Abstract
Abstract. The dorsal and ventral epithelia on the murine tongue exhibit very pronounced circadian rhythms in terms of the cell cycle. These rhythms are such that three injections of tritiated thymidine 3 h apart spanning the circadian peak in S phase cells labelled between 40 and 50% of the basal cells. Injection of bromodeoxyuridine generally gave slightly lower labelling indices. Approximately the same proportion (54% of the basal cells) could be accumulated in metaphase over a 24‐h period using vincristine as a stathmokinetic agent. The experiments reported here using mouse ventral tongue epithelium use double‐labelling approaches to address the question: what proportion of the approximately 50% of the basal cells that are proliferating have a 24‐h cell cycle and can therefore be labelled by a similar labelling protocol the following day? The results suggest a heterogeneity amongst the proliferating basal cells, similar to the heterogeneity proposed for the dorsal tongue epithelium. Although not all the basal component has been accounted for, the data presented here suggest that about 20% of the basal cells may have a cell cycle time of 24 h, about 30% appear to have a longer cell cycle time (48 or 72 h), while about 20% of the basal cells appear to be postmitotic maturing G1 cells, awaiting the appropriate signals for migration into the suprabasal layer.
Keywords: cell cycle, cell kinetics, double labelling, mouse tongue, vincristine
Introduction
In the first paper in this series of cell kinetic studies of the epithelium of the mouse ventral tongue, we presented data, using vincristine‐induced stathmokinetics, throughout a 24‐h period. These showed that up to 54% of the basal cells entered mitosis each day (Potten et al. 2002). Here we attempted to investigate what proportion of these mitotic basal cells re‐entered the cell cycle and also whether the remaining basal cells (those that did not enter mitosis) showed any evidence of cell cycle progression. This was achieved by a series of single‐ and double‐labelling experiments using tritiated thymidine (3HTdR), bromodeoxyuridine (BrdUrd) and Ki67 markers.
Materials and methods
The animals used, housing conditions, tissue fixation, histology, autoradiography and 3HTdR administration were all as described in the preceding paper in this series. Four animals were used per group.
Bromodeoxyuridine (BrdUrd) (Sigma, Poole, UK) was administered at a dose of 3.3 mg per injection per mouse every 3 h for three injections (a total dose of 9.9 mg over 6 h) and the immunohistochemical detection of nuclei that had incorporated bromodeoxyuridine was performed using an anti‐BrdUrd monoclonal antibody (Mas 250b, Harlan Sera Laboratories, Loughborough, UK) following standard immunohistochemical procedures (Hume & Thompson 1990; Thomson et al. 1999).
After dewaxing the slides in xylene overnight and then washing in absolute alcohol, endogenous peroxidase activity was blocked with hydrogen peroxide. The slides were then hydrolysed in 1 m HCl for 8 min at 60 °C, followed by neutralization in boric acid buffer for 6 min. The slides were then washed in phosphate buffered saline (PBS) and incubated in 5% normal rabbit serum for 30 min (Sigma) to block nonspecific binding. This was followed by incubation for 1 h at room temperature in a 1 : 5 dilution of the anti‐BrdUrd antibody. Following further washing in PBS, rabbit antirat peroxidase (Dako, Cambridge, UK) was diluted 1 : 100 in 10% normal mouse serum and applied at room temperature for 1 h. The slides were then washed three times in PBS and developed using diaminobenzidine (DAB). In the case of samples to be processed for autoradiography as well as BrdUrd staining, the slides were washed for 24 h after immunohistochemistry prior to dipping in nuclear emulsion.
A rat monoclonal antibody to Ki67 protein (kindly supplied by Johannes Gerdes, Borstel, Germany) was used to detect the Ki67‐positive fraction of cells in the epithelium. Slides were hydrated, microwaved in citric acid buffer and endogenous peroxidase activity blocked by incubating in 0.3% hydrogen peroxide for 15 min. Following washing in PBS, nonspecific binding was blocked by incubating the slides in 10% goat serum (Sigma, Poole, Dorset, UK) for a period of 30 min.
