Abstract
Ubiquitously distributed in different plant species, plant lectins are highly diverse carbohydrate‐binding proteins of non‐immune origin. They have interesting pharmacological activities and currently are of great interest to thousands of people working on biomedical research in cancer‐related problems. It has been widely accepted that plant lectins affect both apoptosis and autophagy by modulating representative signalling pathways involved in Bcl‐2 family, caspase family, p53, PI3K/Akt, ERK, BNIP3, Ras‐Raf and ATG families, in cancer. Plant lectins may have a role as potential new anti‐tumour agents in cancer drug discovery. Thus, here we summarize these findings on pathway‐ involved plant lectins, to provide a comprehensive perspective for further elucidating their potential role as novel anti‐cancer drugs, with respect to both apoptosis and autophagy in cancer pathogenesis, and future therapy.
Abbreviations
- Apaf‐1
apoptosis‐associated factor‐1
- Atg
autophagy‐related gene
- BNIP3
Bcl2/E1B‐19kDa protein‐interacting protein 3
- ConA
Concanavalin A
- ConBr
Canavaliabrasiliensis
- cyto. c
cytochrome c
- DISC
death‐inducing signalling complex
- DLA
Dalton's Lymphoma Ascites
- ERK
extracellular‐signal regulated kinase
- FADD
Fas‐associated death domain
- FIP200
focal adhesion kinase family interacting protein of 200 kDa
- MAPK
mitogen‐activated protein kinases
- MCL
Momordica charantia lectin
- ML
Mistletoe lectin
- MLL
mulberry leaf lectin
- MMP‐9
matrix metallopeptidase 9
- MT1
membrane type‐1
- mTORC1
mammalian target of rapamycin complex 1
- NMR
nuclear magnetic resonance
- NO
nitric oxide
- NPC
nasopharyngeal carcinoma
- PCL
P. cyrtonema lectin
- PI3K
phosphoinositide 3‐kinase
- PPI
protein‐protein interaction
- PTA
Pinelliaternata agglutinin
- RBA
Rice bran agglutinin
- ROS
reactive oxygen species
- SFL
S. flavescens lectin
Introduction
Lectins are a ubiquitously distributed group of highly diverse plant, animal and fungus non‐immune origin proteins, containing at least one non‐catalytic domain 1. Their non‐catalytic domain enables them to selectively recognize and reversibly bind to specific free sugars or glycans, present on glycoproteins and glycolipids, without altering structure of the carbohydrate 2. Lectins have already been recognised for many years, for their ability to agglutinate red blood cells 72, but their roles played in programmed cell death of apoptosis and autophagy, have not yet been fully elucidated. In the 1980s, first studies suggested that lectin‐like molecules, constitutively expressed on surfaces of macrophages, selectively recognized changes to glycans decorating plasma membranes of apoptotic thymocytes 74. Some years later, more compelling evidence was obtained when Griffiths and co‐workers identified apoptotic changes within lymphoid tissues after injection of plant lectins, in vivo 73, followed by additional studies documenting cell shrinkage and DNA fragmentation in lymphocytes exposed to plant lectins, in vitro. This approach set the basis for therapeutic strategies aimed at eliminating aberrantly glycosylated cancer cells 61. Plant lectins have been demonstrated that specifically bind to various sugar structures, and trigger several important cell processes. Regarding their biochemical properties, carbohydrate‐binding specificities and biological functions, hundreds of plant lectins have been purified and characterized. Carbohydrate‐binding specificities can be either polyspecific or monospecific, thus, plant lectins are classified into 12 families: Agaricusbisporus agglutinin homologues 3, amaranthins 4, Class V chitinase homologues with lectin activity 5, cyanovirin family members, the EEA family 6, GNA family members, proteins with hevein domains 7, jacalins 8, proteins with legume lectin domains 9, LysM domain bearers 10, Nictaba family members 11, and Ricin‐B family members 12.
On the one hand, according to their specific binding characteristics, plant lectins have already been used as recognition tools to study subtle distinctions between malignant and non‐malignant cells 13. In microarrays, plant lectins have been used to analyze high throughput protein glycosylation, or profile global changes on surfaces of mammalian cells 14. In addition, activity of plant lectins has been demonstrated in different tissues and processes, demonstrating their widespread importance as potential therapeutic agents, especially for cancer 15. Some plant lectins are also able to reduce treatment‐associated side effects as adjuvant agents during chemotherapy and radiotherapy.
On the other hand, plant lectins have been found to target apoptotic‐ and autophagy‐related signalling pathways in a variety of tumour cells. Apoptosis is a complex and highly defined type of programmed cell death, while autophagy is an evolutionarily conserved and multi‐step lysosomal degradation process 16. In the context of cancer, apoptosis and autophagy may work together to jointly determine the destiny of cancer cells, thus, the ability of plant lectins to influence both apoptosis and autophagy could conceivably be used to treat cancer in the future. For example, mistletoe lectins (MLs) and ricin may exert both anti‐proliferative and apoptosis‐inducing influence on cancer cells 17. Concanavalin A (ConA) and P. cyrtonema lectin (PCL) lead to autophagy after internalization or binding to certain sugar‐containing receptors on the surfaces of cancer cells 18, 19. Accordingly, these recently reported data of anti‐tumour activities of plant lectins and their molecular mechanisms need to be discussed to reveal their potential role in future cancer therapy.
Here, we present an account of current modern research on plant lectins inducing cell death, by targeting apoptotic and autophagic signalling pathways involved in Bcl‐2 family, caspase family, p53, PI3K/Akt, ERK, BNIP3, Ras‐Raf and the ATG family, in different types of cancer cells. These important data help reveal the potential therapeutic role of plant lectins, which may shed new light on future cancer drug discovery.
Plant lectins and apoptosis in cancer
Apoptotic mechanisms
Apoptosis is an evolutionarily conserved process that can remove superfluous cells that have outlived their usefulness, or are dangerous for the survival of an organism. It has been demonstrated to occur generally via two major pathways, namely a death‐receptor pathway triggered by Fas or other plasma membrane receptor ligation, and a mitochondria‐dependent pathway releasing cytochrome c (cyto. c) 20.
Activation of cell surface death receptors induces the death‐receptor pathway, by binding to specific ligands including TNF, TRAIL or FasL. After this intracellular domains of the receptors interact with Fas‐associated death domain, resulting in assembly of a death‐inducing signalling complex and recruitment of initiator caspase‐8/caspase‐10 21.
