Abstract
Abstract. Embryonal carcinoma and embryonic stem cells have served as models to understand basic aspects of neuronal differentiation and are promising candidates for regenerative medicine. Besides being well characterized regarding the capability of embryonal carcinoma and embryonic stem cells to be precursors of different tissues, the molecular mechanisms controlling neuronal differentiation are hardly understood. Neuropeptide and neurotransmitter receptors are expressed at early stages of differentiation prior to synaptogenesis, triggering transient changes in calcium concentration and inducing neurone‐specific gene expression. In vitro neuronal differentiation of embryonal carcinoma and embryonic stem cells closely resembles early neuronal development in vivo. Murine P19 EC cells are a well‐characterized model for in vitro differentiation, which upon treatment with retinoic acid differentiate into neurones. Expression and activity of various receptor proteins is regulated during their differentiation. Stimulation of kinin‐B2, endothelin‐B, muscarinic acetylcholine, and N‐methyl‐D‐aspartate receptors results in transient increases of intracellular free calcium concentration [Ca2+]i in P19 cells undergoing neuronal differentiation, whereas embryonal cells do not respond or show a smaller change in [Ca2+]i than differentiating cells. Receptor inhibition, as studied with the example of the kinin‐B2 receptor, aborts neuronal maturation of P19 cells, demonstrating the crucial importance of B2 receptors during the differentiation process. Future success in obtaining desired neuronal phenotypes from pluripotent cells in vitro may offer new therapeutic perspectives for curing genetic and acquired dysfunctions of the developing and adult nervous system.
INTRODUCTION
Development of the nervous system entails the formation of trillions of connections between outgrowing nerve cells and their targets of innervation. Neurones in the central nervous system (CNS) are polarized cells with a single axon and multiple dendrites, which are connected to neighbouring cells by synapses. During this process, neurones express voltage‐gated channels, generate action potentials, and release and detect neurotransmitters at their synapses for signal transmission.
In order to ensure the functionality of neuronal connections, the differentiation process from an undifferentiated cell to a fully developed neurone needs to be highly coordinated. Excitability of neurones and glial cells is known to be the basis of signalling and cell communication in the adult nervous system, but recent evidence has been obtained indicating that processes such as induction to proliferate and differentiate are controlled by ion fluxes and subsequent changes of the membrane potential. This requires stage‐specific expression of neuropeptides, neurotransmitters and their respective receptors. Calcium spikes trigger neuronal differentiation, and alterations in pattern of Ca2+‐spike activity in embryonic neurones during differentiation leads to a different neuronal phenotype (Borodinsky et al. 2004). In addition to their well‐established actions in the mature CNS, neuropeptide and neurotransmitter receptors, and their respective ligands are supposed to play fundamental roles during maturation of the nervous system. Therefore, these receptors and their ligands are already often expressed early when synaptic connections are still immature.
In order to understand the roles of neuropeptides, neurotransmitters and their receptors involved in development at the molecular level, as well as the time sequence of these events of expression and activity, in vitro models for development such as embryonic carcinoma (EC) and stem (ES) cells have been implemented in order to study differentiation processes in a simplified environment. The study of molecular processes of differentiation are essential for understanding the molecular and cellular basis of development‐related disease and also subsequently for the development of strategies for regenerative medicine. The present review will focus on applications of pluripotent EC and ES cells to study the mechanism of neurotransmitter receptor expression and activity during neuronal differentiation.
EC AND ES CELLS AS MODELS FOR NEURONAL DIFFERENTIATION
The EC and ES cells that are isolated from the inner cell mass of blastocysts are characterized by their capacity for long‐term self‐renewal without loss of pluripotency and by their ability to differentiate into one or more specialized cell types.
The EC cells derived from human and murine germ cell tumours have, to some extent, the capability of reverting their malignant phenotype during the process of differentiation. ES cells can be precursors of cells of all three germ layers and can differentiate into various mature cell types when they are exposed to appropriate external stimuli. Cell differentiation, such as the ultimate formation of neurones following formation of three‐dimensional spherical cell aggregates, denominated as embryonic or embryoid body stage (EB), is induced by culturing cells as a suspension or in methylcellulose‐containing medium. This stage is similar to the blastula stage of early in vivo development and represents a prerequisite for further differentiation, which takes place following plating cells onto adherent culture dishes, finally yielding neurones and glial cells (Stavridis & Smith 2003). In vitro differentiated EC and ES cells can develop into neurones with functional synapses, which are able to integrate into the brain (Morassutti et al. 1994; Barberi et al. 2003; Chiba et al. 2003), making regeneration therapy feasible. As EC cells are of malignant origin, they could never be used in regeneration therapy; nevertheless, this does not deny them a tremendous potential as a model system for in vitro studies. ES cells, however, are promising candidates for replacement therapy. Evans and Kaufman (Evans & Kaufman 1981) recognized that ES cells isolated from the inner cell mass of blastocysts are the non‐carcinogenic counterpart of EC cells. Murine ES cells were first isolated from the inner cell mass of the developing blastocysts and cultured in vitro (Evans & Kaufman 1981; Martin 1981). Human ES cells were first isolated in 1998 (Thomson et al. 1998), and besides their importance as a model system for studying early differentiation events, they are promising tools for disease therapy by regenerating damaged tissue (reviewed by Kume 2005). The generation and in vitro differentiation of ES cells have been already extensively reviewed by Rippon and Bishop (2004).
Cellular therapy in disease
Both mouse and human ES cells are totipotent and have the capacity to be the origin of all downstream kinds of cells and even (for example, neural stem cells), to differentiate into tissue‐specific progenitor cells that maintain this pluripotency in adulthood. These tissue‐specific stem cells are employed for tissue maintenance and repair during life. Neural stem cells can be found for instance in the subventricular zone in the adult brain (Ishibashi et al. 1994). In addition to their importance in basic research, ES and neural stem cells have gained increasing interest as tools for therapy. Transplantation of stem cells or the mobilization of endogenous stem cells in the adult brain are promising future treatments for neurodegenerative disease. As different neurodegenerative conditions affect more than one type of neuronal tissue, various phenotypes of neurone will be needed for respective replacement therapy. However, various obstacles yet need to be overcome. Instead of differentiating into neurones, in some cases transplanted cells have formed aggregates of an undifferentiated mass, and subsequently have not differentiated at all. Moreover, differentiation of pluripotent cells typically results in the appearance of neurones with more than one phenotype as well as of glial cells (Rathjen & Rathjen 2001; Stavridis & Smith 2003). Exogenous factors, such as the co‐culture of differentiating cells with other cell types (known as feeder layers), growth factors and transcription factors have already been shown to determine the phenotype of the mature neurone (Wichterle et al. 2002). Activation of transcription factors such as Nurr 1 and co‐culture with feeder layers have markedly increased the percentage of dopaminergic neurones in differentiating cell cultures (Barberi et al. 2003), whereas extrinsic factors such as sonic hedgehog activation augmented the number of motor neurones (Caldwell et al. 2001). As results of these studies, distinct differentiation protocols have been established for obtaining dopaminergic and motor neurones (Barberi et al. 2003). Importantly, motor neurones generated by in vitro differentiation have functionally integrated into host tissue following transplantation (Caldwell et al. 2001).
In view of these pioneering studies, replacement therapies using neurones with a defined phenotype derived from stem cells in vitro are promising in the near future. Replacement therapies for lost neurones have been suggested for epilepsy, Parkinson's disease, amyotrophic lateral sclerosis (ALS) and Huntington's disease.
Epilepsy is a neurological disorder resulting from dysregulation of the balance between excitatory and inhibitory forces in the brain. Increased excitation or insufficient inhibition, as a result of the loss of inhibitory neurones, trigger excessive discharge of neurones, which cause epileptic seizures and subsequent brain damage (reviewed by Holmes & Ben‐Ari 2003). Most patients suffer from focal epilepsy, that is temporal lobe epilepsy, which then spreads to other regions of the brain. Conventional medication and surgery fail in many cases. Thus, therapeutic approaches aim at use of stem cells for replacement of lost neurones and inhibition of hyper‐excitability and propagation of electrical discharges. Neural stem‐ and ES‐cell‐derived neurones have been transplanted into rats in a model for chronic temporal‐lobe epilepsy (Chu et al. 2004; Ruschenschmidt et al. 2005). ES cell‐derived neurones grafted into the hippocampus of epileptic rats developed a dense network with the host tissue and revealed intrinsic and synaptic properties characteristic of neurones (Ruschenschmidt et al. 2005). Neural stem cells that had been transplanted intravenously into chronically epileptic rats differentiated into χ‐aminobutyric acid (GABA)‐immunoreactive inter‐neurones in the damaged hippocampus, thus decreasing neuronal excitability and suppressing recurrent epileptic seizures (Chu et al. 2004). The intracerebral implantation of ES‐cell‐derived EBs and glial cell precursors that had been genetically engineered for paracrine adenosine release as an attempt to inhibit neuronal activity and seizure activity, resulted in a transient protection against epileptic seizures. However, as a result of the short survival time of the grafted cells, long‐term seizure suppression could not be achieved (Güttinger et al. 2005).
Parkinson's disease is based on the degeneration of nigrostriatal dopaminergic neurones (reviewed by Fernandez‐Espejo 2004). First successes in cell replacement therapy resulted from transplantation of human foetal mesencephalic tissue. As there are serious limitations in the availability of this human tissue, stem cell technology has been proposed for the generation of large numbers of dopaminergic neurones in vitro under controlled conditions. Success with neurones of dopaminergic phenotype from mouse and monkey has been described extensively (Lindvall et al. 2004). Following in vitro differentiation and transplantation into animals, these cells innervated the denervated striatum and restored dopamine release, thereby improving Parkinson‐like symptoms.
