Abstract
Abstract. Focal adhesion kinase (FAK) was heavily phosphorylated as a function of differentiation of C2C12 mouse skeletal muscle cells. Insulin caused increases in FAK phosphorylation before stabilization in proliferated cells, while in differentiated cells there was a consistent transient inhibition of FAK phosphorylation before stimulation. The expression level of FAK was unaltered. Specific inhibition of insulin receptor tyrosine kinase activity abolished the insulin‐mediated dephosphorylation of FAK. The data strongly indicate that FAK tyrosine phosphorylation, necessary for skeletal muscle differentiation, is modulated by insulin. Thus, for the first time, we report the differential regulation of FAK tyrosine phosphorylation by insulin during skeletal muscle differentiation.
Introduction
In skeletal muscle, the balance between myogenic cell proliferation and terminal differentiation is strongly influenced by peptide growth factors and cytokines (Olson 1992). These factors elicit signalling cascades, predominantly by protein phosphorylation as a result of ligand–receptor interaction, and terminate in the nucleus with the activation of specific genes (Hill & Triesman 1995). However, little information exists regarding the intracellular signalling cascades that mediate the effects of growth factors in myogenesis.
The insulin receptor is a member of the receptor tyrosine kinase family of cell surface receptor (Ebina et al. 1985). Binding of insulin to its receptor results in autophosphorylation of the insulin receptor and its substrates, IRS‐1 and‐2, Shc and Gab‐1 protein (Sun et al. 1991; Pelicci et al. 1992; Holgado‐Madrga et al. 1996). Tyrosine phosphorylation of IRS’s and Shc facilitates the binding of SH2‐domain–containing proteins (Songyang et al. 1993). These include the p85 regulatory subunit of phosphatidylinositol 3‐kinase, Grb2 and the phosphotyrosine phosphatase SHP‐2 (Keller & Lienhard 1994; Myers et al. 1994; White & Kahn 1994). Insulin is known to stimulate differentiation of skeletal muscle cells (Haba et al. 1966) but little is known about the exact mechanism of differentiation.
For most of the cells, cooperation of adhesion‐mediated and growth factor mediated signalling pathways is required for proper growth control. It is now largely accepted that p125 focal adhesion kinase (FAK) may be a point of convergence in the actions of integrins and growth factors, like insulin, platelet‐derived growth factors, and hepatocyte growth factors (Zachary & Rozengurt 1992; Chen & Guan 1994; Vuori & Rouslathi 1994; Clark & Brugge 1995; Ouwens et al. 1996; Baron et al. 1998). FAK phosphorylation and dephosphorylation have been shown to undergo specific alterations by insulin and its receptor, insulin‐like growth factor receptor tyrosine kinases (Baron et al. 1998; Pillay et al. 1995) and by several phosphatases (Konstantopoulos & Clark 1996; Ouwens et al. 1996). Whether there is any interaction between insulin and FAK in muscle cells in terms of their proliferation and/or differentiation, is not known.
To elucidate signal transduction regulated by protein phosphorylation in myogenesis and any possible effect of insulin, we sought to determine comparative tyrosine phosphorylation of proteins in the presence and absence of insulin in proliferative and differentiated C2C12 mouse skeletal muscle cells in culture. We have shown that FAK is a major protein, which undergoes tyrosine phosphorylation in skeletal muscle cells as a function of differentiation. Insulin regulates FAK phosphorylation; however, the effect of insulin on FAK phosphorylation tends to depend upon the morphological and functional status of the cells.
Materials and Methods
Materials
Mouse skeletal muscle cell line, C2C12 was kindly provided by Dr H. Blau, Stanford University School of Medicine, Stanford, CA, USA and Dr J. Dhawan, CCMB, India. Dulbecco’s modified Eagle’s medium (DMEM), horse serum, Protein A‐agarose conjugate was purchased from Gibco BRL, Grand Island, NY, USA. Fetal calf serum (FCS) was purchased from Biological Industries, Kibbutz Beit Haemek, Israel. Bovine insulin and AG1024 were purchased from Calbiochem, San Diego, CA, USA. Mouse monoclonal phosphotyrosine antibody, rabbit polyclonal p125FAK antibody and anti mouse immunoglobulin G isotype (IgG) conjugated to biotin were purchased from Santa Cruz Biotechnology, Sauta Cruz, CA, USA. Other reagents were obtained from Sigma, St. Louis, MO, USA and Biorad, Hercules, CA, USA.
