Abstract
Objectives
To explore effects of hepatocyte growth factor (HGF) combined with insulin‐like growth factor 1 (IGF‐1) on transplanted bone marrow mesenchymal stem cells (BMSCs), for treatment of acute myocardial ischaemia.
Materials and methods
After ligation of the left anterior descending artery, rabbits were divided into a Control group, a Factors group (HGF+IGF‐1), a BMSC group and a Factors+BMSCs group. Allogenous BMSCs (1 × 107) and/or control‐released microspheres of 2 μg HGF+2 μg IGF‐1 were intramyocardially injected into infarcted regions. Apoptosis and differentiation of implanted BMSCs, histological and morphological results, and cardiac remodelling and function were evaluated at different time points. In vitro, BMSCs were exposed to HGF, IGF‐1 and both (50 ng/ml) and subsequently proliferation, migration, myocardial differentiation and apoptosis induced by hypoxia, were analysed.
Results
Four weeks post‐operatively, the above indices were significantly improved in Factors+BMSCs group compared to the others (P < 0.01), although Factors and BMSCs group also showed better results than Control group (P < 0.05). In vitro, HGF promoted BMSC migration and differentiation into cardiomyocytes, but inhibited proliferation (P < 0.05), while IGF‐1 increased proliferation and migration, and inhibited apoptosis induced by hypoxia (P < 0.05), but did not induce myocardial differentiation. Combination of HGF and IGF‐1 significantly promoted BMSCs capacity for migration, differentiation and lack of apoptosis (P < 0.05).
Conclusions
Combination of HGF and IGF‐1 activated BMSCs complementarily, and controlled release of the two factors promoted protective potential of transplanted BMSCs to repair infarcted myocardium. This suggests a new strategy for cell therapies to overcome acute ischemic myocardial injury.
Abbreviations
- MI
Myocardial infarction
- ANOVA
Analysis of variance
- BMSCs
Bone marrow mesenchymal stem cells
- DiI
1′‐dioctadecyl‐3,3,3′,3′‐tetramethylindocarbocyanine perchlorate
- cTnT
Cardiac Troponin T
- EDV
End‐diastolic volume
- EF
Ejection fraction
- FACS
Fluorescence activated cell sorting
- FITC
Fluorescein isothiocyanate
- HGF
hepatocyte growth factor
- IA
infarction area
- IGF‐1
insulin‐like growth factor 1
- LAD
left anterior descending artery
- LV
Left ventricle
- OD
Optical density
- RT‐PCR
Reverse transcription polymerase chain reaction
- SPSS
Statistical Product and Service Solutions
- TUNEL
Terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end labelling
- VD
Vessel density
- WT
Wall thickness
Introduction
Bone marrow mesenchymal stem cell (BMSC) transplantation has emerged to be a promising method for treatment of ischaemic heart disease, due to potential of these cells to promote myocardial regeneration after myocardial injury 1, 2. However, some experimental and clinical studies have strongly suggested that effects of cell transplantation might be largely impacted by hostile microenvironment of the ischaemic region 3, 4. Thus, it may be critical to improve the microenvironment of transplanted zones and potential of transplanted BMSCs from hypoxia, for enhancement of efficiency of such therapy.
It has been demonstrated that hepatocyte growth factor (HGF) and insulin‐like growth factor 1 (IGF‐1) can increase angiogenesis of ischaemic regions and improve their hostile microenvironment, consequently promoting myocardial repair, following acute myocardial infarction (AMI) 5, 6, 7, 8. However, it is unclear whether or not HGF and IGF‐1 would enhance protective effects of BMSC transplantation after AMI. To explore the effects of these two factors on BMSCs, the present study set to assess the following parameters: proliferation, migration, differentiation and apoptosis of BMSCs in vitro, transplanted BMSC survival and differentiation in vivo, left ventricular function and remodelling after combined administration of HGF+IGF‐1 and BMSCs.
Materials and methods
The project protocol was approved by the Liaoning Administrative Committee for Laboratory Animals. According to the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health in 1996, all animals received humane care and all procedures were carried out strictly in accordance with best practice.
Isolation and identification of BMSCs
Rabbit BMSCs were prepared as described previously 9. Briefly, BMSCs were isolated from bone marrow using the method of whole BM adherent cultures, and subsequently incubated with DMEM/F12 medium containing 10% foetal bovine serum, 100 μg/ml penicillin and 100 U/ml streptomycin sulphate (Gibco Life Technologies, Paisley, UK) in a 95% humidified incubator at 37 °C and 5% CO2. Forty‐eight hours later, adherent cells were collected, maintained and cultured continuously.
Fluorescence‐activated cell sorting (FACS) was performed for analysis of BMSC immunophenotype with anti‐rabbit CD34, CD44 and CD45 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Results showed they positively expressed CD44 (92.15%) and did not significantly express CD34 (1.79%) or CD45 (1.94%). Before implantation, BMSCs were stained with cross‐linkable membrane dye 1′‐dioctadecyl‐3,3,3′,3′‐tetramethylindocarbocyanine perchlorate (DiI, Invitrogen Corporation, Carlsbad, CA, USA) for cell labelling.