The characteristics of ALS are progressive degeneration of upper and lower motor neurones in the spinal cord, brain stem and cerebral cortex (reviewed by Strong 2004). Success of stem‐cell‐based therapy does not only depend on transplantation of motor neurones but also on their functional integration into cortical circuits and re‐establishment of neurotransmission. As a promising step towards ALS therapy, transplantation of hNT neurones obtained by in vitro differentiation of the human teratocarcinoma cell line NTERA‐2/D1 has been shown to ameliorate dysfunction in a mouse model of familial ALS (Garbuzova‐Davis et al. 2002).
The neurodegenerative disorder Huntington's disease is characterized by chorea and progressive dementia, resulting from a loss of medium spiny neurones in the striatum caused by the mutation of the Huntington gene. Abnormal calcium signalling has been suggested as being one of the causes of this disease (Bezprozvanny & Hayden 2004). Replacement of defective neurones with grafts of foetal striatal tissue containing projection neurones in order to re‐establish connections with the globus pallidus was promising (Watts et al. 2000). The transplanted cell population initially survived and contained striatal projection neurones and inter‐neurones. Grafted cells received afferent signals, and were able to substitute damaged neuronal connections. As on‐going loss of motor neurones complicates stem‐cell based therapy of Huntington's disease, it is particularly desirable to obtain defined progenitor cells that continuously give rise to known progeny, thereby increasing the number of available striatal neurones. Conditions for neurone survival can be studied in vitro and cells can be genetically manipulated in order to improve survival. Neurones with improved survival rates, obtained by in vitro differentiation and genetic engineering can be transplanted and become functionally integrated in host CNS. They are expected to constitutively improve motor and cognitive function in Huntington's disease. Thus, although a genetically predetermined process causes neuronal death within the Huntington's striatum, implanted foetal neurones lacking the mutant Huntington's gene could be used as replacement therapy for damaged neuronal connections (Freeman et al. 2000).
In summary, increased demand for neurones obtained by in vitro differentiation of stem cells as tools for the treatment of neurodegenerative diseases will rely on distinct in vitro differentiation protocols for the desired neuronal phenotypes.
Extrinsic factors and pattern of gene expression
Retinoic acid (RA) is the most used epigenetic factor for triggering differentiation from undifferentiated EC or ES cells to a neuronal phenotype in vitro, as it promotes neuroectodermal and represses mesodermal gene expression (Boudjelal et al. 1997). RA binds to cellular RA‐binding proteins (RAR, RXR), which interact with nuclear RA‐response elements (RARE), initiating transcription of target genes. RARE induces transcription of developmental‐relevant genes, for instance sonic hedgehog, transcription factors Pax6 and mash‐1 and the signalling molecule wnt‐1 in ES cells, and transcription factors Neuro D, MATH‐1 and NSCL‐2 in differentiating mouse EC cells (reviewed by Guan et al. 2001). Evidence exists that not only transcriptional activation by RA, but also the exposure of the cells to extrinsic factors, such as basic fibroblast growth factor (b‐FGF) induces a neurone or glial specific pattern of gene expression which determines the final phenotype of the cell (Brustle 1999). The combination of extrinsic and intrinsic factors determines the pattern of calcium spikes and oscillations and is the determinant for final neuronal phenotype (Borodinsky et al. 2004). Expression of various receptor proteins is regulated in the developing brain (for reviews see Herlenius & Lagercrantz 2001; Lujan et al. 2005).
In vitro models are indicated when the exact function of a cell‐surface receptor shall be studied. These models are of crucial importance for investigation of how to direct differentiation towards glial or neuronal tissue. Mechanisms of neural differentiation are difficult to examine in complex animal systems. In order to evaluate the function of the protein of interest during differentiation, transgenic animals can be bred that either over‐express or carry a functional knockout of this protein (reviewed by Wilson & Tonegawa 1997). However, the creation of genetically engineered animal models is in itself labour intensive. In addition to the fact that alteration in gene expression may be lethal, the induced mutation may affect expression and activity of other proteins that are not directly involved in the process of interest.
Therefore, in vitro models are desired in which conditions of differentiation can be controlled and the activity and function of the protein of interest can be dissected in a relatively simple environment. For instance, the expression pattern and functionality of a neurotransmitter receptor protein can be analysed by quantification of its gene expression and activity during various stages of neuronal differentiation.
Here we will focus on the expression and activity of muscarinic acetylcholine, N‐methyl‐D‐aspartate (NMDA)‐glutamate, endothelin‐B (ET‐B), and kinin‐B2 receptors during neuronal differentiation in vivo and in vitro.
Muscarinic acetylcholine receptors
Muscarinic acetylcholine receptors (mAChRs) are widely distributed in the CNS and also in the peripheral nervous system. Muscarinic metabotropic, G‐protein‐coupled receptors, consisting of structurally related m1‐m5 subtypes, increase cytosolic calcium concentration [Ca2+]i by acting on intracellular calcium stores and l‐type calcium channels on the cell surface as well as activating potassium currents (Jerusalinsky et al. 1997). Members of the mAChR family have central roles in the regulation of many fundamental physiological functions in the brain. Cholinergic modulation of hippocampal and cortical function plays an important role in memory and attention.
The m1 mAChR is abundant in the hippocampus and cerebral cortex. Marino et al. (1998) showed that the m1 receptor modulates NMDA receptor‐mediated excitatory synaptic transmission in rat hippocampal CA1 pyramidal cells. Dean et al. (2002) suggested that changes in m1 receptor expression in the dorsolateral prefrontal cortex may have a role in the pathology of schizophrenia. The m1 mAChR is expressed in the neuroepithelium of the rat forebrain, where it is found on both nestin‐positive progenitor cells and TuJ1‐positive newly differentiated neurones, suggesting participation of this receptor in the development of the nervous system prior to the onset of synaptogenesis (Williams et al. 2004). The m2 subtype is involved in both developing and regenerating of the retina, and the discrepancy in distribution of the mAChR and choline acetyltransferase suggested that the mAChRs may play more roles than just cholinergic signal transmission during neuronal development (Cheon et al. 2001).
Muscarinic activation of proliferation and differentiation of neural precursors has been suggested, based on the observation that treatment of neural progenitor cells with acetylcholine or its stable analogue carbamoylcholine has led to increased DNA synthesis and neurogenesis (Ma et al. 2000). A possible role of acetylcholine as a modulator of neuronal differentiation has been suggested based on the following observation. A neuroblastoma cell line, which by itself does not synthesize acetylcholine, was transfected with a construct coding for choline acetyltransferase. The activation of acetylcholine synthesis resulted in a higher expression of neuronal‐specific traits compared to untransfected control cells. The expression of muscarinic receptors in these transfected cells indicates the presence of an autocrine loop that may be responsible for the advanced differentiation state (Biagioni et al. 2000). During early development of dorsal root ganglia, choline acetyltransferase as well as muscarinic receptors have been detected. The activation of acetylcholine synthesis at distinct time points of neuronal development may be necessary for communication between differentiating neurones and their neighboring cells.
NMDA‐glutamate receptors
Glutamate acts as the main excitatory neurotransmitter in the CNS. Glutamate and other glutamate receptor agonists bind to ionotropic and metabotropic receptors. Metabotropic receptors act via G‐proteins on intracellular second messenger systems, such as intracellular calcium stores. Ionotropic receptors are ligand‐gated ion channels, which are classified based on their electrophysiological and pharmacological properties as α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionate (AMPA) and as NMDA‐ and kainate‐receptor channels (Le Novere & Changeux 1999). These ionotropic receptors are highly expressed in the mammalian adult brain (Bettler & Mulle 1995) and during early embryogenesis of the nervous system (Gallo et al. 1995). NMDA receptors, whose functions in neuronal differentiation will be discussed in succeeding sections, are composed of NMDA R1, NMDA R2 and NMDA R3 subtypes. The hippocampus is the most intensively studied region because of the importance of NMDA receptors in synaptic plasticity and memory. Lesions of the hippocampus in humans and in other mammals produce severe amnesia. Disruption of NMDA receptors in the hippocampus leads to blockade of synaptic plasticity and also to memory malfunction.
Experimental data suggest that ionotropic glutamate receptors are involved during different stages of neuronal development in cell survival, proliferation, differentiation and in the formation of synaptic plasticity (Scheetz & Constantine‐Paton 1994). A crucial function for NMDA receptors for in vivo synaptogenesis has been suggested. Toluene abuse during pregnancy results in neurobehavioural dysfunction of the offspring, affecting synaptogenesis during development, probably resulting from NMDA receptor inhibition (Chen et al. 2004). Following perinatal administration of the NMDA‐receptor antagonist phencyclidine, development of the frontal cortex and corticostriatal neurones was affected, and increased cell death was detected (Wang & Goldman‐Rakic 2004).
Participation of NMDA‐glutamate receptors in synaptogenesis was also evident in in vitro studies. The expression of functional NMDA receptors also coincided with the appearance of neurone‐specific synapsin I and synaptophysin during differentiation of the NE‐4C neuroectodermal progenitor cell line (Jelitai et al. 2002). The presence of heteromeric NMDA receptors constitutively expressed in neural progenitor cells of the murine hippocampus suggests that these receptors play a crucial role in commitment of these cells to neuronal differentiation (Kitayama et al. 2004). NMDA receptors have been implicated in excitation‐coupled neurogenesis (Deisseroth et al. 2004). Excitation‐coupled neurogenesis has been studied in neural progenitor cells from adult hippocampus where it is sensed via voltage‐dependent calcium l‐type channels and NMDA receptors. When stem cells were transplanted into neuronal tissue, cells started expressing NMDA receptors. Presence of NMDA receptors indicated ongoing differentiation to neurones (Zhang et al. 2004).