Cell culture
C2C12 cells were proliferated in DMEM supplemented with 15% FCS and antibiotics for 2 days. Differentiation was initiated by shifting 70% confluent cells to DMEM supplemented with 2% horse serum for 3 days (fully differentiated) at 37 °C in 5% CO2 incubator. Under these conditions, proliferated cells had fused to form long multinucleated differentiated myotubes. Cells were washed with phosphate buffered saline (PBS), pH 7.4, and incubated in serum free DMEM overnight before giving 5 min of insulin stimulation (250 nm), unless otherwise indicated. Differentiated myotubes were treated with or without 10 µm AG1024 for 1 h before insulin stimulation (250 nm) for 5 min, as indicated in the text.
Western immunoblotting
Proteins were resolved in SDS‐7%PAGE, transferred to nitrocellulose paper (NC) and blocked overnight at 4 °C in 5% bovine serum albumin (BSA) in TBST (10 mm Tris‐HCl, pH 8.0, 150 mm NaCl, 0.05% Tween‐20). The NC paper was washed and incubated with anti phosphotyrosine antibody or anti‐FAK antibody for 1 h at room temperature. Proteins were detected by alkaline phosphatase conjugated secondary antibody using 5‐bromo‐4‐chloro‐3‐indolylphosphate/nitro blue tetrazolium (BCIP/NBT) as substrate.
Immunoprecipitation
The cells were lysed in Triton‐Hepes lysis buffer (50 mm Hepes, pH 7.5, 1% Triton X‐100, 150 mm NaCl, 1.5 mm MgCl2, 1 mm ethelene glycol bis (2‐aminoethyl ether)‐N,N,N′N′ tetraacetic acid (EGTA), 200 µm Na3VO4, 1 mm phenylmethylsulphonyl fluoride (PMSF), 10 mm sodium pyrophosphate, 30 µg/ml benzamidine, aprotinin and leupeptin, 10 µg/ml each) for 20 min at 4 °C. Lysates were clarified by centrifugation at 60 000 × g for 10 min at 4 °C and pre‐cleared by incubation with normal IgG‐Agarose conjugate for 1 h at 4 °C. FAK was immunoprecipitated with antip125FAK antibody and Protein A‐agarose for 3 h at 4 °C. Precipitates were washed three times with lysis buffer, extracted in 2× sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS‐PAGE) sample buffer (Laemmli 1970) and proteins were separated in one‐dimensional SDS‐7% PAGE.
Immunofluorescence microscopy
The cells, cultured on cover slips, were rinsed with Mes buffer (MB) containing 10 mm Mes (pH 6.1), 150 mm NaCl, 5 mm EGTA, 5 mm MgCl2, 5 mm glucose. Cells were permeabilized in 3% paraformaldehyde containing 0.3% Triton X‐100 for 5 min, washed with MB and fixed in 3% paraformaldehyde for 30 min. Washed cells were incubated in blocking solution (20 mm Tris‐HCl (pH 7.5), 150 mm NaCl, 2 mm EGTA, 2 mm MgCl2, 1% BSA, 2% normal goat serum) for 5 min followed by incubation with anti phosphotyrosine antibody for 30 min at room temperature. The cells were washed in TBS (20 mm Tris (pH 7.5), 154 mm NaCl, 2 mm EGTA, 2 mm MgCl2) and incubated with anti mouse IgG conjugated to biotin, and, extravidin conjugated to fluoroscene isothiocyanate (FITC). The cells were observed under fluorescence microscope (Leica, DMIL, Wetzlar, Germany) and photographed.