Surgical procedures
The rabbit (2.5 ± 0.3 kg) model of AMI was established by ligation of the mid‐third of the left anterior descending artery (LAD). Thereafter, animals were randomly assigned to four groups: Control group (no treatment, n = 8), Factors group (controlled release of HGF and IGF‐1, n = 8), BMSC group (BMSC transplantation, n = 12) and Factors+BMSCs group (controlled release of HGF and IGF‐1 and BMSC transplantation, n = 12).
In the Control group, 200 μl saline was injected by sterile microinjection at two sites within the infarcted region. For the other three groups, allogenous BMSCs (1 × 107) and/or biodegradable microspheres containing 2 μg IGF‐1 and 2 μg HGF were administrated in the same way. Antibiotics were administrated intramuscularly for 3 days postoperatively. As described in our previous paper 10, the biodegradable microspheres (diameter 1–3 μm and release period of 2–3 weeks) were made from biodegradable polymer, poly D, L‐lactic/glycolic acid (PLGA), which has no effect on activation of BMCSs 11, 12, 13, 14; this has been widely used as delivery material in wide ranges of BMSCs studies 11, 12, 15, 16.
After assessment of left ventricular wall thickness (LVWT) of infarcted regions, end‐diastolic volume (EDV) and ejection fraction (EF) by echocardiography, animals were sacrificed by overdose of potassium chloride. Tissues of ischaemic areas were harvested at different time points, for PCR, western blotting and histological analysis.
In vitro measurement of BMSC proliferation
To further explore mechanisms of effects of HGF and IGF‐1 on BMSCs, the following in vitro experiment was designed to investigate the BMSC proliferation, migration, differentiation and apoptosis after direct exposure to HGF (50 ng/ml) and/or IGF‐1 (50 ng/ml), not released from biodegradable microspheres.
Passage 3 BMSCs growing on glass slides were serum‐starved in serum‐free DMEM/F12 supplemented with 0.5% BSA (wt/vol) for 14 h. Then they were cultured in the above medium containing 0.25% BSA (Control group) and stimuli (in HGF, IGF‐1 or HGF+IGF‐1 groups) for 6 h. Before the end of experiment, the BMSCs were incubated with BrdU (30 μg/l) for 40 min, and anti‐BrdU staining was performed to evaluate cell proliferation.
In addition, BMSCs were seeded on to 96‐well plates at initial density of 500 cells/well. After 14 h serum starvation in serum‐free DMEM/F12 supplemented with 0.5% BSA (wt/vol), the cells were cultured in the above medium containing 0.25% BSA, meanwhile ‘stimuli’ and Alamar blue (1:10 vol/vol ratio, Invitrogen, DAL1025) were added to the culture system. Absorbance was monitored at 570 nm and 600 nm for 5 days to evaluate proliferation rate.
In vitro determination of BMSC migration
Transwell migration chambers (Millipore, Billerica, MA, USA), pore size 8 μm, were used to assess BMSC migration. After serum starvation for 14 h, the cells were plated in upper chambers and stimuli were added to lower chambers. Eight hours after treatment, cells remaining on upper faces of Transwell chambers were removed carefully using cotton swabs, and subsequently crystal violet staining was performed. Quantitative analysis was performed with both cell counting and measurement of absorbance.
BMSC differentiation
After exposure to HGF and/or IGF‐1 for 1 week and HGF+IGF‐1 for 6 h, the BMSCs were continually cultured for the next 2 weeks. To evaluate their differentiation into cardiomyocytes, expressions of cardiac Troponin T (cTnT), GATA4, CX43, Nkx2.5 were determined, using β‐actin as internal control, and immunofluorescence was performed with anti‐cTNT antibodies (Abcam Ltd, Cambridge, UK).
Apoptosis induced by extreme hypoxia
BMSC apoptosis was induced by extreme hypoxia (0.2%), in a sealed GENbox hypoxic chamber 17, and serum deprivation for 24 h. Subsequently, apoptotic cells were evaluated using flow cytometry (Cytoron Absolute; Ortho) with fluorescein isothio‐cyanate (FITC)‐labelled annexin V and propidium iodide (PI, Roche Applied Science, Basel, Switzerland) staining.
Histological analysis
Immunofluorescence staining of lamella of migrating cells was carried out using anti‐BrdU antibodies (Abcam Ltd, Cambridge, UK) to determine their proliferation, and with anti‐cTNT antibodies to identify their differentiation. For tissues of ischaemic areas, vWF counterstaining was performed to observe angiogenesis, Masson's trichrome stain was used to assess MI, terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end labelling (TUNEL) was carried out for analysis of apoptosis of implanted BMSCs, and anti‐cTNT immunofluorescence staining was performed to identify their myocardial differentiation. For each sample, five non‐overlapping fields at 100× magnification in transverse sections were randomly captured using a video camera, which were stored in TIFF format. New vessels (NV) and infarcted areas (IA) were quantified using Image Pro Plus (IPP) 6.0 software package (IPP, Media Cybernetics, Silver Spring, MD, USA). Positively stained regions were padded with single colour and converted to pixels by optical density (OD) calibration.