Endothelin receptors
G‐protein‐coupled endothelin receptors can be divided to endothelin‐A and ‐B receptor subtypes based on structural and pharmacological similarities, and are activated by their endothelin‐1–3 peptide ligands. The ET‐B receptor subtype is expressed in neurones. ET‐B receptors are activated by their peptide ligands, endothelins‐2 and ‐3, whereas endothelin‐1 is a specific ligand for the endothelin‐A receptor subtype (Battistini et al. 1993). Expression of ET‐B receptors has been detected in ventricular and subventricular zones, as well as postnatally in ependymal and subependymal cells. ET‐B receptor mRNA was also detected prenatally in dorsal root ganglia, as well as postnatally in cerebellar Bergmann glial cells and epithelial cells of choroid plexus (Tsaur et al. 1997). These areas contain neural stem cells, which are crucial for embryogenesis of the CNS (Reynolds et al. 1992) and for neuroregeneration in adulthood (Gritti et al. 2002). It is also possible that ET‐B receptors play a role in the activity of glia in directing migration of neurones, in addition to regulation of cell differentiation and proliferation. During cerebellar development, Bergmann glia cells expressing ET‐B receptors direct granule neurone precursors to migrate along their long processes from the external germinal layer to the granular layer (Tsaur et al. 1997). The expression of ET‐B receptors in glial cells arises after CNS injury in rats, rabbits and humans (Rogers et al. 1997), such as during the initiation of gliosis in adulthood after brain damage (Hama et al. 1997). This supports the notion that ET‐B receptors are crucial for directing neurite proliferation. However, functions of ET‐B receptors are different during the development of the enteric nervous system. Previously, ET‐B receptors have been believed to act in an autocrine way activated by its ligand endothelin‐3 to trigger differentiation and survival of enteric neurones (Baynash et al. 1994). However, recently it became clear that ET‐B receptors inhibit differentiation of crest‐derived cells into neurones (reviewed by Gershon 1999). ET‐B receptors are also present in differentiating EC and neural stem cells (Monge et al. 1995; Shinohara et al. 2004).
P19 EC cells as model system for studying sequential expression of neurotransmitter receptor during neuronal differentiation
P19 murine EC cells have now been used for more than 20 years as model for in vitro differentiation and for the study of differentiation underlying processes (Jones‐Villeneuve et al. 1982). The sequential expression of neuronal surface markers, neurotransmitters and their receptors resemble processes observed during early in vivo development. For example, the abundance of GABA, neuropeptide Y and somatostatin in P19‐derived neurones as well as in embryonic neurones in rostral regions of the mammalian CNS suggests that developmental events in P19 cells closely resemble those of the embryonic neuroectoderm (Staines et al. 1994). P19 cells that have been grafted into adult rat striatum survived and matured into functional neurones and glial cells (Morassutti et al. 1994), justifying the suitability of this cell line as an in vitro model to study neurotransmitter receptor expression and function during differentiation.
Figure 1 illustrates the steps of differentiation from embryonic P19 cells to neurones. In their undifferentiated stage, P19 cells are characterized by high proliferation rates, and the cells possess gene expression patterns of stem cells. Differentiation of pluripotent cells to neurones is stimulated by addition of RA to the cell culture. Following 2 days of culture as cell suspension, differentiating cells form irregular three‐dimensional spherical cell aggregates, such as EBs, which assemble themselves in the form of a primordial embryo. The cells located on the outside of the EBs differentiate into ectoderm‐like cells that then surround the undifferentiated core.
Figure 1.
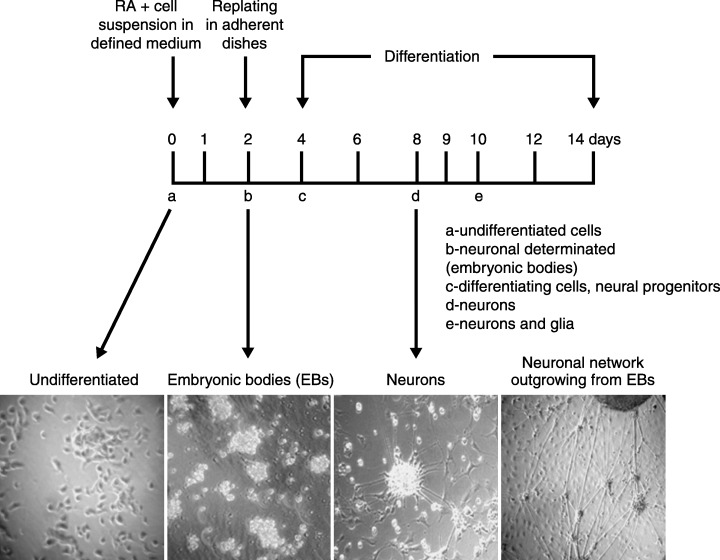
Neuronal differentiation of P19 embryonal carcinoma cells. In vitro differentiation of P19 murine embryonal cells resembles processes during early development in vivo. Scheme of differentiation: Pluripotent P19 cells are treated with all‐trans RA (10−6 m) in defined serum‐free medium and plated on to non‐adherent culture dishes to induce formation of embryonic bodies as described by Martins et al. (2005). Following 48 h of induction to neuronal differentiation, embryonic bodies are collected and replated in adherent cell culture dishes in order for neuronal differentiation to take place. Around day 5 of differentiation, neuronal precursors become post‐mitotic and undergo neuronal maturation that is completed on days 7 and 8. Beginning on day 5, the developing neurones express high molecular weight neurofilaments, such as NF‐200, that reach maximal expression on day 8. The lower panels show phase‐contrast images of undifferentiated P19 cells, the EB stage, P19 neurones and P19 neuronal networks.
Following the disaggregation of EBs, P19 cells are placed in adherent cell culture dishes. From then on cells undergo neuronal differentiation, become neural progenitor cells and finally mature neurones. Termination of neuronal differentiation in this cell culture is confirmed by the identification of expression of neurone‐specific markers such as high weight neurofilaments, β‐3 tubulin, neurite‐specific proteins such as MAP2, Tau, neurocan, neurone‐specific enolase and synaptophysin. Glial cells appear in higher numbers during later differentiation, beginning from day 10, expressing the glial marker protein glial fibrillary acid protein. Evidence has been collected, showing that glial cells guide the migration of P19 neurones from EB cell aggregates, giving rise to a network of P19 cells (Santiago et al. 2005).
P19 neurones form functional synapses and establish neuronal polarity (Finley et al. 1996); are capable of causing membrane depolarization and liberation of GABA, glutamate, neuropeptide Y, somatostatin and other neurotransmitters (Parnas & Linial 1995; Lin et al. 1996; MacPherson et al. 1997). Neuronal phenotypes are similar to those of embryonic neurones of the rostrum region in the mammalian nervous system (Staines et al. 1994). The pattern of expression of neurotransmitter receptors and of liberation of neurotransmitters vary between individual P19 cells (Staines et al. 1994). Consequently, P19 neurones form different types of synapses, inhibitory as well as excitatory ones.
In contrast to their mouse counterparts, many of human EC culture cell lines have shown only little capacity for differentiation, justifying the preference of mouse EC cells as a model for neuronal development. Human EC lines, which finally could be differentiated into neurones, include the TERA2 and GCT27 cell lines (reviewed by Pera et al. 2000).
Examples for using P19 cells as the in vitro model to elucidate molecular bases of differentiation and signalling include the characterization of the action of calcium‐signalling proteins calretinin and calbindin in transfected P19 cells (D’Orlando et al. 2001), and the study of neuroprotection by inhibitors of excitotoxicity p44/42 pathways induced by NMDA (Grant et al. 2001). Cholinergic properties as well as the presence of mechano‐sensitive ion channel, neurotransmitter and neuropeptide receptors such as ionotropic glutamate, acetylcholine, kinin‐B2 and ET‐B receptors have been studied during differentiation of P19 cells (Monge et al. 1995; Tarnok & Ulrich 2001; Lee et al. 2003a; Martins et al. 2005). In vitro models for neuronal differentiation also include ES cells as well as neural progenitor cells. During in vitro maturation of neural progenitor cells from rat hippocampus, cells became electrically excitable, and expressed voltage‐gated ion channels as well as functional neurotransmitter receptors, such as GABA, glycine and purinergic P2X receptors (Hogg et al. 2004). It is generally accepted that calcium signal transduction is crucial for in vitro differentiation as well as for control of cell proliferation.
One expects that neuropeptide and neurotransmitter receptors are either not expressed or are present only in low numbers on pluripotent stem cells; or perhaps if present, they are not functional. Upon stimulation, cells are committed to neuronal differentiation, and sequentially, receptor proteins are required in order to respond to binding of specific receptor ligands with transient elevations of [Ca2+]i. Highest expression levels of neurotransmitter receptors are expected when cells become mature neurones, forming functional synapses.
We have used P19 cells as an in vitro model to demonstrate the sequential rise of activity of two neurotransmitter and two neuropeptide receptors such as mAchR, NMDA, and ET‐B, and kinin‐B2 receptors at three stages during the course of neuronal differentiation in vitro:
-
•
at the pluripotent stage using embryonal P19 teratocarcinoma cells (McBurney & Rogers 1982). This stage is characterized by cells with a high proliferation rate, and the expression of stem‐cell specific antigens;
-
•
at the stage of neural progenitor P19 cells on days 4–6 of differentiation. By this time the cells are committed to neuronal differentiation, become or are already postmitotic, and express cell surface proteins, specific for early development, such as low or intermediate weight neurofilaments.
-
•
in P19 neurones following neuronal maturation on days 8 and 9 of differentiation. Differentiated P19 neurones express surface proteins of mature neurones such as high molecular weight neurofilaments, β‐3 tubulin and neurone‐specific enolase and form functional synapses able to generate action potentials.