Protein estimation
The protein concentration was estimated using the bicinchoninic acid method (Smith et al. 1985), using BSA as a standard protein.
Densitometric analysis
Densitometric analysis of the Western blots was done using a GS‐670 Imaging Densitometer (Bio‐Rad) and Molecular Analyst software (version 1.3, Biorad). FAK phosphotyrosine phosphorylation of samples obtained from proliferated cells were given an arbitrary value of 1.0 for calculating the relative phosphorylation of the other samples.
Results
FAK undergoes enhanced tyrosine phosphorylation during differentiation of C2C12 skeletal muscle cells
To determine the differences in the profile of tyrosine phosphorylation, proliferated and differentiated C2C12 cells were lysed, resolved in SDS‐PAGE and immunoblotted with antiphosphotyrosine antibody. Several proteins were found to be differentially phosphorylated between the proliferated and differentiated cells. As shown in Fig. 1, out of all the phosphorylated proteins, one protein of apparent molecular weight between 120 kDa to 130 kDa (p120–130) was found to undergo increases in phosphorylation from the day 1 as a function of differentiation. The maximum increase in phosphorylation was approximately up to 3‐fold in fully differentiated cells, as compared to the proliferated ones (Fig. 1a and b; lanes 1 and 4). Considering the apparent molecular weight, in an attempt to identify the protein, whole cell lysates obtained from proliferated and (fully) differentiated cells were western immunoblotted with anti p125FAK antibody. Figure 1c shows that the anti FAK antibody identified the protein band as FAK.
Figure 1.

Western immunoblot analysis of tyrosine phosphorylation during differentiation of C2C12 skeletal muscle cells. (a) Proteins were western immunoblotted with antiphosphotyrosine antibody. (b) The relative quantity of tyrosine phosphorylation on p120–130 was measured by densitometer. (c) Proteins were western immunoblotted with anti‐FAK antibody. Lane 1: proliferative cells (control), lane 2: 1‐day differentiated cells, lane 3: 2‐day differentiated cells, lane 4: 3‐day differentiated cells.
To further confirm the differential tyrosine phosphorylation of the protein as FAK, similar samples were immunoprecipitated with anti p125FAK antibody and immunoblotted, either with anti phosphotyrosine antibody or with anti p125FAK antibody. As shown in Fig. 2a and b, FAK tyrosine phosphorylation was found to be approximately up to 2.8‐fold more in differentiated muscle cells as compared to the proliferated sample. However, there was no change in the level of expression of FAK between the proliferated and differentiated muscle cells (Fig. 2c).
Figure 2.
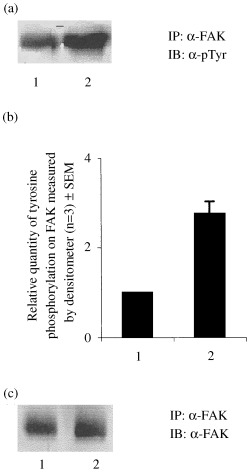
Tyrosine phosphorylation of FAK as a function of differentiation. FAK was immunoprecipitated (IP) from proliferated and differentiated (fully differentiated) C2C12 cells. The immunoprecipitates were resolved in SDS‐7%PAGE and immunoblotted (IB) with antiphosphotyrosine antibody (α‐pTyr) (a) or anti‐FAK (α‐FAK) (c) antibody. (b) Relative quantity of tyrosine phosphorylation on FAK was measured by densitometer. Lane 1: proliferative cells (control), Lane 2: differentiated cells.
To confirm enhanced tyrosine phosphorylation at focal adhesions, proliferated and differentiated cells were probed with antiphosphotyrosine antibody and subjected to immunofluorescence microscopy. Data showed that there was more tyrosine phosphorylation of proteins associated with focal adhesions in differentiated cells as compared to proliferated ones (Fig. 3), which further confirms that FAK tyrosine phosphorylation is associated with differentiation of C2C12 skeletal muscle cells.
Figure 3.

Determination of localization of tyrosine phosphorylations in proliferated and differentiated C2C12 cells. (a) Proliferative cells (b) Differentiated cells.
