Abstract
Objectives: Age‐related changes are common in many tissues and organs. However, cell‐related causes in human alveolar bone remain unclear. This study has been carried out to explore the possibility that advancing age might change the biological characteristics of alveolar osteoblasts (AOBs) in women.
Materials and methods: Alveolar osteoblasts from women donors (five women aged 33–38 years and five women aged 62–68 years) were cultured in vitro. The cells were serially passaged and maximal lifespan evaluated. Cell viability, ultramicrostructure and osteogenic differentiation ability were determined respectively, using MTT assay, transmission electron microscopy, alkaline phosphatase (ALP) activity assay and von Kossa staining assay. These parameters of the two groups of AOBs were evaluated.
Results: When compared with cells from young adult donors, AOBs from elderly women exhibited lower maximal lifespan (P < 0.05). Mean rate of population doubling was lower in elderly donor cells compared to those from young adult cells (P < 0.05). Organelles from AOBs of elderly donors were much fewer than those from young donors. MTT value of elderly donor cells was significantly lower than those of young adult donors from day 2 (P < 0.05). Relative ratio of ALP activity in elderly donor cells was significantly lower than those of the young womens’ cells at 8, 12, 16 and 20 days (P < 0.05). Calcium nodules of young adult donors’ specimens were significantly more numerous and larger than those from elderly donors.
Conclusions: Comparison of biological characteristics of AOBs from young adult women with those from elderly women in vitro revealed differences in proliferative capacity and bone formation functions, which decreased with aging. These data indicate that aging may play an important role in pathogenesis of human AOBs loss.
Introduction
Age plays an important role in bone modelling processes during the whole life span. Two important types of cells are involved, osteoblasts (OB) are responsible for bone formation and originate from bone marrow cells. Osteoclasts (OC) responsible for bone resorption, originate from haematopoietic cells. In general, bone turnover is the balance between bone formation function by OBs and the bone resorption function of OCs, which are results of interactions between multiple factors including quantity and activity of OB and OC, and presence of hormones, cytokines and growth factors (1, 2, 3, 4). According to some studies in bone research, balancing the process is affected by reduction in osteoblast proliferation, differentiation, activity, and lifespan, and increase in osteoclast activity with aging (5, 6, 7, 8, 9, 10, 11, 12, 13). Age‐related bone loss results from unbalanced bone turnover, which universally occurs in animal and humans.
In animal models, previous research has shown marked reduction in numbers and activity of stromal osteogenic cells, from old animals in vitro (5, 6, 7, 8, 9) increase in numbers of bone marrow cells capable of forming osteoclasts, in old mice (5). In vivo, research has investigated formation of new bone after expansion in palatal sutures, in rats of four different ages, and found that new bone induced by expansion, decreased in an age‐dependent manner and increase in alkaline phosphatase (ALP) activity was markedly less in old rats (11). In a fracture model in mice, bone formation has been found to be delayed in middle‐aged and elderly animals (12). Age‐related changes also exist in osteogenic cells of humans. Osteogenic cells from human calvaria and vertebral bodies of thoracic and lumbar spine, show decline in osteoblastic cell proliferation and osteogenic potential, during aging (10, 13, 14). Bone marrow stromal cells (BMSCs) from those of different ages have different maximal lifespans, and those from old donors have lower maximal lifespan compared to cells from young donors (15). Likewise, there was an increase in level of apoptosis and decrease in cell proliferation and osteoblast differentiation in human marrow stromal cells, which appeared age‐dependent (16).
Bone microenvironment is also responsible for impaired osteoprogenitor cell recruitment and differentiation. With aging, extracellular fluid around osteogenic cells might exhibit increased inhibitory effects on osteoblasts (17, 18). The bone microenvironment contains a considerable variety of elements including hormones, growth factors and cytokines, secreted by osteoblasts and other cells, specially hormones in post‐menopausal women. Obvious changes occur in women during their lifetimes, and they are highly prone to developing osteoporosis and other bone loss diseases with increasing age. Numbers of colony‐forming units expressing alkaline phosphatase (CFU‐APs) harvested from human bone marrow decrease significantly with age in women, but not in men (19). Decrease in osteogenic cells contributes to decrease in bone mineralization in elderly women.