Statistical analysis
Blind methods were used to measure all offline data. Results are presented as mean ± SD. Statistical analysis was performed using SPSS 18.0 software package (SPSS Inc, Chicago, IL, USA) and Student's t‐test and one‐way or two‐way analysis of variance (ANOVA) with Bonferroni post hoc correction, used for group‐comparison. P < 0.05 was regarded as statistically significant.
Results
Of the 44 rabbits initially studied, four were excluded from further experiments due to intractable ventricular fibrillation after ligation of the LAD. No severe events such as malignant arrhythmias, embolization, bleeding, or haemodynamic abnormality occurred during the Factors and Cells implantation procedure.
Combination of HGF and IGF‐1 inhibited implanted BMSC apoptosis and promoted their differentiation into cardiomyocytes
TUNEL assays (Fig. 1a,b) revealed many apoptotic BMSCs implanted into ischaemic regions in the BMSCs group 24 h after implantation (91.6 ± 3.3%, n = 4), but this was largely attenuated in the Factors + BMSCs group (77.4 ± 2.7%, n = 4, P = 0.004, Fig. 1c). OD of DiI was higher (33601 ± 824 pixels/hpf, P < 0.001, Fig. 1d) in the Factors+BMSCs group, compared to the BMSCs group (33.2 ± 4.8 cell/hpf and 16127 ± 248 pixels/hpf respectively). As shown in fluorescence images 4 weeks postoperatively (Fig. 1e,f), there were more surviving BMSCs within ischaemic regions in Factors+BMSCs group (OD = 26937 ± 1017 pixels/hpf) than BMSCs group (OD = 10931 ± 346 pixels/hpf, P < 0.001, Fig. 1g). The following anti‐cTNT staining found more cTNT+ BMSCs in Factors+BMSCs group (9.9 ± 2.4 cell/hpf) than BMSCs group (2.6 ± 2.2 cell/hpf, P < 0.001, Fig. 1h), revealing that HGF+IGF‐1 promoted BMSC differentiation into cardiomyocytes.
Figure 1.
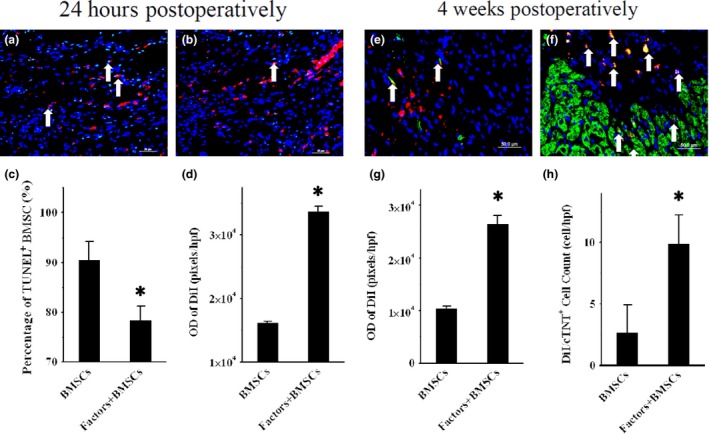
Survival and differentiation of transplanted cells. (a) and (b) representative TUNEL‐staining images of 24‐h postoperative myocardial frozen sections in BMSCs and Factors+BMSCs groups (200× magnification). DiI (Red fluorescence)‐ and TUNEL (green fluorescence)‐positive cells indicate apoptotic transplanted BMSCs (white arrows). Comparisons of percentage of DiI+/TUNEL + cells and OD of DiI in two groups are shown in (c) and (d). Four weeks postoperative anti‐cTNT‐staining images of the above two groups are shown in e and f (200× magnification). DiI (Red fluorescence)‐ and cTNT (green fluorescence)‐positive cells indicate transplanted BMSCs differentiating into cardiomyocytes (white arrows). (g) and (h) respectively show comparisons of amounts of DiI+/cTNT + cells and OD of DiI in two groups. TUNEL, Terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end labelling; BMSCs, Bone marrow mesenchymal stem cells; DiI, 1′‐dioctadecyl‐3,3,3′,3′‐tetramethylindocarbocyanine perchlorate; OD, Optical density; cTNT, Cardiac Troponin T. *P < 0.01 versus Control group.
Combined therapy increased angiogenesis, reduced IA and improved LV function
Representative images (Fig. 2a–d) of vWF‐stained sections of different groups revealed that OD of NV was significantly higher in the Factors+BMSCs group (81328 ± 7729 pixels/hpf, P < 0.001), compared to other groups, although there also was an increase in Factors (59455 ± 5709 pixels/hpf, P < 0.001) and BMSCs groups (45456 ± 4319 pixels/hpf, P < 0.001) compared to controls (25820 ± 2934 pixels/hpf, Fig. 2e).