Controlled by the Bcl‐2 protein family, the mitochondrial pathway regulates mitochondrial membrane permeability, thereby releasing specific mitochondrial proteins, such as cyto. c, into the cytosol 22. Anti‐apoptotic Bcl‐2 proteins including Bcl‐2, Bcl‐XL and Mcl‐1 prevent permeabilization of the outer membrane of mitochondria by inhibiting action of pro‐apoptotic proteins such as cytosolic Bax and Bak, whereas other pro‐apoptotic Bcl‐2 family members including BH3‐only proteins Bad, Bik, Bid, Bim, PUMA and NOXA, are cytosolic sensors of cell damage or stress 23 (Fig. 1).
Figure 1.
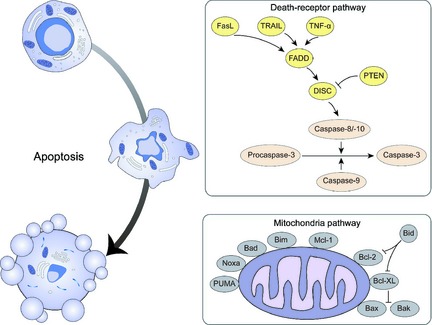
Mechanisms of apoptosis in cancer.
Widely accepted as a crucial cellular molecular mechanism, apoptosis is targeted and regulated by numerous cell signalling pathways, leading to cell death, in normal as well as tumour cells. With identification of required apoptosis‐related molecules and pathways that suppress cancer, modulation of apoptosis will emerge as a modern target for cancer therapy. Thus, targeting key molecular components of the cell death machinery induced by apoptosis is an attractive strategy for use of plant lectins 24.
Interaction between apoptosis and well‐studied plant lectins
Various types of plant lectin modulate several apoptosis‐related signalling pathways in different cancer cells. On the one hand, representative plant lectins such as ConA, mistletoe (V. album) lectins and PCL have been extensively studied. ConA, the first reported legume lectin, can lead to potential collapse of mitochondrial transmembrane, cyto.c release and caspase activation, eventually triggering mitochondria‐mediated apoptosis, in both human melanoma A375 cells and human hepatocellular carcinoma HepG2 cells 25, 26. This lectin directly binds to the extracellular region of SHPS‐1, a multifunctional transmembrane glycoprotein, the interaction mediating ConA‐dependent activation of AKT and secretion of matrix metallopeptidase 9 (MMP‐9). Thereby, both AKT and extracellular‐signal regulated kinase (ERK) are required for increased secretion of MMP‐9 by ConA 27. In addition, ConA up‐regulates expression of COX‐2 and down‐regulates AKT expression via an IKK/NF‐κB‐dependent pathway, in glioblastoma U87 cells 28. ConA selectively induces apoptosis by rendering the ratio of Bax/Bcl‐2, inhibiting AKT expression, and activating the Foxo1a‐Bim signalling pathway, in ovarian cancer SKOV3 cells 29.
As type II ribosome‐inactivating proteins (RIPs II), MLs, can be divided into three distinct types including ML‐I, ML‐II and ML‐III, among which ML‐I and ML‐II have been demonstrated to hold anti‐proliferative and apoptosis‐inducing mechanisms in different cancer cells. ML‐I induces apoptosis by activating caspase‐8 via the extrinsic apoptotic pathway, in Jurkat leukemic T cells, with sensitivity to apoptotic induction in cancer cell death and cooperating with TNF‐α from the TNF‐family death receptors through Bcl‐2 down‐regulation 30, 31. ML‐I can also break down MMP and activate caspase‐3, thus inducing apoptosis through the apoptosis‐associated factor‐1(Apaf‐1)‐dependent pathway, in human p53‐mutated adenocarcinoma cells 31. Since ML‐I alters levels of MMP, it releases cyto.c and increases reactive oxygen species (ROS), in heptacelluar carcinoma Hep3B cells 17. Contrary to ML‐I, ML‐II activates mitogen‐activated protein kinase signalling involved in ERK and p38, then changes cell signalling pathways that modulate responses related to apoptosis. It has been reported that ML‐II induces apoptosis by activating signalling pathways involved in SAPK/JNK and p38, or by inhibiting the ERK1/2 pathway, in human monoblastic leukaemia U937 cells 32.
P. cyrtonema lectin, a mannose/sialic acid lectin belonging to the GNA family, was first isolated from P. cyrtonema, Hua 33. This lectin induces apoptosis in melanoma A375 cells, the mechanism of which involves regulation of Bax, Bcl‐XL and Bcl‐2 proteins as well as collapse of MMP, leading to release of cyto.c and activation of caspases 25. PCL has anti‐proliferative and apoptosis‐inducing activities on cervical cancer HeLa cells, or induces human breast cancer MCF‐7 cell apoptosis, via caspase activation 34, 35. PCL also induces apoptosis accompanied with activation of caspases‐3/‐8/‐9 in fibrosarcoma L929 cells, through a caspase‐dependent apoptotic mechanism 36.
Interaction between apoptosis and new emerging plant lectins
On the other hand, other previously less studied plant lectins are now widely attracting more and more attention from researchers. S. flavescens lectin, a legume lectin, induces apoptotic tumour cell death through a death‐receptor pathway, which is dependent on caspase activation 37. With specificity to sialic acid, a typical legume lectin purified from Phaseoluscoccineus L. (Phaseolus. Multiflorus wild) seed products activate caspase‐dependent apoptosis in murine fibrosarcoma L929 cells 38. As a monocotyledenous mannose‐binding lectin, Pinelliaternata agglutinin has been demonstrated, by nuclear staining assay and DNA fragmentation, to induce apoptosis in human hepatocarcinoma Bel‐7404 cells, further revealing its biological and pharmacological activities 39. Pea (Pisum sativum L.) lectin treatment results in cell cycle arrest in the G2/M phase of Ehrlich ascites carcinoma cells. In addition, intensive increase of Bax gene expression with reduction in Bcl‐2 and Bcl‐X L gene expression, is observed in cells treated with Pea lectin 40. Apoptotic cell death induction by mulberry leaf lectin of human breast cancer MCF‐7 cells and colon cancer HCT‐15 cells, has also been verified to be in a caspase‐dependent manner 41. VAA‐I, a specific plant lectin found in Viscum album, induces the phosphoinositide 3‐kinase (PI3K) pathway, thus leading to apoptosis in human hepatocellular carcinoma SMMC‐7721 cells 4. Similar to ConA, Canavaliabrasiliensis (ConBr) inhibits tumour cell proliferation by inducing death mechanisms of apoptosis in both human leukaemia MOLT‐4 and HL‐60 cells 43. Momordica charantia lectin (MCL) increases cyto.c release, activates caspases‐3/‐8/‐9 and enhances production of cleaved PARP, with regulation of mitogen‐activated protein kinase phosphorylation and promotion of downstream nitric oxide production. This results in apoptosis of nasopharyngeal carcinoma 69 cells 44. Purified from a Chinese herb, galactose‐binding lectin AML induces apoptosis in a caspase‐dependent manner in chronic myeloid leukaemia K562 cells 45. Belonging to the ricin‐B family, ricin has been reported to lead to apoptotic cell death by up‐regulating expression of caspase‐8 and its downstream caspases‐3/‐7 in Hodgkin's lymphoma L540cells 46. Rice bran agglutinin (RBA) is able to inhibit cell proliferation via cytotoxic mechanisms involved in caspase activation and apoptotic induction in human promyelocytic leukemia HL‐60 cells 47. Abrin also has been found to increase caspase‐3 expression and block Bcl‐2, thereby inducing apoptosis in murine Dalton's lymphoma ascites cells 48. In addition, AMML, from the roots of Astragalusmongholius, has been reported to induce apoptosis in various kinds of cancer cells including HeLa cervical cancer cells, human osteoblast‐like MG63 cells and human leukemia K562 cells 49 (Table 1).