The rat anti‐Ki67 was then diluted to a working concentration of 1 : 500 and applied to the slides for 1 h at room temperature. The slides were then once again washed in PBS and biotinylated goat antirat antibody applied at 1 : 200 in 2% mouse serum at room temperature for 30 min. Following further washing in PBS, the slides were incubated for 30 min at room temperature in avidin‐peroxidase solution (Elite ABC Vectorstain kit, Vector Laboratories, Peterborough, UK), washed and developed using DAB (Sigma). Samples that were to be processed for autoradiography were then rinsed in running deionized water for 24 h prior to dipping.
Three separate experimental protocols were used (see Fig. 1). In the first experiment, mice were injected with 3HTdR at 03.00, 06.00 and 09.00 h GMT, and were sampled 40 min after the last injection. This represents a 400‐min continuous‐labelling protocol that includes an injection at the time of the maximum in the circadian rhythm in S phase cells. For ease of use in all of these experiments the animals were acclimatized to a reverse light cycle room for a minimum of 2 weeks prior to use (see Potten et al. 2002). The tissue in this experiment was then analysed for 3HTdR labelling in conjunction with Ki67 expression.
Figure 1.
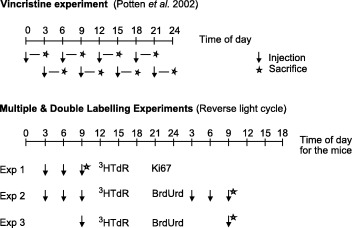
Schematic description of the vincristine experiment presented in Potten et al. 2002 and the three double‐labelling experiments reported in this paper.
In a second experiment mice received three injections of tritiated thymidine at 03.00, 06.00 and 09.00 h as above, and 24 h later at the same times of the day they received three injections of BrdUrd. The tissue was analysed to determine the proportion of single 3HTdR labelled cells (3HTdR only), single BrdUrd (BrdUrd only) and the proportion of double‐labelled cells (3HTdR + BrdUrd).
In a third experiment a single injection of 3HTdR was given at 09.00 h, followed by a single injection of BrdUrd 24 h later at the same time of day. As in the second experiment, single and double labelled cells were analysed (3HTdR only, BrdUrd only and double labelled 3HTdR + BrdUrd).
Areas of the ventral tongue 2 mm from the tip of the tongue were defined using the Zeiss Axiohome interactive microscope and all nuclei in the defined area were allocated a code according to their labelling characteristics as defined in the preceding paper (see Potten et al. 2002).
Results and discussion
The previous paper presented data using vincristine‐induced stathmokinetic accumulation that indicated that 54% of the basal cells entered mitosis and, hence, divided each day. Here we attempted to address the question of what proportion of this 54% re‐entered the cell cycle and the fate of the remaining 46% of the cells that were not trapped by the vincristine accumulation. In the first experiment, cells were labelled for 6 h 40 min (400 min) with 3HTdR (injections at 03.00, 06.00 and 09.00 h) and then also analysed for Ki67 expression. The results of this experiment are shown in Fig. 2. Only 1.9 ± 0.5% of the basal cells were solely labelled with the 3HTdR (i.e. were Ki67 negative). These were therefore cells that had passed beyond the Ki67 G2 expression threshold at the time the animals were culled, but are presumably some of the cells that were labelled by the first of the 3HTdR injections, i.e. at 03.00 h. This would suggest that the minimum duration of G2 is approximately 6 h assuming that none of the 3HTdR‐only labelled cells are