Figure 2.
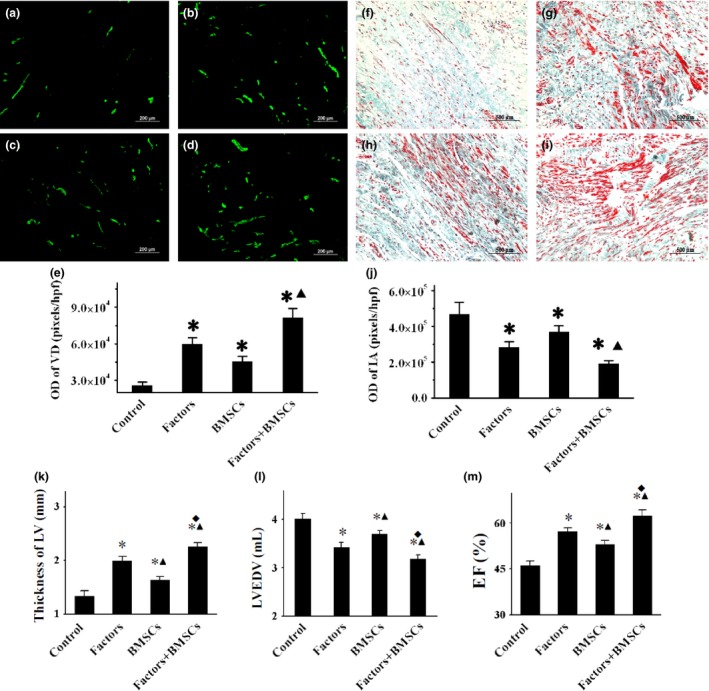
Analysis of angiogenesis, infarction and cardiac function. Representative anti‐vWF and Masson's trichrome‐stained images of myocardial sections (5 μm) within ischaemic territory from control, Factors, BMSCs and Factors+BMSCs groups are shown in (a)–(d) (200× magnification) and (f)–(i) respectively (100× magnification). With Masson's staining, red indicates viable myocardium, blue indicates collagen. Vessel density (VD) and infarcted area (IA) were quantified as OD values by using IPP software, then compared in (e) and (j) respectively. WT, EDV and EF of LV are compared in (k)–(m), respectively. VD, vessel density; IA, infarcted area; WT, wall thickness; EDV, end‐diastolic volume; EF, ejection fraction; LV, left ventricle. *P < 0.001 versus Control group. ▲ P < 0.01 versus Factors groups. ◆ P < 0.01 versus BMSCs groups.
Representative Masson's trichrome staining images of the four groups are shown in Fig. 2f–i. Qualitative analysis (Fig. 2j) demonstrated significant reduction in IA in Factors+BMSCs group (OD = 190220 ± 18280 pixels/hpf) relative to Factors group (OD = 282323 ± 32672 pixels/hpf, P = 0.001) and BMSCs group (OD = 368295 ± 36314 pixels/hpf, P < 0.001), although the two latter also had smaller IA than control (OD = 468828 ± 66349 pixels/hpf, P < 0.001).
No differences in LVWT, EDV or EF were found between the four groups prior to treatment; mean EF value was 49%. Four weeks after treatment, Echo detected significant elevation of LVEF in Factors group (57 ± 2%, P < 0.001) and BMSCs groups (52 ± 2%, P = 0.004) compared to the Control group (47 ± 3%). Importantly however, there was further improvement in Factors+BMSCs group (64 ± 3%, P < 0.001) relative to Factors and BMSCs groups (Fig. 2m). Similar results were found in EDV and WT; the most significant improvement was in the Factors+BMSCs group (P < 0.05, Fig. 2k,l).
Assessment of BMSC proliferation in vitro
Representative anti‐BrdU staining images of BMSCs in Control, HGF, IGF‐1 and HGF+IGF‐1 groups are respectively shown in Fig. 3a–d. Six hours after exposure to the factors, there was a significant increase in percentage of BrdU+ BMSCs in the IGF‐1 group (9.13 ± 0.41%, P = 0.022), and reduction in the HGF group (7.15 ± 0.25%, P = 0.04), but there was no difference in HGF+IGF‐1 group (8.13 + 0.35%, P = 1), compared to the control (8.04 ± 0.22%, Fig. 3e). Growth curves showed similar changes (P < 0.05, Fig. 3f). These results suggest that IGF‐1 promoted BMSC proliferation, but HGF inhibited it, resulting in an offsetting effect of each to the other.
Figure 3.