Gene expression of m1–m3 and m5 subunits of mAChRs is present during neuronal differentiation of P19 EC cells (Parnas et al. 1998; Martins et al. 2005). We have verified mAChR activity in differentiating P19 cells, using single‐cell calcium imaging, following stimulation with the acetylcholine analogue, carbamoylcholine. Undifferentiated P19 cells responded to stimulation by the mAChR‐agonist carbamoylcholine with a small elevation in [Ca2+]i, confirming the presence of muscarinic and possibly also nicotinic acetylcholine receptor activity already in pluripotent stem cells. The agonist‐induced calcium response was largely increased during differentiation and was highest in P19 neurones, confirming that mAChR activity is regulated during differentiation (Fig. 2a and Martins et al. 2005). The carbamoylcholine‐induced calcium response was abolished following pre‐incubation with thapsigargin (Martins et al. 2005), indicating mobilization of intracellular calcium stores during carbamoylcholine‐induced increase in [Ca2+]i, and participation of muscarinic receptor action. The activity of mAChRs during the course of neuronal differentiation in our in vitro model resembles in vivo conditions of neuronal development in which muscarinic receptor expression augments as soon as cells are committed to neuronal development (LoTurco et al. 1995).
Figure 2.
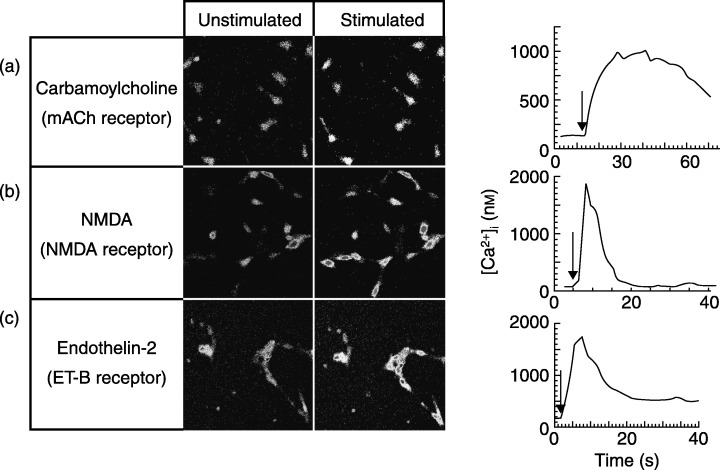
Imaging of receptor‐induced elevation of free intracellular calcium concentration ([Ca2+]i) in undifferentiated and P19‐derived neurones. Cells were loaded with fluo‐3AM, and single‐cell calcium imaging was performed by confocal microscopy as detailed by Martins et al. (2005). Calcium fluxes were stimulated by application of (a) 1.5 mm of carbamoylcholine, (b) 100 µm NMDA, or (c) 50 nm endothelin‐2. Maximal and minimal fluorescence values (Fmax and Fmin) were determined in the presence of calcium ionophore and EGTA, respectively. Transient elevations in [Ca2+]i were quantified using the formula: Kd*(F‐Fmin)/(Fmax − F), assuming a dissociation constant (Kd) of 450 nm for fluo‐3 AM calcium binding. Arrows indicate time points of ligand application. The data represented are mean values of changes in [Ca2+]i of three independent experiments. During each experiment, the calcium responses of at least 10 individual cells in a selected area of the cell culture dish were quantified (right panels).
In undifferentiated P19 cells, transcripts encoding the NMDA 2A and 2B receptor units were identified, whereas the NMDA R1 subunit, critical to receptor function, was expressed together with the 2A and 2B subtypes, only following induction of the neuronal phenotype (McPherson et al. 1997). Glutamate receptor channels are highly permeable for calcium ions, and differentiating P19 cells are known to express NMDA and non‐NMDA receptors (AMPA and kainate receptors) (Canzoniero et al. 1996; Lee et al. 2003b). Glutamate‐induced ion flux into the cell depolarizes the cell membrane and results in activation of voltage‐dependent calcium channels (Morley et al. 1995; Canzoniero et al. 1996). A calcium response to NMDA application was not visible in undifferentiated cells. P19 progenitor cells responded with an increase of [Ca2+]i that was smaller than the one obtained in fully differentiated P19 neurones. P19 neurones responded to NMDA application with a short, rapidly decaying increase in [Ca2+]i (Fig. 2b). This experiment indicated that, as in neuronal development in vivo, NMDA receptor activity is already present in neural progenitor cells, and it is up‐regulated during final differentiation of P19 cells. Besides being crucial for neuronal differentiation and development of synapses in P19 cells (Staines et al. 1994), these receptors make P19 neurones – like CNS neurones – vulnerable to glutamate‐induced toxicity (Turetsky et al. 1993). In fact, glutamate receptor blockage either decreased or increased the survival rate of differentiating neocortical neurones depending on the stage of maturation (Drian et al. 2001). In summary, NMDA receptor activity is not present at the very early stage of differentiation of P19 cells, but is needed as soon as neural progenitor cells begin neuronal maturation, and is maximal when differentiated P19 neurones form functional synapses.
In P19 EC cells, ET‐B receptors are only functionally expressed in P19 neurones (Monge et al. 1995). Activation of this G‐protein coupled seven transmembrane receptor in mature P19 neurones by its ligand endothelin‐2 results in a significant transient, rapidly decaying elevation of [Ca2+]i (Fig. 2c). This response upon application of endothelin‐2 was not observed in embryonal P19 cells or in P19 progenitor cells. The data indicate that ET‐B receptor function is only present during later in vitro differentiation when cells already express neurone‐specific proteins, whereas carbamoylcholine‐ and NMDA‐activated calcium fluxes are already observed in P19 cells at the initial stage of differentiation or at the stage of neural progenitor cells, respectively (Fig. 3). Expression and activity patterns of mAch, NMDA and ET‐B receptors demonstrate that P19 neuronal‐differentiating cells behave similarly as do developing neurones in vivo. They start interacting with neighbouring neurones by the formation of synapses and express neuropeptide and neurotransmitter receptors and pressure‐ or voltage‐activated ion channels (Table 1).
Figure 3.
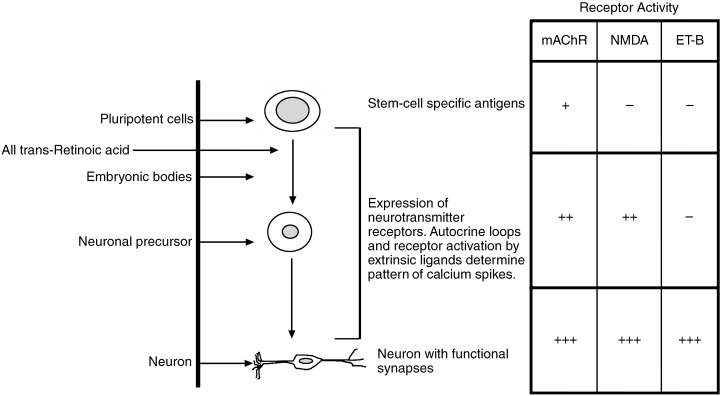
Stage‐specific activity of neurotransmitter receptors during neuronal differentiation of P19 EC cells. Receptor activity was detected by ligand‐induced calcium flux assays using confocal microscopy (see Fig. 2). Amplitudes of transient calcium variants increased during the course of differentiation (–, no response observed; +, 100–300 nm; ++, 300–600 nm; +++, > 600 nm). Calcium measurements were performed at day 0 (pluripotent cells), day 5 (neuronal precursors) and day 7 or 8 (differentiated neurone).
Table 1.
Receptor and ion channel expression in differentiating P19 cells. The presence (+) or absence (–) of the respective receptor or ion channel protein was verified by identifying its gene expression or protein activity
| P19 EC | P19 neurones | Reference | |
|---|---|---|---|
| Ionotropic receptors | |||
| NMDA | R2A/2B | R1/R2A/2B | Morley et al. (1995) |
| AMPA/kainate | GluR5/6/7 | GluR2/3/4/5/6/7 | MacPherson et al. (1997) |
| Turetsky et al. (1993) | |||
| Canzoniero et al. (1996) | |||
| Lee et al. (2003b) | |||
| Ray & Gottlieb (1993) | |||
| Nicotinic acetylcholine | n.d. | α3, α4, β2 | Cauley et al. (1996) |
| GABA | γ 2S | γ 2S, γ 2L | 1994, 1996 |
| Glycine | β | α1, α2, β | Heck et al. (1997a) |
| Metabotropic receptors | |||
| Glutamate | mGlu 2/4 | mGlu1/2/3/4/5/7/8 | Heck et al. (1997b) |
| Muscarinic acetylcholine | m3 | m1, m2, m3, m5 | Parnas et al. (1998) |
| Kinin | Kinin‐B2 | Kinin‐B2 | Martins et al. (2005) |
| Endothelin | – | ET‐B | Monge et al. (1995) |
| Opioid | κ, δ | κ, δ, µ | Chen et al. (1999) |
| Head‐activator | – | + | Niemann & Schaller (1996) |
| Voltage‐dependent and mechano‐ sensitive ion channels | |||
| Calcium | – | + | Canzoniero et al. (1996) |
| van der Heyden et al. (2003) | |||
| Potasssium | – | + | Cheun & Yeh (1991) |
| Sodium | – | + | Cheun & Yeh (1991) |
| Mechano‐sensitive cation | – | + | Tárnok & Ulrich (2001) |
n.d., not determined.
