Abstract
Objectives: Resveratrol, with its robust antioxidant activity, has frequently been suggested as potentially having activity in cancer prevention and some recent reports have indicated that it has cancer treatment potential for several types of neoplasia. It has been found to block p‐glycoprotein and to protect against several chemotherapeutic agents’ side effects. In this study, we assessed interactive characteristics of resveratrol with docetaxel and doxorubicin and further investigated molecular bases of this interaction in cells of three different solid tumour lines (MCF‐7, HeLa and HepG2).
Materials and methods and results: Resveratrol per se was found to have anti‐cancer properties, but with relatively low potency in all tested cell lines (IC50 ranged from 35.1 to 83.8 μM). Doxorubicin and docetaxel showed IC50 ranging from 0.48 to 0.72 μM and from 25.9 to 77.8 nM, respectively. Resveratrol in combination with doxorubicin and docetaxel significantly increased potencies of both chemotherapeutic agents showing IC50 ranging from 0.12 to 0.34 μM and from 7.2 to 53.02 nM, respectively. The combination index showed synergistic interaction between resveratrol and doxorubicin or docetaxel on MCF‐7 cells, and additive interactions on HeLa and HepG2 cells. Real time PCR revealed that expression of Bax and Bcl‐2 was simultaneously elevated on combination of resveratrol with doxorubicin or docetaxel in all tested cell lines, whereas p53 exhibited marginal elevation in MCF‐7 and HepG2 cells. In addition, p‐glycoprotein efflux activity was significantly inhibited, with subsequent accumulation of p‐glycoprotein substrate in intracellular compartments. Expression level of mdr1 gene was downregulated after resveratrol combined with doxorubicin or docetaxel in all tested cell lines.
Conclusion: Resveratrol potentiates cytotoxic properties of both cancer drugs used in the study through increasing their intracellular level due to p‐glycoprotein inhibition and downregulation of mdr1 gene.
Introduction
Cancer is both a national and international health problem. WHO reports show constant rates of mortality from cancer over more than the last five decades (1950–2000) caused by various types of malignancy (1). Cancer is still the leading cause of death worldwide, regardless of the discovery of several dozens of novel anti‐cancer drugs both natural and synthetic. There have been wide gaps between disease incidence (0.16%), disease burden (5.6%) and mortality caused by cancer (12.5%) (2, 3, 4), these gaps clearly denoting lack of effective treatment for cancer compared to other causes of mortality. Accordingly (amongst others), screening of natural products for promising anti‐cancer activity is a pertinent field in several countries that have diversity of the gardenia flower. Many plants are widely used medicinally in different countries and are a source of many potent and powerful drugs (5).
Resveratrol (RES) (trans‐3,4′,5,‐trihydroxystilbene) is a natural polyphenolic molecule found in red grapes (6). Traditionally, RES of red grape wine is considered a general anti‐aging material with classical antioxidant properties, due to its polyphenolic composition (7). Further studies have revealed anti‐inflammatory, vascular endothelial protective and lipid metabolism enhancement effects of Resveratrol (8). RES has been found to interfere with expression/activity of several inflammatory/immunoregulatory cytokines and enzymes such as cyclo‐oxegenase, TNF‐α, NF‐κB, INF‐γ and IL‐1, IL‐4 and IL‐6 (9). The cancer preventive property of RES has mainly been attributed to be due to its antioxidant and anti‐inflammatory/immunoregulatory profiles (6). In addition, several reports have indicated potential anti‐proliferative or anti‐cancer effects of RES per se against many types of human cancer (10, 11, 12, 13).
Most resistance of solid tumours to anti‐cancer treatment has currently been attributed to pharmacokinetic reasons rather than to tissue resistance at the cellular level. Anti‐cancer agents need to be introduced into each single tumour cell at a cytotoxic concentration to generate tumour‐killing effects (14, 15, 16) and intracellular transport of anti‐cancer agents is currently receiving a great deal of attention as a major determinant of anti‐tumour efficacy (17, 18). P‐glycoprotein (P‐gp) is a membrane ATPase efflux pump that naturally occurs on membranes of cells of many excretory organs such as the kidney, salivary gland and GIT (19). In addition, P‐gp is overexpressed in cells of several types of solid tumour and significantly reduces intracellular concentration of anti‐cancer agents (20). Several P‐gp blockers have been used in adjuvant cancer therapy to increase intracellular concentration of anti‐cancer agents (21). RES has a P‐gp blocking effect that might improve anti‐cancer activity of several cytotoxic agents (10). In addition, RES might be a potentially successful adjuvant candidate for combination therapy against malignant tumours due to its antioxidant properties (22). Our current study described here was designed to investigate potential improvement effects of RES on docetaxel’s and doxorubicin’s anti‐cancer properties and its relationship to P‐gp expression and activity, in three different human solid tumour cell lines.
Materials and methods
Drugs and chemicals
Doxorubicin (DOX), docetaxel (DCX), sulpharhodamine, RES and verapamil (VRP) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). RPMI‐164 media, foetal bovine serum and other cell culture materials were purchased from AATC (Houston, TX, USA). Other reagents were of the highest analytical grade.
Cell culture
Human hepatocellular carcinoma cell line (HepG2), breast cancer cell line (MCF‐7) and cervical cancer cell line (HeLa) were obtained from Vaccera (Giza, Egypt). Cells were maintained in RPMI‐1640 supplemented with 100 μg/ml streptomycin, 100 units/ml penicillin and 10% heat‐inactivated foetal bovine serum in a humidified, 5% (v/v) CO2 atmosphere at 37 °C.
Cytotoxicity assays
Cytotoxicity of DOX, DCX and RES were tested on MCF‐7, HeLa and HepG2 cells by SRB assay, as previously described (23). Exponentially proliferating cells were collected using 0.25% trypsin‐EDTA and were plated in 96‐well plates at 1000–2000 cells/well. Cells were exposed to DOX, DCX and RES for 72 h and subsequently fixed in TCA (10%) for 1 h at 4 °C. After several washings, cells were exposed to 0.4% SRB solution for 10 min in the dark and subsequently were washed in 1% glacial acetic acid. After being dried overnight, Tris‐HCl was used to dissolve the SRB‐stained cells and colour intensity was measured at 540 nm (23).
