Abstract
Objectives
Icariin, a prenylated flavonol glycoside isolated from traditional Chinese medicinal herb of the genus Epimedium, has been demonstrated to be a potential alternative therapy for osteoporosis, and its action mechanism so far has been mainly attributed to its phytoestrogenic property. As blood supply to bone is considerably reduced with ageing and by the menopause, we hypothesized that icariin treatment would reduce bone loss by preventing ischaemia‐induced hypoxic damages to bone.
Materials and methods
To investigate effects of icariin treatment on cultured rat calvarial osteoblasts exposed to hypoxic conditions (2% oxygen).
Results
Compared to normoxic control, cell viability decreased with time to 50% by 48 h in the hypoxic group, and icariin attenuated the reduction, dose dependently, with 10−6 and 10−5 m concentrations showing significant protective effects. Icariin also inhibited increase of lactate dehydrogenase activity in culture media. Measurements on oxidative stress, cell cycling and cell survival indicated that icariin protected osteoblasts by reducing production of reactive oxygen species and malondialdehyde, increasing superoxide dismutase activity, arresting the cell cycle and inhibiting apoptosis. Icariin also preserved osteogenic differentiation potential of the hypoxic cells in a dose‐dependent manner, compared to the hypoxia alone group, as revealed by increased levels of RUNX‐2, OSX and BMP‐2 gene expression, alkaline phosphatase activity, and formation of mineralized nodules.
Conclusions
Our results demonstrated that icariin attenuated oxidative stress and apoptosis and preserved viability and osteogenic potential of osteoblasts exposed to hypoxia in vitro, and suggested that its anti‐osteoporotic effect may be attributed to its anti‐hypoxic activity and phytoestrogenic properties.
Introduction
With contempoary demographic changes and longer life expectancy, osteoporosis is becoming increasingly prevalent. In particular, post‐menopausal osteoporosis affects half of women over the age of 60. It has long been considered that post‐menopausal oestrogen deficiency is the pathogenic cause for this major form of osteoporosis, and the positive clinical effects of oestrogen replacement in preventing bone loss, provides strong support for this notion. Thus, this oestrogen‐centric paradigm of osteoporosis has led to an enthusiastic search for compounds (either from natural sources or by chemical synthesis) with oestrogenic activity 1. However, oestrogen deficiency may not be the only pathogenic mechanism for osteoporosis. It has been reported that bone loss begins immediately after attainment of peak bone mass, which is long before any decline in sex steroid production in both men and women 2. In addition, volumetric bone mineral density analyses of the tibia and spine, have demonstrated that there is substantial trabecular bone loss during sex steroid sufficiency 3. These findings have led us to believe that non‐oestrogen‐related pathogenic mechanisms for osteoporosis exist, and that advances in understanding them will help in development of new anti‐osteoporosis drugs.
Recently, increasing evidence has shown that osteoporosis is associated with reduced blood supply to bones 4, 5. During senescence, bone marrow becomes ischaemic with diminished medullary blood supply to the cortex 6. MRI perfusion imaging studies indicate that vertebral marrow perfusion is reduced in older subjects 7. Consistently, women with osteoporosis, or have low bone mass, have significantly impaired endothelial function compared to those with normal bone mineral density 8. Similarly, in experimental‐aged male rats, endothelium‐dependent vasodilatation in femoral nutrient arteries is in the order of 20–25% lower than in young adult controls 9. Ding et al. found that blood supply to the tibial metaphysis of rats is significantly reduced after ovariectomy (OVX), indicating that the oestrogen deficiency‐induced osteoporosis seemed to be related to a reduced blood supply to the bone 10. Similarly, glucocorticoid treatment‐induced secondary osteoporosis has also been found to share this bone ischaemic condition. After 28 days prednisolone administration to 8 month old female mice, vascular area per cancellous bone tissue area was reduced by an average of 74.5% 11. Taken together, these previous studies have demonstrated the association between osteoporosis and reduced blood supply to (or hypoxia in) bones; we thus hypothesize that some compounds which have anti‐osteoporosis activity may also act through their protective effect against hypoxic damage to bone‐forming cells, osteoblasts.
The genus Epimedium is the one that most frequently provides medicinal herbs used to treat bone fractures and osteoporosis, in traditional Chinese medicine 12. It has now been demonstrated that icariin, a prenylated flavonol glycoside contained in the herb, is highly related to its osteotrophic therapeutic effects. In a rat OVX model of oestrogen deficiency‐induced osteoporosis, icariin has been found to be effective in preventing bone loss, suggesting its potential for treating oestrogen deficiency‐induced osteoporosis 13. Similarly, icariin has been found to suppress loss of bone mass and strength, in the distal femur, and to increase mRNA expression ratio of OPG/RANKL in tibia, of mice following OVX 14. Li et al. recently reported that icariin prevented bone loss with comparable potency to 17β‐estradiol, and that it lowered OVX‐induced marrow adipogenesis 15. A randomized, double‐blind, placebo‐controlled clinical trial indicated that Epimedium‐derived phytoestrogen flavonoids (mainly containing icariin) exerted significant effects on bone turnover markers, in post‐menopausal women, compared to placebo controls 16. These animal and clinical studies suggest that icariin can prevent oestrogen deficiency‐induced bone loss without inducing uterotrophic effects, and that icariin could potentially be used as an alternative regimen for management of post‐menopausal osteoporosis.
However, mechanisms of icariin action in preventing bone loss are still elusive. While icariin has been found to promote osteoblast proliferation and mineralization, and to inhibit osteoclast formation and bone resorption, these effects can be attenuated or blocked by oestrogen receptor antagonist ICI 182780 17, 18. The findings indicate that these activities of icariin are oestrogen receptor (ER)‐dependent – similar to those of genistein, another well‐known phytoestrogen (isolated from soybeans). On the other hand, icariin has been reported to have greater ability than genistein to improve osteoblast differentiation and osteogenic function, although it has a substantially lower affinity for ER than genistein 19, 20. It is therefore suggested that there exists a non‐oestrogenic mechanism for icariin osteogenic action which needs to be clarified. In the present study, our hypothesis was that icariin (apart from being a phytoestrogen), protects bone from hypoxic damage caused by reduced blood supply. To verify this, we investigated effects and icariin mechanisms of action, on cultured osteoblasts maintained under hypoxia.