Insulin causes transient decreases in tyrosine phosphorylation of FAK in differentiated C2C12 skeletal muscle cells
To determine the effect of insulin on tyrosine phosphorylation of FAK, the proliferative and differentiated cells were stimulated with or without insulin for varying periods of time and subjected to western immunoblot probed with anti phosphotyrosine antibody. Figure 4 shows that the extent of tyrosine phosphorylation of FAK is generally higher in differentiated cells than the proliferated cells; however, insulin stimulated tyrosine phosphorylation of proliferated cells reached a maximum of up to 40% at 20 min of stimulation and then it became constant (Fig. 4a and e). In contrast, in the differentiated cells, insulin stimulation gradually decreased tyrosine phosphorylation of FAK to a maximum of 50% at 5 min of stimulation before returning gradually to the initial level of phosphorylation (Fig. 4b and e). The observed change in phosphorylation was not due to change in the concentration of FAK because the same samples immunoblotted with anti FAK antibody revealed no change in the expression of FAK (Fig. 4c and d).
Figure 4.
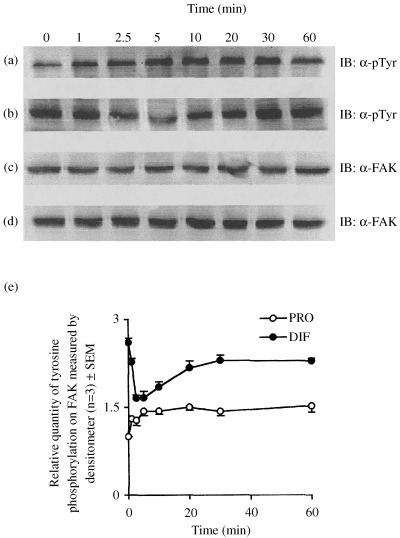
Effect of insulin stimulation on tyrosine phosphorylation of FAK as a function of differentiation. (a) and (c) Proliferated cells (control), (b) and (d) differentiated cells, (e) Relative quantity of tyrosine phosphorylation on FAK was measured by densitometer. IB: immunoblot; α‐pTyr: antiphosphotyrosine antibody; α‐FAK: anti‐FAK antibody; PRO: proliferated cells; DIF: differentiated cells.
To further confirm the above, proliferative and differentiated cells were stimulated with or without insulin and immunoprecipitated with anti‐FAK antibody and immunoblotted with antiphosphotyrosine antibody. The results showed that insulin causes a 30% increase in tyrosine phosphorylation in proliferative cells (Fig. 5a and b; lanes 1 and 2); whereas in differentiated cells the effect was opposite, causing an approximately 45%–50% decrease in tyrosine phosphorylation as compared to non‐stimulated differentiated cells (Fig. 5a and b; lanes 3 and 4). The same sample immunoprecipitated with anti FAK antibody and then immunoblotted with anti FAK antibody revealed no change in the expression of FAK (Fig. 5c).
Figure 5.
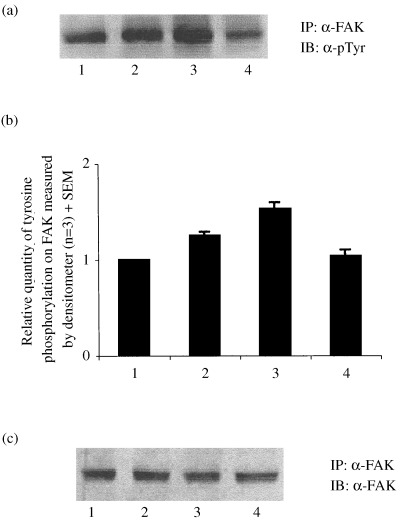
Insulin mediated tyrosine dephosphorylation of FAK as a function differentiation. Proliferative and differentiated cells were stimulated with or without insulin (250 nm) for 5 min and FAK were immunoprecipitated (IP) with anti‐FAK antibody and western immunoblotted (IB) with antiphosphotyrosine (α‐pTyr) antibody (a) and anti‐FAK (α‐FAK) antibody (c). (b) The relative quantity of tyrosine phosphorylation on FAK was measured by densitometer. Lane 1: proliferative cells (control), lane 2: proliferative cells stimulated with insulin, lane 3: differentiated cells, lane 4: differentiated cells stimulated with insulin.
