Abstract
Objectives: To evaluate the effect of raloxifene on Ki‐67 and Bcl‐2 antigen expression in operable, stage II, oestrogen‐receptor‐positive invasive ductal breast carcinomas.
Materials and methods: Twenty post‐menopausal women who had taken 60 mg of raloxifene daily for 28 days prior to definitive surgery were enrolled in the investigation. Two tumour samples were obtained by incisional biopsy during the study, one at the time of confirmation of diagnosis of invasive ductal carcinoma and evaluation of oestrogen receptor status, and the other 29 days later, at the time of definitive surgery. Immunohistochemistry was performed on tumour samples, prior to and after raloxifene treatment, to evaluate Ki‐67 and Bcl‐2 expression. Friedman and McNemar tests were used for statistical analysis of the data, significance being established at 5%.
Results: Mean percentage of Ki‐67‐stained nuclei was 24.86 ± 2.95 prior to raloxifene treatment and 13.33 ± 1.52 after treatment (P < 0.001). Prior to raloxifene treatment, only 9/20 cases (45%) were classified as Bcl‐2‐positive, whereas after treatment, 17/20 (85%) were classified as Bcl‐2‐positive (P < 0.013).
Conclusions: Raloxifene treatment significantly reduced Ki‐67 antigen expression and increased Bcl‐2 expression in breast carcinomas of post‐menopausal women.
Introduction
Selective oestrogen receptor modulators (SERMs) are considered important tools in combating oestrogen‐dependent breast cancer (1, 2). Tamoxifen was the first SERM clinically approved for chemoprevention and treatment of breast cancer; however, when used over long periods, tamoxifen may result in a 2‐ to 7‐fold increase in the patient’s risk of developing endometrial cancer (3). Recent clinical trials have shown raloxifene, a second‐generation SERM approved for the prevention and treatment of osteoporosis (1, 2, 4, 5, 6), to be as effective as tamoxifen in reducing invasive breast carcinoma, while reducing incidence of endometrial carcinoma (4, 6). Nevertheless, a few studies have evaluated raloxifene for primary treatment of breast cancer (7, 8).
Drugs can be administered for shorter period of time (2 to 4 weeks) evaluating the alterations induced in biomolecular markers, such as those linked to the cell proliferation and apoptosis, with the advantage of suffering alterations before any clinical response of the tumor to the treatment (9). Bcl‐2 is a protein that belongs to a family of apoptosis‐related proteins codified by a proto‐oncogene, Bcl‐2, and is considered to be anti‐apoptotic. However, various studies have found that in breast cancer, Bcl‐2 expression is paradoxically associated with a better prognosis (10, 11).
Some studies have shown anti‐proliferative and proapoptotic effects of tamoxifen in breast cancer (12, 13, 14); however, studies involving raloxifene are few (7, 8). One study evaluating the effect of raloxifene on Ki‐67 expression and apoptosis by quantifying apoptotic bodies, showed reduction in cell proliferation and no alteration in apoptosis (7). Nevertheless, in spite of studies evaluating effects of raloxifene on Ki‐67 protein expression of breast tumours (7, 8), to the best of our knowledge, no study has yet been carried out to evaluate Bcl‐2 expression in invasive, oestrogen‐receptor‐positive breast carcinomas of post‐menopausal women, after primary treatment with raloxifene, which led us to design the present study.
Materials and methods
Patients
This study was approved by the Internal Review Board of the Federal University of Piauí and all patients gave their signed informed consent prior to study initiation. Twenty women receiving medical care at the Mastology Division of the Getúlio Vargas Hospital of the Federal University of Piauí, who had been menopausal for at least 1 year and who had invasive ductal, oestrogen‐receptor‐positive Her2‐negative, operable, stage II (≥3 cm) breast carcinoma and no previous history of treatment for breast cancer or hormonal replacement therapy, were included in the study. Tumour size varied from 3 to 5 cm (mean 3.8 cm) and mean age of patients was 60.5 years (range 49–72 years). The majority of patients were multiparas (90%) (Table 1).
Table 1.