Data analysis
Dose response curves of compounds were analysed using the Emax model (Eqn 1).
| (1) |
where R is the residual unaffected fraction (resistance fraction), [D] is drug concentration used, K d is drug concentration that produces 50% reduction in maximum inhibition rate and m is a Hill‐type coefficient. IC50 was defined as drug concentration required to reduce fluorescence to 50% of that of the appropriate control (i.e. K d = IC50 when R = 0 and Emax = 100 −R) (24).
The combination index (CI) was calculated as previously described (25). Briefly, exponentially dividing cells were exposed to equitoxic concentrations of RES and DOX or RES and DCX in 96‐well plates for 72 h and were subsequently subjected to SRB assay. CI was calculated from the formula:
 |
(2) |
The nature of drug interaction is defined as synergism if CI <0.8; as antagonism if CI >1.2; and additive, if CI ranges from 0.8 to 1.2.
RNA extraction and real time PCR analysis and quantification of gene expression
To assess expressions of RES, DOX and DCX coding genes, and their combinations, total RNA extraction from cells was performed using RNeasy Mini Kit® (Qiagen Inc. Valencia, CA, USA). Reverse transcription was undertaken to construct a cDNA library from different treatments, using High‐Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Archived cDNA libraries were then subjected to quantitative real time PCR reactions (26) using cyber green fluorophore (Fermentas Inc., Glen Burnie, MD, USA). Primer sequences were as follows: Bcl‐2 forward primer GGG‐TAC‐GAT‐AAC‐CGG‐GAG‐AT and reverse primer CTG‐AAG‐AGC‐TCC‐TCC‐ACC‐AC; BAX forward primer TCT‐GAC‐GGC‐AAC‐TTC‐AAC‐TG and reverse primer TGG‐GTG‐TCC‐CAA‐AGT‐AGG‐AG; p53 forward primer CCT‐CAC‐CAT‐CAT‐CAC‐ACT‐GG and reverse primer CTG‐AGT‐CAG‐GCC‐CTT‐CTG‐TC; mdr1 forward primer GCT‐GGG‐AAG‐ATC‐GCT‐ACT‐GA and reverse primer GGT‐ACC‐TGC‐AAA‐CTC‐TGA‐GCA. GAPDH was used as reference housekeeping gene with forward primer TGC‐ACC‐ACC‐AAC‐TGC‐TTA‐G and reverse primer GAT‐GCA‐GGG‐ATG‐ATG‐TTC (26).
Assessment of P‐gp efflux activity in tumour cell lines
To assess effects of RES on efflux pumping activity of P‐gp in tumour cell lines, P‐gp substrate, DOX, was used as probe and VRP was used as standard P‐gp blocker. Briefly, exponentially proliferating cells were plated in 6‐well plates at plating density of 105 cells/well. Cells were exposed to DOX (300 μg/ml), DOX (300 μg/ml) and RES (10 μg/ml) or DOX (300 μg/ml) and VRP (10 μg/ml) for 2 h at 37 °C and subsequently, extracellular DOX‐containing medium was washed three times in ice cold PBS. Intracellular DOX was extracted after cell lysis by incubation with SDS (2% w/v), saturated aqueous solution of ZnSO4 (100 μl), Acetonitril (500 μl) and acetone (500 μl) for 30 min at 37 °C. After centrifugation, supernatant was collected and DOX concentration was measured spectroflourometrically at λex/em 482/550 nm (27).
Determination of caspase‐3 activity
To assess the effective phase of apoptosis, caspase‐3 activity was assessed after treatment with DCX, DOX, RES and their combinations. Active caspase‐3 ELISA uses a biotinylated caspase inhibitor to covalently modify the large subunit of caspase‐3. Inhibitor is added directly to the culture medium where it enters apoptotic cells and forms a stable thio‐ether bond with cysteine at its active sites. The inhibitor does not covalently modify inactive caspase‐3, which is the basis for discrimination between its active and inactive forms. Cells were then solubilized in denaturing extraction buffer and diluted to reduce denaturant concentration.
Briefly, cells were harvested after treatment for 72 h with the pre‐determined IC50 of DCX, DOX and RES. Caspase‐3 activity was determined using Quantikine® immunoassay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Standard purified active caspase‐3 was used to plot a standard curve. Concentrations of caspase‐3 were normalized based on protein concentration.
Statistical analysis
Data are presented as mean ± SD. Analysis of variance (ANOVA) with LSD test post hoc was used for testing significance using SPSS® for windows, version 17.0.0. P < 0.05 was taken as being statistically significant.
Results
Influence of RES on efficacy profile of DCX and DOX
To study the effect of RES on cytotoxic profiles of two cornerstone chemotherapeutic agents (DCX and DOX), dose–response curves of both reagents alone were assessed relative to their combinations with RES, in cells of the three different solid tumour lines (Table 1). IC50 and resistance fraction were calculated as described in the Materials and methods section by compensation in an Emax model. In MCF‐7 cells, DCX exerted gradient cytotoxic activity with increasing concentration up to a resistant fraction of 27.0 ± 1.2%. Cell logarithmic kill was gradual in profile with IC50 of 22.3 ± 8.2 ng/ml. Equitoxic combinations of RES significantly improved cytotoxic profile of DCX in MCF‐7 cells, and instead of abolishing the resistant fraction down to 0.9 ± 0.2 %, IC50 of DCX after combination with RES was significantly reduced to one quarter of its original value after single DCX treatment (Fig. 1a). Similar to DCX, DOX showed a gradient cytotoxic effect with increasing concentration until resistant fraction of 2.6 ± 0.8%. Cell logarithmic kill composed a gradient with IC50 of 0.4 ± 0.1 μg/ml. Equitoxic combinations of RES improved the cytotoxic profile of DOX on MCF‐7 cells, reducing the resistant fraction to 0.7 ± 0.2%. IC50 of DOX, after combination with RES, was markedly reduced to around one‐sixth of its value after single DOX treatment (Fig. 1b). The calculated combination indices for DCX and DOX with RES were 0.34 and 0.23, respectively, which is indicative of strong synergistic interactions characteristics in MCF‐7 cells (Table 1).
