Abstract
Abstract. In order to gain more insight into mechanisms operating on the haematopoietic activity of the T‐cell‐derived cytokine, interleukin‐17 (IL‐17) and target cells that first respond to its action in vivo, the influence of a single intravenous injection of recombinant mouse IL‐17 on bone marrow progenitors, further morphologically recognizable cells and peripheral blood cells was assessed in normal mice up to 72 h after treatment. Simultaneously, the release of IL‐6, IL‐10, IGF‐I, IFN‐γ and NO by bone marrow cells was determined. Results showed that, in bone marrow, IL‐17 did not affect granulocyte‐macrophage (CFU‐GM) progenitors, but induced a persistant increase in the number of morphologically recognizable proliferative granulocytes (PG) up to 48 h after treatment. The number of immature erythroid (BFU‐E) progenitors was increased at 48 h, while the number of mature erythroid (CFU‐E) progenitors was decreased up to 48 h. In peripheral blood, white blood cells were increased 6 h after treatment, mainly because of the increase in the number of lymphocytes. IL‐17 also increased IL‐6 release and NO production 6 h after administration. Additional in vitro assessment on bone marrow highly enriched Lin− progenitor cells, demonstrated a slightly enhancing effect of IL‐17 on CFU‐GM and no influence on BFU‐E, suggesting the importance of bone marrow accessory cells and secondary induced cytokines for IL‐17 mediated effects on progenitor cells. Taken together, these results demonstrate that in vivo IL‐17 affects both granulocytic and erythroid lineages, with more mature haematopoietic progenitors responding first to its action. The opposite effects exerted on PG and CFU‐E found at the same time indicate that IL‐17, as a component of a regulatory network, is able to intervene in mechanisms that shift haematopoiesis from the erythroid to the granulocytic lineage.
INTRODUCTION
The production and activity of haematopoietic cells are both regulated by the interplay of a number of molecules and key amongst them are the cytokines. The recently identified family of interleukin‐17 (IL‐17)‐related cytokines and cognate receptors for these molecules, as a distinct signalling system highly conserved across vertebrate evolution, has pointed to the important role of IL‐17 signalling pathways in the homeostasis of tissues in health and disease (Mosely et al. 2003; Witowski et al. 2004). IL‐17 has been characterized as a pro‐inflammatory cytokine secreted predominantly by activated CD4+ memory T lymphocytes, while the expression of the IL‐17 receptor is ubiquitous in most tissues. Accumulating evidence has confirmed multiple biological activities of IL‐17 on a variety of cells including haematopoietic cells (Fossiez et al. 1998; Schwarzenberger & Kolls 2002). IL‐17 can stimulate the production of different kinds of biologically active molecules, among which are the haematopoietic cytokines and mediators involved in inflammation. As a part of a complex cytokine network, it appears that IL‐17 links the immune system and haematopoiesis, exhibiting a regulatory function on haematopoiesis via secretion of both stimulatory and inhibitory cytokines.
However, the haematopoietic effects of IL‐17, particularly in vivo, are not completely known. First reports have shown that IL‐17 could sustain proliferation of CD34+ haematopoietic progenitors and their maturation into neutrophils in vitro only in the presence of a fibroblast feeder layer (Fossiez et al. 1996). Since these effects were associated with the increased release of haematopoietic active cytokines, such as granulocyte colony‐stimulating factor (G‐CSF) and IL‐6 from the fibroblast feeder layer, it was suggested that IL‐17 achieved its effect on primitive precursor cells via secondarily released cytokines. Data obtained in vivo, using adenovirus‐mediated delivery of mIL‐17 cDNA to the liver, showed the stimulation of haematopoiesis, specifically of granulopoiesis, in mice with the expansion of committed and immature haematopoietic progenitors, neutrophilia and the rapid rise in serum G‐CSF levels (Schwarzenberger et al. 1998). In a previous study, we demonstrated in vitro the influence of IL‐17 on haematopoietic progenitor cells growth and cytokine release in normal and post‐irradiated murine bone marrow (Jovčićet al. 2001). The observed effects were lineage dependent and related to the progenitors’ stage of differentiation, as well as being dependent on the physiological/pathological status of the organism.