Table 1.
Plant lectins targeting apoptotic/autophagic signalling pathways in cancer
| Name | Apoptosis/autophagy | Target | Cancer cell type | Specific mechanism | References |
|---|---|---|---|---|---|
| Concanavalin A | Apoptosis | Caspase, Akt, MMP‐9, ERK | Human melanoma A375 cell | Trigger mitochondria‐mediated apoptosis in A375 and HepG2 cells | 25, 26, 27, 28, 29 |
| – | Hepatocellular liver carcinoma HepG2 cell | ||||
| COX‐2, Bax/Bcl‐2, | Glioblastoma U87 cell | Modulate IKK/NF‐κB‐dependent pathway in U87 cells | |||
| – | Human ovarian cancer SKOV3 cells | Activate Foxo1a‐Bim signaling | |||
| Autophagy | BNIP‐3, | Glioblastoma U87 cell | Induce autophagy via the interplay between JAK2/STAT3 and MT1‐MMP signaling pathway | 28, 57, 58, 59, 60 | |
| Atg3, Atg12, PI3K/AKT/mTOR, MEK/ERK | Cervical cancer HeLa cell | Activate autophagy by lowering PI3K/AKT/mTOR expression and induce the MEK/ERK pathway | |||
| Mistletoe lectin | Apoptosis | Caspase‐3, caspase‐8, TNF‐α, Bcl‐2, | Leukemia U937 cell | Induce apoptosis via the extrinsic apoptotic pathway in Jurkat leukemic T cells | 17, 30, 31, 32 |
| MMP, MAPK, p38 | Adenocarcinoma | Induce apoptosis through Apaf‐1‐dependent pathway | |||
| – | Human hepatic carcinoma Hep3B cells | Release cyto. c and increase ROS | |||
| – | Leukemia U937 cell | Activating SAPK/JNK and p38 pathways, or inhibiting ERK1/2 pathway | |||
| Autophagy | mTORC1, PI3KCIII, Beclin‐1, Atg12 | hepatocellular liver carcinoma SK‐Hep‐1 & Hep 3B cell | Reduce cell death by increasing expressions of phosphorylated mTORC1, PI3KCIII, Beclin‐1, Atg12 and active LC3 form | 63 | |
| P. cyrtonema lectin | Apoptosis | Bax, Bcl‐XL, Bcl‐2, MMP | Melanoma A375 cell | Induce apoptosis cyto. c release and caspase activation | 25, 33, 34, 35, 36 |
| Caspase‐3/‐8/‐9 | Cervical cancer HeLa cell | Induce apoptosis via activation of caspase | |||
| – | Breast cancer MCF‐7 | – | |||
| – | Murine fibrosarcoma L929 cell | induce apoptosis via a caspasedependent pathway | |||
| Autophagy | P38, p53, Ras‐Raf, PI3KCI/Akt | Melanoma A375 cell | Induce autophagy via a ROS‐p38‐p53 pathway | 61, 62 | |
| Murine fibrosarcoma L929 cell | Induce autophagy by inhibiting Ras‐Raf signaling pathway or PI3KCI/Akt signaling pathway | ||||
| S. flavescens lectin | Apoptosis | Caspase | Cervical cancer HeLa cell | Induce apoptosis via a death‐receptor pathway | 37 |
| Phaseolus. Multiflorus willd | Apoptosis | Caspase | Murine fibrosarcoma L929 cell | Induce caspase‐dependent apoptosis | 38 |
| Pinellia ternata agglutinin | Apoptosis | – | Hepatocarcinoma Bel‐7404 Cell | Induce apoptosis | 39 |
| Pea (Pisum sativum L.) lectin | Apoptosis | Bax, Bcl‐2, Bcl‐XL | Ehrlich ascites carcinoma (EAC) Cell | Result in cell cycle arrest at G2/M phase of EAC cells | 40 |
| Mulberry leaf lectin | Apoptosis | Caspase | Breast cancer MCF‐7 Cell | Induce apoptotic cell death via a caspase‐dependent manner | 41 |
| Colon cancer HCT‐15 Cell | |||||
| VAA‐I | Apoptosis | PI3K | Hepatocellular carcinoma SMMC7721 cell | Lead to apoptosis by inducing PI3K pathway | 42 |
| Canavalia brasiliensis | Apoptosis | – | Leukemia U937 cell | Inhibit tumor cell proliferation by inducing apoptosis | 43 |
| Momordica charantia lectin | Apoptosis | Caspase‐3/‐8/‐9, MAPK | Nasopharyngeal carcinoma NP‐69 cell | Increase cyto. c release, activate caspases‐3/‐8/‐9, enhance production of cleaved PARP, regulate MAPK phosphorylation and promote downstream NO production | 44 |
| Caspase‐8/‐9 | Human hepatocellular carcinoma Hep G2 cell | Induce apoptosis through caspase‐8 regulated extrinsic and caspase‐9 regulated intrinsic caspase cascades | 45 | ||
| AML | Apoptosis | Caspase | Chronic myeloid leukemia cell | Induce apoptosis in a caspase‐dependent manner | 46 |
| Ricin | Apoptosis | Caspase‐3/‐7/‐8 | L540 human Hodgkin's lymphoma‐derived cell | Induce apoptosis by up‐regulating caspase‐8 and caspase‐3/‐7 | 47 |
| Rice bran agglutinin | Apoptosis | Caspase | Promyelocytic leukemia U937cell | Inhibit cell proliferation via cytotoxic mechanisms involving caspase activation and apoptosis | 48 |
| Abrin | Apoptosis | Caspase‐3, Bcl‐2 | Murine Dalton's Lymphoma Ascites cells | Induce apoptosis by increasing caspase‐3 expression and blocking Bcl‐2 expression | 49 |
| AMML | Apoptosis | – | Human cervical carcinoma HeLa cell line Leukemia | Induce apoptosis | 50 |
| Human osteoblast‐like MG63 cell | |||||
| Human leukemia K562 cell |
Plant lectins and autophagy in cancer
Distinct from apoptosis, autophagy is an evolutionarily conserved, multi‐step lysosomal degradation process, in which a cell degrades long‐lived proteins and damaged organelles 50. In cancer cells, autophagy might act as a physiological mechanism of temporary survival, however, cell death may occur if cellular stresses result in continuously or excessively induced autophagy. Autophagy may play an important role in attacking cancer by regulating a limited number of autophagy‐related gene (ATG) family members 51. The complete autophagic flow is divided into the following five stages: induction, vesicle nucleation, vesicle elongation and completion, docking and fusion, as well as degradation and recycling 52.