Table 1.
Effect of RES on cytotoxicity parameters of DCX and DOX on the three different cell lines
| MCF‐7 | HeLa | HepG2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IC50* | R fraction (%) | CI value | IC50* | R fraction (%) | CI value | IC50* | R fraction (%) | CI value | |
| DCX | 22.3 ± 8.2 | 27.0 ± 1.2 | – | 76.1 ± 14.2 | 18.1 ± 0.8 | – | 40.1 ± 5.2 | 16.2 ± 0.96 | – |
| DCX+RES | 6.2 ± 1.3 | 0.9 ± 0.2 | 0.34 | 45.7 ± 4.5 | 4.1 ± 0.5 | 1.17 | 22.4 ± 0.6 | 7.0 ± 0.34 | 1.06 |
| DOX | 0.42 ± 0.11 | 2.6 ± 0.8 | – | 0.28 ± 0.07 | 7.7 ± 0.5 | – | 0.42 ± 0.05 | 5.3 ± 2.2 | – |
| DOX+RES | 0.07 ± 0.02 | 0.73 ± 0.2 | 0.23 | 0.19 ± 0.01 | 2.2 ± 0.2 | 1.13 | 0.2 ± 0.01 | 7.0 ± 0.44 | 0.92 |
*The IC50 is calculated in ng/ml for DCX and in μg/ml for DOX.
Docetaxel, DCX; doxorubicin, DOX.
Figure 1.
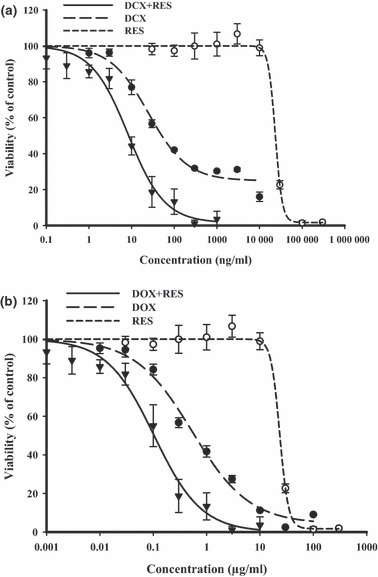
Effect of resveratrol (RES) on dose–response curve of docetaxel (DCX) (a) and doxorubicin (DOX) (b) on MCF‐7 breast cancer cells, was assessed. Cells were exposed to serial dilution of DCX/DOX (•), RES (○) or combination of RES with DCX/DOX () for 72 h. Cell viability was determined using SRB‐U assay; data are expressed as mean ± SD (n = 3).
In the HeLa cervical cancer cell line, an increasingly steep dose–response curve was achieved on treatment with DCX until a resistant fraction of 18.1 ± 0.8% was reached. IC50 of DCX treatment for 72 h was found to be 76.1 ± 14.2 ng/ml. Equitoxic combinations of RES marginally improved the IC50 of DCX on HeLa cells to about half the level in single treatment conditions. However, RES combination resulted in significant reduction in the resistant fraction of HeLa cells down to 4.1 ± 0.5% (Fig. 2a). DOX had a gradient cytotoxic effect with increasing concentration until resistant fraction of 7.7 ± 0.5% was reached. Cell logarithmic kill was a gradient with IC50 of 0.3 ± 0.1 μg/ml. Equitoxic combination of RES slightly improved the cytotoxic profile of DOX on HeLa cells, reducing the resistant fraction to 2.2 ± 0.2%. IC50 of DOX after combination with RES was marginally reduced to around half its value after single DOX treatment (Fig. 2b). The calculated combination indices for DCX and DOX with RES were 1.17 and 1.13, respectively, which is indicative of weak additive interactions on HeLa cells (Table 1).
Figure 2.
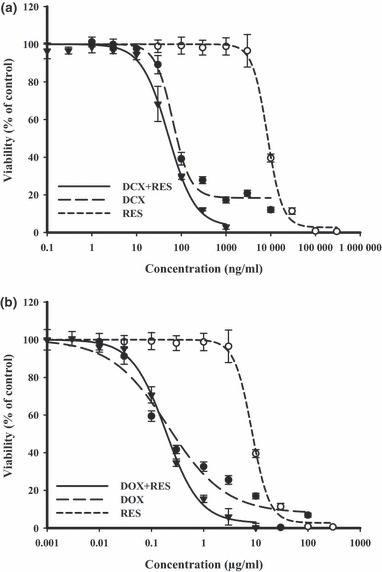
Effect of resveratrol (RES) on dose–response curve of docetaxel (DCX) (a) and doxorubicin (DOX) (b) on HeLa cervical cancer cells was assessed. Cells were exposed to serial dilution of DCX/DOX (•), RES (○) or combination of RES with DCX/DOX () for 72 h. Cell viability was determined using SRB‐U assay; data are expressed as mean ± SD (n = 3).
With respect to HepG2 liver cancer cells, DCX treatment for 72 h resulted in a sharp dose–response curve with IC50 of 40.1 ± 5.2 ng/ml reaching resistant fraction of 16.2 ± 1.0%. Equitoxic combination of RES enhanced the cytotoxic profile of DCX in HepG2 cells; IC50 was decreased to about half its level per single treatment and the resistant fraction was significantly reduced to 7.0 ± 0.3% (Fig. 3a). DOX had gradual cytotoxic effect with increasing concentration until resistant fraction of 5.3 ± 2.2% was reached. Cell logarithmic kill was gradual with IC50 of 0.4 ± 0.1 μg/ml. Equitoxic combination of RES slightly improved the cytotoxic profile of DOX on HepG2 cells, reducing IC50 to 0.2 ± 0.01%, but, the resistant fraction was not significantly changed after combination with RES (Fig. 3b). Calculated combination indices for DCX and DOX with RES were 1.1 and 0.92, respectively, indicative of an additive interaction on HepG2 cells (Table 1).