To further confirm whether or not transient dephosphorylation of FAK was insulin mediated, differentiated myotubes were treated with AG1024, a specific inhibitor of insulin and IGF‐1 receptor tyrosine kinase (Parrizas et al. 1997), before insulin stimulation. Results showed that treatment with AG1024 abolished the insulin‐mediated dephosphorylation of FAK (Fig. 6a and b).
Figure 6.

Effect of AG1024 on insulin mediated tyrosine dephosphorylation of FAK in differentiated myotubes. Differentiated cells were stimulated with or without insulin (250 nm) for 5 min after being treated with or without 10 µm AG1024 for 1 h and FAK was immunoprecipitated (IP) with anti‐FAK antibody. (a) Proteins were western immunoblotted (IB) with antiphosphotyrosine antibody (α‐pTyr). (b) The relative quantity of tyrosine phosphorylation on FAK was measured by densitometer. Lane 1: differentiated cells (control), lane 2: differentiated cells stimulated with insulin, lane 3: differentiated cells stimulated with insulin after 1 h treatment with AG1024.
Discussion
Among the various signalling proteins that accumulate at focal adhesions, FAK plays a central role in transmitting the integrin‐mediated signals (Schlaepfer & Hunter 1998). FAK has been implicated in controlling cellular responses to the engagement of cell surface integrins, including cell spreading, survival and proliferation (Schlaepfer et al. 1999). By ectopic expression of integrin subunits, it has been elegantly demonstrated that FAK tyrosine phosphorylation is associated with myoblast cell cycle withdrawal (Sastry et al. 1999). In smooth muscle, FAK has been associated with contraction (Tang & Gunst 2001) and proliferation (Taylor et al. 2001). But no study was undertaken relating FAK with differentiation of smooth muscle cells. Moreover, the smooth and skeletal muscle tissues are composed of distinct cell types of different embryological origin that express related but distinct isoforms of the structural genes used for contraction (Graves & Yoblanka‐Reuveni 2000) and MyoD, one of the most important transcription factors is not involved in smooth muscle differentiation (Davidson & Morenge 2000). Therefore, data obtained in smooth muscle may not be comparable with skeletal muscle. We have reported for the first time that there is an increase in tyrosine phosphorylation of FAK during differentiation, which signifies the involvement of FAK in myogenic differentiation.
FAK is known to play important roles in signalling by interacting with tyrosine kinase receptors, such as PDGF and hepatocyte growth factor (Matsummato et al. 1994; Rankin & Rozengurt 1994). In contrast to other tyrosine kinase receptors, which induce tyrosine phosphorylation of FAK, it has been reported that in fibroblasts, insulin promotes a decrease in tyrosine phosphorylation (Knight et al. 1995; Pillay et al. 1995; Konstantopoulos & Clark 1996). Recently, it has been reported that insulin causes FAK phosphorylation in non‐attached cells, whereas dephosphorylation occurred in attached cells (Baron et al. 1998). In this paper, we are demonstrating for the first time, regulation of differentiation specific FAK tyrosine phosphorylation by insulin.
Skeletal muscle is one of the major targets of insulin and IGF. It is a well‐established fact that in skeletal muscle insulin has a dual effect. It promotes proliferation as well as differentiation involving different signalling pathways (Conejo & Lorenzo 2001; Conejo et al. 2001). Recent reports suggest synergistic effects of insulin and IGF‐1 in promoting myogenesis (Pirskanen, Kiefer & Hauschka 2000). Effects of insulin and IGF‐1 on integrin‐stimulated pathways are different (Fujita et al. 1998). In one such report, insulin has been shown to stimulate dephosphorylation of FAK (Pillay et al. 1995), whereas, in another report, IGF‐1 has been shown to induce phosphorylation of FAK (Casamassima & Rozengurt 1998). At this point there is not enough data to draw homology or infer anything conclusive regarding their function, however, it is evident that both insulin and IGFs have very important role(s) to play in regulating myogenic proliferation and differentiation.