Induction of autophagy is initiated by the ULK complex composed of ULK1/2, mAtg13, focal adhesion kinase family interacting protein of 200 kDa (FIP200) and ATG101, which can be inhibited by a negative regulator, mammalian target of rapamycin complex 1 (mTORC1) 53.
Subsequently, proteins and lipids are recruited for construction of the autophagosomal membrane for vesicle nucleation, which is induced by activation of the class III PI3K/Beclin‐1 complex 54. Numerous binding partners of this complex function as positive or negative regulators, including Bif‐1, Atg14L, UVRAG, Ambra1 and Rubicon 55.
In vesicle elongation and completion, two unique ubiquitin‐like conjugation systems may take place. The first pathway involves the covalent conjugation of ATG12 to ATG5 in a reaction that requires ATG7 and ATG10, respectively, while the other occurs via activation of LC3/ATG4.
Docking and fusion of the autophagosome may cause autolysosome maturation, eventually leading to the breakdown of autophagosomal contents 56 (Fig. 2).
Figure 2.
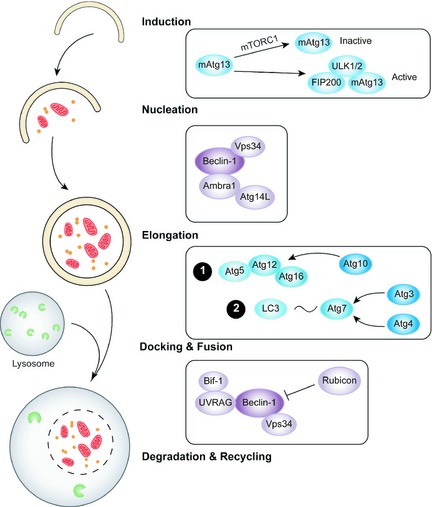
Mechanisms of autophagy in cancer.
Compared to plant lectins involved in apoptosis, there are limited numbers of plant lectins demonstrated to target autophagic signalling pathways in different types of cancer cells. For example, ConA induces autophagy in a Bcl2/E1B‐19kDa protein‐interacting protein 3 (BNIP3)‐mediated way 57. ConA also induces autophagy in hepatoma cells through a pathway mediated by internalization and mitochondria, which also involves mitochondrial interacting protein BNIP3 58. In addition, ConA‐treated glioblastoma U87 cells have shown up‐regulation of BNIP3, ATG3 and ATG12, but this induction can be reversed when membrane type‐1 matrix metalloproteinase (MT1‐MMP) gene is silenced 28. Since the cytoplasmic domain of MT1‐MMP is necessary for transducing STAT3 phosphorylation, ConA‐induced autophagy by BNIP3 calls for interplay between JAK2/STAT3 and MT1‐MMP signalling pathways in U87 cells 59. Moreover, ConA treatment reduces expression of PI3K/AKT/mTOR then induces the MEK/ERK pathway. This activates autophagy in human cervical cancer HeLa cells 60.
Compared to mechanisms involved in PCL‐induced apoptosis, PCL also induces autophagy by a similar mitochondria‐related ROS‐p38‐p53 pathway, in human melanoma A375 cells 61. In fibrosarcoma L929 cells, PCL induces autophagy by inhibiting the Ras‐Raf and PI3KCI/Akt signalling pathways 62. It is interesting to note that PCL‐induced autophagy and PCL‐induced apoptosis may connect with each other to participate in determining the type of cancer cell death, since PCL promotes the mitochondria‐mediated ROS‐p38‐p53 pathway or blocks Ras‐Raf and PI3K‐Akt pathways in both these types of phenomena.
Until recently, there has been only one report indicating that ML can also induce autophagy. Viscum album L. var. coloratum agglutinin (VCA), an agalactose‐ and N‐acetyl‐d‐galactosamine‐specific lectin isolated from Korean mistletoe, increases expression of phosphorylated mTORC1, PI3KCIII, Beclin‐1, ATG12 and active LC3. Thus, VCA can induce survival factors and reduce cell death by autophagy modulation in human hepatocellular carcinoma HepG2 cells 63 (Table 1).
Plant lectins as potential new emerging anti‐cancer drugs
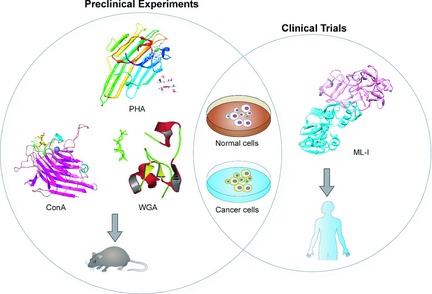
Hitherto, most types of plant lectin have properties of binding distinct sugar‐containing receptors on surfaces of various cancer cells, and this special characteristic has enabled them to determine the ultimate fate of cancer cells. For example, it has recently been shown that PCL binds most of unbound EGFR. It is a key sugar‐containing receptor on surfaces of cancer cells. In this way PCL can block EGFR‐mediated survival pathways involved in BCR‐ABL, PI3K‐AKT‐mTORCI and Ras‐Raf signalling pathways 64. Thus, due to their specific structures and abilities of binding sugar receptors, plant lectins can induce death in many kinds of cancer cell by targeting apoptotic or autophagic cell death involved in many key signalling pathways. Moreover, there are various connections between apoptotic and autophagic processes that can jointly seal the fate of cancer cells. As mentioned above, with deepening research on mechanisms of plant lectins in cancer, they are currently regarded as candidate anti‐cancer drugs for humans, some of which have also been further applied up to pre‐clinical or clinical trials for cancer treatment (Fig. 3, 4).
Figure 3.