Materials and methods
Reagents
Icariin (purity ≥99%) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). α‐minimal essential medium (α‐MEM) was obtained from Invitrogen (Auckland, UK) and foetal bovine serum (FBS) was the product of Lanzhou National Hycolone Bio‐Engineering Co (Lanzhou, China). Collagenase II and trypsin were purchased from Gibco BRL (Gaithersburg, MD, USA) and RNase A, MTT [3‐(4,5‐dimethylthiazol‐2‐yl) ‐2,5‐diphenyl tetrazolium bromide], Triton X‐100, β‐glycerophosphate, dexamethasone, ASAP (ascorbic acid 2‐phosphate), DMSO (dimethyl sulfoxide) and alizarin red S were all from Sigma China (Shanghai, China). All other chemicals were of analytical grade.
Isolation and culture of neonatal rat calvarial osteoblasts
Neonatal rat calvarial osteoblasts (ROBs) were isolated and cultured as previously reported 19. Calvarias were dissected aseptically from 10 newborn Sprague–Dawley (SD) rats obtained from the Laboratory Animal Center (Lanzhou University, Lanzhou, China). Animal care and experiments were approved and conducted in accordance with accepted standards of animal care and use as deemed appropriate by the Animal Care and Use Committee of Lanzhou University, Lanzhou, China. Frontal and parietal bones were cleaned of adhering soft tissues and cut into pieces of approximately 1 mm3. They were digested at 37 °C for 20 min by shaking with enzymatic solution containing 1 mg/ml collagenase II and 0.5 mg/ml trypsin in α‐MEM. This procedure was repeated three times, then pieces were further digested with 1 mg/ml collagenase II, twice each, for 60 min. The two supernatants were pooled and centrifuged at 700 g for 5 min for cell collection. Collected cells were suspended in α‐MEM containing 10% FBS, plated in 100 mm tissue culture dishes (Nunc, Roskilde, Denmark), and cultured in the above medium supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C in 5% CO2 under humid conditins, culture medium being changed every 3 days. After reaching confluence, cells were detached by treatment with 1 mM EDTA and 0.25% trypsin, and subcultured as described below, for the subsequent different assays. ROBs at Passage 1–3 were used in the following procedures.
Hypoxia intervention and icariin treatment
A specially designed cell incubator was used to establish the humidified hypoxic conditions, 30 × 30 × 20 cm3 in size, and was placed inside a conventional CO2 incubator (Thermo Revco, Ashevill, NC, USA). Hypoxia was created by infusion of 5% CO2 and 95% nitrogen gas mixture; oxygen level was maintained at 2% and monitored, using an oxygen detector. Normal atmospheric pressure was ensured by monitoring gas infusion with a standardized pressure gauge. For the normal oxygen (normoxic) control (20% oxygen), cells were incubated in a normal incubator with 5% CO2 and 95% air at 37 °C, with humidification.
Rat osteoblasts were subcultured in 96‐well culture plates (Corning, Union City, CA, USA) at 5 × 103 cells/well. When they reached 70% confluence, icariin was added at 10−7, 10−6, 10−5 or 10−4 m concentrations (n = 6 wells for each concentration). An equal volume of vehicle (medium with 1 μl DMSO/ml) was added to controls, including normoxia control (NC) and hypoxia control (HC). The NC group was incubated under normal conditions, while the HC group and icariin‐supplemented groups were incubated in the hypoxia incubator.
Cell viability assay
Treatment effects on cell viability were assessed by MTT assay at 12, 24, 36 and 48 h after treatment as previously described 19. Briefly, 10 μl of 5 mg/ml MTT solution was added to each well and incubated for 4 h, followed by addition of 100 μl of DMSO, to dissolve the dark blue crystals. Plates were read on a micro‐plate reader at 570 nm wavelength, with 630 nm used as reference wavelength for calibration. Dosage effects on cell viability were determined by comparing absorbance readings of cultures treated with different icariin concentrations.
Assays for LDH release, MDA level and SOD activity
Release of lactate dehydrogenase (LDH), used as a marker of cell membrane permeability and cell injury, was estimated by measuring LDH levels in culture conditioned medium as previously described 21; it was expressed as U/ml. Intracellular levels of malondialdehyde (MDA) and activity of superoxide dismutase (SOD), both biomarkers of oxidative stress, were measured using commercial kits as instructed (Nanjing Jiancheng Bioengineering Ltd, Nanjing, China). To normalize data with protein concentration in each sample, protein concentrations of the conditioned media were determined using a BCA protein assay kit (Thermo, Rockford, IL, USA). MDA levels were expressed as nmol/mg protein and SOD activity as U/mg protein.
Evaluation of intracellular ROS levels
Levels of intracellular reactive oxygen species (ROS) were assessed by fluorescent probe 2′,7′‐dichlorodihydrofluorescein‐diacetate (DCFH‐DA), using a ROS assay kit (KenGen Biotech, Beijing, China). DCFH‐DA is easily transported across the cell membrane and deacetylated by esterases to form DCFH, which is oxidized by ROS to form DCF; this is highly fluorescent. Intracellular ROS levels were examined after 36 h hypoxic culture. Fluorescent signal of images was recorded by fluorescence microscope photography and signal intensity was measured as integrated optical density by Image‐Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA).
Analyses of cell cycle and apoptosis
To analyse the effects of treatment on cell cycling, cell cycle assays were performed after 36 h hypoxic culture. Cells were collected from cultures, resuspended (1 × 106 cells/ml) and fixed in 70% ice‐cold ethanol for 30 min, followed by washing and resuspending in 500 μl PBS containing 1 mg/ml DNase‐free RNase A. After 30‐min incubation, pyridine iodide (PI, 0.05 mg/ml) was added to the solution followed by incubation for an additional 15 min, in the dark. Fluorescence of cells stained by PI was measured using by flow cytometry (Beckman Coulter, Fullerton, CA, USA). Cell subpopulations in G0/G1, S and G2/M phases were calculated by gating analysis, based on differences in DNA content.