Insulin signal transduction is known to be extremely rapid. Reports had established that insulin‐mediated physiologically‐relevant stimulation of phosphorylation and dephosphorylation of the insulin receptor and some of its known down‐stream substrates, occurs within seconds (White et al. 1985; Kuhne et al. 1995; Yamauchi et al. 1995). We have observed transient dephosphorylation of FAK in differentiated cells after between 2.5 and 5 min of insulin stimulation; however, similar dephosphorylation did not take place in differentiated cells not stimulated with insulin. Moreover, we also observed, that in the presence of a specific inhibitor of insulin receptor tyrosine kinase activity, AG1024, insulin failed to cause any decrease in tyrosine phosphorylation of FAK. Similar reports showing the transient effects of insulin were also available in fibroblasts and CHO cells (Baron et al. 1998; Knight et al. 1995). Therefore, our observations suggest that the rapid transient dephosphorylation is specific and physiologically relevant, and, raises the possibility of involvement of FAK in insulin signalling cascade regulating skeletal muscle differentiation.
The process of cellular differentiation is controlled by soluble factors and by the microenvironment (Dexter et al. 1984). Little is known about the signalling pathways by which substrate elements in the microenvironment influence cell differentiation. Recently, specific cell surface receptors have been described which mediate the interaction between cells and specific components of the extracellular matrix (Carpenter 1984; Horwitz et al. 1985; Pytela et al. 1985). Moreover, it is known that the interaction of integrin, present on the membrane of replicating myoblasts, with extracellular matrix on the substrate, is essential to initiate the terminal stages of myogenic differentiaition (Menko & Boettiger 1987). The major contribution of our study is that we have provided evidence that tyrosine phosphorylation of FAK (and perhaps focal adhesions in general) increase as a function of skeletal muscle differentiation, and this functionality is regulated specifically by insulin.
Acknowledgements
We thank Dr C. L. Kaul, Director, NIPER, for his support in this work. Mr Naresh Kumar and Mr Ashwani Khurana are being acknowledged for their constant support. Mr Ranvir Singh is acknowledged for his assistance in the laboratory. This work supported by Department of Biotechnology (DBT), Ministry of Science and Technology, New Delhi. HLG is a recipient of a junior research fellowship from DBT.
References
- Baron V, Calleja V, Ferrari P, Alengrin F, Obberghen EV (1998) p125FAK, focal adhesion kinase is a substrate for the insulin and insulin‐like growth factor‐1 receptors. J. Biol. Chem. 273, 7162. [DOI] [PubMed] [Google Scholar]
- Carpenter G (1984) Properties of the receptor for epidermal growth factor. Cell 37, 357. [DOI] [PubMed] [Google Scholar]
- Casamassima A, Rozengurt E (1998) Insulin‐like growth factor 1 stimulates tyrosine phosphorylation of p130cas, focal adhesion kinase, and paxillin. J. Biol. Chem. 273, 26149. [DOI] [PubMed] [Google Scholar]
- Chen HC, Guan JL (1994) Stimulation of phosphatidylinositol 3′–kinase association with focal adhesion kinase by platelet‐derived growth factor. J. Biol. Chem. 269, 31229. [PubMed] [Google Scholar]
- Clark EA, Brugge JS (1995) Integrins and signal transduction pathways: the road taken. Science 268, 233. [DOI] [PubMed] [Google Scholar]
- Conejo R., Lorenzo M (2001) Insulin signaling leading to proliferation, survival, and membrane ruffling in C2C12 myoblasts. J. Cell Physiol. 187, 96. [DOI] [PubMed] [Google Scholar]