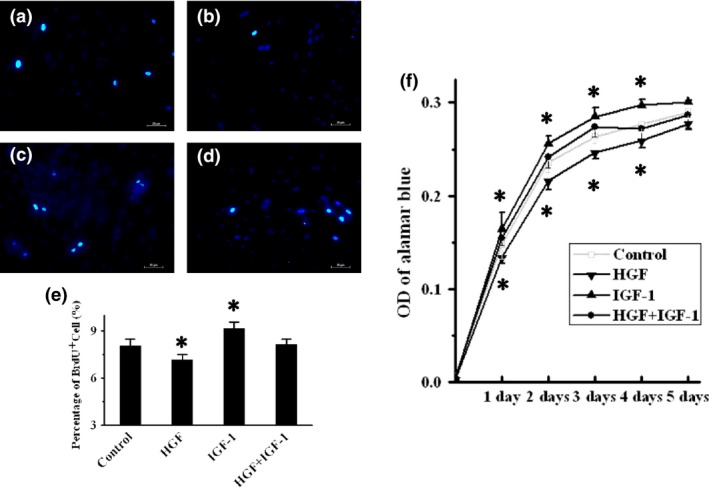
Assessment of BMSC proliferation in vitro. (a)–(d) Representative BrdU‐staining images of BMSCs in control, HGF, IGF‐1 and HGF+IGF‐1 groups respectively. BrdU‐positive cells (green fluorescence) in each group were counted and compared in (e). Cell proliferation measured by the Alamar blue assay, and growth curves for each group are shown in (f). BrdU, 5‐bromo‐2‐deoxyuridine; HGF, hepatocyte growth factor; IGF‐1, insulin‐like growth factor‐1. *P < 0.05 versus Control group.
Measurement of BMSC migration in vitro
Representative images of BMSC migration are shown, Fig. 4a–d. Quantitative analysis of absorbance (Fig. 4e) revealed significantly enhanced migration after exposure to HGF (OD = 0.29 ± 0.01, P < 0.001) and IGF‐1 (OD = 0.37 ± 0.01, P < 0.001) compared to Control cells (OD = 0.21 ± 0.01). Combined exposure further increased BMSC migration (OD = 0.41 ± 0.02), relative to single exposure to HGF (P < 0.001) or IGF‐1 (P = 0.019), indicating a superimposed effect.
Figure 4.
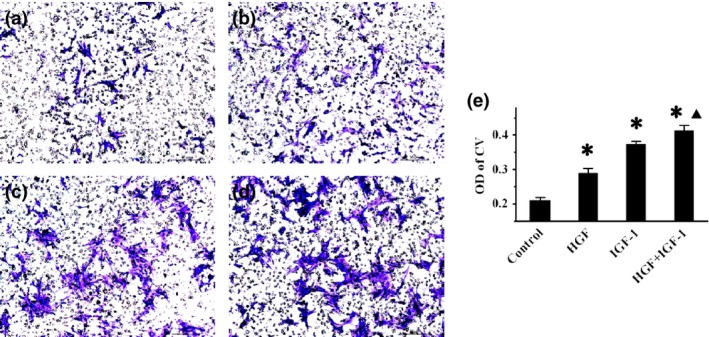
Effects of combined treatment on BMSC migration. (a)–(d) Representative crystal violet staining images of BMSCs in control, HGF, IGF‐1 and HGF+IGF‐1 groups respectively. (e) – comparison of OD value of crystal violet among all groups. *P < 0.001 versus Control group. ▲ P < 0.05 versus HGF and IGF‐1 groups.
Identification of BMSC differentiation into cardiomyocytes in vitro
Images of immunofluorescence staining (Fig. 5a–d) reveal that compared to control cells (2.37 ± 0.45%), HGF (11.52 ± 1.03%, P < 0.001) and HGF+IGF‐1 (11.73 ± 1.06%, P < 0.001) significantly increased percentage of cTNT+ BMSCs, without marked differences between the two groups (P = 1), but single IGF‐1 did not increase cTNT expression of BMSCs (2.9 ± 0.4%, P = 1). A similar trend was found in mRNA and protein expressions of GATA4, cTNT, NKx2.5, CX43 (P < 0.05), as shown by RT‐PCR (Fig. 5g) and western blotting (Fig. 5h,i).
Figure 5.