Characteristics of patients
| Characteristics | n | % |
|---|---|---|
| Age (years) | ||
| 40–49 | 2 | 10 |
| 50–59 | 6 | 30 |
| 60–69 | 10 | 50 |
| ≥70 | 2 | 10 |
| Parity | ||
| Nullipara | 2 | 10 |
| Multipara | 18 | 90 |
| Size of tumour (cm) | ||
| 3.0–3.9 | 14 | 70 |
| 4.0–5.0 | 6 | 30 |
| Histological grade | ||
| 1 | 9 | 45 |
| 2 | 8 | 40 |
| 3 | 3 | 15 |
| Staging | ||
| IIa | 10 | 50 |
| IIb | 10 | 50 |
| Her2/neu positive | 0 | 0 |
Study design
Patients received 60 mg of raloxifene/day for 28 days prior to definitive surgery, beginning immediately after receiving results of a diagnostic incisional biopsy. Two tumour samples were obtained by incisional biopsy during the study, one at the time of confirmation of diagnosis of invasive ductal carcinoma and evaluation of oestrogen receptor status, and the other 29 days later, at the time of definitive surgery. Tumours with nuclear staining for oestrogen receptor (ER) measured semi‐quantitatively as ‘high’ (>10% immunoreactive cells) were considered positive.
Immunohistochemistry for Ki‐67 and Bcl‐2
For immunohistochemical evaluation of Ki‐67 and Bcl‐2 expression, tumour samples were fixed in buffered formalin for 12–24 h and then cut into 3 μm sections. Next, the samples were processed and stained with haematoxylin & eosin for confirmation of diagnosis of ductal invasive carcinoma, after which, sections were deparaffinized in xylol for 5 min, dehydrated in absolute ethanol and washed in buffered saline solution at pH 7.4 for 5 min. Next, the sections were treated with 3% hydrogen peroxide (H2O2), diluted in buffered solution, for 5 min to block endogenous peroxide. To recover antigens, slides were placed in racks containing 0.21% citric acid (pH 6.0) and were heated in a microwave oven for 15 min at maximum power. Phosphate‐buffered saline containing Tween (PBS‐Tween) was added to the slides after they had been cooled for 20 min. Tissue samples were incubated with primary mouse anti‐Ki‐67 monoclonal antibody (clone MIB1, Ref. M7240; Dako, Carpinteria, CA, USA/1:4800) and mouse anti‐Bcl‐2 monoclonal antibody (clone 124, Ref. M0887; Dako/1:2000) overnight at 4–8 °C. Then, slides were washed with PBS‐Tween and instilled with secondary reagent (Anti‐mouse BA 2000; Vector, Burlingame, CA, USA), incubated for 60 min at room temperature, washed again with PBS‐Tween and incubated with ABC Elite detection system (PK 6100; Vector, Burlingame, CA, USA), for 45 min at room temperature, washed once again with PBS‐Tween, treated with DAB (Diaminobenzidine tetra‐hydrochloride, Ref. D5637; Sigma, St. Louis, MO, USA) and incubated for 5 min. Finally, slides were washed in distilled water, counterstained with haematoxylin, stained with ammoniacal solution, dehydrated with absolute ethanol, passed through xylol series and mounted in Permount resin. Cells that expressed Ki‐67 and Bcl‐2 proteins were identified by dark brown staining of the nucleus and cytoplasm respectively.
Quantitation
Quantification was carried out by two observers blind to patients’ identity and had no previous knowledge of any of the cases. It was performed using a light microscope (Nikon Eclipse E‐400, optical microscope, Tokyo, Japan) connected to a colour video camera (Samsung digital camera CHC‐370N, Seoul, Korea), which captured the image and transmitted it to a computer equipped with the Imagelab® software program, version 2.3, developed by Softium Informática Ltda. (São Paulo, Brazil) for image analysis. For Ki‐67 expression, 1000 cells were counted in each slide, whether stained by anti‐Ki‐67 antibody or not, using 400× magnification, beginning from the area with the greatest Ki‐67 expression. In each case, percentage of stained cells was obtained from ratio of the number of cells with stained nuclei and total number of cells multiplied by 100. Likewise, the Bcl‐2 immunoreaction was evaluated semi‐quantitatively according to the criteria established by van Slooten et al. (15) taking the following parameters into consideration: intensity of cell colouration (I) and the fraction of stained neoplastic cells (F). Intensity of cell staining was classified as: 0 (negative), 1 (weakly stained), 2 (moderately stained) or 3 (strongly stained). The fraction of stained cells was classified as I (0–25%), II (25–75%) or III (75–100%). Final score was the result of combination of the two parameters (I and F) and ranged from 0 to 6. Cases with a final score ≥3 were classified as positive for Bcl‐2. In all cases, brownish staining in the cytoplasm was adopted as standard of positivity (15).