However, age‐related changes in osteoblasts remain controversial. There have been conflicting reports on effects of age on human marrow‐derived osteogenic cells. Previous research has shown that there were no differences in cell colony formation and osteogenic gene markers between young donors (aged 18–42 years) and elderly healthy donors (aged 66–78 years) (20). A further group has reported that BMSCs from two age groups (aged 68–81 years and 18–29 years) formed similar amounts of mineralized matrix in vitro and of normal lamellar bone in vivo (15), although lifespan was different in different age groups. Thus, these authors pointed out that there may be other mechanisms responsible for age‐related decrease in bone formation.
Human alveolar bone is part of the periodontal tissues and plays an important role in periodontal disease, periodontal regeneration and implantation. Throughout life, alveolar bone in the maxilla and mandible continuously remodels as a result of tooth eruption, forces of mastication and periodontal inflammation. Although alveolar bone appears to resemble skeletal bone, it has its own specific characteristics; its origin is from ectomesenchyme and is formed by intramembranous ossification (21, 22, 23). Thus it is speculated that, with respect to aging, alveolar bone possesses biological characteristics different from those of skeletal bone. To date, changes in human alveolar osteoblasts (AOBs) with aging remain unclear. The present investigation was designed to test the hypothesis that aging is associated with significant changes in cell ultramicrostructure, cell viability and bone‐formation ability of AOBs in the alveolar bone of women.
Materials and methods
Subjects
Approval for conducting experiments on human tissue specimens was obtained from the Committee of Ethics in Research, School of Medicine, Shanghai Jiao Tong University. After informed consent was obtained from each dental patient undergoing oral surgical procedures, discarded alveolar bone was collected. There were five samples from young adult women (aged 33–38), self‐reportedly non‐menopausal, and five samples from elderly women group (aged 62–68) self‐reportedly post‐menopausal. All donors were non‐smokers, were of normal health without systemic disease, not using contraceptives or nor oestrogen substitution, and without overt clinical signs of inflammation in periodontal tissues.
Cell culture
After obtaining alveolar osteoblast explants, they were immersed in sterile Hank’s balanced saline (HBS) (Gibco, Rockville, MD, USA), cleaned of adherent soft tissues, washed in sterile HBS and placed in 60 mm culture plates (Corning Costar, Lowell, MA, USA) containing α‐minimum essential medium (α‐MEM) with l‐glutamine (2 mmol/ml) (Gibco), supplemented with 10% foetal bovine serum (FBS) (Gibco), antibiotics–antimycotics (100 U/ml penicillin G, 100 mg/ml streptomycin sulphate and 0.25 mg/ml amphotericin B) (Gibco). When the cells had migrated out of the explants and reached around 80% confluence, they were collected and serially passaged.
Cell lifespan
The method for determining lifespan of cells was modified from Stenderup et al. (15). In brief, AOBs from young adult and old donors were seeded at 5 × 104/ml in 24‐well plates. Every 7 days, cells were trypsinized in 0.25% trypsin–EDTA (Gibco), counted and reseeded at 5 × 104/ml in 24‐well plates. Cell population growth was determined as number of population doublings (PD). Formula for PD: log N/log 2, where N is the number of cells after culture, divided by initial number of cells seeded. This procedure was repeated until the cells reached their maximal lifespan. Mean PD rate formula: maximal number of PD reached/number of days in culture. Growth arrest was detected as lower number of cells after culture, than initial number of cells seeded.
Cell viability
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) (Sigma‐Aldrich, St Louis, MO, USA) was used to compare cell viability between the two groups. In brief, cells from passage 3–4 were seeded into 96‐well plates (Corning Costar) and detected continuously 8d. MTT at concentration of 0.5 mg/ml was added to each well and culture plates were incubated at 37 °C for 4 h. MTT was converted to its purple formazan product by viable cells. Then, medium was decanted, and 150 μl dimethyl sulphoxide (DMSO) (Sigma‐Aldrich) was added to dissolve the formazan salts. Finally, absorbance of colour present was measured at 590 nm.
Transmission electron microscopy
Cells were fixed in 2% glutaraldehyde for 2 h at 4 °C. After washing twice in phosphate‐buffered saline (PBS), they were then post‐fixed in 1% OsO4 for 2 h. After washing twice in PBS again, cells were then dehydrated (using ethanol in a series of dilutions) before being embedded in epoxy resin 618. Ultrathin sections were then cut using an LKB V ultramicrotome. Sections were stained with saturated uranyl acetate and lead citrate. Specimens were observed using a CM‐120 TEM (Philips, Eindhoven, The Netherlands).