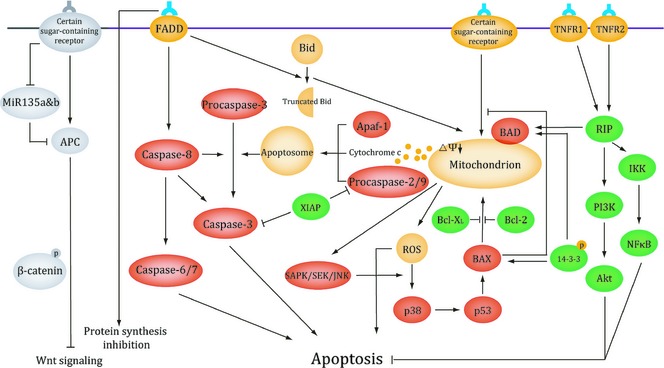
Signalling pathway of plant lectins involved in apoptosis.
Figure 4.
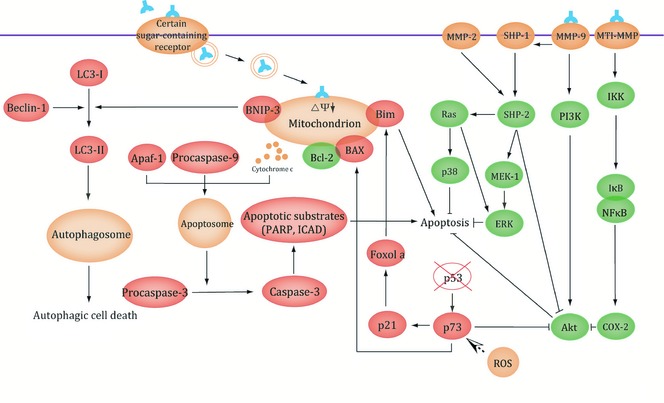
Signalling pathway of plant lectins involved in autophagy.
Recent studies have shown that ConA can be used as a therapeutic anti‐hepatoma agent since it induces autophagic and immuno‐modulatory activities in vivo; thus ConA can inhibit tumour nodule formation in the liver 65. It has also been found that chemical modification of ConA (PEG‐ConA) significantly enhances anti‐tumour cytotoxicity of peripheral lymphocytes against B16 melanoma cells 66. In addition, Phaseolus vulgaris lectin (PHA), a lectin present in kidney beans remarkably reduces growth of murine non‐Hodgkin lymphoma 67. As a typical chitin‐binding lectin composed of hevein domains, wheat germ agglutinin is also effective in controlling tumour growth by activating the host immune response 68. It has been shown that alterations exist in carbohydrate structures of cell glycol‐conjugates, and these differences can be related to goblet‐cell differentiation in normal, benign and malignant tissues. Accordingly, these results and information offer guidance for subsequent clinical trials.
With regard to clinical trials, ML‐I has widely been utilized as a potential anti‐neoplastic drug or as an adjuvant therapeutic agent during chemotherapy and radiotherapy, and has been used to reduce treatment‐associated side effects 69. Viscum fraxini‐2 has been used to treat hepatocellular carcinoma. Fourteen of 23 patients (median age of 54) were reported to have survived treatment; tumour status having been bilateral lobe 8, 34.8%; unilateral lobe 15, 65.2%; distant metastasis 4, 17.4%; hepatic portal vein thrombosis 2, 8.7%; ascites 4, 17.4%, when European mistletoe had been used to treat malignant melanoma. In a further study, two hundred and twelve of 329 patients (median age of 51.4) survived from with no evidence of tumour enhancement, neither indication of increased frequency nor earlier onset of brain metastases. Recently, further researchers have tested safety and efficacy of European MLs, and European mistletoe (V. album L.) extract Iscador (FME) has been tested to be safe during postsurgical aftercare of primary intermediate to high‐risk malignant melanoma (UICC/AJCC stage II–III) patients 70. MLs are also used in aqueous injectable solution, benefiting survival rates of patients, anti‐tumour activities and low toxicity profiles in clinical trials 71. Further studies in combination with other active agents would be required to determine any the survival rate, tumour remission, overall quality of life and quality of life correlated to side effects, during cyto‐reductive therapies.
Previously, plant lectins have been considered to be toxic, however, they have recently been demonstrated to possess inhibitory effects on cancer development. Currently, researchers are working to switch use of plant lectins from detection to actual use for fighting cancer. Hitherto, plant lectins, formerly regarded as simple recognition tools for identifying malignant tumours, have become crucial biomarkers and potential agents for cancer diagnosis and prognosis. Accumulating lines of evidence have revealed that targeting important key apoptotic and autophagic signalling pathways may be a promising avenue for potential therapeutic purposes. Interestingly, some plant lectins such as PCL have been shown to induce apoptosis and autophagy via the same mechanisms, thus dealing with matters related to carcinogenesis. Due to plant lectins having natural toxicity and drug‐resistant production, direct mutations could be used to modify them, designing them into synthetic ‘ideal’ candidate anti‐tumour drugs with higher efficiency and lower toxicity for their therapeutic purposes (Fig. 5).
Figure 5.
Potential therapeutic roles of plant lectins in cancer.
Conclusions
Plant lectins, highly diverse carbohydrate‐binding proteins of non‐immune origins, are widely distributed in different plant species. They have previously been used as simple recognition tools for identifying malignant tumours, but have gradually developed into important biomarkers for cancer diagnosis and prognosis and potential agents for cancer therapeutics. Moreover, due to their widely accepted anti‐proliferative activities, plant lectins may exhibit complicated mechanisms, especially involved in apoptotic and autophagic signalling pathways. Plant lectins may also determine the fate of cancer cells mainly in three ways: (i) inactivate ribosomes of cancer cells; (ii) selectively localize to organelles in cancer cells; (iii) bind certain sugar‐containing receptors on surfaces of cancer cells. Thus, plant lectins are able to deal with many types of cancer cells by targeting several core apoptotic and autophagic pathways according to these three possible avenues. However, our understanding of how plant lectins play their important roles in cancer cells still remains limited, and thus there is an urgent need for more additional information. Further discoveries will be prompted by new methods such as X‐ray crystallography and nuclear magnetic resonance, which could harness plant lectins for cancer drug discovery. In addition, protein–protein interaction can be used to screen potential candidate drugs. The best hopes for targeting cancer‐related pathways in potential therapeutic applications lie in the discovery of numerous useful agents to target physiological effects of altered key pathways and the whole cancer network rather than their individual genes or proteins.