Statistical analysis
Friedman non‐parametric test was used to analyse percentages of nuclei stained with anti‐Ki‐67 prior to and after raloxifene treatment (16). McNemar’s test of symmetry was used to evaluate agreement between classification of cells stained positive or negative with anti‐Bcl‐2 prior to and after raloxifene treatment (17). Significance was established at P < 0.05.
Results
By light microscopy, there was greater concentration of Ki‐67‐stained nuclei in samples obtained prior to raloxifene treatment compared to those collected after therapy. The percentage of cells intensely stained for Bcl‐2 was higher in samples obtained after raloxifene treatment compared with that of pre‐treatment samples (Fig. 1). Mean percentage of Ki‐67‐stained nuclei was 24.86 ± 2.95 and 13.33 ± 1.52 (P < 0.001) prior to and after raloxifene treatment respectively (Fig. 2). With respect to Bcl‐2 antigen expression, out of the 11 cases classified as negative for Bcl‐2 expression prior to treatment, eight were classified as positive after raloxifene use and only three remained negative, whereas all nine cases that were classified as positive prior to treatment remained positive for Bcl‐2 expression after treatment. Therefore, only nine out of the 20 patients (45%) were classified as positive prior to treatment, whereas after raloxifene treatment, Bcl‐2 expression was positive in 17 out of the 20 samples (85%) (P < 0.013) (Table 2).
Figure 1.
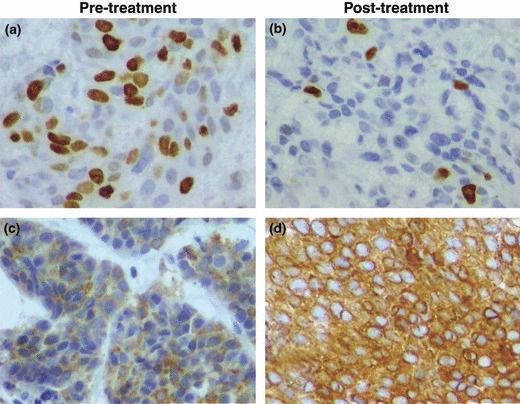
Photomicrographs of histological sections of breast cancer from patient no. 4. Note numerous nuclei stained brown by anti‐Ki‐67 antibody prior to treatment with raloxifene (a), and sparsely stained nuclei post‐treatment (b). Observe negative immunohistochemical reaction for Bcl‐2 protein prior to treatment with raloxifene, as expressed by sparse numbers of cells with cytoplasm weakly stained brown (c), and positive immunohistochemical reaction post‐treatment, as expressed by numerous cells with cytoplasm intensely stained (d) (original magnification 400×).
Figure 2.
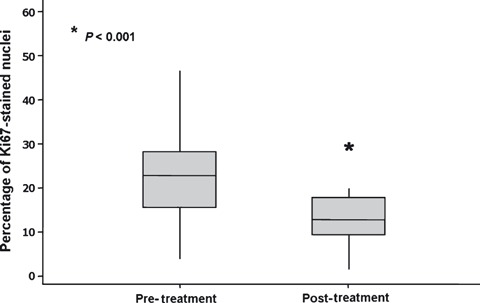
Boxplot of mean percentage of nuclei stained with anti‐Ki‐67 antibody prior to and after raloxifene treatment.
Table 2.
Percentage of breast cancer cases with Bcl‐2‐positive cells prior to and after raloxifene treatment
| Pre‐treatment | Post‐treatment | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 9 | 0 | 9 (45%) |
| Negative | 8 | 3 | 11 (55%) |
| Total | 17 (85%)* | 3 (15%) | 20 (100%) |
*There was a statistically significant increase in Bcl‐2 expression after raloxifene treatment (P < 0.013).