Alkaline phosphatase activity
Activity of ALP was determined in cell lysates. Cells from passage 3–4 of all samples, were cultured in conditional medium (α‐MEM, l‐glutamine, FBS, antibiotics–antimycotics, as before, with 50 mmol/ml ascorbic acid, 10 nmol/ml of dexamethasone and 10 mmol/ml of β‐glycerophosphate). At 0, 4, 8, 12, 16 and 20 days of growth, cells of replicate cultures of each sample were harvested, washed twice in PBS, and lysed using 1% Triton X‐100 at 4 °C for 1 h after culture medium was decanted. Triplicate aliquots from each lysate were added to substrate r‐nitrophenol phosphate, in 2‐amino‐2‐methyl‐1‐propanol buffer (Sigma‐Aldrich) and incubated at 37 °C for 1 h in the dark. Then 0.5 N NaOH was added to stop the reaction and amount of ALP activity was measured by reading absorbance at 405 nm using a microplate reader (Bio‐Tek, Winooski, VT, USA). ALP activities were normalized by cell protein content, and expressed as OD value/h mg protein.
Mineralization assay
The von Kossa assay was used to estimate mineralization in cell layers in a 12‐well plate (Corning Costar); six duplicates were performed for each sample. Fixed cultures were covered with 10 mg/ml silver nitrate solution (Sigma‐Aldrich) and kept for 1 h under UV light. After being rinsed, cultures were added with 50 mg/ml sodium thiosulphate solution (Sigma‐Aldrich) for 2 min and then washed again. Phosphate deposits stained black. Four visual fields in each well were selected and number of stained nodules was counted. Results were expressed as number of stained nodules per well.
Statistical analysis
Each experiment was repeated at least twice. Data are presented as mean ± SEM. Differences between study groups were analysed using Student’s t‐test. P < 0.05 was considered statistically significant.
Results
To differentiate between human alveolar osteoblasts of young adult women and elderly women, biological characteristics of these cells, including their maximal lifespan, ultramicrostructure, cell viability and bone formation activity were investigated.
First, we observed attachment of human alveolar osteoblasts to culture plates, which occurred within minutes after seeding. After 2 h, cytoplasm spread relatively, with apparent bulging in the area of the nucleus. Cell morphology became progressively elongated, and flattened fibroblastic appearance was visible at 24 h. In both groups, AOBs exhibited similar attachment and morphology. Prolonged incubation of cultures was used to detect maximal lifespan of cells of the two groups. Figure 1a shows cumulative PD maximal lifespan was reached at 59 ± 6 PD in young adult donor AOBs and 34 ± 9 PD for cells from elderly donor AOBs (P < 0.05). In addition, mean PD level decreased significantly with advancing age of the donor; that is, 0.31 ± 0.01 PD/day and 0.28 ± 0.02 PD/day for AOBs of young adult and elderly donors, respectively (P < 0.05) (Fig. 1b).
Figure 1.
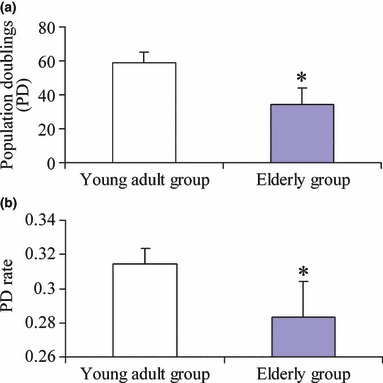
Lifespan difference between two groups of women. (a) Maximum lifespan of human AOBs cultured in vitro measured as maximum number of PDs. (b) Mean PD level of human AOBs cultured in vitro measured as maximal number of PD reached per number of days in culture. Young donors (n = 5) and old donors (n = 5). Results are represented as mean ± SD. *P < 0.05.
Cell viability of AOBs in the young adult group was compared to that of the elderly donor group, during continuous 8d culture. At the beginning, MTT OD value was almost the same in the two groups (P > 0.05). As shown in Fig. 2, from the second day, cell proliferation of AOBs in the young adult group was significantly higher than that of the elderly group (P < 0.05).
Figure 2.
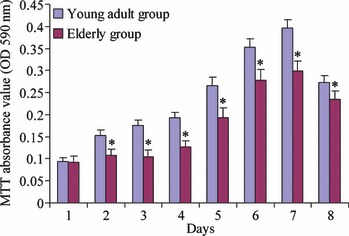
MTT assay. Viability of human alveolar osteoblasts between young adult group and elderly group during continuous 8‐day culture. Young adult donors (n = 5) and elderly donors (n = 5). Results are represented as mean ± SD. *P < 0.05.