- Conejo R., Valverde AM, Benito M, Lorenzo M (2001) Insulin produces myogenesis in C2C12 myoblasts by induction of NF‐kappaB and downregulation of AP‐1 activities. J. Cell Physiol. 186, 82. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Morenge M (2000) MSP 25 and p38 MAPK pathways are involved in differentiation of cardiomyocytes. Dev. Biol. 218, 146. [DOI] [PubMed] [Google Scholar]
- Dexter TM, Simmons P, Purnell RA, Spooncer E, Schofield R (1984) The regulation of hemopoietic cell development by the stromal cell environment and diffusible regulatory molecules. Prog. Clin. Biol. Res. 148, 13. [PubMed] [Google Scholar]
- Ebina Y, Ellis L, Jarnagin K, Edery M, Graf L, Ou J, Masiarz F, Kan YW, Goldfine ID, Roth RA, Rutter WJ (1985) The human insulin receptor cDNA: the structural basis for hormone‐activated transmembrane signaling. Cell 40, 747. [DOI] [PubMed] [Google Scholar]
- Fujita T, Maegawa H, Kashiwagi A, Hirai H, Kikkawa R (1998) Opposite regulation of tyrosine phosphorylation of p130cas by insulin and insulin‐like growth factor 1. J. Biochem. 124, 1111. [DOI] [PubMed] [Google Scholar]
- Graves DC, Yoblanka‐Reuveni Z (2000) Vascular smooth muscle cells spontaneously adopt a skeletal muscle phenotype: a unique Myf5(−)/myoD(+) myogenic program. J. Histochem. Cytochem. 48, 1173. [DOI] [PubMed] [Google Scholar]
- Haba GDL, Cooper GW, Elting V (1966) Hormonal requirements for myogenesis of striated muscle in vitro: insulin and somatotropin. Proc. Natl. Acad. Sci. USA 56, 1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS, Triesman R (1995) Transcriptional regulation by extracellular signals: mechanism and specificity. Cell 80, 199. [DOI] [PubMed] [Google Scholar]
- Holgado‐Madrga M, Elmet DR, Moscattello DK, Godwin AK, Wong AJ (1996) A Grb2‐associated docking protein in EGF‐ and insulin receptor signaling. Nature 379, 560. [DOI] [PubMed] [Google Scholar]
- Horwitz A, Duggan K, Greggs R, Decker C, Buck C (1985) The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J. Cell Biol. 101, 2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SR, Lienhard GE (1994) Insulin signaling: the role of insulin receptor substrate 1. Trends Cell Biol. 4, 115. [DOI] [PubMed] [Google Scholar]
- Knight JB, Yamauchi K, Pessin JE (1995) Divergent insulin and platelet‐derived growth factor regulation of Focal Adhesion Kinase (pp125FAK) tyrosine phosphorylation and rearrangement of Actin stress fibre. J. Biol. Chem. 270, 10199. [DOI] [PubMed] [Google Scholar]
- Konstantopoulos N, Clark S (1996) Insulin and insulin‐like growth factor‐1 stimulate dephosphorylation of paxillin in parallel with focal adhesion kinase. Biochem. J. 314, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhne MR, Zhao Z, Lienhard GE (1995) Evidences against dephosphorylation of insulin‐elicite phosphotyrosine proteins in vivo by the phosphatase PTP2C. Biochem. Biophys. Res. Commun. 211, 190. [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227, 680. [DOI] [PubMed] [Google Scholar]
- Matsummato K, Nakamura T, Kramer RH (1994) Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of Focal Adhesion Kinase (p125FAK) and promotes migration and invasion by oral sequamous cell carcinoma cells. J. Biol. Chem. 269, 31807. [PubMed] [Google Scholar]
- Menko AS, Boettiger D (1987) Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell 51, 51. [DOI] [PubMed] [Google Scholar]
- Myers MGJ, Sun XJ, White MF (1994) The IRS‐1 signaling system. Trends Biochem. Sci. 19, 289. [DOI] [PubMed] [Google Scholar]
- Olson EN (1992) Interplay between proliferation and differentiation within the myogenic lineage. Dev. Biol. 154, 261. [DOI] [PubMed] [Google Scholar]