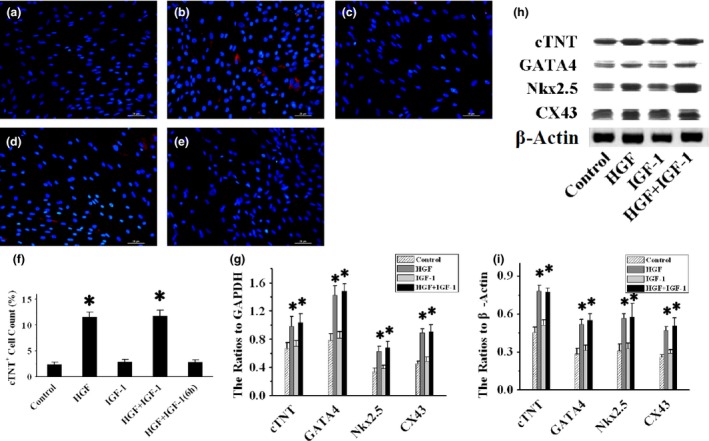
BMSC differentiation into cardiomyocytes. Representative anti‐cTNT (red fluorescence) staining images of BMSCs exposed to blank control, HGF, IGF‐1 and HGF+IGF‐1 for 1 week, as well as HGF+IGF‐1 for 6 h, are shown in (a)–(e) respectively. Numbers of cTNT‐positive cells are compared in (f). Myocyte‐specific factor cTNT, GATA4, Nkx2.5 and CX43 mRNA expressions were measured by RT‐PCR, and ratios to GAPDH are compared in (g). Representative images of western blots are shown in (h), and ratios of these proteins to β‐Actin are compared in (i). CX43, connexin43; GAPDH, Glyceraldehyde‐3‐phosphate dehydrogenase; RT‐PCR, Reverse transcription polymerase chain reaction. *P < 0.001 versus Control group.
Analysis of hypoxia‐induced BMSC apoptosis in vitro
As shown by annexin‐V/PI staining (Fig. 6a–d), there were fewer early‐ and late‐phase apoptotic BMSCs (18.97 ± 1.16%) induced by hypoxia and more surviving BMSCs (80.58 ± 0.86%) after treatment with IGF‐1 than controls (28.39 ± 0.82% and 68.31 ± 0.99% respectively, P < 0.001, Fig. 6e). However, there was no further improvement of BMSC apoptosis (17.74 ± 1.12%, P = 1) and survival (81.58 ± 1%, P = 1) after administration of IGF‐1 combined with HGF. Single exposure to HGF did not inhibit BMSC apoptosis (28.55 ± 1.07%, P = 1) or enhance BMSC survival (70.62 ± 0.98%, P = 0.112), compared to controls.
Figure 6.
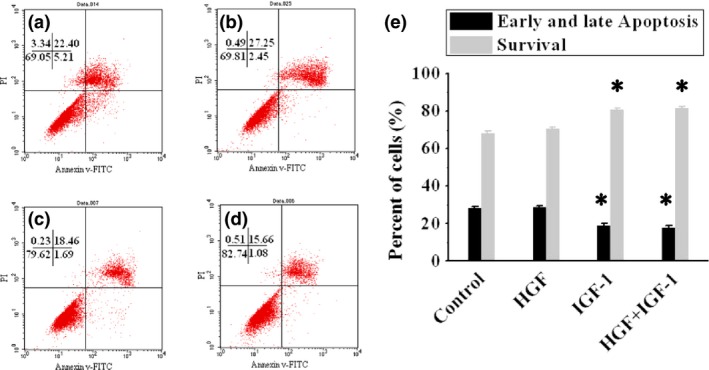
Analysis of BMSC apoptosis in vitro. (a)–(d) show BMSC apoptosis detected by flow cytometry with annexin‐V/PI staining, in control, HGF, IGF‐1 and HGF+IGF‐1 groups respectively. Percentages of early and late apoptotic cells and those surviving, in all group are compared in (e). *P < 0.01 versus Control group.
Discussion
Although BMSC implantation has been advocated as a very promising therapeutic strategy for treatment of patients with MI (due to possible ability to improve cardiac function and reverse ventricular remodelling 3, 18), there are still two crucial problems – survival and differentiation of transplanted BMSCs 13, 19, largely restricting their protective effects on ischaemic heart disease. It has been documented that most implanted BMSCs die under extreme hypoxic conditions experienced by ischaemic regions 20. Moreover, their very low intrinsic capacity to differentiate into cardiomyocytes impacts direct cell participation in myocardial regeneration.
Some in vitro experimental results have shown that HGF significantly promotes myocardial differentiation of BMSCs, but has not provided protective effects for apoptosis induced by hypoxia 21, 22. IGF‐1 has been certified to be an anti‐apoptotic factor, while it is unclear whether it promotes BMSCs to differentiate into cardiomyocytes 12, 23, 24. Due to complementation of function, there have already been studies trying to apply combinations of the two factors for AMI therapy and yield expected results 7, 8. Recently, Savi et al. reported continuous release of HGF and IGF‐1 promoted repairing ability of adipose‐derived stem cells, in healing myocardial infarction in rats 25. This strongly suggests that this combination may promote therapeutic effects of BMSC transplantation for treatment of ischemic myocardial injury. Our previous studies have revealed that HGF+IGF‐1 promoted BMSCs co‐cultured with myocardium, to express cardiac specific transcription factor GATA4, which indicated myocardial differentiation 26. In the present experiment, protective effects of HGF+IGF‐1 on BMSCs were further investigated. The results show that controlled release of HGF and IGF‐1 significantly increased angiogenesis, reduced apoptosis, and enhanced implanted BMSC survival and differentiation into cardiomyocytes, consequently improved LV remodelling and cardiac function. However, it has been identified that single intramyocardial injection did not provide these benefits (published in Chinese). The main reason to account for the different results is that intramyocardially injected cytokines have short biological half‐life in vivo and may be rapidly washed out from large vascular structures in targeted sites. Thus, it is strongly suggested that slow release is very important for administration of HGF and IGF‐16, 8.