Discussion
In the present study, raloxifene administered at the dose of 60 mg/day for 28 days in post‐menopausal women with ER‐positive Her2‐negative invasive ductal breast carcinoma significantly reduced Ki‐67 expression, while significantly increasing Bcl‐2 expression. The 60 mg dose of raloxifene was selected because this is the dose generally used for prevention and treatment of osteoporosis and was also the dose used in the studies in which raloxifene was evaluated for chemoprevention of breast cancer (4, 5, 6). The 28‐day schedule of use of medication was chosen because 28 days is the normal delay incurred by a patient between her first consultation at this institute and surgical treatment; hence, proposed treatment would result in no further delay in carrying out definitive surgery. Studies investigating the effect of drugs on breast cancer have generally used core biopsy to obtain tumour samples; however, taking heterogeneity of the tumours into consideration, the small tumour volume obtained with this type of biopsy results with a fragment removed perhaps not being representative of the tumour as a whole (18); for this reason, we opted to perform incisional biopsies.
To the best of our knowledge, this is the first study in which the effect of raloxifene has been evaluated after immunohistochemical expression of Bcl‐2 in ER‐positive Her2‐negative invasive breast cancer samples from post‐menopausal women. Dowsett et al. (7) used Ki‐67 expression in their study to evaluate the effect of 60 mg of raloxifene administered for 14 days on proliferation of breast cancer cells and also reported significant reduction in expression of this marker. These investigators used the immunohistochemical technique of terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end‐labelling (TUNEL) to calculate the apoptotic index and reported no significant difference prior to and after raloxifene treatment. These results differ from the findings of our present study, in which raloxifene used for 28 days increased expression of anti‐apoptotic protein Bcl‐2. However, direct assessment of apoptosis is a more consistent method, while behaviour of Bcl‐2 may reflect other biological processes within the transformed cell (7). Likewise, previous studies have shown that reduced proliferation is associated with Bcl‐2 expression (13).
The anti‐proliferative effects of SERMs on breast cancer have been well documented in various studies showing their effect in reducing cell proliferation, evaluated by Ki‐67 immunohistochemical expression (8, 12, 13, 19), as confirmed in the present study. The role of the immunohistochemical expression of Ki‐67 has been widely investigated, the majority of studies showing direct association between elevated Ki‐67 immunostaining and negative oestrogen receptor immunohistochemistry, higher grade and highly proliferative tumours, characteristics known to be associated with poorer prognosis (20), although it has not been possible to establish an appropriate and universally accepted cut‐off limit to distinguish between high and low proliferative activities (21). There are workers who use Ki‐67 staining at 20% or more to define a high level of expression, while others advocate segregating high from low staining via the median of positively stained cells as the distinguishing value (20, 22, 23). Additional variations in issues include selection of the appropriate area for evaluation of immunostaining, be it the centre or periphery of the tumour, area of highest cell density, or zone of highest tumour cell immunohistochemical reactivity (10, 20). In this study, percentage of positively stained nuclei and area of highest cell density were the variables used in evaluation of Ki‐67 expression pre‐ and post‐treatment with raloxifene.
Here, the percentage of positivity for Bcl‐2 expression was 45% prior to raloxifene use, which is close to values found in other studies such as those carried out by Silvestrini et al. (24) and Johnston et al. (13), which reported, respectively, around 43% and 32% of positivity for the immunohistochemical expression of Bcl‐2 in invasive breast tumours. After raloxifene use, the percentage of positivity for Bcl‐2 expression practically doubled, increasing from 45% to 85%, an increase proportionally similar to that found by Johnston et al. (13) who reported an increase in percentage of Bcl‐2‐positive tumours from 32% to 65% after use of tamoxifen for 18 days. As we have been unable to find any previous studies evaluating Bcl‐2 expression after raloxifene treatment, a direct comparison between the findings of the present study and previous findings is impossible.
Bcl‐2 protein is considered to be anti‐apoptotic and its tumorigenic potential has been demonstrated in animal models and in some types of tumour such as lymphomas (25). Nevertheless, in many human tumours including breast cancer, Bcl‐2 paradoxically appears to exert a suppressive effect on tumours and its expression is, instead, associated with favourable prognostic characteristics such as low nuclear grade and oestrogen‐receptor positivity (11, 25). A meta‐analysis of 25 studies involving 5892 cases of breast cancer in which the prognostic role of Bcl‐2 immunohistochemical expression was investigated, showed that it is strongly associated with longer disease‐free survival and overall survival, its effect being dependent neither on lymph node status, tumour size nor on histological grade (11).