The aim of the present study was to investigate the in vivo action of IL‐17 on constitutive haematopoiesis and cytokine release in CBA mice, as in vitro data are not sufficient for accurate determination of in vivo biological function, because of the complexity of in vivo cytokine cascades. In order to study the IL‐17 target cell range, the effects of a single intravenous (i.v.) administration of recombinant mouse IL‐17 (rmIL‐17) on bone marrow granulocyte‐macrophage (CFU‐GM) and erythroid (BFU‐E and CFU‐E) progenitor cell compartments, further morphologically recognizable cells, as well as peripheral blood haematological parameters were examined at different time intervals after treatment. Evaluation of target cells that first respond to the action of this T cell‐derived cytokine may also help better understanding of the contribution of T cells in regulation of haematopoiesis and the inflammatory response. At the same time points, the influence of IL‐17 on the release of secondary mediators with haematopoietic effects, such as IL‐6, IL‐10, IGF‐I, IFN‐γ and nitric oxide (NO) by bone marrow cells was analysed. Namely, it is well documented that, within the haematopoietic microenvironment, bone marrow stromal cells are crucial for providing positive and negative signals for proliferation and differentiation of haematopoietic cells, and that IL‐17 stimulates fibroblasts and stromal cells to release pro‐inflammatory and haematopoietic cytokines, chemokines and cell adhesion molecules (Fossiez et al. 1998; Mosely et al. 2003; Witowski et al. 2004). In addition, an effort has been made to resolve the mode of IL‐17 action on progenitor cell compartments, that is to distinguish the direct IL‐17 effects from the those via secondary mediators. In vitro effects of increasing concentrations of IL‐17 on bone marrow highly enriched for Lin− progenitor cells were assessed in the colony formation assays.
MATERIALS AND METHODS
Experimental protocol
The experiments were carried out in normal inbred male CBA mice weighing 20–22 g (Breeding Facilities of the Institute for Medical Research, Military Medical Academy, Belgrade). The animals received 1 µg of rmIL‐17 (R & D Systems, Minneapolis, MN, USA) dissolved in saline and administered i.v. in a volume of 0.2 ml. Mice were killed 6, 12, 24, 48 and 72 h after treatment. To distinguish IL‐17 mediated effects from the effects caused by the injection procedure itself, beside non‐treated mice, saline‐treated mice were used as controls. In each animal, the following haematological parameters were estimated: in the femoral bone marrow, the number of CFU‐GM‐, BFU‐E‐ and CFU‐E‐derived colonies, the total number of nucleate cells and differential count of morphologically recognizable cells were performed. In peripheral blood, haematocrit, the total number and differential count of nucleated cells, the number of erythrocytes and the percentage of reticulocytes were derived. At the same time points after IL‐17 treatment, the release of IL‐6, IL‐10, IGF‐I, IFN‐γ and NO by bone marrow cells was determined. The experiments were performed on 4–6 animals per group for each time point and were replicated at least three times.
Colony forming assays
Methylcellulose cultures for CFU‐GM and BFU‐E were performed using MethoCult GF M3434 methylcellulose medium with recombinant cytokines (50 ng/ml rmSCF, 10 ng/ml rmIL‐3, 10 ng/ml rhIL‐6 and 3 units/ml rhEpo, Stem Cell Technologies, Vancouver, BC, Canada) following the instructions of the manufacturer, while for CFU‐E determination MethoCult M 3334 with recombinant erythropoietin (3 units/ml) only was used. Briefly, 1 × 105 nucleate bone marrow cells for CFU‐GM and BFU‐E and 2 × 105 for CFU‐E were suspended in 1 ml of methylcellulose medium, plated in duplicate in 35‐mm tissue culture dishes and incubated at 37 °C in a humidified atmosphere with 5% CO2 in air. The number of colonies was counted at day 2 for CFU‐E and day 7 for CFU‐GM and BFU‐E using an inverted microscope.
Estimation of morphologically recognizable bone marrow cells
On bone marrow cell smears stained by the May–Grunwald–Giemsa procedure, 1000 morphologically differentiated nucleate cells on each smear were observed and divided into the following compartments: proliferative granulocytes, metamyelocytes, mature granulocytes, monocytes, lymphocytes, erythroblasts and orthochromatic blasts. Myeloblasts, promyelocytes and myelocytes, cells capable of replication, constitute the mitotic or proliferative granulocytes compartment (PG).
Estimation of peripheral blood cells
Differential counts of nucleated cells were made on 100 counted cells on blood smears stained by the May–Grunwald–Giemsa procedure. The reticulocytes were stained with brilliant cresyl blue, counted per 1000 erythrocytes, and results expressed as percentage of the number of red blood cells examined.
Cytokine determination
To evaluate the influence of rmIL‐17 administration on cytokine release and nitrite accumulation by bone marrow cells, at the indicated time points after treatment, femoral bone marrow cell suspensions (5 × 106/ml) were prepared from each animal in Dulbecco's modified Eagle's medium supplemented with 5% foetal calf serum and incubated for 48 h at 37 °C in a humidified atmosphere with 5% CO2 in air. Cell‐derived conditioned media were harvested and stored at −20 °C until cytokine assays were performed. Measurement of IL‐6, IL‐10 and interferon‐gamma (IFN‐γ) concentrations in bone marrow cell‐derived conditioned media was performed using ELISA kits following instructions provided by R & D Systems (Minneapolis, MN, USA). The insulin‐like growth factor‐I (IGF‐I) levels were determined using radioimmunoassay kits (INEP, Zemun, Serbia and Montenegro). The sensitivity of these assays enables detection of cytokine concentrations as low as 1.6 pg/ml for IL‐6, 4 pg/ml for IL‐10, 2 pg/ml for IFN‐γ and 2 pg/ml for IGF‐I.
