Abstract
Abstract. To further explore that hepatic stellate cell (HSC) activation results in physiological protection against environmental insult, the profile of differentiation of HSC has been examined upon treatment with ellagic acid (EA), a plant‐derived antioxidant that shows multiple protective effects during liver disease. Sparse rat liver cell cultures were grown in media containing EA (3, 6, 30 and 100 µg/ml) and, as controls, without EA, and inspected until day 7 in culture. The cells were double‐labelled with antibodies against glial fibrillary acidic protein (GFAP) and smooth muscle alpha‐actin (SMAA), marker proteins of quiescent and activated HSC, respectively. In EA‐free culture conditions, the quiescent (SMAA−/GFAP+) HSC transiently acquired a semi‐activated (SMAA+/GFAP+), phenotype and were further transformed into activated (SMAA+/GFAP−), pleomorphic HSC. Up to a concentration of 30 µg/ml, EA induced an early synthesis of SMAA in all HSC and inhibited their morphologic differentiation and individual growth throughout the culture period. At a concentration of 6 µg/ml, EA supported the semi‐activated (SMAA+/GFAP+) phenotype of HSC throughout the culture period, whereas treatment with high EA concentrations (30 µg/ml) resulted in an early loss of GFAP expression. In conclusion: (i) the uniform response of HSC to EA by mild activation adds functional significance to cellular features preceding the transformation of HSC to myofibroblasts; (ii) the high sensitivity of HSC to EA treatment suggests their involvement in any mechanisms of protection by this antioxidant; (iii) the maintenance of HSC morphology might be one of the factors playing a role in the prevention or slowing down of liver fibrosis; (iv) because the effects of EA are concentration‐ and time‐dependent, an arbitrary usage of this antioxidant is a matter of potential concern; (v) the various patterns of HSC activation observed might correspond to distinct activities of these cells, which, in turn, might lead to different outcomes of liver fibrosis.
INTRODUCTION
Ellagic acid (EA), a gallic acid dimer, is generated by hydrolysis of ellagitannins. This antioxidant is present in fruit berries, edible nuts, grapes, Brazilian and Paraguayan collections of Lafoensia pacari (Lythraceae), green tea, and the stem bark of Eucalyptus globulus (Stoner & Mukhtar 1995; Gastonguay et al. 1998; Solon et al. 2000; Kinjo et al. 2001 , Kim et al. 2001). Due to its high therapeutic potency based on its various metabolic activities, EA is widely used in folk medicine. As a metal‐binding compound, EA suppresses nickel‐induced biochemical alterations in kidney and liver (Ahmed et al. 1999). It attenuates the activity of N‐acetyl transaminase and decreases the N‐acetylation of carcinogens in several tissues (Lin et al. 2000). EA has been reported to inhibit tumorigenesis via several mechanisms including the enhancement of glutathione peroxidase, catalase, quinone reductase and glutathione‐S‐transferase activities (Mukhtar et al. 1984; Stoner & Mukhtar 1995). Examination of chemical analogues of EA showed that different portions of the ellagic acid molecule are responsible for its different putative anti‐carcinogenic activities (Barch et al. 1996). This antioxidant also shows anti‐inflammatory activity (Stoner & Mukhtar 1995) and, along with other antioxidants such as retinoic acid, retinol, beta‐carotene, canthaxanthin, and ascorbic acid, it reduces instability of chromosomes (Stich et al. 1990). It also protects isolated rat hepatocytes by inhibiting t‐butyl hydroperoxide‐induced lipid peroxide formation and the generation of superoxide anions (1999a, 1999b). These influences of EA on regulatory mechanisms (that may break down under stress) seem to be reflected in its various effects shown at cellular and subcellular levels (Ahn et al. 1996; Iakovleva et al. 2001). Though EA has been shown to inhibit carbon tetrachloride‐induced liver fibrosis in rats (Thresiamma & Kuttan 1996; Singh et al. 1999a), the influence of this commonly consumed antioxidant on the phenotypic modulation of hepatic stellate cells (HSC), which are in a strategic position to control the blood‐borne signals, has not previously been investigated.
The general anatomy of HSC is similar to that of other glial fibrillary acidic protein (GFAP)‐positive cells (for example, astrocytes), that are known to protect organ‐specific homeostasis by inducing various protective features of the blood–tissue interface. In healthy brain, astrocytes contribute to the establishment of a prophylactic, protective blood–tissue barrier supporting brain‐specific homeostasis necessary for neuronal function. Glomerular podocytes, the GFAP‐positive cells of the kidney participate in permeability‐limiting ultrafiltration. Though podocytes lack immediate contacts with parenchymal cells, i.e. renal tubule cells, they nonetheless, show protective features common to astrocytes and participate in renal homeostasis via control of the composition of primary urine running into the tubules (Buniatian et al. 2002). By contrast, the fenestrated nature of healthy liver sinusoids and low sensitivity threshold of quiescent HSC to xenobiotics reflect the liver‐specific function of detoxification performed by hepatocytes. In healthy liver, HSC possess features characteristic of differentiated astrocytes. (1999, 2001).