To analyse effects of treatment on apoptosis, the Annexin‐V FITC/PI kit (Molecular Probes, Eugene, OR, USA) was used. Cells retained under hypoxia for 36 h were trypsinized, washed in PBS, resuspended (1 × 106 cells/ml), and examined by flow cytometry as described above. More than 99% non‐specific fluorescence was regarded as background and shown with a 2D point lattice map. By double variance scatterplot, left inferior quadrant represented living cells (FITC−/PI−), right inferior quadrant represented early apoptotic cells (FITC+/PI−), and right superior quadrant represented late apoptotic cells (FITC+/PI+).
Immunohistochemical staining for PCNA
To analyse treatment effects on cell proliferative activity, expression of proliferating cell nuclear antigen (PCNA) was examined immunohistochemically after 36 h hypoxic culture. Cells grown on coverslips were fixed in 3.7% formaldehyde for 10 min, permeabilized with 0.1% Triton X‐100 and blocked with 5% bovine serum. To detect PCNA, primary antibody, polyclonal rabbit anti‐PCNA (at 1:400) and secondary antibody, biotin‐conjugated goat anti‐rabbit IgG (at 1:200) (Boster Biological Technology, Wuhan, China) were used. PCNA protein was revealed using avidin–biotin horseradish peroxidase (HRP) solution (SABC kit) and 3,3‐diaminobenzidine (DAB kit; Boster Biological Technology). For negative controls, primary and/or secondary antibodies were omitted. Coverslips were mounted on slides and stained cells were photographed by light microscopy; signal intensity was measured as integrated optical density by Image‐Pro Plus 6.0.
Osteogenic differentiation induction culture and assay
For osteogenic differentiation induction, ROBs were subcultured in 60 mm dishes (Nunc) at 5 × 103 cells/cm2. At confluence, original medium was replaced with osteogenic medium containing 10−8 m dexamethasone, 10 mm β‐glycerophosphate and 50 μg/ml ASAP. After 3 days, icariin was added at 10−7, 10−6 and 10−5 m respectively, and equal volume of vehicle (1 μl DMSO/ml medium) was added for the NC and the HC. Cells were transferred to hypoxic conditions, as described above, for 12, 24, 36 and 48 h respectively, and then returned to normal (day 0), from which time osteogenic differentiation induction culture continued, for different assays described below, under normal oxygen conditions and without additional icariin treatment.
Cell ALP activity, an early marker of osteogenic differentiation, was measured on day 6 using a commercial kit (Nanjing Jiancheng Bioengineering Ltd), normalized to cell protein concentration determined as described above, and expressed as nmol phenol/15 min/mg protein as previously described 19. Mineralized nodules were stained on day 12 as previously reported 19. Briefly, cultures were fixed in 3.7% formaldehyde for 10 min and stained with 0.1% alizarin red for 1 h. Numbers and total areas of red nodules were measured using Image‐Pro Plus 6.0.
Real‐time PCR quantification of gene expression
As a measure for characterization of molecular mechanisms for the treatment effects, relative levels of the enzyme caspase‐3 mRNA, apoptosis effector, and anti‐apoptotic gene BCL‐2 expression, were quantitatively determined after 12, 24, 36 and 48 h hypoxia. Expression levels of key osteogenic bone morphogenic protein (BMP‐2) and osteogenic transcription factors RUNX‐2 and Osterix (OSX) were determined after 12, 24, 36 or 48 h osteogenic differentiation culture under hypoxia. Total RNA was extracted from the cells using RNAiso Kit (Takara Biotechnology, Dalian, China). Single‐stranded cDNA was synthesized from 1 μg total RNA with a Primescrip™ RT reagent kit (Takara Biotechnology). Real‐time PCR was performed using 7300 Real Time PCR System (Applied Biosystems, Singapore) in 25 μl reaction volume containing SYBR® Premix Ex Taq™ II (Takara Biotechnology), specific primers (Table 1), and 2 μl cDNA as we have previously described 22. Optimal oligonucleotide primers were designed using Primer 5.0 software (Applied Biosystem, Foster City, CA, USA), based on published rat cDNA sequences. GAPDH was used as internal control gene. All reactions were performed in triplicate, and results were calculated using the ΔΔCt method after calibration with GAPDH expression.
Table 1.
Rat primer sequences for real‐time RT‐PCR analyses
| Gene | GenBank No. | Primer sequence | Product length (bp) |
|---|---|---|---|
| BCL‐2 | L_14680.1 |
Forward: 5′‐ATGCGACCTCTGTTTGATTTCTC‐3′ Reverse: 5′‐TCATATTTGTTTGGGGCAGGT‐3′ |
135 |
| CASP‐3 | NM_012922.2 |
Forward: 5′‐GAGACAGACAGTGGAACTGACGATG‐3′ Reverse: 5′‐GGCGCAAAGTGACTGGATGA‐3′ |
147 |
| BMP‐2 | NM_017178 |
Forward: 5′‐ACCGTGCTCAGCT TCCATCAC‐3′ Reverse: 5′‐TTCCTGCATTTGTTCCCGAAA‐3′ |
156 |
| RUNX‐2 | NM_053470.1 |
Forward: 5′‐GCACCCAGCCCATAATAGA‐3′ Reverse: 5′‐TTGGAGCAAGGAGAACCC‐3′ |
165 |
| OSX | NM_001037632.1 |
Forward: 5′‐GCCTACTTACCCGTCTGACTTT‐3′ Reverse: 5′‐GCCCACTATTGCCAACTGC‐3′ |
131 |
| GAPDH | NM_017008.3 |
Forward: 5′‐ TATCGGACGCCTGGTTAC‐3′ Reverse: 5′‐ CTGTGCCGTTGAACTTGC‐3′ |
140 |
Statistical analyses
All data were obtained from six or triplicate parallel experiments and expressed as means ± SD. Statistical analyses were carried out using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA); significance levels were determined by ANOVA. Multiple comparisons were made using the Tukey method; values of P < 0.05 were taken as significant difference and two‐tailed P‐values are presented.