NO determination
Production of NO by bone marrow cells was determined indirectly by measuring accumulation of nitrite, a stable break‐down product of NO, using the Griess reaction (Green et al. 1982). Briefly, 50 µl samples of culture supernatants were mixed with equal volumes of 1% sulphanilamide and 0.1% N‐1 naphtylethylene diamine dihydrochloride in 5% H3PO4. After 10 min at room temperature, the absorbance at 540 nm was measured in a microplate reader.
In vitro effects of IL‐17 on bone marrow Lin−‐enriched colony‐forming cells
A highly enriched murine Lin− haematopoetic progenitor cell population from bone marrow was obtained using SpinSep Cocktail for Negative Selection from Stem Cell Technologies (Vancouver, BC, Canada) following the recommendations of the manufacturer. This depletion cocktail includes the monoclonal antibodies to the following murine cells/cell surface antigens: CD5 (Ly‐1), CD45R (B220), CD11b (Mac‐1), erythroid cells (TER119), Ly‐6G (Gr‐1) and neutrophils (7–4). To assess the influence of IL‐17 on the growth of haematopoietic progenitor cell (CFU‐GM and BFU‐E) ‐derived colonies, Lin− enriched cell populations (1–5 × 103/ml) were cultured in the presence of increasing concentrations of IL‐17 (0–100 ng/ml) using MethoCult GF M3434.
Statistical analysis
The comparison between the results of rmIL‐17‐treated and control, normal, non‐treated and saline‐treated mice, was performed by Student's t‐test using the Origin PC Program with the actual numbers of each investigated parameter. Data were expressed as means ± SEM and a P‐value less than 0.05 was considered to be statistically significant.
RESULTS
In vivo haematopoietic activity of IL‐17 was assessed in normal mice after a single intravenous injection of rmIL‐17 by the analysis of cellular and cytokine release changes at different time intervals (6, 12, 24, 48 and 72 h) after treatment. Because IL‐17 mediated biological effects in vivo could interfere with the response of the organism to stress caused by the injection procedure itself, obtained results were analysed and compared with two control groups, normal, non‐treated and saline‐treated mice, respectively.
Influence of IL‐17 on bone marrow progenitor cells
In the bone marrow of investigated mice, the number of CFU‐GM‐derived colonies was not significantly affected by either IL‐17 or saline treatment at all observed time points and their numbers oscillated within the values found for the normal mice (Fig. 1a). The estimation of immature erythroid progenitor cells (BFU‐E) showed that IL‐17 treatment significantly increased the number of femoral BFU‐E‐derived colonies 48 h after administration (Fig. 1b), in comparison with both control groups, normal, non‐treated (P < 0.001) and saline‐treated mice (P < 0.05). However, at this time point, saline treatment also induced the increase of BFU‐E‐derived colony number, but to a lesser extent than IL‐17 treatment. In contrast to the effect on BFU‐E, IL‐17 treatment resulted in a significant reduction in the number of mature erythroid progenitor cells (CFU‐E) in comparison with both control groups. As early as 6 h after IL‐17 administration, the number of CFU‐E‐derived colonies was decreased, reaching the minimum at 24 h, while at 72 h their number returned to normal values (Fig. 1c). The number of CFU‐E was not significantly altered by saline treatment, and oscillated within the values found for the normal, non‐treated mice.
Figure 1.
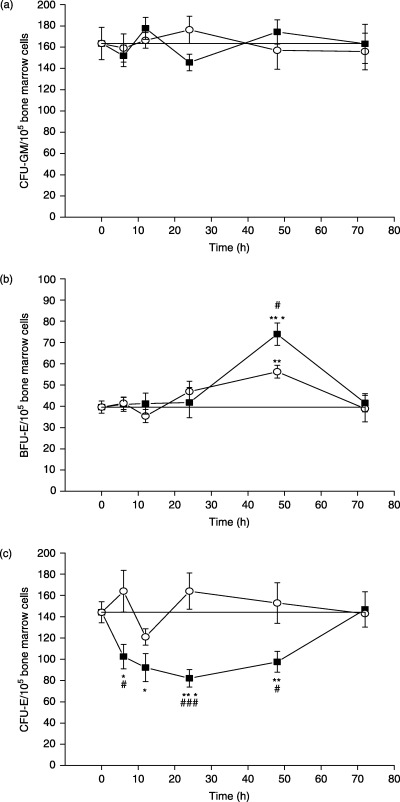
In vivo influence of rmIL‐17 on femoral (a) CFU‐GM, (b) BFU‐E and (c) CFU‐E‐derived colonies growth in normal mice at different time points after treatment. The data points represent means ± SEM of three to five experiments each performed in duplicate. (–▪–) IL‐17‐treated mice; (–○–) saline‐treated mice; (–—–) non‐treated mice. Significance at *P < 0.05, **P < 0.01, ***P < 0.001 for IL‐17‐ and saline‐treated vs. non‐treated mice; and at #P < 0.05, ###P < 0.001 for IL‐17‐treated vs. saline‐treated mice.