Thus, based on their specific structures and binding sugar receptor abilities, plant lectins can kill many types of cancer cells by targeting autophagic death involved in many key signalling pathways. With biochemical and molecular complexities of apoptotic and autophagic pathways becoming better understood, new therapeutic strategies will be developed and initiate other therapeutic strategies. Additional research, such as clinical trials into mechanisms of action at the molecular level, will help cancer scientists and clinicians to further understand the therapeutic effects, nutritional benefits and toxic consequences of plant lectins. Together, these findings provide a comprehensive perspective for further elucidating the role of plant lectins that may target apoptotic and autophagic cell death pathways as potential agents in cancer pathogenesis and therapeutics.
Acknowledgements
This work was supported by grants from the Key Projects of the National Science and Technology Pillar Program (no. 2012BAI30B02), National Natural Science Foundation of China (nos U1170302, 81160543, 81260628, 81303270 and 81172374), and West China Hospital‐Chengdu Science and Technology Department Translational Medicine Innovation Foundation (no. ZH13039).
References
- 1. Liu Z, Luo Y, Zhou TT, Zhang WZ (2013) Could plant lectins become promising anti‐tumour drugs for causing autophagic cell death? Cell Prolif. 46, 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Damme EJ, Lannoo N, Fouquaert E, Peumans WJ (2004) The identification of inducible cytoplasmic/nuclear carbohydrate‐binding proteins urges to develop novel concepts about the role of plant lectins. Glycoconj. J. 20, 449–460. [DOI] [PubMed] [Google Scholar]
- 3. Liu B, Bian HJ, Bao JK (2010) Plant lectins: potential antineoplastic drugs from bench to clinic. Cancer Lett. 287, 1–12. [DOI] [PubMed] [Google Scholar]
- 4. Mody R, Joshi S, Chaney W (1995) Use of lectins as diagnostic and therapeutic tools for cancer. J. Pharmacol. Toxicol. Methods 33, 1–10. [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B (2012) Plant natural compounds: targeting pathways of autophagy as anti‐cancer therapeutic agents. Cell Prolif. 454, 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang SY, Yu QJ, Zhang RD, Liu B (2011) Core signaling pathways of survival/death in autophagy‐related cancer networks. Int. J. Biochem. Cell Biol. 43, 1263–1266. [DOI] [PubMed] [Google Scholar]
- 7. Liu B, Cheng Y, Liu Q, Bao JK, Yang JM (2010) Autophagic pathways as new targets for cancer drug development. Acta Pharmacol. Sin. 31, 1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edelman GM, Cunningham BA, Reeke GN Jr, Becker JW, Waxdal MJ, Wang JL (1972) The covalent and three‐dimensional structure of Concanavalin A. Proc. Natl. Acad. Sci. USA 69, 2580–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinha S, Gupta G, Vijayan M, Surolia A (2007) Subunit assembly of plant lectins. Curr. Opin. Struct. Biol. 17, 498–505. [DOI] [PubMed] [Google Scholar]
- 10. Li WW, Yu JY, Xu HL, Bao JK (2011) Concanavalin A: a potential anti‐neoplastic agent targeting apoptosis, autophagy and anti‐angiogenesis for cancer therapeutics. Biochem. Biophys. Res. Commun. 414, 282–286. [DOI] [PubMed] [Google Scholar]
- 11. Liu B, Cheng Y, Bian HJ, Bao JK (2009) Molecular mechanisms of Polygonatum cyrtonema lectin‐induced apoptosis and autophagy in cancer cells. Autophagy 5, 253–255. [DOI] [PubMed] [Google Scholar]
- 12. Liu B, Peng H, Yao Q, Li J, Van Damme E, Balzarini J et al (2009) Bioinformatics analyses of the mannose‐binding lectins from Polygonatum cyrtonema, Ophiopogon japonicus and Liparis noversa with anti‐proliferative and apoptosis‐inducing activities. Phytomedicine 6, 601–608. [DOI] [PubMed] [Google Scholar]
- 13. Pusztai A, Bardocz S, Ewen SW (2008) Uses of plant lectins in bioscience and biomedicine. Front. Biosci. 13, 1130–1140. [DOI] [PubMed] [Google Scholar]
- 14. Rillahan CD, Paulson JC (2013) Glycan microarrays for decoding the glycome. Annu. Rev. Biochem. 80, 797–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dan XL, Ng TB (2013) Lectins in human cancer: both a devil and an angel? Curr. Protein Pept. Sci. 14, 481–491. [DOI] [PubMed] [Google Scholar]
- 16. Liu JJ, Lin M, Yu JY, Liu B, Bao JK (2011) Targeting apoptotic and autophagic pathways for cancer therapeutics. Cancer Lett. 300, 105–114. [DOI] [PubMed] [Google Scholar]
- 17. Lyu SY, Choi SH, Park WB (2002) Korean mistletoe lectin‐induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch. Pharm. Res. 25, 93–101. [DOI] [PubMed] [Google Scholar]
- 18. Lei HY, Chang CP (2007) Induction of autophagy by concanavalin A and its application in anti‐tumour therapy. Autophagy 3, 402–404. [DOI] [PubMed] [Google Scholar]
- 19. Liu B, Cheng Y, Zhang B, Bian HJ, Bao JK (2009) Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria ‐mediated ROS‐p38‐p53 pathway. Cancer Lett. 275, 54–60. [DOI] [PubMed] [Google Scholar]
- 20. Tait SW, Green DR (2012) Mitochondria and cell signalling. J. Cell Sci. 125, 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Estaquier J, Vallette F, Vayssiere JL, Mignotte B (2012) The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 942, 157–183. [DOI] [PubMed] [Google Scholar]
- 22. Wang K (2014) Molecular mechanisms of liver injury: apoptosis or necrosis. Exp. Toxicol. Pathol. 66, 351–356. S0940‐2993(14)00052‐9 [DOI] [PubMed] [Google Scholar]
- 23. Gómez‐Fernández JC (2014) Functions of the C‐terminal domains of apoptosis‐related proteins of the Bcl‐2 family. Chem. Phys. Lipids 183, 77–90. S0009‐3084(14)00065‐6 [DOI] [PubMed] [Google Scholar]