Results
Icariin preserved osteoblast viability under hypoxia
Effects of hypoxic culture conditions and icariin treatment, on viability of rat calvarial osteoblasts, were examined. Viability of the HC group was significantly reduced compared to the NC group after 12 h (P < 0.05), and further decreased over time (Fig. 1a). After 48 h, the HC group was only half as viable as the NC group (P < 0.01). Icariin supplementation attenuated the reduction in a concentration‐dependent manner. Viability of 10−7 m icariin group was always higher than that of the HC group after 12 h although not statistically significant. Viability of the 10−6 m icariin group was significantly higher than that of the HC group after 36 or 48 h (P < 0.05). The 10−5 m icariin group also had higher viability than the HC group after 24 h (P < 0.05), which became much higher after 36 and 48 h (P < 0.01). However, the 10−4 m icariin group had the lowest viability and was even lower than that of the HC group (P < 0.01), indicating that icariin at this concentration was toxic to osteoblasts, and effects of icariin at this high dose were not examined in further experiments.
Figure 1.

Cell viability and oxidative stress of osteoblasts after hypoxic treatment with or without icariin presence, over different times. The subcultured rat calvarial osteoblasts grown to 70% confluence were supplemented with 10−7, 10−6, 10−5 and 10−4 m icariin and switched to hypoxia (2% oxygen) for 12, 24, 36 and 48 h respectively. The group supplemented with vehicle (1 μl/l DMSO) under normoxia, was used as normal normoxia control (NC). The group with the vehicle under hypoxia was hypoxia control (HC). Cell viability was assayed by the MTT method (a). lactate dehydrogenase activity in culture media (lactate dehydrogenase release) was used as an indicator for degree of cell injury (b). Intracellular MDA content (c) and superoxide dismutase activity (d) represent oxidative and anti‐oxidative levels, respectively. Values for cell viability were means ± SD from six replicate cultures, and the others are means ± SD from triplicate cultures. *P < 0.05, **P < 0.01 versus NC group; #P < 0.05, ##P < 0.01 versus HC group.
Damage caused by hypoxia to the osteoblasts was also evaluated by LDH release, the gold standard marker for cell membrane rupture. As shown in Fig. 1b, LDH level in medium of the HC group was higher than that of the NC group after 12 h (P < 0.05), which continued to increase over time and became four times higher than that of the NC group by 48 h (P < 0.01). Icariin inhibited LDH release in a concentration‐dependent manner. LDH release of the 10−7 m icariin group was always lower than that of the HC group despite not reaching statistical significance. LDH release of the 10−6 m icariin group was significantly lower than that of the HC group after 36 or 48 h (P < 0.05) and LDH release of the 10−5 m icariin group began to be lower than that of the HC group after 24 h (P < 0.05); it became much lower after 36 or 48 h (P < 0.01).
Icariin attenuated hypoxia‐induced oxidative damage
As oxidative stress is a major contributing factor to hypoxic damage, treatment effects were assessed on levels of the oxidative stress maker MDA, anti‐oxidative enzyme SOD and ROS. As shown in Fig. 1c, intracellular MDA content in the HC group increased with a similar tendency to that of LDH release (Fig. 1b), and was attenuated in icariin‐supplemented groups in a similar manner as LDH release. Icariin at 10−5 m yielded earliest (24 h) and highest activity in inhibiting production of MDA (P < 0.05, P < 0.01 and P < 0.01 versus HC groups respectively at 24, 36 and 48 h). Changes in activity of SOD had an opposite tendency as MDA, being reduced in the HC group and increased in the icariin‐supplemented groups, in a concentration‐dependent manner (Fig. 1d). The 10−6 m icariin group had a significantly higher SOD activity than the HC group after 36 h (P < 0.05), and differences became more obvious after 48 h (P < 0.01).
Intracellular ROS level was visually evaluated by fluorescence staining with a ROS probe, after 36 h hypoxic culture. While there only few osteoblasts were stained positively in the NC group, most in the HC group were positively stained (Fig. 2a). Icariin treatment reduced ROS fluorescence staining, with number of positively stained cells and fluorescence signal intensity in icariin‐supplemented groups being reduced in a concentration‐dependent manner (Fig. 2a,b). The 10−7 m icariin group had lower fluorescence signal intensity than the HC group despite not reaching a statistically significant level. The 10−6 m icariin group had significantly lower signal intensity than the HC group (P < 0.05). Number of positive cells in the 10−5 m icariin group was further reduced and the cells more resembled those of the NC group.
Figure 2.

Intracellular reactive oxygen species ( ROS ) stained by fluorescence probe DCFH ‐ DA after hypoxic treatment, with or without icariin for 36 h (a), scale bar = 100 μm. Images of fluorescent signals and signal intensities were analysed by Image‐Pro Plus 6.0 software (b). Results are means ± SD from triplicate cultures. **P < 0.01 versus normoxia control (NC); #P < 0.05, ##P < 0.01 versus hypoxia control (HC).
Icariin inhibited osteoblast proliferation under hypoxia
To investigate whether reduction of osteoblast viability was related to changes in cell proliferation, we analysed effects of hypoxia on cell cycling after 36 h hypoxic culture. Flow cytometric data indicated that hypoxia arrested cell cycling in the G0/G1 phase. While average percentages of cells in G0/G1, S and G2/M phases in the NC group were 65.7%, 20.2% and 14.1% respectively, they were 84.6%, 8.21% and 7.22% in the HC group. Interestingly, icariin did not lessen, but intensified cell cycle arrest, with average percentages of cells at the three phases in the 10−6 m icariin group being 90.2%, 3.43% and 6.32%, and in the 10−5 m icariin group 92.4%, 1.56% and 6.05% respectively. These data indicate that hypoxia retarded proliferation of osteoblasts, and icariin supplementation intensified the retardation in a concentration‐dependent manner.
To verify the inhibitory effect of icariin on proliferation of osteoblasts exposed to hypoxia, we analysed expression of the proliferation marker PCNA, by immunohistochemical staining, after 36 h hypoxic culture. As shown in Fig. 3a and 3b, expression level of PCNA in the HC group was clearly lower than that of the NC group (P < 0.01), and was further reduced in the icariin‐supplemented groups, reaching statistical significance in the 10−6 m icariin group (P < 0.05) and 10−5 m icariin group (P < 0.01).
Figure 3.

Effects of hypoxic treatment with or without icariin, on proliferating cell nuclear antigen ( PCNA ) expression of osteoblasts. PCNA expression was examined by immunohistochemical staining (a), scale bar = 50 μm. Positive signal intensity was analysed by Image‐Pro Plus 6.0 software (b). Results are means ± SD from triplicate cultures. **P < 0.01 versus normoxia control (NC); #P < 0.05, ##P < 0.01 versus hypoxia control (HC).