Influence of IL‐17 on morphologically recognizable bone marrow cells
No significant differences in the bone marrow cellularity were found between IL‐17‐treated mice and both control groups during all observed time points. Examination of the bone marrow morphologically recognizable haematopoietic cells showed that the cells of macrophage, lymphocyte and erythroid lineage were not significantly affected by IL‐17 treatment (data not shown). However, IL‐17 treatment induced significant alterations in the cells of granulocyte lineage. The analysis of changes within different granulocytic cell compartments demonstrated a significant increase in the absolute number of proliferative granulocytes (PG) already 6 h after IL‐17 administration, as compared with both control groups of mice (Fig. 2). The persistent increase in the absolute number of PG was evident up to 48 h after IL‐17 injection, while at 72 h their number returned to normal values. Saline treatment did not induce any changes in the number of PG. At the same time, within post‐mitotic granulocyte compartments, no significant alterations were determined in the number of metamyelocytes and mature granulocytes, by both IL‐17 and saline treatments (data not shown).
Figure 2.
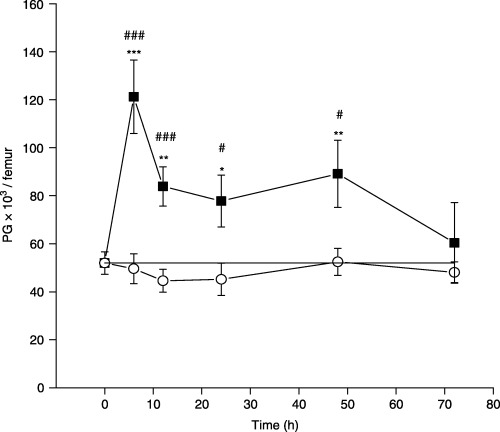
The effect of a single dose of rmIL‐17 on morphologically recognizable proliferative granulocytes (PG) in normal mice at different time points after treatment. The data points represent means ± SEM of five separate experiments. (–▪–) IL‐17 treated mice; (–○–) saline treated mice; (–—–) non‐treated mice. Significance at *P < 0.05, **P < 0.01, ***P < 0.001 for IL‐17‐ and saline‐treated vs. non‐treated mice, and at #P < 0.05, ###P < 0.001 for IL‐17‐treated vs. saline‐treated mice.
Influence of IL‐17 on peripheral blood cells
Analysis of haematological parameters in peripheral blood demonstrated that IL‐17 induced a significant increase of the total number of white blood cells (WBC) 6 h after application in comparison with both control groups, non‐treated (P < 0.001) and saline‐treated mice (P < 0.01) (Table 1). At the same time point, saline treatment also led to some transient increase of WBC, but to a lesser extent than IL‐17 treatment (P < 0.05). Differential counts of nucleated cells showed that the observed increase of WBC in IL‐17 and saline‐treated mice was mainly as a result of the increase in the number of lymphocytes. After 6 h, IL‐17 did not induce any statistically significant changes in the number of peripheral blood cells in comparison with saline‐treated mice, and their values at 24 h were almost normal. The other investigated parameters (the number of erythrocytes, the percentage of reticulocytes and haematocrit) were not affected by IL‐17 treatment (data not shown).
Table 1.
The effect of a single dose of rmIL‐17 on peripheral blood cells
| Time | Group | × 109/l | ||||
|---|---|---|---|---|---|---|
| WBC | GRAN | MONO | LYMPHO | Other cells | ||
| N | 4.96 ± 0.37 | 1.65 ± 0.19 | 0.17 ± 0.02 | 3.03 ± 0.33 | 0.11 ± 0.22 | |
| 6 h | IL‐17 | 7.31 ± 0.31***, † | 1.68 ± 0.13 | 0.25 ± 0.03 | 5.19 ± 0.24*** | 0.19 ± 0.17 |
| Saline | 6.17 ± 0.25* | 1.31 ± 0.11 | 0.20 ± 0.04 | 4.54 ± 0.24* | 0.12 ± 0.32 | |
| 24 h | IL‐17 | 5.16 ± 0.43 | 1.53 ± 0.17 | 0.19 ± 0.02 | 3.43 ± 0.24 | 0.01 ± 0.09 |
| Saline | 4.54 ± 0.40 | 1.41 ± 0.18 | 0.18 ± 0.02 | 2.94 ± 0.25 | 0.01 ± 0.14 | |
Total number of white blood cells (WBC) and the differential counts of peripheral nucleated cells. GRAN, granulocytes; MONO, monocytes; LYMPHO, lymphocytes. Values are expressed as means ± SEM of three separate experiments. Significance at *P < 0.05, ***P < 0.001 for IL‐17‐ and saline‐treated vs. non‐treated mice, and at †P < 0.01 for IL‐17‐treated vs. saline‐treated mice.