The mechanism by which Bcl‐2 may exert its protective effect on breast cancer remains to be fully clarified. In vitro studies have shown that Bcl‐2 exerts an inhibitory effect on the cell cycle, prolonging G0 phase and therefore delaying passage of the cell to G1 phase (26). It is also possible that the capacity of breast cancer cells to proceed to apoptosis may be delayed by the profound anti‐proliferative effects of anti‐oestrogenic therapy. It has also been reported that c‐myc, a proto‐oncogene that is essential in codifying the proliferative machinery and whose unregulated expression has been associated with many neoplasias, is a determinant of both cell proliferation and apoptosis and its expression is accentuated by oestrogen and suppressed by anti‐oestrogens (27). Furthermore, although at least 20 proteins of the Bcl‐2 family have been described, the effect of drugs such as raloxifene on these proteins as a whole, and how they synergistically affect apoptosis remain unknown.
In conclusion, there was a significant reduction in proliferative activity as evaluated by Ki‐67 immunohistochemical expression and a significant increase in Bcl‐2 expression in oestrogen‐receptor‐positive breast carcinoma of post‐menopausal women treated with raloxifene. Our results suggest that raloxifene used for short periods of time, before definitive surgery, may reveal patients who would probably benefit from adjuvant endocrinotherapy with this drug; however, further basic and clinical studies must be carried out to clarify the biomolecular mechanisms of tumour response to raloxifene.
Funding
None.
Conflicts of interest
None.
References
- 1. Saji S, Kuroi K (2008) Application of selective estrogen receptor modulators for breast cancer treatment according to their intrinsic nature. Breast Cancer 15, 262–269. [DOI] [PubMed] [Google Scholar]
- 2. Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E et al. (2009) Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J. Natl Cancer Inst. 101, 384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh MN, Stringfellow HF, Paraskevaidis E, Martin‐Hirsch PL, Martin FL (2007) Tamoxifen: important considerations of a multi‐functional compound with organ‐specific properties. Cancer Treat. Rev. 33, 91–100. [DOI] [PubMed] [Google Scholar]
- 4. Cauley JA, Norton L, Lippman ME, Eckert S, Krueger KA, Purdie DW et al. (2001) Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4‐year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res. Treat. 65, 125–134. [DOI] [PubMed] [Google Scholar]
- 5. Martino S, Disch D, Dowsett SA, Keech CA, Mershon JL (2005) Safety assessment of raloxifene over eight years in a clinical trial setting. Curr. Med. Res. Opin. 21, 1441–1452. [DOI] [PubMed] [Google Scholar]
- 6. Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN et al. (2006) National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P‐2 trial. JAMA 295, 2727–2741. [DOI] [PubMed] [Google Scholar]
- 7. Dowsett M, Bundred NJ, Decensi A, Sainsbury RC, Lu Y, Hills MJ et al. (2001) Effect of raloxifene on breast cancer cell Ki67 and apoptosis: a double‐blind, placebo‐controlled, randomized clinical trial in postmenopausal patients. Cancer Epidemiol. Biomarkers Prev. 10, 961–966. [PubMed] [Google Scholar]
- 8. Da Silva BB, Pires CG, Dos Santos AR, De Castro‐Leão AH, Alencar AP, Lopes‐Costa PV (2009) Effects of raloxifene on Ki‐67 and CD34 antigen expression in breast cancer. Gynecol. Obstet. Invest. 67, 103–108. [DOI] [PubMed] [Google Scholar]
- 9. Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C et al. (2006) Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin. Cancer Res. 12, 1024s–1030s. [DOI] [PubMed] [Google Scholar]
- 10. Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM et al. (2000) Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch. Pathol. Lab. Med. 124, 966–978. [DOI] [PubMed] [Google Scholar]