Figure 3.
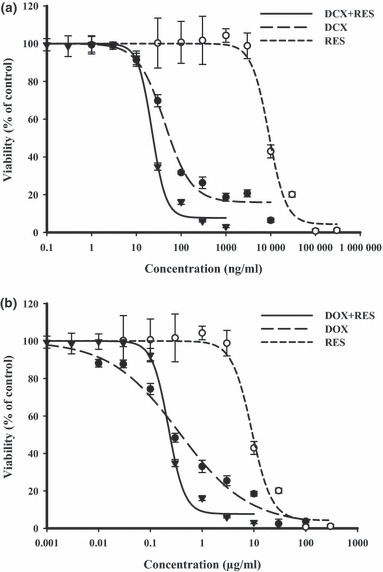
Effect of resveratrol (RES) on dose–response curve of docetaxel (DCX) (a) and doxorubicin (DOX) (b) on HepG2 liver cancer cells was assessed. Cells were exposed to serial dilution of DCX/DOX (•), RES (○) or combination of RES with DCX/DOX () for 72 h. Cell viability was determined using SRB‐U assay; data are expressed as mean ± SD (n = 3).
Effects of RES on DCX‐ and DOX‐induced apoptosis signalling
To explain interaction characteristics of DCX or DOX with RES, quantitative gene expression analysis for apoptotic key elements was performed using real time PCR. In MCF‐7 cells, the gene coding apoptotic protein BAX, was underexpressed in all single treatments. However, combination of RES with DCX or DOX significantly increased expression level of BAX gene by around fold that of untreated cells. Reciprocally, Bcl‐2 anti‐apoptotic gene was not significantly overexpressed compared to untreated cells. However, it was markedly overexpressed up to 4‐ to 5‐fold after combination treatment of RES with either DCX or DOX. The effector apoptotic gene, p53, was downregulated in all single treatments compared with untreated cells. Interestingly, p53 level was significantly overexpressed after exposure to combination of RES with DCX or DOX (Fig. 4a,b). This might be attributed to overweighing the total apoptotic signal to the anti‐apoptotic signal in MCF‐7 cell line.
Figure 4.
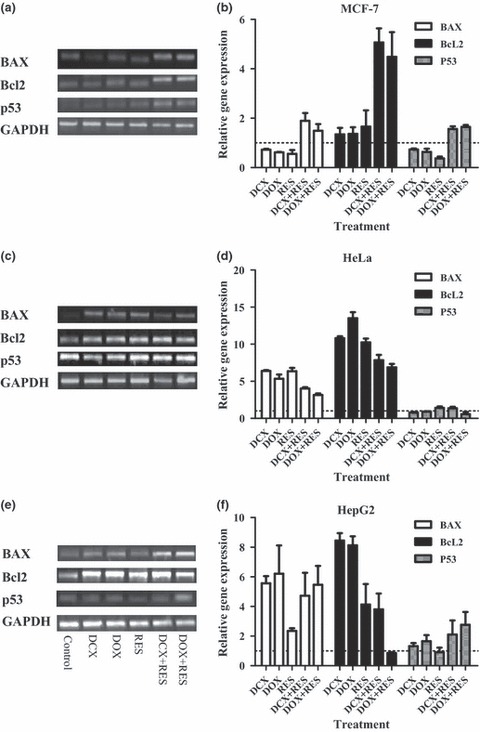
Effect of resveratrol (RES) on apoptotic pathway in docetaxel (DCX) and doxorubicin (DOX)‐treated cells. Gene expression of BAX, Bcl‐2 and p53 using RT‐PCR on MCF‐7 (a and b), HeLa (c and d) and HepG2 (e and f) cells after treatment with DCX, DOX, RES or their combinations, was assessed. Data expressed as mean ± SD (n = 3).
In HeLa cells, apoptotic gene for BAX protein, was 2‐ to 5‐fold overexpressed in all single treatments. Yet, combination of RES with DCX or DOX did not significantly affect expression level of BAX gene. However, RES combination showed a decreasing trend in the gene BAX signal. Bcl‐2 anti‐apoptotic gene was significantly overexpressed (5‐ to 10‐fold) in all treatments of HeLa cells. Similar to BAX gene expression, Bcl‐2 had a reducing expression trend after combination treatment of RES with either DCX or DOX compared to single exposure. In contrast to MCF‐7 cells, the expression level of p53 was not affected in any single or combined treatments under investigation, compared to untreated cells. This might be attributed to potential equilibrium between total apoptotic and anti‐apoptotic signals in the HeLa cells after treatment with RES, DCX, DOX and their combinations (Fig. 4c,d).
In HepG2 cells, the apoptotic gene BAX was 2‐ to 6‐fold overexpressed in all single and combinations of RES, DCX and DOX. However, RES alone showed the weakest effect in inducing BAX gene signals. Bcl‐2 anti‐apoptotic gene was significantly overexpressed (8‐ to 9‐fold) in DCX and DOX treatments and to a lesser extent (4‐ to 5‐fold) after RES and RES+DCX treatments. Interestingly, RES combination abolished DOX‐induced Bcl‐2 overexpression. The expression level of p53 was not affected in any single treatment under investigation compared to untreated cells. However, RES combinations with DCX or DOX significantly induced p53 gene expression (Fig. 4e,f).