Icariin inhibited hypoxia‐induced osteoblast apoptosis
To examine whether the hypoxia‐induced reduction in cell viability was partly due to apoptosis, we performed an apoptosis assay after 36 h hypoxic culture. Flow cytometric analyses of expression of apoptotic marker annexin‐V showed that while there was only 1% of apoptotic cells in the NC group, 25% were apoptotic in the HC group (P < 0.01) (Fig. 4a,b). This was confirmed by expression level changes of CASP‐3, an apoptosis effector gene, which showed hypoxia‐induced increases reaching its peak value by 24 h hypoxic culture (all at P < 0.01 for all time points when compared to NC group), and was in the order of 16 times higher in the HC group over that of NC group (Fig. 4c). Expression level of BCL‐2, an anti‐apoptotic gene, was also significantly increased in the HC group (all at P < 0.01 for all time points) compared to the NC group (Fig. 4d).
Figure 4.

Effects of hypoxic treatment with or without icariin on osteoblast apoptosis and associated gene expression. Flow cytometric analysis of apoptosis (a), showing that hypoxic treatment sharply increased percentage of apoptotic cells, while icariin supplements inhibited increase in a dose‐dependent manner (b). Expression levels of pro‐apoptotic gene caspase‐3 (c) and anti‐apoptotic gene Bcl‐2 (d). Data are means ± SD from triplicate cultures. **P < 0.01 versus normoxia control (NC); #P < 0.05, ##P < 0.01 versus hypoxia control (HC).
Icariin reduced the extent of hypoxia‐induced osteoblast apoptosis in a concentration‐dependent manner. Average levels of apoptosis in the three icariin‐supplemented groups were 23.9%, 21.7% and 16.2% respectively (Fig. 4a,b), with differences reaching statistical significance for the 10−6 m icariin group (P < 0.05) and the 10−5 m icariin group (P < 0.01) compared to the HC group. Consistently, icariin also reduced mRNA expression level of CASP‐3 in a similar tendency as with level of apoptosis (Fig. 4c). Expression level of CASP‐3 in the 10−6 m icariin group was lower than that of the HC group after 12 or 24 h (P < 0.05), and was much lower in the 10−5 m icariin group (P < 0.05). After 36 and 48 h, CASP‐3 levels in icariin‐supplemented groups were still lower than those of the HC group despite not reaching statistical significance.
Expression of BCL‐2 reached its peak level after 36 h hypoxic culture (Fig. 4d), 12 h later than peak expression of CASP‐3. Icariin increased mRNA expression levels of BCL‐2 in a concentration‐dependent manner in the hypoxic groups. The 10−7 m icariin group had higher levels of BCL‐2 than the HC group from 24 h to 48 h, despite differences not reaching a statistically significant level. The 10−6 m icariin group always had higher levels of BCL‐2 than the HC group, reaching statistical significance after 36 h (P < 0.05). Expression of BCL‐2 of the 10−5 m icariin group was also higher than that of the HC group after 24 h (P < 0.05) or 36 h (P < 0.01).
Icariin preserved osteogenic differentiation potential of osteoblasts exposed to hypoxia
To evaluate efficacy of icariin in maintaining osteogenic differentiation potential of osteoblasts exposed to hypoxia, osteoblasts were firstly cultured in osteogenic medium for 3 days under normal oxygen condition, then supplemented with different concentrations of icariin, and cultured under hypoxia (except the NC group) for 12, 24, 36 and 48 h respectively. Immediately after hypoxia, treatment effects on levels of mRNA expression of osteogenic growth factor BMP‐2 and the critical osteogenic transcription factors RUNX‐2 and OSX, were examined. BMP‐2 expression in the NC group peaked after 24 h, and then decreased gradually (Fig. 5a). Levels of BMP‐2 expression in the HC group were much lower than those of the NC group after 12, 24 and 36 h (P < 0.01), and after 48 h (P < 0.05). Icariin supplementation attenuated the reduction in BMP‐2 expression in a concentration‐dependent manner, the highest inhibitory effect being observed after 24 h in the 10−6 m (P < 0.05) and 10−5 m (P < 0.01) icariin groups. The 10−5 m icariin group still had higher BMP‐2 expression level than the HC group after 36 h (P < 0.05).
Figure 5.
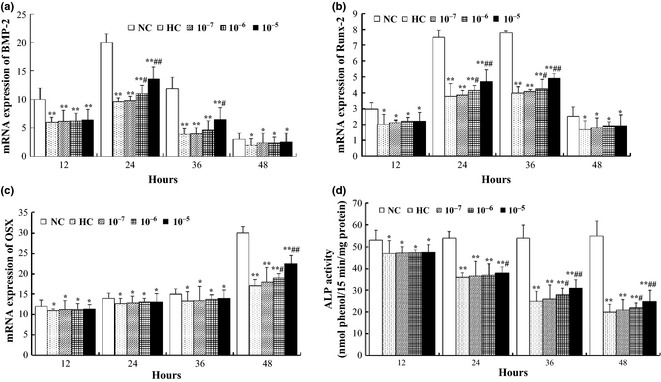
Effects of hypoxic treatment with or without icariin, on osteoblast differentiation potential as analysed by expression levels of genes associated with osteogenesis and alkaline phosphatase ( ALP ) activity. Expression levels of growth factor BMP‐2 (a), transcription factors Runx‐2 (b) and OSX (c). Intracellular ALP activities were measured after 6 days (d). Results are means ± SD from triplicate cultures. *P < 0.05, **P < 0.01 versus normoxia control (NC); #P < 0.05, ##P < 0.01 versus hypoxia control (HC).
Expression of RUNX‐2 under normal conditions began to increase at 12 h and peaked by 36 h (Fig. 5d). However, RUNX‐2 expression was reduced in the hypoxic groups with a similar tendency to that of BMP‐2. Icariin at 10−6 m significantly inhibited reduction in RUNX‐2 expression after 24 and 36 h (P < 0.05), and icariin at 10−5 m showed more obvious inhibitory effects at the same time points (P < 0.01). OSX, a transcription factor downstream of RUNX‐2, was expressed at low levels until 48 h, at which time it was over twice that at 12 h. By 48 h, the HC group significantly inhibited OSX expression compared to the NC group (in the order of 45% reduction, P < 0.01, Fig. 4c), and icariin supplementation had some rescuing effect particularly at 10−6 m (P < 0.05), and more so at 10−5 m (P < 0.01) compared to the HC group.