Functional NMDA, mACh, and ET‐B receptors have also been implicated in neuronal differentiation of progenitor cells obtained as primary cultures. For instance, the addition of an NMDA receptor antagonist to neurospheres of neural progenitor cells from adult murine hippocampus inhibited NMDA‐induced expression of c‐Jun and c‐Fos and therefore has been suggested to play a crucial role in commitment and differentiation towards neurone development in adult murine hippocampus (Kitayama et al. 2004). mAChRs transduce a growth regulatory signal during neurogenesis. When neural precursor cells isolated from the embryonic rat cortical neuroepithelium were treated with muscarinic agonists in the absence of b‐FGF, cell number and DNA synthesis were increased and differentiation to neurones was enhanced (Ma et al. 2000). ET‐B receptor function is implicated in neuronal migration. Neural crest cell differentiation in vitro and in vivo has been linked to both the endothelin peptide and the ET‐B receptor function (Shin et al. 1999; Lee et al. 2003a).
P19 cells as in vitro model to probe receptor participation in neuronal differentiation
A variety of receptors and ion channels are differentially expressed during neuronal development to induce stage‐specific calcium spikes, thereby triggering neuronal differentiation. These spikes differ in the amount of intracellular calcium elevation and inactivation time.
Alterations of patterns of calcium spike activity in vivo have been shown to change the phenotypes of embryonic spinal neurones as studied by the expression of excitatory and inhibitory neurotransmitters in these cells (Borodinsky et al. 2004). A new role of patterned activity based on transient changes in [Ca2+]i is suggested, which is regulated by proteins such as ion channels and neurotransmitter receptors. P19 cells had been proposed to serve as a model system to probe receptor participation in neuronal development, such as for opioid and cholinergic receptors (Parnas & Linial 1995; Chen et al. 1999), but evidence has yet to be collected to connect activity of a receptor to the outcome of differentiation.
In this regard, we will discuss the following work performed in our laboratory (Martins et al. 2005), which demonstrates that a neuropeptide receptor, the kinin‐B2 receptor, actively participates in triggering neuronal differentiation, rather than only being a marker of the differentiation stage. The P19 cell line, already extensively discussed in previous sections of this review, was used as in vitro model for studying kinin‐B2 receptor participation in neuronal differentiation. Kinins are vasoactive oligopeptides generated upon proteolytic cleavage of low and high molecular weight kininogens by kallikreins. These peptides have a well‐established signalling role in inflammation and homeostasis. Nevertheless, emerging evidence suggests that bradykinin and other kinins are stored in the CNS and may act as neuromediators. We have shown that during the course of neuronal differentiation, kinin‐B2 receptor gene expression and activity as well as liberation of bradykinin into the culture medium is increased, reaching maximal values during final neuronal maturation when high molecular weight neurofilaments are expressed (Martins et al. 2005 and Fig. 4). Co‐application of the specific kinin‐B2 receptor antagonist HOE‐140 together with RA inhibits early differentiation as determined by decreased sizes of EBs in the presence of HOE‐140. The presence of HOE‐140 during later neuronal differentiation affected maturation of P19 neurones and the formation of functional cholinergic neurones, as shown by the inhibition of carbamoylcholine‐induced calcium flux and down‐regulation of the gene expression of m1, m2, and m3 subtypes of mAChRs (Martins et al. 2005 and Fig. 5).
Figure 4.
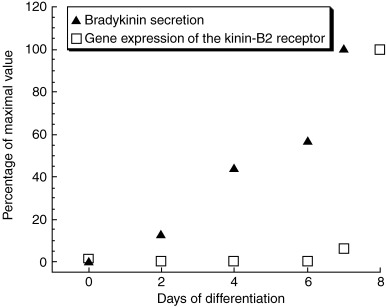
Bradykinin secretion and kinin‐B2 receptor gene expression during the course of neuronal differentiation of P19 EC cells. Both bradykinin secretion and receptor expression increased until neuronal maturation was complete (Martins et al. 2005). Data plotted as percentages of maximal values observed at days 7 or 8 of differentiation.
Figure 5.
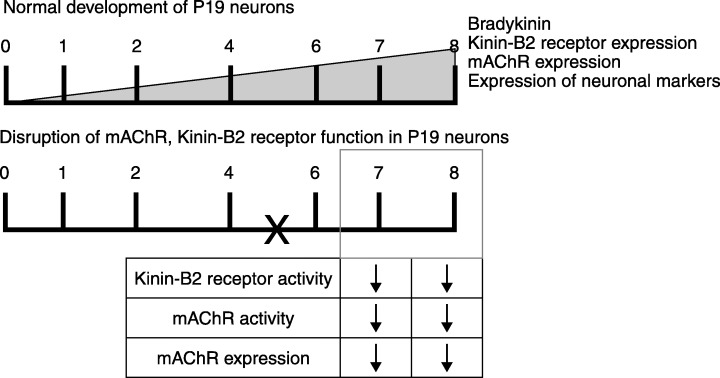
Inhibition of neuronal maturation of P19 cells by the presence of the kinin‐B2 receptor inhibitor HOE‐140. Application of the kinin‐B2 receptor inhibitor HOE‐140 on day 5 resulted in a loss of m1‐m3 mAChR expression on days 7 and 8 of differentiation, and inhibition of carbamoylcholine‐induced calcium fluxes, indicating that bradykinin and its receptor actively participate in neuronal differentiation. 0–8, Days of differentiation following addition of RA. X indicates time point of HOE‐140 addition to differentiating cell cultures.
In summary, this study has indicated that P19 cells secrete bradykinin, which then acts in an autocrine loop on kinin‐B2 receptors in order to produce transient changes in [Ca2+]i triggering neuronal differentiation. Inhibition of kinin‐B2 receptor activity aborted neuronal differentiation and formation of cholinergic neurones.
The observation of a glia‐guided neuronal migration during differentiation of P19 cells (Santiago et al. 2005) gives rise to the suggestion that in addition to autocrine loops as shown for kinin‐B2 receptor activation on differentiating neurones, paracrine stimulation mechanisms exist in which glial cells liberate neurotransmitter molecules into the extracellular space, and thereby participate in the outcome of the phenotype of neuronal differentiation. This hypothesis is supported by the work of Hartley et al. (1999) who showed that the formation of functional synapses during differentiation of human NTera2 EC cells depends on the presence of a supporting glia. The importance of glial cells as participants in forming structural and functional shapes of synapses has been extensively reviewed (Ullian et al. 2004).
Cross‐talk between neurotransmitter receptors affecting their gene expression and activity has been already demonstrated in the mature CNS. Muscarinic receptors interfere with nicotinic acetylcholine, dopamine, adenosine and purinergic receptors (Li et al. 1992; Lukas & Bencherif 1992; Grant et al. 1999; Ferroni et al. 2002). NMDA receptor activity interferes with metabotropic glutamate, GABA, dopamine and opioid receptors (Cai et al. 1997; Pinilla et al. 2001; Bai et al. 2002; Cho et al. 2002; Scott et al. 2002). Further cross‐talk between neuropeptide and neurotransmitter receptors may also exist in developing neurones, and it is suggested that during differentiation, expression and activity of a pattern of neurotransmitter receptors is highly regulated. Dysfunction of a single receptor type will affect the cross‐talk within the entire system and, consequently, change the fate of differentiation. Therefore, it is important to elucidate regulation of receptor action at the cellular level, as CNS pathologies and neurodegenerative diseases may be reflected by abnormalities of receptor function; for instance, Hirschsprung disease is caused by ET‐B receptor dysfunction during neuronal development. A similar mechanism is suggested for the neurodegenerative Alzheimer's disease. The disease state is correlated with a loss of neuronal nicotinic receptors which may be the result of inhibition of nicotinic receptor activity by β‐amyloid peptides (Magdesian et al. 2005). Correcting receptor dysfunction or replacement of lost neurones with neuronal tissue of identical phenotype obtained by in vitro differentiation offer new therapeutic perspectives for curing genetic and acquired dysfunctions of the developing, adult and ageing nervous system.
Neurotransmitter receptor expression during in vivo neuronal development and associated disease states
The hypothesis that the dysfunction of a single receptor species inhibits neuronal differentiation is supported by data obtained from neuronal development of animals. The differential expression of ionotropic glutamate receptors, such as the NMDA, AMPA and kainate subtypes, the A‐type γ‐aminobutyric acid receptor, the glycine receptor, nicotinic acetylcholine, serotonin, dopamine, α‐adrenergic, as well as metabotropic neurotransmitter receptors, such as ET‐B and mAChRs receptors, in neuronal development, are well described in the literature (Nguyen et al. 2001).
Neurotransmitter receptors have been shown to take part in the development of the CNS and in some cases, receptor dysfunction can be directly linked to a certain disease state. Results obtained from a mouse model of Huntington's disease led to the hypothesis that neurodegeneration of striatal medium‐sized spiny neurones results from an alteration of NMDA receptor function (Li et al. 2004). In accordance with the hypothesis of glutamate‐receptor dysfunction related neurodegeneration, Martynyuk et al. (2005), collected evidence that impaired brain development in classical phenylketonuria is the result of defective glutaminergic synaptic transmission.
Abnormal responses to muscarinic m1 agonist treatment in terms of hippocampal amyloid precursor protein, nerve growth factor and brain‐derived neurotrophic factor levels indicate that developmental and age‐related behavioural deficits in Ts65 Down's model mice may be related to disturbed muscarinic receptor function (Seo & Isacson 2005).
Disease states in humans, such as Hirschsprung disease, have been associated with ET‐B receptor mutation (Woodward et al. 2000). Mutations in the ET‐B receptor gene result in pathogenic conditions such as congenital aganglionic megacolon associated with pigment abnormalities in rodents and humans, caused by defects in the development of two neural crest‐derived cell lineages to enteric neurones and epidermal melanocytes (Baynash et al. 1994; Hosoda et al. 1994; Puffenberger et al. 1994; Kapur et al. 1995; Gariepy et al. 1996). Receptor dysfunction and disease are caused by a point mutation of a tryptophan‐276 to a cystein residue at the fifth transmembrane region of the ET‐B receptor (Woodward et al. 2000). Experimental mutation at the ET‐B receptor ligand in animal models resulted in a similar disease state (MacLean et al. 1998). Blockade receptor of the ET‐B receptor produced terminal aganglionosis in both isolated colons and intact guts (Sidebotham et al. 2002). Observations that this disease is based on mutations of the ET‐B receptor or its ligand support the hypothesis that the endothelin receptor‐ligand system participates in the development of the nervous system and formation of the neural crest, thereby contributing to the establishment of a neuronal network.