Concentrations of active caspase‐3 were measured to confirm the apoptotic cascade. In MCF‐7 cells, treatment with DCX, DOX, RES and combination of DOX with RES significantly increased caspase‐3 activity by 4‐ to 14‐fold of its original activity. Combination of RES with DCX did not show any increase in caspase‐3 activity relative to the control group (Fig. 5).
Figure 5.
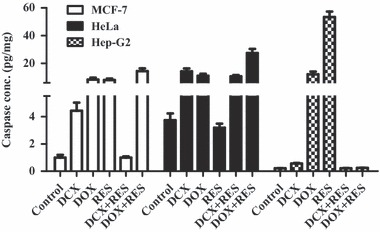
Effect of resveratrol (RES) on apoptotic effective caspase‐3 on docetaxel (DCX)‐ and doxorubicin (DOX)‐treated cells. Caspase‐3 activity was assessed on MCF‐7, HeLa and HepG2 cells after treatment with DCX, DOX, RES or their combinations. Data expressed as mean ± SD (n = 3).
In the HeLa cell line, DCX and DOX increased activity of caspase‐3 by 4‐ and 3‐fold, respectively. RES did not significantly improve the effect of DCX in inducing activity of caspase‐3, whereas it significantly doubled DOX‐induced effect on caspase‐3 induction. RES treatment alone did not have any significant effect on caspase‐3 activity on HeLa cells (Fig. 5).
Liver cancer cells (HepG2) had an elevated level of caspase‐3 activity in response to single treatment of RES and DOX, whereas none of the other treatments induced caspase‐3 activity (Fig. 5).
Effect of RES on efflux pump protein expression and activity
Efflux protein pump, P‐gp encoded by mdr1 gene is known to expel several drug moieties into the extracellular space and to reduce their effective intracellular concentrations to beneath cytotoxic levels. The effect of RES on expression of mdr1 was quantified using real time PCR. In MCF‐7 cells, DOX and DCX significantly downregulated expression level of mdr1. On the other hand, RES did not show any reduction in expression of mdr1. Combinations of DCX and DOX with RES showed mdr1 downregulation to a lesser extent than single DCX or DOX treatments, respectively (Fig. 6a). In HeLa cells, DCX failed to downregulate expression of mdr1. However, DOX, RES and combinations of RES with DCX or DOX reduced expression levels of mdr1 (Fig. 6b). In contrast to both MCF‐7 and HeLa cells, none of the treatments under investigation, as single treatment or in combination with RES, showed any significant change in expression level of mdr1 in HepG2 (Fig. 6c).
Figure 6.
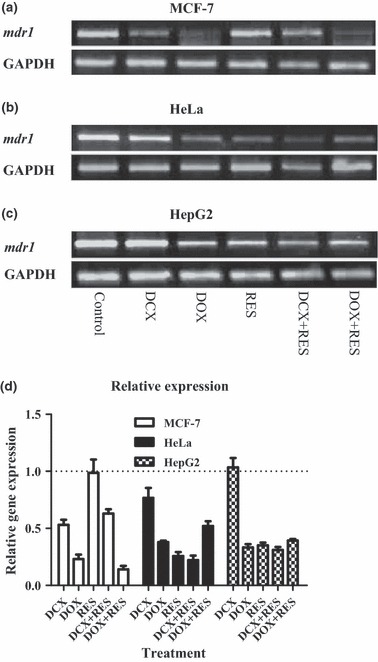
Effect of resveratrol (RES) on expression of ABC‐binding cassette in docetaxel (DCX)‐ and doxorubicin (DOX)‐treated cells. Gene expression of mdr1 using RT‐PCR on MCF‐7 (a), HeLa (b) and HepG2 (c) cells after treatment with DCX, DOX, RES or their combinations was assessed. Expression relative to control group (d). Data expressed as mean ± SD. (n = 3).
Besides expression level of mdr1 gene, P‐gp pump activity was evaluated by determining intracellular concentration of P‐gp substrate (DOX) after exposure to RES and in comparison to positive control P‐gp blocker (VER). RES reduced P‐gp pump activity and significantly increased intracellular effective concentration of the probe used by 15.9%, 119.1% and 40.5% in MCF‐7, HeLa and HepG2 cells, respectively. However, positive control P‐gp blocker, VRP, resulted in increasing intracellular concentrations by 18.5%, 57.3% and 28.1%, respectively (Fig. 7).
Figure 7.
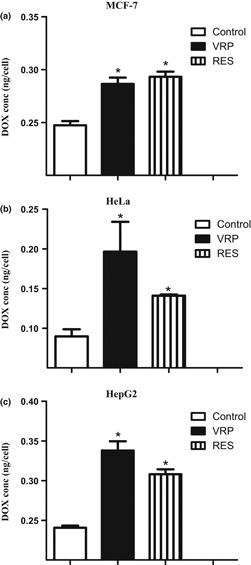
Effect of resveratrol (RES) on efflux pumping activity of P‐gp. Intracellular concentration of P‐gp probe, doxorubicin (DOX), was assessed on MCF‐7 (a), HeLa (b) and HepG2 (c) cells after treatment with RES and VER for 1 hour. Data expressed as mean ± SD (n = 3).
Discussion
Failure to achieve total success in fighting neoplasia has resulted in further research efforts to be made over the past six decades. In several tropical and Middle Eastern countries, using the fountain of domestic natural resources is a very hot issue for onco‐pharmacologists. In this study, we have investigated potential use of RES, a natural polyphenolic compound derived from red grape seeds, as chemosensitizer for docetaxel and doxorubicin, on three different solid tumour cell lines.
Resveratrol has shown diversity of beneficial health effects such as being anti‐oxidant, anti‐aging, cardio‐protective, anti‐inflammatory and neuro‐protective (28). Due to its anti‐oxidant properties, cancer preventive activities of RES have been studied and proven (29). However, the first report mentioning potential anti‐cancer activity of RES was published as late as in 1997 (30, 31). Besides its anti‐leukaemic properties (32), RES has shown promising cytotoxic effects on a wide range of solid tumour cells such as those of breast, liver, prostate, colorectum and pancreas as well as on gliomas (33, 34). In the current study presented here, RES per se had relatively low IC50 against cells of three different solid tumour. Clinically, 0.5–1 g RES administered orally every three hours built up concentrations close to its effective anti‐cancer concentration in tumour and peri‐tumoural regions of colorectal cancer (35).