To further evaluate the protective effect of icariin over the longer term, the osteogenic induction culture was continued after the hypoxic treatment, and without further icariin supplementation. After 6 days, alkaline phosphatase (ALP) activity, an important marker of osteogenic differentiation, was measured and compared between the different groups. As shown in Fig. 5d, the longer the osteoblasts were exposed to hypoxia, the more obvious was the reduction in ALP activity. When exposure time was 12 h, there was a significant reduction in ALP activity (P < 0.05) and icariin supplementation did not show any protective effect. Exposure for 24 h to hypoxia induced a more obvious reduction in ALP activity (P < 0.01) compared to the NC control, and 10−5 m icariin was found to have a protective effect compared to the HC control (P < 0.05). Even more obviously, exposure for 36 or 48 h to hypoxia caused >50% reduction in ALP activity (P < 0.01), and the protective effect of icariin was observed at both 10−6 m (P < 0.05) and 10−5 m (P < 0.01) concentrations compared to HC control.
To visually examine treatment effects on osteogenic differentiation, osteoblasts exposed to hypoxia for 36 h were histochemically stained for mineralized nodules formed after 12 days (Fig. 6a). Number and area of stained mineralized nodules in the HC group were significantly lower than those of the NC group (P < 0.01, Fig. 6b,c); however, these reductions were partially but significantly attenuated in the 10−6 m (P < 0.05) and 10−5 m (P < 0.01) icariin‐supplemented groups compared to the HC group. Measurements of calcium deposition level displayed a similar tendency to the mineralized nodules (data not shown).
Figure 6.

Effects of hypoxic treatment with or without icariin, on osteoblast mineralization potential. Mineralized nodules were stained with alizarin red after 12 days (a). Numbers (b) and areas (c) of mineralized nodules were analysed by Image‐Pro Plus 6.0 software. Results are means ± SD from triplicate cultures. **P < 0.01 versus normoxia control (NC); #P < 0.05, ##P < 0.01 versus hypoxia control (HC).
Discussion
Reduced blood supply to bone is known to lead to lower supplies of oxygen and nutrients, and increased accumulation of toxic metabolites in bone tissue. Shortage of oxygen (hypoxia) or low oxygen tension impairs production of ATP through oxidative phosphorylation in mitochondria, but stimulates anaerobic ATP production by glycolysis; this however, causes increased production of ROS 23. ROS damage all components of cells, including proteins, lipids and DNA, and contribute to hypoxia‐induced apoptosis 24. Association between oxidative stress and reduced bone mass and strength, has been established in mice and observed in human clinical studies 25, 26, 27; preventive effect of antioxidants on bone loss has been reported 28, 29. Icariin is a long recognized antioxidant 30, 31, 32, 33, having been found to protect vascular endothelial cells exposed to hypoxia, inhibit hydrogen peroxide‐mediated neurotoxicity, and protect against cognitive deficits induced by chronic cerebral hypoperfusion, in rats 34, 35, 36. We therefore hypothesized that, apart from its action as a phytoestrogen 20, anti‐osteoporosis effect of icariin may be related to its antioxidant property, and that it may protect bone also by ameliorating reduced blood supply‐induced ischaemic and hypoxic damage to bone. As a step to test this hypothesis, the present study investigated effects of icariin supplementation on viability and osteogenic potential of osteoblasts exposed to hypoxic environments in vitro. We observed that icariin dose‐dependently attenuated hypoxia‐induced oxidative stress and apoptosis in primary osteoblasts and suppressed hypoxia‐induced reduction of their osteogenic differentiation and mineralization potentials.
Our data showed that viability of osteoblasts was significantly reduced under hypoxia, which became more obvious with increasing exposure time. This tendency for cell damage was confirmed by increased LDH release and oxidative stress, as indicated by increased production of ROS and MDA and reduced activity of the anti‐oxidative enzyme SOD. Cell proliferation was clearly inhibited and apoptosis was significantly increased by hypoxia exposure. These results were consistent with previous reports that age‐related bone loss in human and animals is due mainly to a deficit in osteoblasts, due to reduced osteoblastogenesis or increased apoptosis, and is related to increased oxidative stress 1, 37, 38.
Icariin supplementation attenuated the reduction in osteoblast viability, reduced ROS production and intracellular MDA level, and simultaneously increased activity of SOD enzyme in a dose‐dependent manner. This activity of icariin was consistent with the findings of its antioxidant properties in the above‐mentioned previous studies. Interestingly, icariin did not lessen hypoxia‐induced inhibition of osteoblast proliferation, but further arrested the cell cycle in G0/G1 phase, contributing to survival of osteoblasts exposed to hypoxia by reducing consumption of ATP and reducing production of ROS and MDA. Icariin also reduced osteoblast apoptosis induced by hypoxic treatment, in a concentration‐dependent manner, also contributing to maintenance of osteoblast number.
Icariin not only attenuated impact of hypoxia on osteoblast viability but also suppressed hypoxia‐induced reduction in their osteogenic differentiation ability. This was shown by its ability to preserve ALP activity and formation of mineralized nodules in a dose‐dependent manner, which was otherwise reduced by hypoxia. Consistently, icariin was found to increase expression levels of major osteogenic growth factor BMP‐2, early osteogenic transcription factor RUNX‐2 and late osteogenic transcription factor OSX (required for osteoblast maturation) in treated osteoblasts. As icariin was removed after hypoxia treatment and osteogenic differentiation was induced under normal conditions, increased osteogenic ability in icariin‐supplemented groups (compared to the hypoxic groups) seemed to be due to the protective effect of icariin on viability and survival of osteoblasts, and osteogenic activity of icariin itself 19, 39.