Numerous studies have demonstrated that, during liver injury, HSC undergo phenotypic transformation into extracellular matrix‐producing, myofibroblast‐like cells which proliferate in response to signals originating from inflamed regions (for reviews, see Friedman 1999; Gressner 2001; Kmiec 2001; Reeves & Friedman 2002). Activation of HSC during pathological situations raises the question of whether this reaction of HSC supports the protective response of the blood–liver interface to external pathologic influences, or, as is traditionally considered, it appears to be the priming mechanism initiating liver fibrosis. Previous results demonstrating that HSC differentiation leads to an acquisition of features common to undifferentiated smooth muscle alpha‐actin (SMAA)‐positive myofibroblast‐like astrocytes have raised doubts concerning the deleterious influences of activated HSC (for review, see Buniatian 2001). The reaction of HSC to EA, an antioxidant that possesses a protective influence during liver diseases, might provide information concerning the cellular mechanisms of protection underlying the recovery from liver diseases.
MATERIALS AND METHODS
Animals and reagents
Sprague‐Dawley rats were purchased from Interfauna (Tuttlingen, Germany). EA was purchased from Sigma (Deisenhofen, Germany). Rabbit anti‐bovine GFAP antiserum was purchased from Dacopatts (Glostrup, Denmark), monoclonal antibody (MAb) against SMAA, clone ASM‐1 (mouse IgG2a), from Progen (Heidelberg, Germany), and fluorescein isothiocyanate (FITC)‐labelled goat anti‐rabbit IgG from Sigma. Cy3‐conjugated goat anti‐mouse IgG was obtained from Dianova (Jackson Immunoresearch, West Grove, PA, USA). The sources of other reagents are specified elsewhere (Buniatian et al. 2002).
Methods
Preparation of control cultures As a source of HSC, sparse hepatocyte primary cultures were used. Such cultures are advantageous in investigating the profile of cell differentiation over a relatively short period of time and examining the behaviour of HSC and hepatocytes under the same conditions. Hepatocytes were isolated from rat liver by a modification (Gebhardt et al. 1990) of the two‐step collagenase perfusion technique (Seglen 1976) and were purified by differential centrifugation (Seglen 1976; Gebhardt et al. 1990). Such preparations are always contaminated by a small population of HSC, demonstratable by staining for GFAP (Buniatian et al. 1996a). For culture, the cells were suspended in 90% William's Medium E/10% foetal calf serum containing 2 mm glutamine, 50 U/ml penicillin and 50 µg/ml streptomycin sulphate. Three millilitres of cell suspension containing 1 million viable cells/ml were transferred into plastic Petri dishes (60 mm in diameter) containing cover‐slips (18 mm × 18 mm) and incubated at 37 °C in a humidified atmosphere containing 5% CO2 in air. In control cultures, 4 h after inoculation, the medium was changed to serum‐free William's Medium E containing 2 mm glutamine, 0.1 mm dexamethasone and antibiotics as above. The serum‐free medium was renewed daily.
Preparation of EA‐supplemented cultures In this series of experiments, 4 h after inoculation of liver cells, the medium was changed to serum‐free Williams E culture medium supplemented with EA. EA was dissolved in 100% dimethylsulfoxide (DMSO) to give a stock solution concentration of 1 mg/ml. Portions of this solution were transferred to William's Medium E to give final concentrations of EA of 100, 30, 6 and 3 µg/ml. Cell growth was stopped after each 24 h during the next 7 days, by washing the cultures with phosphate‐buffered saline (PBS) at room temperature, followed by methanol fixation at −20 °C for 10 min.
Double‐labelling immunofluorescence analyses
Cell cultures After fixation in ice‐cold methanol, cells were washed with PBS at room temperature and exposed to a mixture of anti‐GFAP antiserum (dilution 1 : 100) and MAb SMAA (dilution 1 : 100). Subsequently, the cells were incubated for 2 h at 4 °C in a humid environment and washed twice for 15 min with PBS – 0.1% (w/v) Triton X‐100. The cells were exposed for 1 h to the mixture of secondary antibodies: fluorescein isothiocyanate‐labelled goat anti‐rabbit IgG (dilution 1 : 100) and Cy3‐conjugated goat anti‐mouse IgG (dilution 1 : 800). All antibodies were diluted with PBS. After washing twice in PBS, the cells were mounted in 50% glycerol. In control procedures, the labelling with primary antibodies was omitted. Microscopy was performed using a Zeiss fluorescence microscope (IM‐35).
RESULTS
Definition of HSC phenotype followed the established functional classification of HSC, i.e. quiescent and activated, corresponding to early and late stages of HSC differentiation. To determine the phenotype of HSC, the presence of established functional marker proteins was investigated: GFAP (Buniatian et al. 1996a) and SMAA (Johnson et al. 1992), the marker proteins of quiescent and activated HSC, respectively. According to this functional classification and because in the intermediary stage of differentiation the cells show a mixture of features characteristic of quiescent and activated HSC (Nanni et al. 2002), the cells expressing both GFAP and SMAA were designated as semi‐activated.