Influence of IL‐17 on cytokine release and NO production by bone marrow cells
Simultaneously with the assessment of cellular changes, at the same time points after administation, the influence of IL‐17 on the production of IL‐6, IL‐10, IGF‐I and IFN‐γ, as well as on nitrite accumulation by bone marrow cells, was determined. In the bone marrow cell‐derived conditioned media of non‐treated mice, detectable amounts of IL‐6, IL‐10, IGF‐I and nitrite were measured, while IFN‐γ was below detectable levels (Table 2). IL‐17 significantly increased the release of IL‐6, 6 h after administration. Although saline treatment also augmented IL‐6 release by bone marrow cells, significantly higher concentrations of IL‐6 were found in IL‐17‐treated mice than in saline‐treated animals. In comparison with constitutive secretion, IL‐10 release by bone marrow cells was significantly increased only 6 h after both IL‐17 and saline administration. However, in this case, there were no differences between IL‐17‐treated and saline‐treated mice. The basal IGF‐I release by bone marrow cells was not affected either by IL‐17 or by saline treatment, while the secretion of IFN‐γ was below detectable levels in all experimental groups in all tested time points. The estimation of NO production by bone marrow cells revealed increased nitrite accumulation also only 6 h after IL‐17 treatment in comparison with the constitutive production, as well as with saline treatment. For all tested molecules, after the initial rise at 6 h after treatment, a decrease in their concentrations was found and already 24 h after both IL‐17 and saline injection the concentrations of all tested cytokines were approaching normal (Table 2).
Table 2.
The effect of a single dose of rmIL‐17 on cytokine release and nitric oxide (NO) production by bone marrow cells
| Time | Group | IL‐6 (pg/ml) | IL‐10 (pg/ml) | IFN‐γ (pg/ml) | IGF‐I (pg/ml) | NO (µmol) |
|---|---|---|---|---|---|---|
| N | 187.9 ± 31.7 | 52.55 ± 4.9 | < 2‡ | 3.62 ± 0.7 | 2.75 ± 0.3 | |
| 6 h | IL‐17 | 470.75 ± 41.3***, † | 121.12 ± 5.9*** | < 2 | 2.2 ± 0.1 | 4.94 ± 0.8**, † |
| Saline | 310.25 ± 16.7* | 142.41 ± 20.6*** | < 2 | 3.23 ± 0.8 | 3.0 ± 0.4 | |
| 24 h | IL‐17 | 204.4 ± 39.9 | 78.6 ± 13.1 | < 2 | 3.77 ± 0.8 | 3.8 ± 0.6 |
| Saline | 192.83 ± 65.0 | 79.5 ± 7.7 | < 2 | 2.15 ± 0.2 | 2.9 ± 0.8 |
The cells were obtained from non‐treated mice (N) and IL‐17‐ and saline‐treated 6 h and 24 h after injection. Cells (5 × 106/ml) were cultured for 48 h at 37 °C and cell‐free supernatants were collected. Values are expressed as means ± SEM. Significance at *P < 0.05, **P < 0.01, ***P < 0.001 for IL‐17‐ and saline‐treated vs. non‐treated mice, and at †P < 0.05 for IL‐17‐treated vs. saline‐treated mice. ‡The secretion of IFNγ was below detectable levels.
In vitro effects of IL‐17 on bone marrow Lin−‐enriched colony‐forming cells
To resolve the direct from indirect effects of IL‐17 on haematopoietic progenitor cells, highly enriched murine Lin− progenitor cells from bone marrow were cultured in the presence of increasing concentrations of IL‐17. The results revealed a slightly enhanced effect of IL‐17 on the growth of CFU‐GM‐derived colonies, while the growth of BFU‐E derived colonies was not affected by IL‐17 (Fig. 3).
Figure 3.

In vitro influence of increasing concentrations of rmIL‐17 on femoral CFU‐GM (▪) and BFU‐E (□)‐derived colonies growth in bone marrow highly enriched Lin− cell fraction obtained from normal mice. The data points represent means ± SEM of four experiments each performed in duplicate.
DISCUSSION
Our previous in vitro study showed that IL‐17 increased CFU‐GM and BFU‐E but reduced CFU‐E‐derived colony number in the bone marrow cells, from normal mice (Jovčićet al. 2001). Based on these findings, the goal of the current study was to gain more insight into mechanisms operating on the IL‐17 action and heterogeneity of its haematopoietic target cells in vivo. For this purpose, we used one well‐established method for the investigation of the in vivo cytokine activity, that is its administration as recombinant protein. The results obtained demonstrated that a single dose of IL‐17 elicited a cascade of biological changes in vivo, affecting the haematopoietic cells from both granulocytic and erythroid lineages, as well as the levels of cytokines released by bone marrow cells, as early as 6 h after administration.