As cell ultramicrostructure is closely related to cell function, we observed ultramicrostructure of cells of the two groups using transmission electron microscopy (TEM). From the TEM study, we found cell organelles were strikingly fewer in osteoblasts from elderly donors than those from young adult donors (Fig. 3). These observations indicate that cell activity was decreasing with advancing age.
Figure 3.
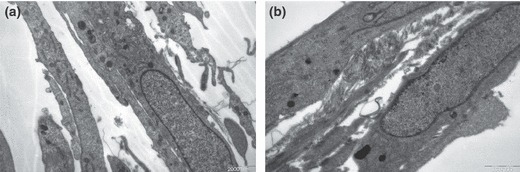
Transmission electron microscopy. Difference between human alveolar osteoblasts of young adult group (a) and elderly group (b) (7500×).
To determine the osteogenic potentials of the AOBs, we tested osteogenic differentiation in vitro. During early phases of culture, ALP activity in the young adult group was similar to that of the elderly group. At day 4, there was still no great difference between the two groups in conditional medium, although activity of ALP increased in both. As shown in Fig. 4, at days 8, 12, 16 and 20, activity of ALP in the young adult group was much higher than that of the elderly group. Activity of ALP then increased very rapidly in AOBs of young adult donors reaching its maximum at day 12. Then it decreased but was still at a higher level than that of the initial culture. AOBs of the elderly group had the same curve over the whole culture period.
Figure 4.
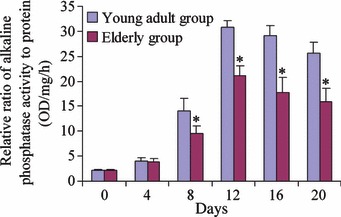
Alkaline phosphatase activity assay. Comparison of ALP activity of human alveolar osteoblasts between young adult donors (n = 5) and elderly donors (n = 5). Differences were significant between the two groups at days 8, 12, 16 and 20 (P < 0.05), labelled *.
Furthermore, we investigated mineralization of AOBs from both age groups. AOBs from both were able to form bone mineralization nodules. However, size of nodules in the young adult group was much larger than those of the elderly group (Fig. 5). Number of mineralization nodules was significantly different between the two groups (P < 0.05), Fig. 6, AOBs from young adult donors formed more mineralization nodules.
Figure 5.
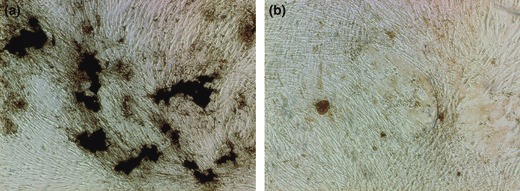
Mineralization assay as shown by von Kossa stain. Comparison of human alveolar osteoblasts bone formation between young adult group (a) and elderly group (b) (40×). Calcium nodules in young adult group were much larger than in elderly group.
Figure 6.
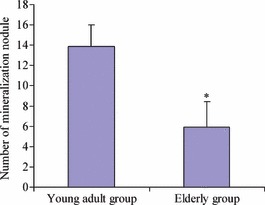
Comparison of calcium nodule number between young adult group and elderly group. Difference was significant between two groups (P < 0.05).
Discussion
In this study, we have compared biological characteristics of human alveolar osteoblasts from young adult subjects with those from elderly subjects. Cell viability and capacity of bone formation, which showed age‐related decreases, were significantly different between the two groups.
In previous studies, number of osteogenic cells has been shown to decrease (5, 13, 15, 24, 25), increase (26), or remain unchanged with advancing age (27). No previous study has examined the relationship between age and maximal proliferative potential of AOBs in vitro. We used the long‐term culture system to detect AOB proliferative ability and found obvious differences in their growth patterns between young and old donors. Maximal lifespan and proliferation rate of the cells decreased as donor age increased, that is negative correlation between age and proliferative potential. To investigate possible mechanisms for decrease in maximal proliferative potential of AOBs of elderly donors, cell viability was examined.