CONCLUSION
Reconstruction of complex neocortical and other CNS circuitry may be possible via transplantation of appropriate neural precursors, guided by cellular and molecular controls. Although cellular repopulation and complex circuitry repair may make possible new avenues of treatment for degenerative, developmental, or acquired CNS diseases, functional integration may depend critically on the specificity of neuronal synaptic integration and the appropriate neurotransmitter/receptor phenotype (Shin et al. 2000). Stem‐cell‐based therapies, such as the therapeutic use of EC, ES and neural stem cells, are promising for the treatment of neurodegenerative disease states. Protocols are being developed to generate a single, desired neuronal phenotype that can then be transplanted to the patient. The exact phenotype of the neurone depends on the combination of intrinsic and extrinsic factors present during neuronal differentiation. The time course and pattern of calcium signalling during neuronal development in vivo has been shown to contribute to the exact phenotype, including the formation of excitatory and inhibitory synapses and the liberation of excitatory and inhibitory neurotransmitters (Borodinsky et al. 2004). Time points and quantities of calcium flux during development in vivo as well as during in vitro differentiation are controlled by the expression and regulation of cell surface receptors and ion channels.
Free intracellular calcium concentration [Ca2+]i, which in resting cells is typically in the range of 10–100 nm, is raised to high nanomolar or low micromolar concentration upon binding of a ligand to its receptor on the cell surface. Besides controlling metabolic function, transient calcium spikes are involved in control of liberation of neurotransmitters and receptor activity in synapses, and it is also implicated in the regulation of trophic processes such as neuronal development and cellular migration, changes in cell architecture, cellular differentiation and organogenesis (Baynash et al. 1994).
As CNS neurones in vivo develop in a complex environment, which is largely inaccessible to experimental manipulation, little is known about the factors that direct differentiation. These difficulties of in vivo experimentation led to the development of in vitro models for neuronal differentiation, such as the murine embryonal EC line P19, which has been used to study differential neuropeptide and neurotransmitter receptor expression and activity.
Dysfunction of neurotransmitter receptor function disturbs the coordination of calcium signalling and can affect the development of the CNS. Receptor expression and activity are coordinated and regulated by successive events of development, determining the ultimate phenotype of the neurone. Dysfunction of a single receptor species leads to change of fate of neuronal differentiation, as demonstrated by the inhibition of mAChR receptor gene expression when kinin‐B2 receptors are not functional during in vitro differentiation of P19 EC cells.
ACKNOWLEDGEMENTS
We would like to thank Dr Antonio Carlos Cassola, Instituto de Biociências, Universidade de São Paulo (USP), São Paulo, Brazil, for letting us use the confocal microscope. H.U. is grateful for a grant from FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo), and financial support from CNPq (Conselho Nacional de Pesquisa), Brazil. P.M. has been supported by a doctoral fellowship from FAPESP.
REFERENCES
- Bai D, Muller RU, Roder JC (2002) Non‐ionotropic cross‐talk between AMPA and NMDA receptors in rodent hippocampal neurones. J. Physiol. 543, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L (2003) Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat. Biotechnol. 21, 1200–1207. [DOI] [PubMed] [Google Scholar]
- Battistini B, Chailler P, D’Orleans‐Juste P, Briere N, Sirois P (1993) Growth regulatory properties of endothelins. Peptides 14, 385–399. [DOI] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M (1994) Interaction of endothelin‐3 with endothelin‐B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell 79, 1277–1285. [DOI] [PubMed] [Google Scholar]
- Bettler B, Mulle C (1995) Review: neurotransmitter receptors. II. AMPA and kainate receptors. Neuropharmacology 34, 123–139. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Hayden MR (2004) Deranged neuronal calcium signaling and Huntington disease. Biochem. Biophys. Res. Commun. 322, 1310–1317. [DOI] [PubMed] [Google Scholar]
- Biagioni S, Tata AM, De Jaco A, Augusti‐Tocco G (2000) Acetylcholine synthesis and neuron differentiation. Int. J. Dev. Biol. 44, 689–697. [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC (2004) Activity‐dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429, 523–530. [DOI] [PubMed] [Google Scholar]
- Boudjelal M, Taneja R, Matsubara S, Bouillet P, Dolle P, Chambon P (1997) Overexpression of Stra13, a novel retinoic acid‐inducible gene of the basic helix‐loop‐helix family, inhibits mesodermal and promotes neuronal differentiation of P19 cells. Genes Dev. 11, 2052–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle O (1999) Building brains: neural chimeras in the study of nervous system development and repair. Brain Pathol. 9, 527–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YC, Ma L, Fan GH, Zhao J, Jiang LZ, Pei G (1997) Activation of N‐methyl‐D‐aspartate receptor attenuates acute responsiveness of delta‐opioid receptors. Mol. Pharmacol. 51, 583–587. [DOI] [PubMed] [Google Scholar]
- Caldwell MA, He X, Wilkie N, Pollack S, Marshall G, Wafford KA, Svendsen CN (2001) Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat. Biotechnol. 19, 475–479. [DOI] [PubMed] [Google Scholar]
- Canzoniero LM, Sensi SL, Turetsky DM, Finley MF, Choi DW, Huettner JE (1996) Glutamate receptor‐mediated calcium entry in neurons derived from P19 embryonal carcinoma cells. J. Neurosci. Res. 45, 226–236. [DOI] [PubMed] [Google Scholar]
- Cauley K, Marks M, Gahring LC, Rogers SW (1996) Nicotinic receptor subunits alpha 3, alpha 4, and beta 2 and high affinity nicotine binding sites are expressed by P19 embryonal cells. J. Neurobiol. 30, 303–314. [DOI] [PubMed] [Google Scholar]
- Chen HC, Wei LN, Loh HH (1999) Expression of ‐, κ‐ and δ‐opioid receptors in P19 mouse embryonal carcinoma cells. Neuroscience 92, 1143–1155. [DOI] [PubMed] [Google Scholar]
- Chen HH, Wei CT, Chan MH (2004) Neonatal toluene exposure alters glutamate‐induced calcium signaling in developing cerebellar granule neurons. Ann. N.Y. Acad. Sci. 1025, 556–560. [DOI] [PubMed] [Google Scholar]
- Cheon EW, Kuwata O, Saito T (2001) Muscarinic acetylcholine receptors in the normal, developing and regenerating newt retinas Dev. Brain Res. 127, 9–21. [DOI] [PubMed] [Google Scholar]
- Cheun JE, Yeh HH (1991) Differentiation of a stem cell line toward a neuronal phenotype. Int. J. Dev. Neurosci. 9, 391–404. [DOI] [PubMed] [Google Scholar]
- Chiba S, Iwasaki Y, Sekino H, Suzuki N (2003) Transplantation of motoneuron‐enriched neural cells derived from mouse embryonic stem cells improves motor function of hemiplegic mice. Cell Transplant. 12, 457–468. [DOI] [PubMed] [Google Scholar]
- Cho K, Brown MW, Bashir ZI (2002) Mechanisms and physiological role of enhancement of mGlu5 receptor function by group II mGlu receptor activation in rat perirhinal cortex. J. Physiol. 540, 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Kim M, Jung K‐H, Jeon D, Lee S‐T, Kim J, Jeong S‐W, Kim SU, Lee SK, Shin HS, Roh J‐K (2004) Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine‐induced status epilepticus in adult rats. Brain Res. 1023, 213–221. [DOI] [PubMed] [Google Scholar]
- D’Orlando C, Fellay B, Schwaller B, Salicio V, Bloc A, Gotzos V, Celio MR (2001) Calretinin and calbindin D‐28k delay the onset of cell death after excitotoxic stimulation in transfected P19 cells. Brain Res. 909, 145–158. [DOI] [PubMed] [Google Scholar]
- Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E (2002) Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 7, 1083–1091. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC (2004) Excitation‐neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42, 535–552. [DOI] [PubMed] [Google Scholar]
- Drian MJ, Bardoul M, Konig N (2001) Blockade of AMPA/kainate receptors can either decrease or increase the survival of cultured neocortical cells depending on the stage of maturation. Neurochem. Int. 38, 509–517. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman M (1981) Establishment in culture of pluripotent cells from mouse embryos. Nature 292, 154–156. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Espejo E (2004) Pathogenesis of Parkinson's disease: prospects of neuroprotective and restorative therapies. Mol. Neurobiol. 29, 15–30. [DOI] [PubMed] [Google Scholar]
- Ferroni S, Marchini C, Ogata T, Schubert P (2002) Recovery of deficient cholinergic calcium signaling by adenosine in cultured rat cortical astrocytes. J. Neurosci. Res. 68, 615–621. [DOI] [PubMed] [Google Scholar]