mitotic. In contrast, 8.8 ± 0.6% of the basal cells were labelled with Ki67 only (Ki67 G1 cells that did not enter S during the labelling period), while 42.0 ± 3.0% of the basal cells were double labelled (3HTdR and Ki67), which means that the 3HTdR labelling protocol of 6 h 40 min detects about 78% (42/54) of the cells that are trapped by the vincristine block over a 24‐h period, i.e. a 6‐h labelling protocol spanning the circadian S phase peak labels three‐quarters of the cells that cycle once a day as seen in the vincristine‐induced arrest experiment (Potten et al. 2002). The implication is that the remaining 22% of the cells that pass through the cycle in any given 24 h either do not re‐enter S phase (and are presumably the cohort of cells that leaves the cycle and enters a postmitotic (G1/G0) compartment awaiting signals for migration suprabasally or the labelling protocol here did not catch all the 24‐h cycling cells. If all the Ki67‐positive cells are considered (double and single labelled), 50.8% of the basal cells were positive. This is very similar to the value of 54% of cells trapped in the vincristine experiment suggesting a growth fraction of at least 51%. It is likely that the solely Ki67‐positive cells (9%) represent part of a cohort of cells with a longer cell cycle time. It seems reasonable to assume that the Ki67 labels all the cycling cell compartments except those cells in a Ki67 refractive phase of the cell cycle (late G2 or early G1). (see Gerdes et al. 1984; Birner et al. 2001; Tsurusawa & Fujimoto 1995).
Figure 2.
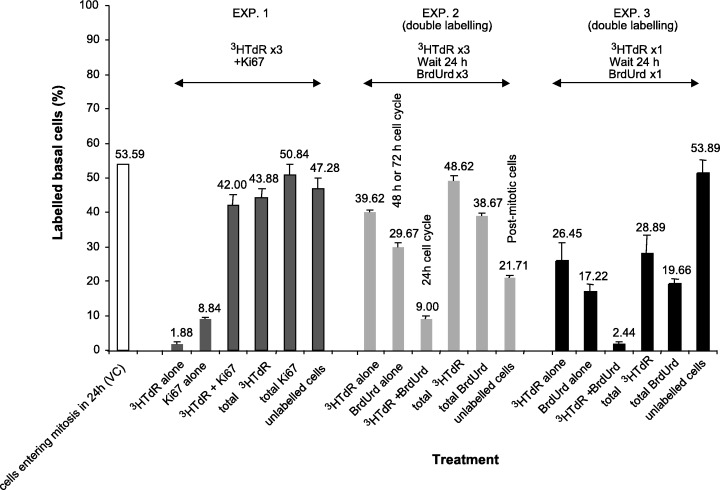
Bar chart showing the results of the three double‐labelling experiments for the percentage of labelled basal cells. The value obtained from the vincristine accumulation experiment is shown on the left for comparison. The bar chart shows the mean values for each experimental group of four animals with the standard errors of the mean. The figures above the bar chart show the rounded values for the percentage in each case. The full values plus their standard errors are shown in the text.
The second experiment involved a somewhat similar protocol of three injections of 3HTdR at 03.00, 06.00 and 09.00 h, followed 24 h later by three injections of BrdUrd. This protocol should clearly detect the proportion of cells that are in S phase on one day and that re‐enter S phase 24 h later. Such experiments are possible because of the strong circadian rhythm in the fraction of S phase cells with its peak values in the early hours of the morning. The results are also summarized in Fig. 2. In this experiment 49% of the basal cells were labelled with 3HTdR, similar to the 44% in experiment 1 (42 + 2%). Of the 49% of 3HTdR labelled cells, 18%, i.e. 9.0 ± 0.8% of the total basal cells, were double‐labelled and had therefore entered a second cell cycle and must have a cell cycle time of 24 h. About 29.7 ± 1.3% of the basal cells were labelled only with BrdUrd, i.e. these cells were not cycling on the previous day. Because of the strong circadian rhythm, this cohort must have a cell cycle time of 48 h or longer (e.g. 72 h). A total of 39% of the basal cells were labelled with BrdUrd (30 + 9%), in comparison with the 49 or 44% that were labelled using 3HTdR delivered in a similar way. About 21‐22% (100 − (40 + 30 + 9) = 21) of the basal cells were not labelled by either labelling protocol. These cells may represent the postmitotic cells awaiting migration into the suprabasal layer.