While the above protective effects of icariin on hypoxic damage may be explained (to a certain degree) by its antioxidant properties as suggested above, accumulating evidence indicates that beneficial action of flavonoids in vivo is unlikely to be merely by outcompeting antioxidants, but may more likely work as potential modulators of intracellular signalling cascades, vital to cell functions and defence mechanisms against oxidative stress 40. It has been reported that icariin protects neurons against injury after oxygen and glucose deprivation by increasing SIRT1 35, a member of the sirtuin family of NAD+‐dependent deacetylase, and that higher SIRT1/FOXO3A axis signalling upon treatment with the SIRT1 agonist resveratrol, promotes osteogenesis of human mesenchymal stem cells 41. Icariin also attenuates cigarette smoke‐mediated oxidative stress in human lung epithelial cells by activation of the PI3K–AKT pathway and signalling of NRF2 42, an essential transcription factor that regulates expression of several antioxidant genes and plays a crucial role in cellular defence against oxidative stress 43. Future studies are required to investigate whether icariin reduces hypoxic damage to osteoblasts by its effects regulating expression and functions of Nrf2 and hypoxia‐induced factor (HIF‐1α, a central regulator of hypoxic response) that regulate transcription of more than 100 genes for survival of cells under low oxygen tension 44, 45.
In conclusion, the present study has demonstrated that icariin attenuated hypoxic damage to osteoblasts. As skeletal hypoxia occurs as a result of reduced blood supply during ageing and after the menopause, and has been proposed to be a cause of both primary and glucocorticoid‐induced osteoporosis, these new findings will widen the breadth of our knowledge concerning potential anti‐osteoporosis mechanisms of icariin. While previous studies have shown that icariin, as a phytoestrogen, can prevent bone loss by inhibiting bone resorption and enhancing bone formation 20, our study suggests that it may prevent bone loss also by protecting osteoblasts from hypoxic damage. Future in vivo studies with different models of osteoporosis are required to test this possibility. If proven positive, this knowledge will provide evidence/guide for development or identification of new anti‐osteoporotic drugs or natural compounds which could possess, not only pro‐osteogenic and/or anti‐resorptive properties, but also anti‐hypoxic benefits.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81270963). CJX is supported by a Senior Research Fellowship of NHMRC Australia.
References
- 1. Manolagas SC (2010) From estrogen‐centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 31, 266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP et al (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos. Int. 8, 468–489. [DOI] [PubMed] [Google Scholar]
- 3. Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L et al (2008) A population‐based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J. Bone Miner. Res. 23, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Griffith JF, Kumta SM, Huang Y (2011) Hard arteries, weak bones. Skeletal Radiol. 40, 517–521. [DOI] [PubMed] [Google Scholar]
- 5. London GM (2011) Soft bone – hard arteries: a link? Kidney Blood Press. Res. 34, 203–208. [DOI] [PubMed] [Google Scholar]
- 6. Bridgeman G, Brookes M (1996) Blood supply to the human femoral diaphysis in youth and senescense. J. Anat. 188, 611–621. [PMC free article] [PubMed] [Google Scholar]
- 7. Baur A, Stabler A, Bartl R, Lamerz R, Scheidler J, Reiser M (1997) MRI gadolinium enhancement of bone marrow: age‐related changes in normals and in diffuse neoplastic infiltration. Skeletal Radiol. 26, 414–418. [DOI] [PubMed] [Google Scholar]
- 8. Sumino H, Ichikawa S, Kasama S, Takahashi T, Sakamoto H, Kumakura H et al (2007) Relationship between brachial arterial endothelial function and lumbar spine bone mineral density in postmenopausal women. Circ. J. 71, 1555–1559. [DOI] [PubMed] [Google Scholar]
- 9. Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM 2nd, Donato AJ, Allen MR et al (2007) Aging reduces skeletal blood flow, endothelium‐dependent vasodilation, and NO bioavailability in rats. J. Bone Miner. Res. 22, 1280–1288. [DOI] [PubMed] [Google Scholar]
- 10. Ding WG, Wei ZX, Liu JB (2011) Reduced local blood supply to the tibial metaphysis is associated with ovariectomy‐induced osteoporosis in mice. Connect. Tissue Res. 52, 25–29. [DOI] [PubMed] [Google Scholar]
- 11. Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien CA et al (2010) Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 9, 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhai YK, Guo X, Pan YL, Niu YB, Li CR, Wu XL et al (2013) A systematic review of the efficacy and pharmacological profile of Herba Epimedii in osteoporosis therapy. Pharmazie 68, 713–722. [PubMed] [Google Scholar]
- 13. Nian H, Ma MH, Nian SS, Xu LL (2009) Antiosteoporotic activity of icariin in ovariectomized rats. Phytomedicine 16, 320–326. [DOI] [PubMed] [Google Scholar]
- 14. Mok SK, Chen WF, Lai WP, Leung PC, Wang XL, Yao XS et al (2010) Icariin protects against bone loss induced by oestrogen deficiency and activates oestrogen receptor‐dependent osteoblastic functions in UMR 106 cells. Br. J. Pharmacol. 159, 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li GW, Xu Z, Chang SX, Nian H, Wang XY, Qin LD (2014) Icariin prevents ovariectomy‐induced bone loss and lowers marrow adipogenesis. Menopause 21, 1007–1016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16. Zhang G, Qin L, Shi Y (2007) Epimedium‐derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24‐month randomized, double‐blind and placebo‐controlled trial. J. Bone Miner. Res. 22, 1072–1079. [DOI] [PubMed] [Google Scholar]