The measurement of cytokine production by bone marrow cells revealed a rapid increase in IL‐6 and IL‐10 levels, as well as elevated nitrite accumulation, 6 h after IL‐17 administration. Elevation of IL‐6 and IL‐10 in the saline‐treated animals, indicates that the injection itself activated host defence mechanisms known to induce cellular changes and elevation of pro‐inflammatory cytokines during an immediate stress‐like response. However, IL‐17 led to much higher release of IL‐6 than the saline treatment, and this difference could be ascribed to the effect of IL‐17 itself. This is consistent with the well‐known ability of IL‐17 to trigger the production of IL‐6 in a variety of cells. As regards IL‐10, both IL‐17 and saline treatments augmented its release to the same extent. Concomitant elevation of this anti‐inflammatory cytokine in both groups of animals indicates an effort of the organism to overcome the disturbance caused by the host defence reaction to the injection procedure itself and change in blood volume. The concentrations of elevated mediators decreased afterwards and 24 h after injection were within normal values. Investigated cytokine profiles of IGF‐I and IFN‐γ did not change during the whole examined period. The lack of more significant changes in cytokine levels could be also as a result of the experimental procedure used, as the production of cytokines by bone marrow cells was analysed following 48‐h incubation in vitro. The possibility that the in vitro manipulation caused alterations in bone marrow cells’ ability to release cytokines could not be excluded. Cytokine levels in peripheral blood serum and/or bone marrow extracts should be further analysed in order to provide better insight in the consequences of IL‐17 in vivo action.
Assessment of the IL‐17‐induced changes within different compartments of granulocytic lineage demonstrated that one injection of IL‐17 did not significantly affect femoral CFU‐GM, but induced considerable increase in morphologically recognizable neutrophil precursors, proliferative granulocytes (PG). The lack of IL‐17 effect on CFU‐GM was unexpected and was contradictory to previously reported in vivo IL‐17 stimulation of granulopoiesis which was accompanied by the expansion of haematopoietic progenitors and neutrophilia (Schwarzenberger et al. 1998). Our experimental approach was under different conditions from those obtained by the usage of the recombinant adenovirus expression system where effective production of IL‐17 was provided. One possible explanation is that the dose of IL‐17 applied was not sufficient to alter the CFU‐GM compartment, as our previous data in vitro showed also the requirement of relatively high concentrations of IL‐17 for stimulation of CFU‐GM growth (Jovčićet al. 2001). Although our additional experiments performed in vitro with the highly enriched Lin− bone marrow progenitor cell fraction revealed a slightly enhancing effect of IL‐17 on the CFU‐GM growth, at present it is difficult to draw any conclusion about the mode of IL‐17 mediated effect on the CFU‐GM. Further investigations are necessary to determine the precise mechanisms and regulatory steps of IL‐17 stimulated granulopoiesis in vivo, especially at the level of progenitor cells. However, the most obvious in vivo response of granulocytic cells to IL‐17 administration was observed at the level of PG. A significant and continuous increase in the number of cells in this compartment was evident from 6 h after IL‐17 injection onward. To our knowledge, this is the first demonstration of the effect exerted by IL‐17 on morphologically recognizable proliferative granulocytes. This finding may be of importance in understanding the mechanisms by which IL‐17 is involved in the regulation of granulopoiesis and the inflammatory response, bearing in mind that neutrophils are the first host defensive cells to be recruited to the site of inflammation. In order to meet the emergency demand for a massive number of granulocytes, it cannot be ruled out that the most rapid and increased production of granulocytes is provided via regulator(s) able to act on the quantitatively largest granulocytic cell compartment capable of proliferating, that is PG. Thus, one can suppose that IL‐17, as mediator and/or modulator of the host defence responses, signalling through its widely expressed cell surface receptors, might participate in the stimulation of granulopoiesis acting at the level of PG, either directly or through enhanced secretion of other cytokines that stimulate granulopoiesis. At the moment it is difficult to distinguish between these two possibilities, particularly in vivo. IL‐17 has been shown to induce G‐CSF production (Fossiez et al. 1996; Schwarzenberger et al. 1998) and, as for the direct effects of IL‐17 on haematopoietic cells, there is little evidence except that IL‐17 can activate various members of the JAK/STAT pathway in human monocytic leukaemia cells (Subramaniam et al. 1999). The absence of significant changes within post‐mitotic granulocyte compartments in the bone marrow indicates that the dose of IL‐17 used was insufficient to increase the production of more mature cells and/or that the mechanisms towards restoring the disturbed homeostasis were activated as predominant, as demands for the excess number of granulocytes did not exist. The same was true for the number of peripheral blood granulocytes. Namely, in peripheral blood, IL‐17 treatment increased the number of WBC 6 h after administration – interestingly, mainly as a result of the increase in the number of lymphocytes. Schwarzenberger et al. (1998) also reported that IL‐17 in vivo can mediate increase in the number of peripheral blood lymphocytes.