- Ouwens DM, Mikkers HMM, Vanderzon GCM, Stein‐Gerlach M, Ullrich A, Massen JA (1996) Insulin induced tyrosine dephosphorylation of paxillin and focal adhesion kinase requires active phosphotyrosine phosphatase 1D. Biochem. J. 318, 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrizas M, Gazit A, Levitzki A, Wertheimer E, Leroith D (1997) Specific inhibition of insulin‐like growth factor‐1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology 138, 1427. [DOI] [PubMed] [Google Scholar]
- Pelicci G, Lanfrancone L, Grignani F, Mcglade J, Cavallo F, Forni G, Nicoletti I, Grignani F, Pawson T, Pelicci PG (1992) A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 70, 93. [DOI] [PubMed] [Google Scholar]
- Pillay TS, Sasaoka T, Olefsky JM (1995) Insulin stimulates tyrosine dephosphorylation of pp125 focal adhesion kinase. J. Biol. Chem. 270, 991. [DOI] [PubMed] [Google Scholar]
- Pirskanen A, Kiefer JC, Hauschka SD (2000) IGFs, insulin, Shh, bFGF, and TGF‐beta1 interact synergistically to promote somite myogenesis in vitro . Dev Biol. 224, 189. [DOI] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Rouslahti E (1985) Identification and isolation of a 140kDa cell surface glycoprotein with properties expected of a fibronectin receptor. Cell 40, 191. [DOI] [PubMed] [Google Scholar]
- Rankin S, Rozengurt E (1994) Platelet‐derived growth factor modulation of Focal Adhesion Kinase (p125FAK) and paxillin tyrosine phosphorylation in swiss 3T3 cells. J. Biol. Chem. 269, 704. [PubMed] [Google Scholar]
- Sastry SK, Lakonishok M, Wu S, Truong TQ, Turner CE, Horwitz AF (1999) Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J. Cell Biol. 144, 1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Seig DJ (1999) Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71, 435. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T (1998) Integrin signaling and tyrosine phosphorylation: just the FAK. Trend Cell Biol. 8, 151. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzana MD, Fuzimato EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem. 150, 76. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser GW, King F, Roberts T, Ratnofsky S, Lechleider RJ, Neel BG, Birge RB, Fajardo JE, Chou MM, Hanafusa H, Schaffhausen B, Cantley LC (1993) SH2 domains recognize specific phosphopeptide sequence. Cell 72, 767. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF (1991) The structure of insulin receptor substrate IRS‐1 defines a unique signal transduction protein. Nature 352, 73. [DOI] [PubMed] [Google Scholar]
- Tang DD, Gunst SJ (2001) Depletion of focal adhesion kinase by antisense depress contractile activation of smooth muscle. Am. J. Physiol. Cell Physiol. 280, C874. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Mack CP, Nolan K, Regan CP, Owens GK, Parsons JT (2001) Selective expression of an endogenous inhibitor of FAK regulates proliferation and migration of vascular smooth muscle cells. Mol. Cell Biol. 21, 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K, Rouslathi E (1994) Association of insulin receptor substrate‐1 with integrins. Science 266, 1576. [DOI] [PubMed] [Google Scholar]
- White MF, Kahn CR (1994) The insulin signaling system. J. Biol. Chem. 269, 1. [PubMed] [Google Scholar]
- White MF, Maron R, Kahn CR (1985) Insulin stimulates tyrosine phosphorylation of a Mr‐185 000 protein in intact cells. Nature 318, 183. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Ribon V, Saltiel AR, Pessin JE (1995) Identification of the major SHPTP2‐binding protein that is tyrosine‐phosphorylated in response to insulin. J. Biol. Chem. 270, 17716. [DOI] [PubMed] [Google Scholar]
- Zachary I, Rozengurt E (1992) Focal Adhesion Kinase (pp125FAK): point of convergence in the action of neuropeptide, integrins and oncogenes. Cell 71, 891. [DOI] [PubMed] [Google Scholar]