- 17. Zhang D, Zhang J, Fong C, Yao X, Yang M (2012) Herba epimedii flavonoids suppress osteoclastic differentiation and bone resorption by inducing G2/M arrest and apoptosis. Biochimie 94, 2514–2522. [DOI] [PubMed] [Google Scholar]
- 18. Song L, Zhao J, Zhang X, Li H, Zhou Y (2013) Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor‐mediated ERK and JNK signal activation. Eur. J. Pharmacol. 714, 15–22. [DOI] [PubMed] [Google Scholar]
- 19. Ma HP, Ming LG, Ge BF, Zhai YK, Song P, Xian CJ et al (2011) Icarrin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro. J. Cell. Biochem. 112, 916–923. [DOI] [PubMed] [Google Scholar]
- 20. Ming LG, Chen KM, Xian CJ (2013) Function and action mechanism of flavoids genistein and icariin in regulating bone remodeling. J. Cell. Physiol. 228, 513–521. [DOI] [PubMed] [Google Scholar]
- 21. Messner B, Ploner C, Laufer G, Bernhard D (2012) Cadmium activates a programmed, lysosomal membrane permeabilization‐dependent necrosis pathway. Toxicol. Lett. 212, 268–275. [DOI] [PubMed] [Google Scholar]
- 22. Zhou J, Ming LG, Ge BF, Wang JQ, Zhu RQ, Wei Z et al (2011) Effects of 50Hz sinusoidal electromagnetic fields of different intensities on proliferation, differentiation and mineralization potentials of rat osteoblasts. Bone 49, 753–761. [DOI] [PubMed] [Google Scholar]
- 23. Miura T, Tanno M (2010) Mitochondria and GSK‐3β in cardioprotection against ischemia/reperfusion injury. Cardiovasc. Drugs Ther. 24, 255–263. [DOI] [PubMed] [Google Scholar]
- 24. Kim JY, Park JH (2003) ROS‐dependent caspase‐9 activation in hypoxic cell death. FEBS Lett. 549, 94–98. [DOI] [PubMed] [Google Scholar]
- 25. Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H (2001) Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 288, 275–279. [DOI] [PubMed] [Google Scholar]
- 26. Jagger CJ, Lean JM, Davies JT, Chambers TJ (2005) Tumor necrosis factor‐α mediates osteopenia caused by depletion of antioxidants. Endocrinology 146, 113–118. [DOI] [PubMed] [Google Scholar]
- 27. Altindag O, Erel O, Soran N, Celik H, Selek S (2008) Total oxidative/anti‐oxidative status and relation to bone mineral density in osteoporosis. Rheumatol. Int. 28, 317–321. [DOI] [PubMed] [Google Scholar]
- 28. Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA (2006) Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J. Womens. Health (Larchmt) 15, 295–300. [DOI] [PubMed] [Google Scholar]
- 29. Sanders KM, Kotowicz MA, Nicholson GC (2007) Potential role of the antioxidant N‐acetylcysteine in slowing bone resorption in early post‐menopausal women: a pilot study. Trans. Res. 150, 215. [DOI] [PubMed] [Google Scholar]
- 30. Liu ZQ (2006) Icariin: a special antioxidant to protect linoleic acid against free‐radical‐induced peroxidation in micelles. J. Phys. Chem. A 110, 6372–6378. [DOI] [PubMed] [Google Scholar]
- 31. Wang YK, Huang ZQ (2005) Protective effects of icariin on human umbilical vein endothelial cell injury induced by H2O2 in vitro. Pharmacol. Res. 52, 174–182. [DOI] [PubMed] [Google Scholar]
- 32. Liu ZQ, Luo XY, Sun YX, Wu W, Liu CM, Liu ZQ et al (2004) The antioxidative effect of icariin in human erythrocytes against free‐radical‐induced haemolysis. J. Pharm. Pharmacol. 56, 1557–1562. [DOI] [PubMed] [Google Scholar]
- 33. Zhao F, Tang YZ, Liu ZQ (2007) Protective effect of icariin on DNA against radical‐induced oxidative damage. J. Pharm. Pharmacol. 59, 1729–1732. [DOI] [PubMed] [Google Scholar]
- 34. Xu RX, Wu Q, Luo Y, Gong QH, Yu LM, Huang XN et al (2009) Protective effects of icariin on cognitive deficits induced by chronic cerebral hypoperfusion in rats. Clin. Exp. Pharmacol. Physiol. 36, 810–815. [DOI] [PubMed] [Google Scholar]
- 35. Zhang L, Huang S, Chen Y, Wang Z, Li E, Xu Y (2010) Icariin inhibits hydrogen peroxide‐mediated cytotoxicity by up‐regulating sirtuin type 1‐dependent catalase and peroxiredoxin. Basic Clin. Pharmacol. Toxicol. 107, 899–905. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Q, Li H, Wang S, Liu M, Feng Y, Wang X (2013) Icariin protects rat cardiac H9c2 cells from apoptosis by inhibiting endoplasmic reticulum stress. Int. J. Mol. Sci. 14, 17845–17860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 21, 115–137. [DOI] [PubMed] [Google Scholar]
- 38. Jilka RL, Bellido T, Almeida M, Plotkin LI, O'Brien CA, Weinstein RS et al (2008). Apoptosis and bone cells In: Bilezikian JP, Raisz LG, Martin T, eds. Principles of Bone Biology, pp. 235–259. San Diego, CA: Academic Press. [Google Scholar]
- 39. Ma XN, Zhou J, Ge BF, Zhen P, Ma HP, Shi WG et al (2013) Icariin induces osteoblast differentiation and mineralization without dexamethasone in vitro. Planta Med. 79, 1501–1508. [DOI] [PubMed] [Google Scholar]
- 40. Williams RJ, Spencer JPE, Rice‐Evans C (2004) Flavonoids: antioxidants or signaling molecules? Free. Radical. Bio. Med. 36, 838–849. [DOI] [PubMed] [Google Scholar]
- 41. Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF, Kuo ML et al (2011) Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating Runx2 gene expression via the Sirt1/Foxo3A axis. J. Bone Miner. Res. 26, 2552–2563. [DOI] [PubMed] [Google Scholar]
- 42. Wu J, Xu H, Wong PF, Xia S, Xu J, Dong J (2014) Icaritin attenuates cigarette smoke‐mediated oxidative stress in human lung epithelial cells via activation of PI3K‐AKT and Nrf2 signaling. Food Chem. Toxicol. 64, 307–313. [DOI] [PubMed] [Google Scholar]
- 43. Huang XS, Chen HP, Yu HH, Yan YF, Liao ZP, Huang QR (2014) Nrf2‐dependent upregulation of antioxidative enzymes: a novel pathway for hypoxic preconditioning‐mediated delayed cardioprotection. Mol. Cell. Biochem. 385, 33–41. [DOI] [PubMed] [Google Scholar]
- 44. Kaelin WG Jr, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402. [DOI] [PubMed] [Google Scholar]
- 45. Kiani AA, Kazemi A, Halabian R, Mohammadipour M, Jahanian‐Najafabadi A, Roudkenar MH (2013) HIF‐1α confers resistance to induced stress in bone marrow‐derived mesenchymal stem cells. Arch. Med. Res. 44, 185–193. [DOI] [PubMed] [Google Scholar]