The final experiment involved a single injection of 3HTdR at 09.00 h, followed by a single injection of BrdUrd 24 h later, again at 09.00 h. In this experiment 28.9 ± 5.4% of the basal cells were labelled by the single injection of tritiated thymidine (single‐plus double‐labelled), i.e. a little over half (66%) of the total cells labelled by the three‐injection protocol could be detected by a single injection of tritiated thymidine at 09.00 h (29% as a fraction of 44%: the mean of 44, 49 and 39% as detected by the three labelling protocols). In comparison, 19.7 ± 1.8% of the cells were labelled by BrdUrd (single plus double). BrdUrd consistently gave a slightly lower labelling index. 2.44 ± 0.49% of the basal cells were double‐labelled. Thus, approximately 7.1% of the cells labelled at 09.00 h with 3HTdR could be labelled 24 h later by BrdUrd, and therefore have a 24‐h cell cycle. 17.2 ± 2.2% of the basal cells were labelled by BrdUrd alone and 26.45 ± 5.10% were labelled with tritiated thymidine alone.
These data suggest that the basal layer of the ventral tongue epithelium may contain at least three distinct cohorts of cells and the proliferation of these cells is highly synchronous within the framework of the circadian rhythm. The data from two different experiments suggest that approximately 20% of the basal cells may have exited the cell cycle and are postmitotic cells awaiting the appropriate signals for an ordered migration from the basal layer into the suprabasal compartments. It is possible that this compartment may also contain some cells that have longer cell cycle times (48‐72 h). The data suggest that a small component of the basal cells have a cell cycle time of 24 h (about 20%). Finally, there is a cohort (of about 30%) that may have a cell cycle time of 48 h or more.
These interpretations are consistent with the observation that there is considerable synchrony amongst proliferative cells within the circadian rhythm. Indeed, as has been suggested elsewhere, based on studies in dorsal epithelium of the tongue (Hume & Potten 1976), the rhythm may be generated by an absolute daily synchronization of the stem cells (Potten et al. 1977). However, the influence of variations in the cell cycle times within each of the cohorts on the interpretation remains unclear. Indeed it may be possible to explain the data largely on the basis of such variations, i.e. that the basal layer consists of a homogeneous cell population with a large latent variance in the cell cycle which is modulated via a gate‐control system by the circadian rhythm regulatory factors.
These data suggest that the proliferation kinetics may be hierarchical and show many similarities with the dorsal surface of the tongue (Hume & Potten 1976). In the dorsal surface, the evidence suggested that cells at the stem cell location were cycling with a 24‐h cycle time and that these cells showed the most dramatic circadian rhythm (i.e. greatest amplitude). It was assumed that the circadian rhythm in the tissue was generated as a result of the highly synchronous daily cycle of these stem cells (Hume & Potten 1976; Potten et al. 1977). This implies that the stem cell population is the most sensitive component of the basal layer to the signals that generate circadian fluctuations. They produce daughter cells that enter a dividing transit population only at one time of the day, and these dividing transit cells then gradually de‐synchronize as they pass through the transit generations, thus accounting for the spread in circadian synchronization seen when the tissue as a whole is analysed.
Similar proliferative cohorts have been detected by others (Dörr & Kummermehr 1991) based on studies such as percentage labelled mitosis analyses, with cohorts of cells having distinct cell cycle times differing by integers of 24 h. However, there is a surprising difference between the data generated by Dörr and those presented here in that the cell cycle time in the present data appears to be shorter by a factor of 2 overall, possibly due to mouse strain differences (see Dörr et al. 2002 in this supplement).