Many suggested mechanisms have been studied for RES‐induced cytotoxicity; yet, its exact mode of action is still controversial (36). RES‐induced apoptosis has been attributed to interference with the Bcl‐2, BAX, apoptosis cascade (11). Furthermore, p53 and caspase‐3 dependence and independent cell death has been attributed to RES in many solid tumour cell lines (37, 38). According to our observation, RES did not induce BAX gene overexpression; however, it significantly enhanced DCX‐ and DOX‐induced BAX gene expression in MCF‐7 cells. In HeLa and HepG2 cells, BAX gene was substantially high after all treatments. On the other hand, Bcl‐2 was overexpressed in MCF‐7 cells, which might partly explain the elevation in p53 expression profile in RES in combination with DCX and DOX compared to single exposures of them. Strong synergistic interaction of RES with DCX and DOX in MCF‐7 cells cannot be attributed to the afore‐mentioned marginal genetic enhancement.
Resveratrol and other stilbene analogues are known for their anti‐oestrogenic properties, thus useful in treatment of hormone‐dependent tumours such as breast cancer (39). RES inhibits oestrogen‐mediated proliferation of MCF‐7 cells and interferes with oestrogen–DNA adduct formation (40). In addition, RES and its analogues downregulate oestrogen receptors and eventually can behave like selective oestrogen receptor modulators (SERM) (41). Supra‐synergistic interaction of RES with DCX and DOX in MCF‐7 cells might be attributed to these anti‐oestrogenic criteria relative to marginal additive interaction in HepG2 and HeLa cells. Bcl‐2 overexpression is known to counteract RES‐induced cytotoxic effects (42); the substantial Bcl‐2 overexpression in HeLa and HepG2 cells might explain borderline additive interaction characteristics of RES with DCX and DOX. In all cell lines in the present study, combination of RES with DCX was less promising than combination with DOX. Consistent with this finding, taxanes and other spindle toxins such as vinblastin are known to target the cell cycle in metaphase. RES has shown high tendency to abort cell cycle progression in S‐phase, reducing the chance of mitotic toxins reaching their temporal metaphase target (43). Previous studies have shown antagonistic interactions between RES and several mitotic targeting chemotherapeutic agents (44); our observation of negative or absence of interaction between RES and DCX in inducing caspase‐3 activity strongly supports these reports. Other research groups have shown caspase‐9 rather than caspase‐3 dependence of RES‐induced cell death. Furthermore, in a genome‐wide proteomic analysis of RES‐induced apoptosis in Jeko‐1 mantle cell lymphoma, caspase‐3 was not found to be among the key molecules (45). Autophagy via a beclin‐1 pathway has also been attributed to RES and RES combinations with chemotherapeutic agents in apoptotic deficit cells. In other words, RES induces caspase‐dependent and caspase‐independent cell death in MCF‐7 breast cancer cells (46). RES induced autophagy in human U251 glioma cells and autophagy (47).
P‐gp and other efflux proteins have been thoroughly investigated concerning any increase in intracellular level of several chemotherapeutic agents such as DCX and DOX. This might positively influence the pharmacokinetic aspect of these chemotherapeutic agents, building cytotoxic concentrations with relatively lower doses (20). RES has been assumed to possess potential P‐gp inhibiting properties that encourage its combination protocol with various chemotherapeutic agents (48, 49). In the present study, RES per se or in combination with DCX and DOX downregulated mdr1 gene expression (gene coding for P‐gp protein), in all solid tumour cells investigated. In addition and regardless of protein level of P‐gp efflux pump, RES administered at IC50 level inhibited efflux activity in all cell lines tested comparable to VRP after only one hour exposure. RES inhibited efflux activity on the three cell lines up to 18–57%. Other efflux proteins such as MRP1, MRP2 and BCRP have been postulated to affect pharmacokinetics of RES itself (50). Herein, MRP1 and BCRP genes did not have results as significant as those of mdr1 (data not shown). Diversity of the effect of RES against mdr1 expression and P‐gp activity between different cell lines might be attributed to the multifaceted nuclear targets of RES (51).
In terms of RES’s multisystem protective properties, RES may constitute additive value when combined with chemotherapeutic agents with known toxic profiles such as DOX. Cardiac toxicity of DOX though, is the main limiting hurdle for chemotherapeutic course completion and patient compliance, with this therapy (52). RES is assumed to alleviate DOX‐induced cardiac toxicity due to its cardioprotective effects (53). Not only DOX, but a vast majority of chemotherapeutic agents is known to dramatically shoot up oxidative stress and reactive oxygen species. RES uniquely possesses anti‐oxidant and cytotoxic properties in an unexplainable cocktail. This mixed action locates RES to a high rank as candidate adjuvant therapy for anti‐cancer agents.
Conclusion
In conclusion, RES has shown very promising pharmacodynamic and pharmacokinetic interactive characteristics with two important chemotherapeutic agents (DCX and DOX) on three different solid tumour cell lines, nonetheless with DOX in MCF‐7 hormone‐dependent breast cancer cells. Safety profile and antioxidant protective activity of RES will encourage us to recommend it for clinical studies regarding combination of RES with DOX in treatment of breast cancer patients.
Acknowledgements
This research was fully funded by grants from the National CFIDS Foundations Inc, Needham, MA 02492‐3931, USA.