Following in vitro experiments revealed that HGF significantly enhanced myocardial differentiation of BMSCs and promoted their migration, but reduced their proliferation and did not inhibit apoptosis induced by hypoxia. However, IGF‐1 significantly increased BMSC proliferation and inhibited hypoxia‐inducing apoptosis. Price et al. demonstrated that IGF‐1 is a comitogen for HGF in a rat model of hepatocellular carcinoma 27. However, it is disappointing that similar effects were not found in rabbit BMSCs. Although it is unclear whether there is any synergistic effect between HGF and IGF‐1, complementary activity has been confirmed in the present study. Combination of HGF and IGF‐1 not only promoted differentiation of BMSCs into cardiomyocytes but also inhibited apoptosis induced by hypoxia, which is very important for cells implanted in ischaemic areas, to survive and provide protective effects after AMI. It has also been revealed in the present study that effects of the two factors on differentiation were significantly time‐dependent; short‐term exposure (6 h) did not work, further emphasizing the importance of controled‐release.
Despite encouraging results, there are still questions and considerations that need to be further addressed. For instance, in this study it has not been investigated whether there were ranges of effects of different doses of cytokines on BMSCs. In addition, due to dissimilarities between human and rabbit hearts, animal experiment results may not contribute precisely to clinical application.
In summary, it was demonstrated that in a rabbit model of AMI, controlled release of HGF and IGF‐1 reduced implanted BMSC apoptosis and promoted their myocardial differentiation, consequently reducing IA, attenuating LV remodelling and improving cardiac function. In vitro experimentation revealed that combined administration of HGF and IGF‐1 increased BMSC migration, inhibited hypoxia‐induced apoptosis and time‐dependently promoted differentiation into cardiomyocytes. It is strongly suggested that combination of HGF and IGF‐1 may be a new therapeutic method in BMSCs transplantation.
Conflict of interest
None declared.
Acknowledgements
This work was supported by The National Natural Science Foundation of China (grant number: 81400196), Science Technology Research Project of Education Department of Liaoning Province (grant number: L2011140, L2013297 and L2013316) and the Scientific Research Foundation of the First Hospital of China Medical University (fsfh1311). Technical assistance of cardiac surgeons from Yong‐Hua Bi is gratefully acknowledged.
References
- 1. Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H et al (2006) Improved clinical outcome after intracoronary administration of bone‐marrow‐derived progenitor cells in acute myocardial infarction: final 1‐year results of the REPAIR‐AMI trial. Eur. Heart J. 27, 2775–2783. [DOI] [PubMed] [Google Scholar]
- 2. Uemura R, Xu M, Ahmad N, Ashraf M (2006) Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ. Res. 98, 1414–1421. [DOI] [PubMed] [Google Scholar]
- 3. Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H et al (2006) Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 12, 459–465. [DOI] [PubMed] [Google Scholar]
- 4. Lu G, Haider HK, Jiang S, Ashraf M (2009) Sca‐1 + stem cell survival and engraftment in the infarcted heart: dual role for preconditioning‐induced connexin‐43. Circulation 119, 2587–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang X, Li Q, Hu Q, Suntharalingam P, From AH, Zhang J (2014) Intra‐myocardial injection of both growth factors and heart derived Sca‐1 + /CD31‐ cells attenuates post‐MI LV remodeling more than does cell transplantation alone: neither intervention enhances functionally significant cardiomyocyte regeneration. PLoS ONE 9, e95247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koudstaal S, Bastings MM, Feyen DA, Waring CD, van Slochteren FJ, Dankers PY et al (2014) Sustained delivery of insulin‐like growth factor‐1/hepatocyte growth factor stimulates endogenous cardiac repair in the chronic infarcted pig heart. J. Cardiovasc. Transl. Res. 7, 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellison GM, Torella D, Dellegrottaglie S, Perez‐Martinez C, Perez de Prado A, Vicinanza C et al (2011) Endogenous cardiac stem cell activation by insulin‐like growth factor‐1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J. Am. Coll. Cardiol. 58, 977–986. [DOI] [PubMed] [Google Scholar]
- 8. Ruvinov E, Leor J, Cohen S (2011) The promotion of myocardial repair by the sequential delivery of IGF‐1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 32, 565–578. [DOI] [PubMed] [Google Scholar]