- 11. Callagy GM, Webber MJ, Pharoah PD, Caldas C (2008) Meta‐analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer 8, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke RB, Laidlaw IJ, Jones LJ, Howell A, Anderson E (1993) Effect of tamoxifen on Ki67 labelling index in human breast tumours and its relationship to oestrogen and progesterone receptor status. Br. J. Cancer 67, 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnston SR, MacLennan KA, Sacks NP, Salter J, Smith IE, Dowsett M (1994) Modulation of Bcl‐2 and Ki‐67 expression in oestrogen receptor‐positive human breast cancer by tamoxifen. Eur. J. Cancer 30A, 1663–1669. [DOI] [PubMed] [Google Scholar]
- 14. Millen EC, Da Silva BB, Gebrim LH (2006) Apoptotic index in breast carcinoma cells following tamoxifen treatment. Int. J. Gynaecol. Obstet. 95, 64–65. [DOI] [PubMed] [Google Scholar]
- 15. Van Slooten HJ, Clahsen PC, Van Dierendonck JH, Duval C, Pallud C, Mandard AM et al. (1996) Expression of Bcl‐2 in node‐negative breast cancer is associated with various prognostic factors, but does not predict response to one course of perioperative chemotherapy. Br. J. Cancer 74, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conover WJ (1980) Practical Nonparametric Statistics, 2nd edn New York: John Wiley & Sons Inc. [Google Scholar]
- 17. Agresti A (2002) Categorical Data Analysis, 2nd edn New York: John Wiley & Sons Inc. [Google Scholar]
- 18. Jacobs TW, Siziopikou KP, Prioleau JE, Raza S, Baum JK, Hayes DF et al. (1998) Do prognostic marker studies on core needle biopsy specimens of breast carcinoma accurately reflect the marker status of the tumor? Mod. Pathol. 11, 259–264. [PubMed] [Google Scholar]
- 19. Makris A, Powles TJ, Allred DC, Ashley S, Ormerod MG, Titley JC et al. (1998) Changes in hormone receptors and proliferation markers in tamoxifen treated breast cancer patients and the relationship with response. Breast Cancer Res. Treat. 48, 11–20. [DOI] [PubMed] [Google Scholar]
- 20. Tan PH, Bay BH, Yip G, Selvarajan S, Tan P, Wu J et al. (2005) Immunohistochemical detection of Ki67 in breast cancer correlates with transcriptional regulation of genes related to apoptosis and cell death. Mod. Pathol. 18, 374–381. [DOI] [PubMed] [Google Scholar]
- 21. Spyratos F, Ferrero‐Poüs M, Trassard M, Hacène K, Phillips E, Tubiana‐Hulin M et al. (2002) Correlation between MIB‐1 and other proliferation markers: clinical implications of the MIB‐1 cutoff value. Cancer 94, 2151–2159. [DOI] [PubMed] [Google Scholar]
- 22. Petit T, Wilt M, Velten M, Millon R, Rodier JF, Borel C et al. (2004) Comparative value of tumour grade, hormonal receptors, Ki‐67, HER‐2 and topoisomerase II alpha status as predictive markers in breast cancer patients treated with neoadjuvant anthracycline‐based chemotherapy. Eur. J. Cancer 40, 205–211. [DOI] [PubMed] [Google Scholar]
- 23. Talley LI, Grizzle WE, Waterbor JW, Brown D, Weiss H, Frost AR (2002) Hormone receptors and proliferation in breast carcinomas of equivalent histologic grades in pre‐ and postmenopausal women. Int. J. Cancer 98, 118–127. [DOI] [PubMed] [Google Scholar]
- 24. Silvestrini R, Veneroni S, Daidone MG, Benini E, Boracchi P, Mezzetti M et al. (1994) The Bcl‐2 protein: a prognostic indicator strongly related to p53 protein in lymph node‐negative breast cancer patients. J. Natl Cancer Inst. 86, 499–504. [DOI] [PubMed] [Google Scholar]
- 25. Dos Santos LG, Lopes‐Costa PV, Dos Santos AR, Facina G, Da Silva BB (2008) Bcl‐2 oncogene expression in estrogen receptor‐positive and negative breast carcinoma. Eur. J. Gynaecol. Oncol. 29, 459–461. [PubMed] [Google Scholar]
- 26. Pietenpol JA, Papadopoulos N, Markowitz S, Willson JK, Kinzler KW, Vogelstein B (1994) Paradoxical inhibition of solid tumor cell growth by bcl2. Cancer Res. 54, 3714–3717. [PubMed] [Google Scholar]
- 27. Evan G, Harrington E, Fanidi A, Land H, Amati B, Bennett M (1994) Integrated control of cell proliferation and cell death by the c‐myc oncogene. Philos. Trans. R. Soc. Lond. B Biol. Sci. 345, 269–275. [DOI] [PubMed] [Google Scholar]