- 24. Souza MA, Carvalho FC, Ruas LP, Ricci‐Azevedo R, Roque‐Barreira MC (2013) Theimmunomodulatory effect of plant lectins: a review with emphasis on ArtinM properties. Glycoconj. J. 30, 641–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu B, Li CY, Bian HJ, Min MW, Chen LF, Bao JK (2009) Antiproliferative activity and apoptosis‐inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch. Biochem. Biophys. 482, 1–6. [DOI] [PubMed] [Google Scholar]
- 26. Liu ZY, Li XF, Ding XP, Yang Y (2010) In silico and experimental studies of Concanavalin A: insights into its antiproliferative activity and apoptotic mechanism. Appl. Biochem. Biotechnol. 162, 134–145. [DOI] [PubMed] [Google Scholar]
- 27. Biswas MH, Hasegawa HH, Rahman MA, Huang P, Mon NN, Ruhul Amin AR et al (2006) SHP‐2‐ERK signaling regulates Concanavalin A‐dependent production of TIMP‐2. Biochem. Biophys. Res. Commun. 348, 1145–1149. [DOI] [PubMed] [Google Scholar]
- 28. Pratt J, Roy R, Annabi B (2012) Concanavalin‐A‐induced autophagy biomarkers requires membrane type‐1 matrix metalloproteinase intracellular signaling in glioblastoma cells. Glycobiology 22, 1245–1255. [DOI] [PubMed] [Google Scholar]
- 29. Ruhul Amin AR, Paul RK, Thakur VS, Agarwal ML (2007) A novel role for p73 in the regulation of Akt‐Foxo1a‐Bim signaling and apoptosis induced by the plant lectin, Concanavalin A. Cancer Res. 67, 5617–5621. [DOI] [PubMed] [Google Scholar]
- 30. Pryme IF, Bardocz S, Pusztai A, Ewen SWB (2006) Suppression of growth of tumor cell lines in vitro and tumors in vivo by mistletoe lectins. Histol. Histopathol. 21, 285–299. [DOI] [PubMed] [Google Scholar]
- 31. Hostanska K, Vuong V, Rocha S, Soengas MS, Glanzmann C, Saller R et al (2003) Recombinant mistletoe lectin induces p53‐independent apoptosis in tumor cells and cooperates with ionizing radiation. Br. J. Cancer 88, 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pae HO, Oh GS, Kim NY, Shin MK, Lee HS, Yun YG et al (2001) Roles of extracellular signal‐regulated kinase and p38 mitogen‐activated protein kinase in apoptosis of human monoblastic leukemia U937 cells by lectin‐II isolated from Korean mistletoe. In Vitr. Mol. Toxicol. 14, 99–106. [DOI] [PubMed] [Google Scholar]
- 33. Li CY, Luo P, Liu JJ, Wang EQ, Li WW, Ding ZH et al (2011) Recombinant expression of Polygonatum cyrtonema lectin with anti‐viral; apoptosis‐inducing activities and preliminary crystallization. Process Biochem. 46, 533–542. [Google Scholar]
- 34. Liu B, Xu XC, Cheng Y, Huang J, Liu YH, Liu Z et al (2008) Apoptosis‐inducing effect and structural basis of Polygonatum cyrtonema lectin and chemical modification properties on its mannose‐binding sites. BMB Rep. 41, 369–375. [DOI] [PubMed] [Google Scholar]
- 35. Liu B, Peng H, Yao Q, Li J, Van Damme EJ, Balzarini J et al (2009) Bioinformatics analyses of the mannose‐binding lectins from Ophiopogon japonicus and Liparis noversa with antiproliferative and apoptosis‐inducing activities. Phytomedicine 16, 601–608. [DOI] [PubMed] [Google Scholar]
- 36. Zhang ZT, Peng H, Li CY, Liu JJ, Zhou TT, Yan YF et al (2010) Polygonatum cyrtonema lectin induces murine fibrosarcoma L929 cell apoptosis via a caspasedependent pathway as compared to Ophiopogon japonicus lectin. Phytomedicine 18, 25–31. [DOI] [PubMed] [Google Scholar]
- 37. Liu Z, Liu B, Zhang ZT, Zhou TT, Bian HJ, Min MW et al (2008) A mannose‐binding lectin from Sophora flavescens induces apoptosis in HeLa cells. Phytomedicine 15, 867–875. [DOI] [PubMed] [Google Scholar]
- 38. Chen J, Liu B, Ji N, Zhou J, Bian JH, Li CY et al (2009) A novel sialic acid‐specific lectin from Phaseolus coccineus seeds with potent antineoplastic and antifungal activities. Phytomedicine 16, 352–360. [DOI] [PubMed] [Google Scholar]
- 39. Zhou W, Gao Y, Xu S, Yang Z, Xu T (2014) Purification of a mannose‐binding lectin Pinellia ternata agglutinin and its induction of apoptosis in Bel‐7404 cells. Protein Expr. Purif. 93, 11–17. [DOI] [PubMed] [Google Scholar]
- 40. Kabir SR, Nabi MM, Haque A, Zaman Rokon Uz, Mahmud ZH, Reza MA (2013) Pea lectin inhibits growth of Ehrlich ascites carcinoma cells by inducing apoptosis and G2/M cell cycle arrest in vivo in mice. Phytomedicine 20, 1288–1296. [DOI] [PubMed] [Google Scholar]
- 41. Deepa M, Sureshkumar T, Satheeshkumar PK, Priya S (2012) Purified mulberry leaf lectin (MLL) induces apoptosis and cell cycle arrest in human breast cancer and colon cancer cells. Chem. Biol. Interact. 200, 38–44. [DOI] [PubMed] [Google Scholar]
- 42. Yang X, Jiang S, Liu Y, Zhang P, Xie S, Wang G (2012) Recombinant VAA‐I from viscum album induces apoptotic cell death of hepatocellular carcinoma SMMC7721 cells. Molecules 17, 11435–11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faheina‐Martins GV, da Silveira AL, Cavalcanti BC, Ramos MV, Moraes MO, Pessoa C et al (2012) Antiproliferative effects of lectins from Canavalia ensiformis and Canavalia brasiliensis in human leukemia cell lines. Toxicol. In Vitro 26, 1161–1169. [DOI] [PubMed] [Google Scholar]
- 44. Fang EF, Zhang CZ, Wong JH, Shen JY, Li CH, Ng TB (2012) The MAP30 protein from bitter gourd (Momordica charantia) seeds promotes apoptosis in liver cancer cells in vitro and in vivo. Cancer Lett. 324, 66–74. [DOI] [PubMed] [Google Scholar]
- 45. Fang EF, Zhang CZ, Ng TB, Wong JH, Pan WL, Ye XJ et al (2012) Momordica charantia lectin, a type II ribosome inactivating protein, exhibits antitumor activity toward human nasopharyngeal carcinoma cells in vitro and in vivo. Cancer Prev. Res. (Phila.) 5, 9–21. [DOI] [PubMed] [Google Scholar]
- 46. Polito L, Bortolotti M, Farini V, Battelli MG, Barbieri L, Bolognesi A (2009) Saporin induces multiple death pathways in lymphoma cells with different intensity and timing as compared to ricin. Int. J. Biochem. Cell Biol. 41, 1055–1061. [DOI] [PubMed] [Google Scholar]