References
- 1. World Health Organization (2008) The Global Burden of Disease: 2004 Update. Geneva: WHO; Available at http://www.who.int/evidence/bod (accessed 6 May 2011). [Google Scholar]
- 2. Mathers CD, Shibuya K, Boschi‐Pinto C, Lopez AD, Murray CJ (2002) Global and regional estimates of cancer mortality and incidence by site: I. Application of regional cancer survival model to estimate cancer mortality distribution by site. BMC Cancer 2, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray CJ, Lopez AD, Black R, Mathers CD, Shibuya K, Ezzati M, et al. (2007) Global burden of disease 2005: call for collaborators. Lancet 370, 109–110. [DOI] [PubMed] [Google Scholar]
- 4. Shibuya K et al. (2002) Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer 2, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dev S (2010) Impact of natural products in modern drug development. Indian J. Exp. Biol. 48, 191–198. [PubMed] [Google Scholar]
- 6. Brisdelli F, D’Andrea G, Bozzi A (2009) Resveratrol: a natural polyphenol with multiple chemopreventive properties. Curr Drug Metab 10, 530–546. [DOI] [PubMed] [Google Scholar]
- 7. Marques FZ, Markus MA, Morris BJ (2009) Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int. J. Biochem. Cell Biol. 41, 2125–2128. [DOI] [PubMed] [Google Scholar]
- 8. Resveratrol Monograph (2010) Resveratrol. Monograph. Altern Med Rev. 15, 152–158. [PubMed] [Google Scholar]
- 9. Han J, Liu W, Bi Y (2008) Advances in resveratrol studies. Sheng Wu Gong Cheng Xue Bao 24, 1851–1859. [PubMed] [Google Scholar]
- 10. Alfaras I, Pèrez M, Juan ME, Merino G, Prieto JG, Planas JM et al. (2010) Involvement of breast cancer resistance protein (BCRP1/ABCG2) in the bioavailability and tissue distribution of trans‐resveratrol in knockout mice. J. Agric. Food. Chem. 58, 4523–4528. [DOI] [PubMed] [Google Scholar]
- 11. Cui J, Sun R, Yu Y, Gou S, Zhao G, Wang C (2010) Antiproliferative effect of resveratrol in pancreatic cancer cells. Phytother Res. 24, 1637–1644. [DOI] [PubMed] [Google Scholar]
- 12. Cui X, Jin Y, Hofseth AB, Pena E, Habiger J, Chumanevich A et al. (2010) Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res (Phila). 3, 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gagliano N, Aldini G, Colombo G, Rossi R, Colombo R, Gioia M et al. (2010) The potential of resveratrol against human gliomas. Anticancer Drugs 21, 140–150. [DOI] [PubMed] [Google Scholar]
- 14. Tredan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J. Natl Cancer Inst. 99, 1441–1454. [DOI] [PubMed] [Google Scholar]
- 15. Hicks KO, Pruijn FB, Sturman JR, Denny WA, Wilson WR (2003) Multicellular resistance to tirapazamine is due to restricted extravascular transport: a pharmacokinetic/pharmacodynamic study in HT29 multicellular layer cultures. Cancer Res. 63, 5970–5977. [PubMed] [Google Scholar]
- 16. Subramanian L, Youssef S, Bhattacharya S, Kenealey J, Polans AS, van Ginkel PR (2010) Resveratrol: challenges in translation to the clinic‐‐a critical discussion. Clin. Cancer Res. 16, 5942–5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minchinton AI, Tannock IF (2006) Drug penetration in solid tumours. Nat. Rev. Cancer 6, 583–592. [DOI] [PubMed] [Google Scholar]
- 18. Tannock IF, Lee CM, Tunggal JK, Cowan DSM, Egorin MJ (2002) Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin. Cancer Res. 8, 878–884. [PubMed] [Google Scholar]
- 19. Zhou SF (2008) Structure, function and regulation of P‐glycoprotein and its clinical relevance in drug disposition. Xenobiotica 38, 802–832. [DOI] [PubMed] [Google Scholar]
- 20. Krishna R, Mayer LD (2000) Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci 11, 265–283. [DOI] [PubMed] [Google Scholar]
- 21. Tunggal JK, Melo T, Ballinger JR, Tannock IF (2000) The influence of expression of P‐glycoprotein on the penetration of anticancer drugs through multicellular layers. Int. J. Cancer 86, 101–107. [DOI] [PubMed] [Google Scholar]
- 22. Mikstacka R, Rimando AM, Ignatowicz E (2010) Antioxidant effect of trans‐resveratrol, pterostilbene, quercetin and their combinations in human erythrocytes in vitro. Plant Foods Hum. Nutr. 65, 57–63. [DOI] [PubMed] [Google Scholar]
- 23. Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D et al. (1990) New colorimetric cytotoxicity assay for anticancer‐drug screening. J. Natl Cancer Inst. 82, 1107–1112. [DOI] [PubMed] [Google Scholar]
- 24. Al‐Abd AM, Lee JH, Kim SY, Kun N, Kuh HJ (2008) Novel application of multicellular layers culture for in situ evaluation of cytotoxicity and penetration of paclitaxel. Cancer Sci. 99, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chou TC, Talalay P (1984) Quantitative analysis of dose‐effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22, 27–55. [DOI] [PubMed] [Google Scholar]
- 26. Longo MC, Berninger MS, Hartley JL (1990) Use of uracil DNA glycosylase to control carry‐over contamination in polymerase chain reactions. Gene 93, 125–128. [DOI] [PubMed] [Google Scholar]
- 27. Neyfakh AA (1988) Use of fluorescent dyes as molecular probes for the study of multidrug resistance. Exp. Cell Res. 174, 168–176. [DOI] [PubMed] [Google Scholar]
- 28. Li L, Henry GE, Seeram NP (2009) Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J. Agric. Food. Chem. 57, 7282–7287. [DOI] [PubMed] [Google Scholar]
