietic rescue via T‐cell‐dependent, endogenous granulocyte‐macrophage colony‐stimulating factor induced by the pineal neurohormone melatonin in tumor‐bearing mice. Cancer Res. 54, 2429–2432. [PubMed] [Google Scholar]
- 33. Lissoni P, Rovelli F, Brivio F, Brivio O, Fumagalli L (1998) Circadian secretions of IL‐2, IL‐12, IL‐6 and IL‐10 in relation to the light/dark rhythm of the pineal hormone melatonin in healthy humans. Nat. Immun. 16, 1–5. [DOI] [PubMed] [Google Scholar]
- 34. Morrey KM, McLachlan JA, Serkin CD, Bakouche O (1994) Activation of human monocytes by the pineal hormone melatonin. J. Immunol. 153, 2671–2680. [PubMed] [Google Scholar]
- 35. Sanchez‐Sanchez AM, Martin V, Garcia‐Santos G, Rodriguez‐Blanco J, Casado‐Zapico S, Suarez‐Garnacho S et al (2011) Intracellular redox state as determinant for melatonin antiproliferative vs cytotoxic effects in cancer cells. Free Radic. Res. 45, 1333–1341. [DOI] [PubMed] [Google Scholar]
- 36. Fic M, Podhorska‐Okolow M, Dziegiel P, Gebarowska E, Wysocka T, Drag‐Zalesinska M et al (2007) Effect of melatonin on cytotoxicity of doxorubicin toward selected cell lines (human keratinocytes, lung cancer cell line A‐549, laryngeal cancer cell line Hep‐2). In Vivo 21, 513–518. [PubMed] [Google Scholar]
- 37. Feighery C, Whelan CA, Weir DG, Greally JF (1978) In vitro studies of suppressor cell function in human peripheral blood mononuclear cells. Clin. Exp. Immunol. 32, 459–465. [PMC free article] [PubMed] [Google Scholar]
- 38. Gomes NE, Brunialti MK, Mendes ME, Freudenberg M, Galanos C, Salomao R (2010) Lipopolysaccharide‐induced expression of cell surface receptors and cell activation of neutrophils and monocytes in whole human blood. Braz. J. Med. Biol. Res. 43, 853–858. [DOI] [PubMed] [Google Scholar]
- 39. Yeap SK, Alitheen NB, Ali AM, Omar AR, Raha AR, Suraini AA et al (2007) Effect of Rhaphidophora korthalsii methanol extract on human peripheral blood mononuclear cell (PBMC) proliferation and cytolytic activity toward HepG2. J. Ethnopharmacol. 114, 406–411. [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Zhu Y, Zhang L, Tian W, Hua S, Zhao J et al (2012) Insulin promotes proliferation, survival, and invasion in endometrial carcinoma by activating the MEK/ERK pathway. Cancer Lett. 322, 223–231. [DOI] [PubMed] [Google Scholar]
- 41. Park WH, Han YW, Kim SH, Kim SZ (2007) An ROS generator, antimycin A, inhibits the growth of HeLa cells via apoptosis. J. Cell. Biochem. 102, 98–109. [DOI] [PubMed] [Google Scholar]
- 42. Wan XS, Zhou Z, Kennedy AR (2003) Adaptation of the dichlorofluorescein assay for detection of radiation‐induced oxidative stress in cultured cells. Radiat. Res. 160, 622–630. [DOI] [PubMed] [Google Scholar]
- 43. Bishayee K, Ghosh S, Mukherjee A, Sadhukhan R, Mondal J, Khuda‐Bukhsh AR (2013) Quercetin induces cytochrome‐c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: signal cascade and drug‐DNA interaction. Cell Prolif. 46, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sliwkowski MX, Mellman I (2013) Antibody therapeutics in cancer. Science 341, 1192–1198. [DOI] [PubMed] [Google Scholar]
- 45. Drazen DL, Nelson RJ (2001) Melatonin receptor subtype MT2 (Mel 1b) and not mt1 (Mel 1a) is associated with melatonin‐induced enhancement of cell‐mediated and humoral immunity. Neuroendocrinology 74, 178–184. [DOI] [PubMed] [Google Scholar]
- 46. Gonzalez‐Haba MG, Garcia‐Maurino S, Calvo JR, Goberna R, Guerrero JM (1995) High‐affinity binding of melatonin by human circulating T lymphocytes (CD4+). FASEB J. 9, 1331–1335. [DOI] [PubMed] [Google Scholar]
- 47. Carrillo‐Vico A, Lardone PJ, Fernandez‐Santos JM, Martin‐Lacave I, Calvo JR, Karasek M et al (2005) Human lymphocyte‐synthesized melatonin is involved in the regulation of the interleukin‐2/interleukin‐2 receptor system. J. Clin. Endocrinol. Metab. 90, 992–1000. [DOI] [PubMed] [Google Scholar]
- 48. Carrillo‐Vico A, Guerrero JM, Lardone PJ, Reiter RJ (2005) A review of the multiple actions of melatonin on the immune system. Endocrine 27, 189–200. [DOI] [PubMed] [Google Scholar]
- 49. Finkel T (2011) Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Panieri E, Gogvadze V, Norberg E, Venkatesh R, Orrenius S, Zhivotovsky B (2013) Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radic. Biol. Med. 57, 176–187. [DOI] [PubMed] [Google Scholar]
- 51. Lo YL, Wang W (2013) Formononetin potentiates epirubicin‐induced apoptosis via ROS production in HeLa cells in vitro. Chem. Biol. Interact. 205, 188–197. [DOI] [PubMed] [Google Scholar]
- 52. Deponte M (2013) Glutathione catalysis and the reaction mechanisms of glutathione‐dependent enzymes. Biochim. Biophys. Acta 5, 2. [DOI] [PubMed] [Google Scholar]
- 53. Menon SG, Sarsour EH, Spitz DR, Higashikubo R, Sturm M, Zhang H et al (2003) Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 63, 2109–2117. [PubMed] [Google Scholar]
- 54. Gotoh Y, Noda T, Iwakiri R, Fujimoto K, Rhoads CA, Aw TY (2002) Lipid peroxide‐induced redox imbalance differentially mediates CaCo‐2 cell proliferation and growth arrest. Cell Prolif. 35, 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang S, Ingber DE (2002) A discrete cell cycle checkpoint in late G(1) that is cytoskeleton‐dependent and MAP kinase (Erk)‐independent. Exp. Cell Res. 275, 255–264. [DOI] [PubMed] [Google Scholar]
- 56. Benitez‐King G (2006) Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. J. Pineal Res. 40, 1–9. [DOI] [PubMed] [Google Scholar]