While the effects of IL‐17 on granulopoiesis have been intensively investigated, the effects of IL‐17 on erythropoiesis have received relatively scant attention. Our results demonstrated the profound in vivo effects of IL‐17 on the erythroid progenitor cell compartments. In accordance with its pleiotropic nature, as well as our in vitro data (Jovčićet al. 2001), IL‐17 exerted opposite effects on erythroid progenitors; stimulatory on immature progenitors, BFU‐E and inhibitory on mature progenitors, CFU‐E. The number of femoral BFU‐E increased significantly 48 h after IL‐17 treatment in comparison with both normal and saline‐injected animals. Because saline treatment also temporarily increased BFU‐E, the observed IL‐17 effect on BFU‐E was, in a part, non‐specific and interfered with the action of secondary mediators elevated in response to the stress caused by the injection and, at least partly, was specific to IL‐17. The effect of IL‐17 on BFU‐E was probably mediated through its ability to induce the release of secondary cytokines, particularly IL‐6. In favour of this assumption is the fact that in IL‐17‐treated mice the levels of IL‐6 produced by bone marrow cells were significantly higher than in controls. It is well known that IL‐6 exhibits stimulatory effects on haematopoiesis, specifically erythropoiesis (Kishimoto 1989). Moreover, it was demonstrated that in vivo IL‐6 can increase the number of femoral BFU‐E in normal mice (Pojda & Tsuboi 1990). Also, it cannot be excluded that the reduced number of CFU‐E which persisted up to 48 h after IL‐17 administration was a signal for the more extensive BFU‐E proliferation and differentiation observed in IL‐17‐treated mice, in order to restore the dynamic equlibrium between BFU‐E and CFU‐E. In any case, the results suggest that the observed in vivo effects of IL‐17 on BFU‐E were predominantly indirect via secondarily induced cytokines. This is additionally supported with our data obtained in vitro. Namely, in our previous experiments, when we used unseparated bone marrow cells, in order to mimic in vivo conditions and to provide the presence of accessory cells, IL‐17 enhanced the growth of BFU‐E‐derived colonies, and simultaneously augmented the release of IL‐6. In contrast to these data, as presented here, no in vitro effect of IL‐17 was observed on the formation of BFU‐E colonies by highly enriched Lin− bone marrow cells, suggesting the necessity of the presence of accessory cells for IL‐17 mediated effects on BFU‐E.
Concerning the in vivo effect of IL‐17 on femoral CFU‐E, the obtained results again confirmed our previous in vitro finding that IL‐17 induces a decrease in their number. At present the mechanism(s) by which IL‐17 suppressed the number of CFU‐E is unknown. However, increased nitrite accumulation determined only in IL‐17‐treated animals, led us to assume that the suppressing effect of IL‐17 on CFU‐E might be, at least partly, mediated by NO generation. It has been previously reported that at low concentrations NO can actually enhance CFU‐GM colony growth, while inhibiting erythroid cell (CFU‐E) growth from human bone marrow CD34+ cells (Shami & Weinberg 1996). In contrast, IL‐17 has been shown to induce NO production in a variety of cells (Miljković & Trajković 2004). In support of this assumption, that the reducing effect of IL‐17 on CFU‐E might be mediated by NO, are our in vitro results that IL‐17 can stimulate low level production of NO in murine bone marrow cells and that iNOS inhibitors can reverse the in vitro CFU‐E suppression by IL‐17 in a dose‐dependent manner (Bugarski et al. 2004). However, because an increased production of NO by bone marrow cells was not detected after 6 h post‐IL‐17 treatment, the possibility that other negative factor(s) and mechanisms are involved in the prolonged IL‐17 reducing effect on CFU‐E could not be excluded. In any case, this in vivo IL‐17 mediated inhibitory effect on CFU‐E might be of special importance in view of the role of this cytokine in the inflammatory response of organism. Namely, one can suppose that, during inflammation, signalling pathways activated by IL‐17 might selectively switch bone marrow cell production from the erythroid to the granulocyte lineage by attenuating CFU‐E proliferation and/or differentiation.