- 29. Luther DJ, Ohanyan V, Shamhart PE, Hodnichak CM, Sisakian H, Booth TD et al. (2009) Chemopreventive doses of resveratrol do not produce cardiotoxicity in a rodent model of hepatocellular carcinoma. Invest. New Drugs 29, 380–391. [DOI] [PubMed] [Google Scholar]
- 30. Signorelli P, Ghidoni R (2005) Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem 16, 449–466. [DOI] [PubMed] [Google Scholar]
- 31. Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW et al. (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218–220. [DOI] [PubMed] [Google Scholar]
- 32. Puissant A, Auberger P (2010) AMPK‐ and p62/SQSTM1‐dependent autophagy mediate resveratrol‐induced cell death in chronic myelogenous leukemia. Autophagy 6, 655–657. [DOI] [PubMed] [Google Scholar]
- 33. Choi HY, Chong SA, Nam MJ (2009) Resveratrol induces apoptosis in human SK‐HEP‐1 hepatic cancer cells. Cancer Genomics Proteomics 6, 263–268. [PubMed] [Google Scholar]
- 34. Harper CE, Cook LM, Patel BB, Wang J, Eltoum IA, Arabshahi A et al. (2009) Genistein and resveratrol, alone and in combination, suppress prostate cancer in SV‐40 tag rats. Prostate 69, 1668–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel KR, Brown VA, Jones DJL, Britton RG, Hemingway D, Miller AS et al. (2011) Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 70, 7392–7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oi N, Jeong C‐H, Nadas J, Cho Y‐Y, Pugliese A, Bode AM et al. (2010) Resveratrol, a red wine polyphenol, suppresses pancreatic cancer by inhibiting leukotriene a4 hydrolase. Cancer Res. 70, 9755–9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chow SE, Wang JS, Chuang SF, Chang YL, Chu WK, Chen WS et al. (2010) Resveratrol‐induced p53‐independent apoptosis of human nasopharyngeal carcinoma cells is correlated with the downregulation of DeltaNp63. Cancer Gene Ther. 17, 872–882. [DOI] [PubMed] [Google Scholar]
- 38. Gatouillat G, Balasse E, Joseph‐Pietras D, Morjani H, Madoulet C (2010) Resveratrol induces cell‐cycle disruption and apoptosis in chemoresistant B16 melanoma. J. Cell. Biochem. 110, 893–902. [DOI] [PubMed] [Google Scholar]
- 39. Papoutsis AJ, Lamore SD, Wondrak GT, Selmin OI, Romagnolo DF (2010) Resveratrol prevents epigenetic silencing of BRCA‐1 by the aromatic hydrocarbon receptor in human breast cancer cells. J. Nutr. 140, 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zahid M, Saeed M, Beseler C, Rogan EG, Cavalieri EL (2010) Resveratrol and N‐acetylcysteine block the cancer‐initiating step in MCF‐10F cells. Free Radic. Biol. Med. 50, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boehme K, Simon S, Mueller SO (2009) Gene expression profiling in Ishikawa cells: a fingerprint for estrogen active compounds. Toxicol. Appl. Pharmacol. 236, 85–96. [DOI] [PubMed] [Google Scholar]
- 42. Jeong SH, Jo WS, Song S, Hongsuk S, Seol S‐Y, Leem S‐H et al. (2009) A novel resveratrol derivative, HS1793, overcomes the resistance conferred by Bcl‐2 in human leukemic U937 cells. Biochem. Pharmacol. 77, 1337–1347. [DOI] [PubMed] [Google Scholar]
- 43. Zhou R, Fukui M, Choi HJ, Zhu BT (2009) Induction of a reversible, non‐cytotoxic S‐phase delay by resveratrol: implications for a mechanism of lifespan prolongation and cancer protection. Br. J. Pharmacol. 158, 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fukui M, Yamabe N, Zhu BT (2010) Resveratrol attenuates the anticancer efficacy of paclitaxel in human breast cancer cells in vitro and in vivo . Eur. J. Cancer 46, 1882–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cecconi D, Zamò A, Parisi A, Bianchi E, Parolini C, Timperio AM, et al. (2008) Induction of apoptosis in Jeko‐1 mantle cell lymphoma cell line by resveratrol: a proteomic analysis. J. Proteome Res. 7, 2670–2680. [DOI] [PubMed] [Google Scholar]
- 46. Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R (2008) Role of non‐canonical Beclin 1‐independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 15, 1318–1329. [DOI] [PubMed] [Google Scholar]
- 47. Li J, Qin Z, Liang Z (2009) The prosurvival role of autophagy in Resveratrol‐induced cytotoxicity in human U251 glioma cells. BMC Cancer 9, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee SC, Chan JY, Pervaiz S (2010) Spontaneous and 5‐fluorouracil‐induced centrosome amplification lowers the threshold to resveratrol‐evoked apoptosis in colon cancer cells. Cancer Lett. 288, 36–41. [DOI] [PubMed] [Google Scholar]
- 49. Osmond GW, Augustine CK, Zipfel PA, Padussis J, Tyler DS (2010) Enhancing melanoma treatment with resveratrol. J. Surg. Res. doi:10.1016/j.jss.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 50. van de Wetering K, Burkon A, Feddama W, Bot A, de Jonge H, Somoza V et al. (2009) Intestinal breast cancer resistance protein (BCRP)/Bcrp1 and multidrug resistance protein 3 (MRP3)/Mrp3 are involved in the pharmacokinetics of resveratrol. Mol. Pharmacol. 75, 876–885. [DOI] [PubMed] [Google Scholar]
- 51. Kovacic P, Somanathan R (2010) Multifaceted approach to resveratrol bioactivity: focus on antioxidant action, cell signaling and safety. Oxid Med Cell Longev. 3, 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Licata S, Saponiero A, Mordenta A, Minotti (2000) Doxorubicin metabolism and toxicity in human myocardium: role of cytoplasmic deglycosidation and carbonyl reduction. Chem. Res. Toxicol. 13, 414–420. [DOI] [PubMed] [Google Scholar]
- 53. Das M, Das DK (2010) Resveratrol and cardiovascular health. Mol. Aspects Med. 31, 503–512. [DOI] [PubMed] [Google Scholar]