Previously, Cabral & Fernandes have observed that proliferation of human alveolar bone cells at passage 1 from young adult patients (mean age of 34 years) increased until day 21, decreased during the fourth week and increased again (28). However, we found that cell proliferation of AOBs increased until day 7 and decreased at day 8. It is possible that these inconsistent results were due to use of different methods. However, we still found differences between two groups, which is consistent with human osteoblasts from other anatomical bone areas and with animal bone results (5, 10). We also observed utlramicrostructure of AOBs of two groups. In the same in vitro environment, cells from elderly donors contained fewer cell organelles, indicating that cell activities were much lower in the old people than in young adult donors. These decreases with advancing age contribute to bone loss in elderly humans.
Then, we investigated effects of elevated age on function of alveolar osteoblasts. ALP is considered to be a marker of osteogenesis, which represents bone matrix formation ability of osteoblasts. ALP can hydrolyse organic phosphate to increase local concentration of phosphate ions and restrain inhibitors of calcification, which in turn aids processes of calcification. Cabral & Fernandes demonstrated that alveolar bone cells produced high levels of ALP, and activity of the enzyme increased until day 21 and decreased afterwards when cells were at the first passage (28). Perinpanayagam found that ALP activity was maintained during 28 days in differentiation culture and increased rapidly by more than 3‐fold during the first 7 days; it then declined to the 28‐day level which was still 2‐fold higher than that of the initial culture, when using passage 7–9 alveolar bone osteoblasts (29). According to our data, ALP activity increased during the first 12 days and decreased afterwards which still remained at higher levels than those of initial cultures, when using passages 3–4 of alveolar bone osteoblasts. From these findings the summit of ALP activity appeared earlier than high passage number, which indicated that subculture may change the time point of ALP activity and induce osteogenic cell maturation earlier. In our study, ALP activity of AOBs from elderly donors at the same passage reached maximum on the same day as those of AOBs from young adult donors. It seems that senescence caused by subculture, was, to some extent, different from that induced in vivo. At the beginning of conditional culture, there was almost the same level of ALP in the two groups, which was consistent with the study of Justesen et al. (20); however, ALP activity levels of AOBs from elderly donors were much lower than those from young adult donors after 4 days of culture. The possibility is that ability of secretion of ALP or ALP‐positive cell formation in aged AOBs decreases. Similar results on human osteogenic cells have been reported by other groups (11, 15, 19). These data support our further research on mineralization data.
Number and size of calcium nodules provide a rough estimate of capability of the cells osteogenesis. Since cell density when original plating was identical, mineralization was determined by osteoblast function. Calcium nodules in AOBs from young adult donors were more numerous and were larger than those from elderly donors; this suggests that capacity of bone mineralization of AOBs from young adult donors was higher than that from elderly donors. The results are consistent with other studies in vitro and in vivo on humans and animals (8, 10, 11, 30). Age‐related decrease in osteoprogenitor cells and osteogenic potential have been shown in animal models in other studies (5, 6, 7, 31). The possibility, that mineralization decreased in the elderly group, may be related to decrease in osteoprogenitor cells and osteogenic potential, as described in our study.
In addition, another possibility, that AOBs from elderly women have lower ability to form bone, may be related to reduced oestrogen levels in such women in vivo. Oestrogen receptors (ERs) are present on human osteoblasts and mediate anti‐resorptive effects on bone. Also, oestrogen has been shown to prevent glucocorticoid‐induced apoptosis and thus to prolong life of normal functioning osteoblasts (32). One previous piece of research has demonstrated lower induction of ligand concentration‐dependent ER‐alpha in aged women, which indicates that receptor regulation and ligand‐receptor signal transduction diminishes with increasing donor age (33). Another piece of research has found that post‐menopausal and pre‐menopausal bone cells have differences in genes controlled by oestrogen receptor‐alpha (34). In an animal model, there was 73% reduction in cancellous bone at the metaphysis after ovariectomy (35). Meanwhile, oestrogen could inhibit RANKL enhancing function of osteoclastic differentiation of human monocytes, which might contribute to bone formation (36). According to these studies, the oestrogen pathway plays an important role in bone metabolism in post‐menopausal women. In our study, we recorded pre‐menopausal and post‐menopausal status simply according to self‐reporting. There is limitation to interpretation of our results whether or not biological changes are related to menopause and/or oestrogen pathway. While future studies involving relationship between alveolar osteoblasts of women and the oestrogen pathway are required, this study demonstrates that differences in human alveolar osteoblasts between young adult women and elderly women may contribute to our understanding of osteogenic cell characteristics of human alveolar bone, which is important in periodontal treatment, but not yet well understood.