In the dorsal tongue, the proliferative populations and the lineages could clearly be related to structural units resembling the epidermal proliferative units (Potten 1974; Hume & Potten 1976). The ventral tongue has no clear structural organization that enables the proliferative units to be recognized but the hierarchical organization, together with the circadian synchronization indicated in the present studies, could account for the clustering of labelled cells that are seen in the histological preparations following the various labelling protocols. A stem cell dividing at a particular time of day would generate daughter cells with a tendency to enter S phase at approximately the same time of day and these daughter cells would be in close proximity and would have a high probability of being labelled. It is possible that the cohort of cells in the ventral tongue with a 24‐h cell cycle represent the stem cells and that the transit compartment has a longer cell cycle. This is also the situation postulated for the dorsal tongue (Hume & Potten 1976).
The proposed hierarchical organization places this epithelium, together with the dorsal tongue epithelium, as yet another example of a renewing cell population with a hierarchical proliferative organization. It would appear that such lineages are a common feature of cell renewal systems. It is not possible from the data available to predict the number of generations in the dividing transit compartment of the ventral tongue epithelium, but the lineage is unlikely to be longer than 2‐3 generations.
Acknowledgements
This work was supported by the Cancer Research Campaign (UK) and partially by Amgen Inc., California. We are grateful to Marcus Loeffler for helpful comments.
References
- Birner P, Ritzi M, Musahl C, Knippers R., Gerdes J, Voigtlander T, Budka H, Hainfellner JA (2001) Immunohistochemical detection of cell growth fraction in formalin‐fixed and paraffin‐embedded murine tissue. Am. J. Pathol. 158, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörr W, Kummermehr J (1991) Proliferation kinetics of mouse tongue epithelium under normal conditions and following single dose irradiation. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 60, 287. [DOI] [PubMed] [Google Scholar]
- Dörr W, Spekl K, Martin M (2002) Radiation induced oral mucositis in mice: Strain differences. Cell Prolif. 35 (Suppl. 1) 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baish H, Wacker H‐H, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation associated human nuclear antigen defined by the monoclonal antibody Ki‐67. J. Immunol. 133, 1710. [PubMed] [Google Scholar]
- Hume WJ, Potten CS (1976) The ordered columnar structure of mouse filiform papillae. J. Cell Sci. 22, 149. [DOI] [PubMed] [Google Scholar]
- Hume WJ, Thompson J (1990) Double labelling of cells with tritiated thymidine and bromodeoxyuridine reveals a circadian rhythm‐dependent variation in duration of DNA synthesis and S‐phase flux rates in rodent oral epithelium. Cell Tissue Kinetics 23, 313. [DOI] [PubMed] [Google Scholar]
- Potten CS, Al‐Barwari SE, Hume WJ, Searle J (1977) Circadian rhythms of presumptive stem cells in three different epithelia of the mouse. Cell Tissue Kinetics 10, 557. [DOI] [PubMed] [Google Scholar]
- Potten CS (1974) The epidermal proliferative unit: the possible role of the central basal cell. Cell Tissue Kinetics 7, 77. [DOI] [PubMed] [Google Scholar]
- Potten CS, Booth D, Cragg N, Tudor GL, O'Shea JA, Appleton D, Barthel D, Meineke FA, Loeffler M, Booth C (2002) Cell kinetic studies in the murine ventral tongue epithelium: thymidine metabolism studies and circadian rhythm determination. Cell Prolif. 35 (Suppl. 1) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson PJ, McGurk M, Potten CS, Walton GM, Appleton DR (1999) Tritiated thymidine and bromodeoxyuridine double‐labelling studies on growth factors and oral epithelial proliferation in the mouse. Arch. Oral Biol. 44, 721. [DOI] [PubMed] [Google Scholar]
- Tsurusawa M, Fujimoto T (1995) Cell cycle progression and phenotypic modification of Ki67 antigen‐negative G1 and G2 phase cells in phorbol ester‐treated Molt‐4 human leukemia cells. Cytometry 20, 146. [DOI] [PubMed] [Google Scholar]