- 9. Zhang W, Zhang F, Shi H, Tan R, Han S, Ye G et al (2014) Comparisons of rabbit bone marrow mesenchymal stem cell isolation and culture methods in vitro. PLoS ONE 9, e88794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qi X, Okamoto Y, Murakawa T, Wang F, Oyama O, Ohkawa R et al (2010) Sustained delivery of sphingosine‐1‐phosphate using poly(lactic‐co‐glycolic acid)‐based microparticles stimulates Akt/ERK‐eNOS mediated angiogenesis and vascular maturation restoring blood flow in ischemic limbs of mice. Eur. J. Pharmacol. 634, 121–131. [DOI] [PubMed] [Google Scholar]
- 11. Kim H, Kim HM, Jang JE, Kim CM, Kim EY, Lee D et al (2013) Osteogenic Differentiation of Bone Marrow Stem Cell in Poly(Lactic‐co‐Glycolic Acid) Scaffold Loaded Various Ratio of Hydroxyapatite. Int. J. Stem Cells 6, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jayasuriya AC, Shah C (2008) Controlled release of insulin‐like growth factor‐1 and bone marrow stromal cell function of bone‐like mineral layer‐coated poly(lactic‐co‐glycolic acid) scaffolds. J. Tissue Eng. Regen. Med. 2, 43–49. [DOI] [PubMed] [Google Scholar]
- 13. Zhang GW, Liu XC, Li‐Ling J, Luan Y, Ying YN, Wu XS et al (2011) Mechanisms of the protective effects of BMSCs promoted by TMDR with heparinized bFGF‐incorporated stent in pig model of acute myocardial ischemia. J. Cell Mol. Med. 15, 1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Liu XC, Zhang GW, Zhao J, Zhang JM, Shi RF et al (2009) A new transmyocardial degradable stent combined with growth factor, heparin, and stem cells in acute myocardial infarction. Cardiovasc. Res. 84, 461–469. [DOI] [PubMed] [Google Scholar]
- 15. Cenni E, Granchi D, Avnet S, Fotia C, Salerno M, Micieli D et al (2008) Biocompatibility of poly(D, L‐lactide‐co‐glycolide) nanoparticles conjugated with alendronate. Biomaterials 29, 1400–1411. [DOI] [PubMed] [Google Scholar]
- 16. Lee YS, Lim KS, Oh JE, Yoon AR, Joo WS, Kim HS et al (2015) Development of porous PLGA/PEI1.8k biodegradable microspheres for the delivery of mesenchymal stem cells (MSCs). J. Control. Release 205, 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen J, Baydoun AR, Xu R, Deng L, Liu X, Zhu W et al (2008) Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation‐induced apoptosis. Stem Cells 26, 135–145. [DOI] [PubMed] [Google Scholar]
- 18. Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H et al (2006) Intracoronary bone marrow‐derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 355, 1210–1221. [DOI] [PubMed] [Google Scholar]
- 19. Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S et al (2006) Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow‐up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST‐elevation infarct regeneration) trial. Circulation 113, 1287–1294. [DOI] [PubMed] [Google Scholar]
- 20. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105, 93–98. [DOI] [PubMed] [Google Scholar]
- 21. Son BR, Marquez‐Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J et al (2006) Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal‐derived factor‐1‐CXCR4 and hepatocyte growth factor‐c‐met axes and involves matrix metalloproteinases. Stem Cells 24, 1254–1264. [DOI] [PubMed] [Google Scholar]
- 22. Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V et al (2006) Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells 24, 23–33. [DOI] [PubMed] [Google Scholar]
- 23. Chen C, Xu Y, Song Y (2014) IGF‐1 gene‐modified muscle‐derived stem cells are resistant to oxidative stress via enhanced activation of IGF‐1R/PI3K/AKT signaling and secretion of VEGF. Mol. Cell. Biochem. 386, 167–175. [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Shelat H, Geng YJ (2012) IGF‐1 prevents oxidative stress induced‐apoptosis in induced pluripotent stem cells which is mediated by microRNA‐1. Biochem. Biophys. Res. Commun. 426, 615–619. [DOI] [PubMed] [Google Scholar]
- 25. Savi M, Bocchi L, Fiumana E, Karam JP, Frati C, Bonafe F et al (2015) Enhanced engraftment and repairing ability of human adipose‐derived stem cells, conveyed by pharmacologically active microcarriers continuously releasing HGF and IGF‐1, in healing myocardial infarction in rats. J. Biomed. Mater. Res. A 103, 3012–3025. doi: 10.1002/jbm.a.35442. [DOI] [PubMed] [Google Scholar]
- 26. Li Z, Gu TX, Zhang YH (2008) Hepatocyte growth factor combined with insulin like growth factor‐1 improves expression of GATA‐4 in mesenchymal stem cells cocultured with cardiomyocytes. Chin. Med. J. (Engl) 121, 336–340. [PubMed] [Google Scholar]
- 27. Price JA, Kovach SJ, Johnson T, Koniaris LG, Cahill PA, Sitzmann JV et al (2002) Insulin‐like growth factor I is a comitogen for hepatocyte growth factor in a rat model of hepatocellular carcinoma. Hepatology 36, 1089–1097. [DOI] [PubMed] [Google Scholar]