In summary, we clearly observed age‐related changes in alveolar osteoblasts of two groups of women. These decreases in cell proliferation and osteogenic differentiation could be related to advancing age, which may in turn contribute to osteoporosis. Moreover, that the menopause might have an influence on alveolar osteoblasts of women as shown in this study. Further investigations are needed to detect whether the oestrogen pathway is involved in these changes.
Acknowledgements
This study was funded by National Nature Science Foundation of China (30672315), Science and Technology Commission of Shanghai (08DZ2271100) and Shanghai Leading Academic Discipline Project (S30206).
References
- 1. Boyce BF, Hughes DE, Wright KR, Xing L, Dai A (1999) Recent advances in bone biology provide insight into the pathogenesis of bone diseases. Lab. Invest. 79, 83–94. [PubMed] [Google Scholar]
- 2. Aubin JE (1998) Advances in the osteoblast lineage. Biochem. Cell Biol. 76, 899–910. [PubMed] [Google Scholar]
- 3. Manolagas SC (1995) Role of cytokines in bone resorption. Bone 17, 63–67. [DOI] [PubMed] [Google Scholar]
- 4. Manolagas SC, Weinstein RS (1999) New developments in the pathogenesis and treatment of steroid‐induced osteoporosis. J. Bone Miner. Res. 14, 1061–1066. [DOI] [PubMed] [Google Scholar]
- 5. Kahn A, Gibbons R, Perkins S, Gazit D (1995) Age‐related bone loss. A hypothesis and initial assessment in mice. Clin. Orthop. Relat. Res. 313, 69–75. [PubMed] [Google Scholar]
- 6. Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ (1996) Age‐related changes in osteogenic stem cells in mice. J. Bone Miner. Res. 11, 568–577. [DOI] [PubMed] [Google Scholar]
- 7. Quarto R, Thomas D, Liang CT (1995) Bone progenitor cell deficits and the age‐associated decline in bone repair capacity. Calcif. Tissue Int. 56, 123–129. [DOI] [PubMed] [Google Scholar]
- 8. Gazit D, Zilberman Y, Ebner R, Kahn A (1998) Bone loss (osteopenia) in old male mice results from diminished activity and availability of TGF‐beta. J. Cell. Biochem. 70, 478–488. [DOI] [PubMed] [Google Scholar]
- 9. Zhang W, Ou G, Hamrick M, Hill W, Borke J, Wenger K et al. (2008) Age‐related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J. Bone Miner. Res. 7, 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Pollak C, Arnaud E, Renier D, Marie PJ (1997) Age‐related changes in bone formation, osteoblastic cell proliferation, and differentiation during postnatal osteogenesis in human calvaria. J. Cell. Biochem. 64, 128–139. [PubMed] [Google Scholar]
- 11. Kanekawa M, Shimizu N (1998) Age‐related changes on bone regeneration in midpalatal suture during maxillary expansion in the rat. Am. J. Orthod. Dentofacial Orthop. 114, 646–653. [DOI] [PubMed] [Google Scholar]
- 12. Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C et al. (2005) Cellular basis for age‐related changes in fracture repair. Orthop. Res. 23, 1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA (1999) Age‐related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J. Bone Miner. Res. 14, 1115–1122. [DOI] [PubMed] [Google Scholar]
- 14. Mueller SM, Glowacki J (2001) Age‐related decline in the osteogenic potential of human bone marrow cells cultured in three‐dimensional collagen sponges. J. Cell. Biochem. 82, 583–590. [DOI] [PubMed] [Google Scholar]
- 15. Stenderup K, Justesen J, Clausen C, Kassem M (2003) Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33, 919–926. [DOI] [PubMed] [Google Scholar]
- 16. Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS et al. (2008) Age‐related intrinsic changes in human bone marrow‐derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 7, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Egrise D, Vienne A, Martin D, Chaboteaux C, Bergmann P, Schoutens A (1999) Age‐related inhibitory activity of rat bone marrow supernatant on osteoblast proliferation. J. Bone Miner. Res. 14, 2099–2106. [DOI] [PubMed] [Google Scholar]