- 47. Miyoshi N, Koyama Y, Katsuno Y, Hayakawa S, Mita T, Ohta T et al (2001) Apoptosis induction associated with cell cycle dysregulation by rice bran agglutinin. J. Biochem. 130, 799–805. [DOI] [PubMed] [Google Scholar]
- 48. Ramnath V, Rekha P, Kuttan G, Kuttan R (2009) Regulation of caspase‐3 and Bcl‐2 expression in Dalton's lymphoma ascites cells by abrin. Evid. Based Complement. Alternat. Med. 6, 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yan Q, Li Y, Jiang Z, Sun Y, Zhu L, Ding Z (2009) Antiproliferation and apoptosis of human tumor cell lines by a lectin (AMML) of Astragalus mongholicus. Phytomedicine 16, 586–593. [DOI] [PubMed] [Google Scholar]
- 50. Schneider JL, Cuervo AM (2014) Autophagy and human disease: emerging themes. Curr. Opin. Genet. Dev. 26, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu B, Wen X, Cheng Y (2013) Survival or death: disequilibrating the oncogenic and tumor suppressive autophagy in cancer. Cell Death Dis. 4, e892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wen X, Wu J, Wang F, Liu B, Huang C, Wei Y (2013) Deconvoluting the role of reactive oxygen species and autophagy in human diseases. Free Radic. Biol. Med. 65, 402–410. [DOI] [PubMed] [Google Scholar]
- 53. Cheng Y, Ren X, Hait WN, Yang JM (2013) Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol. Rev. 65, 1162–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fu LL, Cheng Y, Liu B (2013) Beclin‐1: autophagic regulator and therapeutic target in cancer. Int. J. Biochem. Cell Biol. 45, 921–924. [DOI] [PubMed] [Google Scholar]
- 55. He C, Levine B (2010) The Beclin 1 interactome. Curr. Opin. Cell Biol. 22, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nagelkerke A, Sweep FC, Geurts‐Moespot A, Bussink J, Span PN (2014) Therapeutic targeting of autophagy in cancer. Part I: molecular pathways controlling autophagy. Semin. Cancer Biol. pii: S1044‐579X(14)00073‐X. doi: 10.1016/j.semcancer.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 57. Lei HY, Chang CP (2009) Lectin of Concanavalin A as an anti‐hepatoma therapeutic agent. J. Biomed. Sci. 16, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chang CP, Yang MC, Liu HS, Lin YS, Lei HY (2007) Concanavalin A induces autophagy in hepatoma cells and has a therapeutic effect in a murine in situ hepatoma mode. Hepatology 45, 286–296. [DOI] [PubMed] [Google Scholar]
- 59. Pratt J, Annabi B (2014) Induction of autophagy biomarker BNIP3 requires a JAK2/STAT3 and MT1‐MMP signaling interplay in Concanavalin‐A‐activated U87 glioblastoma cells. Cell. Signal. 5, 917–924. [DOI] [PubMed] [Google Scholar]
- 60. Roy B, Pattanaik AK, Das J, Bhutia SK, Behera B, Singh P et al (2014) Role of PI3K/Akt/mTOR and MEK/ERK pathway in Concanavalin A induced autophagy in HeLa cells. Chem. Biol. Interact. 210, 96–102. [DOI] [PubMed] [Google Scholar]
- 61. Fu LL, Zhou CC, Yao S, Yu JY, Liu B, Bao JK (2011) Plant lectins: targeting programmed cell death pathways as anti‐tumor agents. Int. J. Biochem. Cell Biol. 43, 1442–1449. [DOI] [PubMed] [Google Scholar]
- 62. Liu B, Wu JM, Li J, Liu JJ, Li WW, Li CY et al (2010) Polygonatum cyrtonema lectin induces murine fibrosarcoma L929 cell apoptosis and autophagy via blocking Ras‐Raf and PI3K‐Akt signaling pathways. Biochimie 92, 1934–1938. [DOI] [PubMed] [Google Scholar]
- 63. Choi JH, Lyu SY, Lee HJ, Jung J, Park WB, Kim GJ (2012) Korean mistletoe lectin regulates self‐renewal of placenta‐derived mesenchymal stem cells via autophagic mechanisms. Cell Prolif. 45, 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fu LL, Zhao X, Xu HL, Wen X, Wang SY, Liu B et al (2012) Identification of microRNA‐regulated autophagic pathways in plant lectin‐induced cancer cell death. Cell Prolif. 45, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chang CP, Lei HY (2008) Autophagy induction in T cell‐independent acute hepatitis induced by concanavalinA in SCID/NOD mice. Int. J. Immunopathol. Pharmacol. 21, 817–826. [DOI] [PubMed] [Google Scholar]
- 66. Ueno T, Ohtawa K, Kimoto Y, Sakurai Y, Kodera Y, Hiroto M et al (2000) Polyethylene glycol‐modified Concanavalin A as an effective agent to stimulate anti‐tumour cytotoxicity. Cancer Detect. Prev. 24, 100–106. [PubMed] [Google Scholar]
- 67. Remmelink M, Darro F, Decaestecker C, De R, Decker Bovin NV, Gebhart M et al (1999) In vitro influence of lectins and neoglycoconjugates on the growth of three human sarcoma cell lines. J. Cancer Res. Clin. Oncol. 125, 275–285. [DOI] [PubMed] [Google Scholar]
- 68. Mayer AF, Hoffmann A, Hidvegi M (2000) First clinical data of a natural immunomodulator in colorectal cancer. Hepatogastroenterology 47, 393–395. [PubMed] [Google Scholar]
- 69. Kirsch A, Hajto T (2011) Case reports of sarcoma patients with optimized lectin‐oriented mistletoe extract therapy. J. Altern. Complement. Med. 10, 973–979. [DOI] [PubMed] [Google Scholar]
- 70. Büssing A, Raak C, Ostermann T (2012). Quality of life and related dimensions in cancer patients treated with mistletoe extract (iscador): a meta‐analysis. Evid. Based Complement. Alternat. Med. 2012, 219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Podlech O, Harter PN, Mittelbronn M, Pöschel S, Naumann U (2012). Fermented mistletoe extract as a multimodal antitumoral agent in gliomas. Evid. Based Complement. Alternat. Med. 2012, 501796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sharon N, Lis H. (2004) History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 14, 53R–62R. [DOI] [PubMed] [Google Scholar]
- 73. Griffiths GD, Leek MD, Gee DJ (1987) The toxic plant proteins ricin and abrin induce apoptotic changes in mammalian lymphoid tissues and intestine. J. Pathol. 151, 221–229. [DOI] [PubMed] [Google Scholar]
- 74. Duvall E, Wyllie AH, Morris RG. (1985) Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology 56, 351–358. [PMC free article] [PubMed] [Google Scholar]