Taken together, our results suggest that IL‐17, as a cross‐talk cytokine between the immune and haematopoietic systems, can influence haematopoiesis in vivo at different levels, as well as the release of secondary mediators with haematopoietic effects. The results are basically in agreement with the hypothesis that IL‐17 is involved in the regulation of granulopoiesis and may play an important role in the initiation and/or maintenance of the inflammatory response. Evaluation of target cell range pointed to the heterogeneity of haematopoietic cells affected by IL‐17. The most profound and rapid effects of IL‐17 were observed on PG and CFU‐E, suggesting that, in vivo, in the bone marrow more mature haematopoietic progenitors are the first to respond to its action. Additionally, the opposite effects exerted on PG and CFU‐E imply that IL‐17 may be one of the key cytokines that coordinate and control haematopoiesis, as in this way IL‐17 can enable the switch in cell production from the erythroid to the granulocyte lineage during inflammation or infection when enhanced defences are required. The capacity of IL‐17 to significantly stimulate proliferation of PG after a single application indicates its therapeutical potential and encourages further investigation in vivo towards development of new modalities in therapy of patients with compromised granulopoiesis. Novel experimental approaches are required, especially suitable animal experimental models with disturbed haematopoiesis, and also varying dose schedules. Namely, the requirements for and the effects of cytokines are not equal during constitutive and inducible haematopoiesis and, according to our studies, cytokines express only some of their potential effects in the steady state, either on haematopoietic cells or cytokine release (Jovčićet al. 1996; Bugarski et al. 2000).
ACKNOWLEDGEMENTS
The excellent technical assistance of Mrs K. Božanić, Mrs Z. Dimitrijević and Mrs S. Marković is appreciated. This work was supported by a grant from the Ministry of Science and Environmental Protection, Republic of Serbia (project # 1742 ‘Regulation of Haematopoiesis and Biologically Active Molecules’).
REFERENCES
- Bugarski D, Jovčić G, Kataranovski M, Ivanović Z, Petakov M, Stojanović N, Milenković P (2000) Effects of treatment with interleukin‐1 receptor antagonist on endogenous interleukin‐1 levels in normal and irradiated mice. Physiol. Res. 49, 355. [PubMed] [Google Scholar]
- Bugarski D, Krstić A, Vlaški M, Petakov M, Jovčić G, Stojanović N, Milenković P (2004) IL‐17 induced inhibitory effect on late stage murine erythroid bone marrow progenitors. Eur. Cytokine Netw. 15, 247. [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores‐Romo L, Ait‐Yahia S, Maat C, Pin J, Garcia E, Saeland S, Blanchard D, Galliard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S (1996) T cell interleukin‐17 induces stromal cell to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecgue S (1998) Interleukin‐17. Int. Rev. Immunol. 16, 541. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok SJ, Tannenbaum SR (1982) Analysis of nitrate, nitrite and (15N) nitrite in biological fluids. Annal. Biochem. 126, 131. [DOI] [PubMed] [Google Scholar]
- Jovčić G, Ivanović Z, Biljanović‐Paunović L, Bugarski D, Stošić‐Grujičoć S, Milenkovoć P (1996) In vivo effects of interleukin‐1 receptor antagonist on hematopoietic bone marrow progenitor cells in normal mice. Eur. Cytokine Netw. 7, 71. [PubMed] [Google Scholar]
- Jovčić G, Bugarski D, Petakov M, Stanković J, Stojanović N, Milenković P (2001) Effect of IL‐17 on in vitro hematopoietic progenitor cells growth and cytokine release in normal and post‐irradiated murine bone marrow. Growth Factors 19, 61. [DOI] [PubMed] [Google Scholar]
- Kishimoto T (1989) The biology of IL‐6. Blood 74, 1. [PubMed] [Google Scholar]
- Miljković D, Trajković V (2004) Inducible nitric oxide synthase activation by interleukin‐17. Cytokine Growth Factor Rev. 15, 21. [DOI] [PubMed] [Google Scholar]
- Mosely TA, Haudenschild DR, Rose L, Reddi AH (2003) Interleukin‐17 family and IL‐17 receptors. Cytokine Growth Factor Rev. 14, 155. [DOI] [PubMed] [Google Scholar]
- Pojda Z, Tsuboi A (1990) In vivo effects of human recombinant interleukin‐6 on hematopoietic stem and progenitor cells and circulating blood cells in normal mice. Exp. Hematol. 18, 1034. [PubMed] [Google Scholar]
- Schwarzenberger P, Kolls JK (2002) Interleukin 17: an example for gene therapy as a tool to study cytokine mediated regulation of hematopoiesis. J. Cell Bioch. Suppl. 38, 88. [DOI] [PubMed] [Google Scholar]
- Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK (1998) IL‐17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy‐derived method for in vivo evaluation of cytokines. J. Immunol. 161, 6383. [PubMed] [Google Scholar]
- Shami PJ, Weinberg JB (1996) Differential effects of nitric oxide on erythroid and myeloid colony growth from CD34+ humane bone marrow cells. Blood 87, 977. [PubMed] [Google Scholar]
- Subramaniam SV, Cooper RS, Adunyah SE (1999) Evidence for involvement of JAK/STAT pathway in the signaling mechanism of interleukin‐17. Biochem. Biophys. Res. Commun 262, 14. [DOI] [PubMed] [Google Scholar]
- Witowski J, Ksiazek K, Jorres A (2004) Interleukin‐17: a mediator of inflammatory responses. Cell Mol. Life Sci. 61, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]