- 18. Abdallah BM, Haack‐Sørensen M, Fink T, Kassem M (2006) Inhibition of osteoblast differentiation but not adipocyte differentiation of mesenchymal stem cells by sera obtained from aged females. Bone 39, 181–188. [DOI] [PubMed] [Google Scholar]
- 19. Muschler GF, Nitto H, Boehm CA, Easley KA (2001) Age‐ and gender‐related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J. Orthop. Res. 19, 117–125. [DOI] [PubMed] [Google Scholar]
- 20. Justesen J, Stenderup K, Eriksen EF, Kassem M (2002) Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif. Tissue Int. 71, 36–44. [DOI] [PubMed] [Google Scholar]
- 21. Saffar JL, Lasfargues JJ, Cherruau M (1997) Alveolar bone and the alveolar process: the socket that is never stable. Periodontol 2000 13, 76–90. [DOI] [PubMed] [Google Scholar]
- 22. Sodek K, McKee MD (2000) Molecular and cellular biology of alveolar bone. Periodontol 2000 24, 99–126. [DOI] [PubMed] [Google Scholar]
- 23. Zernik JH, Nowroozi N, Liu YH, Maxson R (1997) Development, maturation, and aging of the alveolar bone. New insights. Dent. Clin. North Am. 41, 1–15. [PubMed] [Google Scholar]
- 24. Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE (1999) Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner. Metab. 17, 171–177. [DOI] [PubMed] [Google Scholar]
- 25. Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF (1997) Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J. Orthop. Res. 15, 546–557. [DOI] [PubMed] [Google Scholar]
- 26. Xu CX, Hendry JH, Testa NG, Allen TD (1983) Stromal colonies from mouse marrow: characterization of cell types, optimization of plating efficiency and its effect on radiosensitivity. J. Cell Sci. 61, 453–466. [DOI] [PubMed] [Google Scholar]
- 27. Oreffo RO, Bennett A, Carr AJ, Triffitt JT (1998) Patients with primary osteoarthritis show no change with ageing in the number of osteogenic precursors. Scand. J. Rheumatol. 27, 415–424. [DOI] [PubMed] [Google Scholar]
- 28. Cabral CT, Fernandes MH (2007) In vitro comparison of chlorhexidine and povidone‐iodine on the long‐term proliferation and functional activity of human alveolar bone cells. Clin. Oral Invest. 11, 155–164. [DOI] [PubMed] [Google Scholar]
- 29. Perinpanayagam H, Martin T, Mithal V, Dahman M, Marzec N, Lampasso J et al. (2006) Alveolar bone osteoblast differentiation and Runx2/Cbfa 1 expression. Arch. Oral Biol. 51, 406–415. [DOI] [PubMed] [Google Scholar]
- 30. Zhang H, Lewis CG, Aronow MS, Gronowicz GA (2004) The effects pf patient age on human osteoblasts’ response to Ti‐6Al‐4V implants in vitro. J. Orthop. Res. 22, 30–38. [DOI] [PubMed] [Google Scholar]
- 31. Tsuji T, Hughes FJ, McCulloch CA, Melcher AH (1990) Effects of donor age on osteogenic cells of rat bone marrow in vitro. Mech. Ageing Dev. 51, 121–132. [DOI] [PubMed] [Google Scholar]
- 32. Gohel A, McCarthy MB, Gronowicz G (1999) Estrogen prevents glucocorticoid‐induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology 140, 5339–5347. [DOI] [PubMed] [Google Scholar]
- 33. Ankrom MA, Patterson JA, D’Avis PY, Vetter UK, Blackman MR, Sponseller PD et al. (1998) Age‐related changes in human oestrogen receptor alpha function and levels in osteoblasts. Biochem. J. 333, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kósa JP, Balla B, Speer G, Kiss J, Borsy A, Podani J et al. (2009) Effect of menopause on gene expression pattern in bone tissue of nonosteoporotic women. Menopause 16, 367–377. [DOI] [PubMed] [Google Scholar]
- 35. Baldock PA, Need AG, Moore RJ, Durbridge TC, Morris HA (1999) Discordance between bone turnover and bone loss: effects of aging and ovariectomy in the rat. J. Bone Miner. Res. 14, 1442–1448. [DOI] [PubMed] [Google Scholar]
- 36. García Palacios V, Robinson LJ, Borysenko CW, Lehmann T, Kalla SE, Blair HC (2005) Negative regulation of RANKL‐induced osteoclastic differentiation in RAW264.7 cells by estrogen and phytoestrogens. J. Biol. Chem. 280, 13720–13727. [DOI] [PubMed] [Google Scholar]