- Finley MF, Kulkarni N, Huettner JE (1996) Synapse formation and establishment of neuronal polarity by P19 embryonic carcinoma cells and embryonic stem cells. J. Neurosci. 16, 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TB, Cicchetti F, Hauser RA, Deacon TW, Li XJ, Hersch SM, Nauert GM, Sanberg PR, Kordower JH, Saporta S, Isacson Q (2000) Transplanted fetal striatum in Huntington's disease: phenotype development and lack of pathology. Proc. Natl. Acad. Sci. USA 97, 13877–13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Pende M, Scherer S, Molne M, Wright P (1995) Expression and regulation of kainate and AMPA receptors in uncommitted and committed neural progenitors. Neurochem. Res. 20, 549–560. [DOI] [PubMed] [Google Scholar]
- Garbuzova‐Davis S, Willing AE, Milliken M, Saporta S, Zigova T, Cahill DW, Sanberg PR (2002) Positive effect of transplantation of hNT neurons (NTera 2/D1 cell‐line) in a model of familial amyotrophic lateral sclerosis. Exp. Neurol. 174, 169–180. [DOI] [PubMed] [Google Scholar]
- Gariepy CE, Cass DT, Yanagisawa M (1996) Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc. Natl. Acad. Sci. USA 93, 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD (1999) Endothelin and the development of the enteric nervous system. Clin. Exp. Pharmacol. Physiol. 26, 985–988. [DOI] [PubMed] [Google Scholar]
- Grant ER, Errico MA, Emanuel SL, Benjamin D, McMillian MK, Wadworth SA, Zivin RA, Zhong Z (2001) Protection against glutamate toxicity through inhibition of the p44/42 mitogenic‐activated protein kinase pathway in neuronally differentiated P19 cells. Biochem. Pharmacol. 62, 283–296. [DOI] [PubMed] [Google Scholar]
- Grant MK, Christopoulos A, El‐Fakahany EE (1999) Regulation of acetylcholine binding by ATP at the muscarinic M(1) receptor in intact CHO cells. Brain Res. 839, 94–99. [DOI] [PubMed] [Google Scholar]
- Gritti A, Vescovi AL, Galli R (2002) Adult neural stem cells: plasticity and developmental potential. J. Physiol. Paris. 96, 81–90. [DOI] [PubMed] [Google Scholar]
- Guan K, Chang H, Rolletschek A, Wobus AM (2001) Embryonic stem cell‐derived neurogenesis. Retinoic acid induction and lineage selection of neuronal cells. Cell Tissue Res. 305, 171–176. [DOI] [PubMed] [Google Scholar]
- Güttinger M, Fedele D, Koch P, Padrun V, Pralong WF, Brüstle O, Boison D (2005) Suppression of kindled seizures by paracrine adenosine release from stem cell‐derived brain implants. Epilepsia 46, 1162–1169. [DOI] [PubMed] [Google Scholar]
- Hama H, Kasuya Y, Sakurai T, Yamada G, Suzuki N, Masaki T, Goto K (1997) Role of endothelin‐1 in astrocyte responses after acute brain damage. J. Neurosci. Res. 47, 590–602. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Margulis M, Fishman PS, Lee VM‐Y, Tang C‐M (1999) Functional synapses are formed between human NTera2 (NT2N, hNT) neurons grown on astrocytes. J. Comp. Neurol. 407, 1–10. [DOI] [PubMed] [Google Scholar]
- Heck S, Enz R, Richter‐Landberg C, Blohm DH (1997a) Expression and mRNA splicing of glycine receptor subunits and gephyrin during neuronal differentiation of P19 cells in vitro, studied by RT‐PCR and immunocytochemistry. Brain Res . Dev. Brain Res. 98, 211–220. [DOI] [PubMed] [Google Scholar]
- Heck S, Enz R, Richetr‐Landsberg C, Blohm DH (1997b) Expression of eight metabotropic glutamate receptor subtypes during neuronal differentiation of P19 embryocarcinoma cells: a study by RT‐PCR and in situ hybridization. Brain Res. Dev. Brain Res. 101, 85–91. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H (2001) Neurotransmitters and neuromodulators during early human development. Early Hum. Dev. 65, 21–37. [DOI] [PubMed] [Google Scholar]
- Van Der Heyden MA, Van Kempen MJ, Tsuji Y, Rook MB, Jongsma HJ, Opthof T (2003) P19 embryonal carcinoma cells: a suitable model system for cardiac electrophysiological differentiation at the molecular and functional level. Cardiovasc. Res. 58, 410–422. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Chipperfield H, Whyte KA, Stafford MR, Hansen MA, Cool SM, Nurcombe V, Adams DJ (2004) Functional maturation of isolated neural progenitor cells from the adult rat hippocampus. Eur. J. Neurosci. 19, 2410–2420. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Ben‐Ari Y (2003) Seizing hold of seizures. Nat. Med. 9, 994–996. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M (1994) Targeted and natural (piebald‐lethal) mutations of endothelin‐B receptor gene produce megacolon associated with spotted coat color in mice. Cell 79, 1267–1276. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Moriyoshi K, Sasai Y, Shiota K, Nakanishi S, Kageyama R (1994) Persistent expression of helix‐loop‐helix factor HES‐1 prevents mammalian neural differentiation in the central nervous system. EMBO J. 13, 1799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelitai M, Schlett K, Varju P, Eisel U, Madarasz E (2002) Regulated appearance of NMDA receptor subunits and channel functions during in vitro neuronal differentiation. J. Neurobiol. 51, 54–65. [DOI] [PubMed] [Google Scholar]
- Jerusalinsky D, Kornisiuk E, Izquierdo I (1997) Cholinergic neurotransmission and synaptic plasticity concerning memory processing. Neurochem. Res. 22, 507–515. [DOI] [PubMed] [Google Scholar]
- Jones‐Villeneuve EM, McBurney MW, Rogers KA, Kalnins VI (1982) Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J. Cell. Biol. 94, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur RP, Sweetser DA, Doggett B, Siebert JR, Palmiter RD (1995) Intercellular signals downstream of endothelin receptor‐B mediate colonization of the large intestine by enteric neuroblasts. Development 121, 3787–3795. [DOI] [PubMed] [Google Scholar]
- Kitayama T, Yoneyama M, Tamaki K, Yoneda Y (2004) Regulation of neuronal differentiation by N‐methyl‐D‐aspartate receptors expressed in neural progenitor cells isolated from adult mouse hippocampus. J. Neurosci. Res. 76, 599–612. [DOI] [PubMed] [Google Scholar]
- Kume S (2005) Stem‐cell‐based approaches for regenerative medicine. Dev. Growth Differ. 47, 393–402. [DOI] [PubMed] [Google Scholar]
- Lee HO, Levorse JM, Shin MK (2003a) The endothelin receptor‐B is required for the migration of neural crest‐derived melanocyte and enteric neuron precursors. Dev. Biol. 259, 162–175. [DOI] [PubMed] [Google Scholar]
- Lee YH, Lin CH, Hsu LW, Hu SY, Hsiao WT, Ho YS (2003b) Roles of ionotropic glutamate receptors in early developing neurons derived from the P19 mouse cell line. J. Biomed. Sci. 10, 199–207. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Changeux J (1999) The ligand gated ion channel database. Nucleic Acids Res. 27, 340–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wing LL, Kirch DG, Wyatt RJ, Chuang DM (1992) Effects of chronic nicotine and haloperidol administration on muscarinic receptor‐mediated phosphoinositide turnover in rat brain slices. Psychopharmacology (Berl.) 109, 248–250. [DOI] [PubMed] [Google Scholar]
- Li L, Murphy TH, Hayden MR, Raymond LA (2004) Enhanced striatal NR2B‐containing N‐methyl‐D‐aspartate receptor‐mediated synaptic currents in a mouse model of Huntington disease. J. Neurophysiol. 92, 2738–2746. [DOI] [PubMed] [Google Scholar]
- Lin P, Kusano K, Zhang Q, Felder CC, Geiger PM, Mahan LC (1996) GABAA receptors modulate early spontaneous excitatory activity in differentiating P19 neurons. J. Neurochem. 66, 233–242. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Martinez‐Serrano A (2004) Stem cell therapy for human neurodegenerative disorders – how to make it work. Nat. Med. 10 (Suppl.), S42–S50. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR (1995) GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 15, 1287–1298. [DOI] [PubMed] [Google Scholar]
- Lujan R, Shigemoto R, Lopez‐Bendito G (2005) Glutamate and GABA receptor signalling in the developing brain. Neuroscience 130, 567–580. [DOI] [PubMed] [Google Scholar]
- Lukas RJ, Bencherif M (1992) Heterogeneity and regulation of nicotinic acetylcholine receptors. Int. Rev. Neurobiol. 34, 25–131. [DOI] [PubMed] [Google Scholar]
- Ma W, Maric D, Li BS, Hu Q, Andreadis JD, Grant GM, Liu QY, Shaffer KM, Chang YH, Zhang L, Pancrazio JJ, Pant HC, Stenger DA, Barker JL (2000) Acetylcholine stimulates cortical precursor cell proliferation in vitro via muscarinic receptor activation and MAP kinase phosphorylation. Eur. J. Neurosci. 12, 1227–1240. [DOI] [PubMed] [Google Scholar]
- MacLean MR, Docherty CC, McCulloch KM, Morecroft I (1998) Effect of novel mixed ETA/ETB antagonists on responses to ET‐1 in human small muscular pulmonary arteries. Pulm. Pharmacol. Ther. 11, 147–149. [DOI] [PubMed] [Google Scholar]
- MacPherson PA, Jones S, Pawson PA, Marshall KC, McBurney NW (1997) P19 cells differentiate into glutamatergic and glutamate‐responsive neurons in vitro . Neuroscience 80, 487–499. [DOI] [PubMed] [Google Scholar]
- Magdesian MH, Nery AA, Martins AH, Juliano MA, Juliano L, Ulrich H, Ferreira ST (2005) Peptide blockers of the inhibition of neuronal nicotinic acetylcholine receptors by amyloid beta. J. Biol. Chem. 280, 31085–31090. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ (1998) Activation of the genetically defined m1 muscarinic receptor potentiates N‐methyl‐D‐aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc. Natl. Acad. Sci. USA 95, 11465–11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 78, 7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins AH, Resende RR, Majumder P, Faria M, Casarini DE, Tarnok A, Colli W, Pesquero JB, Ulrich H (2005) Neuronal differentiation of P19 embryonal carcinoma cells modulates kinin B2 receptor gene expression and function. J. Biol. Chem. 280, 19576–19586. [DOI] [PubMed] [Google Scholar]
- Martynyuk AE, Glushakov AV, Sumners C, Laipis PJ, Dennis DM, Seubert CN (2005) Impaired glutamatergic synaptic transmission in the PKU brain. Mol. Genet. Metab. 86, S34–S42. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Rogers BJ (1982) Isolation of male embryonal carcinoma cells and their chromosome replication patterns. Dev. Biol. 89, 503–508. [DOI] [PubMed] [Google Scholar]
- Monge JC, Stewart DJ, Cernacek P (1995) Differentiation of embryonal carcinoma cells to a neural or cardiomyocyte lineage is associated with selective expression of endothelin receptors. J. Biol. Chem. 270, 15385–15390. [DOI] [PubMed] [Google Scholar]
- Morassutti DJ, Staines WA, Magnuson DS, Marshall KC, McBurney MW (1994) Murine embryonal carcinoma‐derived neurons survive and mature following transplantation into adult rat striatum. Neuroscience 58, 753–763. [DOI] [PubMed] [Google Scholar]
- Morley P, MacPherson P, Whitfield JF, Harris EW, McBurney MW (1995) Glutamate receptor‐mediated calcium surges in neurons derived from P19 cells. J. Neurochem. 65, 1093–1099. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G (2001) Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 305, 187–202. [DOI] [PubMed] [Google Scholar]
- Niemann S, Schaller HC (1996) Head‐activator and the neuroectodermal differentiation of P19 mouse embryonal carcinoma cells. Neurosci. Lett. 207, 49–52. [DOI] [PubMed] [Google Scholar]
- Parnas D, Linial M (1995) Cholinergic properties of neurons differentiated from an embryonal carcinoma cell‐line (P19). Int. J. Dev. Neurosci. 13, 767–781. [DOI] [PubMed] [Google Scholar]
- Parnas D, Heldman E, Branski L, Feinstein N, Linial M (1998) Expression and localization of muscarinic receptors in P19‐derived neurons. J. Mol. Neurosci. 10, 17–29. [DOI] [PubMed] [Google Scholar]
- Pera MF, Reubinoff B, Trounson A (2000) Human embryonic stem cells. J. Cell. Sci. 113, 5–10. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Gonzalez LC, Tena‐Sempere M, Aguilar E (2001) Cross‐talk between excitatory and inhibitory amino acids in the regulation of growth hormone secretion in neonatal rats. Neuroendocrinology 73, 62–67. [DOI] [PubMed] [Google Scholar]
- Puffenberger EG, Hosoda K, Washington SS, Nakao K, Dewit D, Yanagisawa M, Chakravart A (1994) A missense mutation of the endothelin‐B receptor gene in multigenic Hirschsprung's disease. Cell 79, 1257–1266. [DOI] [PubMed] [Google Scholar]
- Rathjen J, Rathjen PD (2001) Mouse ES cells: experimental exploitation of pluripotent differentiation potential. Curr. Opin. Genet. Dev. 11, 587–594. [DOI] [PubMed] [Google Scholar]
- Ray WJ, Gottlieb DI (1993) Expression of ionotropic glutamate receptor genes by P19 embryonal carcinoma cells. Biochem. Biophys. Res. Commun. 197, 1475–1482. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss SA (1992) Multipotent EGF‐responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 12, 4565–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Ryan PJ, Prasad A, Paterno GD (1994) Neurons derived from embryonal carcinoma (P19) cells express multiple GABAA receptor subunits and fully functional GABAA receptors. Neurosci. Lett. 165, 129–132. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Prasad A, Gillespie LL, Paterno GD (1996) Developmental expression of functional GABAA receptors containing the gamma 2 subunit in neurons derived from embryonal carcinoma (P19) cells. Brain Res. Mol. Brain Res. 35, 11–18. [DOI] [PubMed] [Google Scholar]
- Rippon HJ, Bishop (2004) Embryonic stem cells. Cell Prolif. 37, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SD, Demaster E, Catton M, Ghilardi JR, Levin LA, Maggio JE, Mantyh PW (1997) Expression of endothelin‐B receptors by glia in vivo is increased after CNS injury in rats, rabbits and humans. Exp. Neurol. 145, 180–195. [DOI] [PubMed] [Google Scholar]
- Ruschenschmidt C, Koch PG, Brustle O, Beck H (2005) Functional properties of ES cell‐derived neurons engrafted into the hippocampus of adult and chronically epileptic rat. Epilepsia 46 (Suppl. 5), 174–183. [DOI] [PubMed] [Google Scholar]
- Santiago MF, Liour SS, Mendez‐Otero R, Yu RK (2005) Glial‐guided neuronal migration in P19 embryonal carcinoma stem cell aggregates. J. Neurosci. Res. 81, 9–20. [DOI] [PubMed] [Google Scholar]
- Scheetz AJ, Constantine‐Paton M (1994) Modulation of NMDA receptor function: implications for vertebrate neural development. FASEB 8, 745–752. [DOI] [PubMed] [Google Scholar]
- Scott L, Kruse MS, Forssberg H, Brismar H, Greengard P, Aperia A (2002) Selective up‐regulation of dopamine D1 receptors in dendritic spines by NMDA receptor activation. Proc. Natl. Acad. Sci. USA 99, 1661–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Isacson O (2005) Abnormal APP, cholinergic and cognitive function in Ts65Dn Down's model mice. Exp. Neurol. 193, 469–480. [DOI] [PubMed] [Google Scholar]
- Shin MK, Levorse JM, Ingram RS, Tilghman SM (1999) The temporal requirement for endothelin receptor‐B signalling during neural crest development. Nature 402, 496–501. [DOI] [PubMed] [Google Scholar]
- Shin JJ, Fricker‐Gates RA, Perez FA, Leavitt BR, Zurakowski D, Macklis JD (2000) Transplanted neuroblasts differentiate appropriately into projection neurons with correct neurotransmitter and receptor phenotype in neocortex undergoing targeted projection neuron degeneration. J. Neurosci. 20, 7404–7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara H, Udagawa J, Morishita R, Ueda H, Otani H, Semba R, Kato K, Asano T (2004) Gi2 signaling enhances proliferation of neural progenitor cells in the developing brain. J. Biol. Chem. 279, 41141–41148. [DOI] [PubMed] [Google Scholar]
- Sidebotham EL, Woodward MN, Kenny SE, Lloyd DA, Vaillant CR, Edgar DH (2002) Localization and endothelin‐3 dependence of stem cells of the enteric nervous system in the embryonic colon. J. Pediatr. Surg. 37, 145–150. [DOI] [PubMed] [Google Scholar]
- Staines WA, Morassutti DJ, Reuhl KR, Ally AI, McBurney MW (1994) Neurons derived from P19 embryonal carcinoma cells have varied morphologies and neurotransmitters. Neuroscience 58, 735–751. [DOI] [PubMed] [Google Scholar]
- Stavridis MP, Smith AG (2003) Neural differentiation of mouse embryonic stem cells. Biochem. Soc. Trans. 31, 45–49. [DOI] [PubMed] [Google Scholar]
- Strong MJ (2004) Amyotrophic lateral sclerosis: contemporary concepts in etiopathogenesis and pharmacotherapy. Expert Opin. Invest. Drugs 13, 1593–1614. [DOI] [PubMed] [Google Scholar]
- Tárnok A, Ulrich H (2001) Characterization of pressure‐induced calcium response in neuronal cell lines. Cytometry 43, 175–181. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Tsaur ML, Wan YC, Lai FP, Cheng HF (1997) Expression of B‐type endothelin receptor gene during neural development. FEBS Lett. 417, 208–212. [DOI] [PubMed] [Google Scholar]
- Turetsky DM, Huettner JE, Gottlieb DI, Goldberg MP, Choi DW (1993) Glutamate receptor‐mediated currents and toxicity in embryonal carcinoma cells. J. Neurobiol. 24, 1157–1169. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA (2004) Role for glia in synaptogenesis. Glia 47, 209–216. [DOI] [PubMed] [Google Scholar]
- Wang Y, Goldman‐Rakic PS (2004) D2 receptor regulation of synaptic burst firing in prefrontal cortical pyramidal neurons. Proc. Natl. Acad. Sci. USA 101, 5093–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C, Brasted PJ, Dunnett SB (2000) The morphology, integration, and functional efficacy of striatal grafts differ between cell suspensions and tissue pieces. Cell Transplant. 9, 395–407. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Poter JA, Jessell TM (2002) Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397. [DOI] [PubMed] [Google Scholar]
- Williams BP, Milligan CJ, Street M, Hornby FM, Deuchars J, Buckley NJ (2004) Transcription of the M1 muscarinic receptor gene in neurons and neuronal progenitors of the embryonic rat forebrain. J. Neurochem. 88, 70–77. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Tonegawa S (1997) Synaptic plasticity, place cells and spatial memory: study with second‐generation knockouts. Trends Neurosci. 20, 102–106. [DOI] [PubMed] [Google Scholar]
- Woodward MN, Kenny SE, Vaillant C, Lloyd DA, Edgar DH (2000) Time‐dependent effects of endothelin‐3 on enteric nervous system development in an organ culture model of Hirschsprung's disease. J. Pediatr. Surg. 35, 25–29. [DOI] [PubMed] [Google Scholar]
- Zhang H, Lu A, Zhao H, Li K, Song S, Yan J, Zhang W, Wang S, Li L (2004) Elevation of NMDAR after transplantation of neural stem cells. Neuroreport 15, 1739–1743. [DOI] [PubMed] [Google Scholar]