HSC of quiescent SMAA−/GFAP+ phenotype
Previous studies (Buniatian et al. 1996b) showed that, during the first day of culture, HSC expressed GFAP and lacked SMAA (i.e. were SMAA−/GFAP+). A similar result was obtained in this study: whether culture medium were supplemented with EA or not, the HSC possessed well‐developed processes extending from a small cell body and expressed solely GFAP (not shown).
HSC of semi‐activated SMAA+/GFAP+ phenotype
Control cultures After 2 days of culture in EA‐free medium, in addition to the cells containing solely GFAP, a small population of cells was observed that responded to the culture conditions by semi‐activation, illustrated by the maintenance of the GFAP cytoskeleton and de novo expression of SMAA. Thereafter, and up until day 4, a high proportion of GFAP‐positive HSC (Fig. 1a) expressed SMAA (Fig. 1b). The semi‐activated phenotype of HSC dominated during a short period of differentiation in culture (day 3–4) and was characterized by process‐bearing morphology and moderate enlargement of the cell body, visualized not only by staining for GFAP but also for SMAA (cf. Fig. 1a with Fig. 1b). Diverse arrangement of the GFAP cytoskeleton was characteristic of HSC of 3–4‐day‐old‐control cultures: in some cells, GFAP was distributed throughout the cytoplasm, in others the area of GFAP reaction was strongly reduced.
Figure 1.
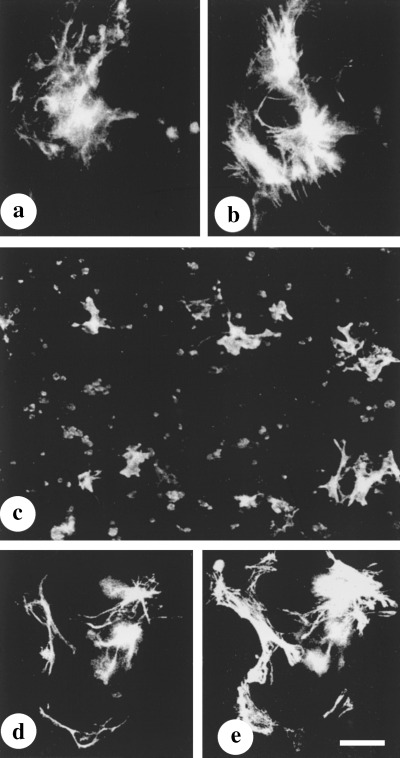
Co‐expression of GFAP and SMAA in early EA‐free control(a–b) and EA‐treated cultures(c–e). (a, b) Staining for GFAP (a) and SMAA (b) in a 3‐day‐old EA‐free culture. (c) De novo expression of SMAA in a 2‐day‐old culture treated with EA (up to 30 µg/ml). (d, e) Co‐expression of GFAP and SMAA in an early, EA‐treated culture. In HSC from 2‐ to 3‐day‐old control cultures, the area of SMAA reaction and the size of the cells are greater, compared with those treated with EA. (cf. b with e). Double‐labelling was performed as follows: antiserum against GFAP and fluorescein isothiocyanate‐conjugated anti‐rabbit IgG from goat (a, d); MAb SMAA and Cy3‐conjugated anti‐mouse IgG from goat (b, d, e). Bar = 50 µm in a, b, d, e, and 100 µm in c.
EA‐treated cultures In 2‐day‐old liver cell cultures grown in EA‐supplemented medium (6 and 30 µg/ml) the GFAP‐positive cells showed de novo expression of SMAA. Figure 1(c) illustrates a 2‐day‐old, EA‐treated cell culture scattered with SMAA‐positive HSC. The SMAA‐positive HSC (Fig. 1e) still contained GFAP filaments (Fig. 1d) and exhibited the original, process‐bearing morphology. The semi‐activated phenotype of HSC induced by 6 µg/ml of EA at day 2 in culture was maintained throughout the culture period of 7 days: the cells expressed both SMAA and GFAP (Fig. 2a and b, respectively). In addition, the culture contained multiple hepatocytes.
Figure 2.
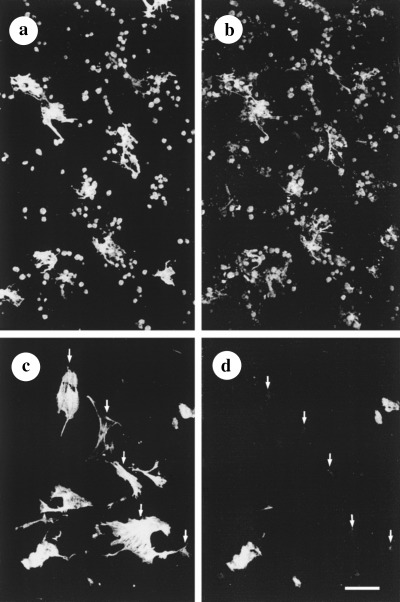
Differences in the morphology and the composition of the cytoskeletal proteins of HSC from 7‐day‐old, EA‐treated (a, b) and control (c, d) cultures. During treatment with EA (6 µg/ml EA), HSC maintained their semi‐activated phenotype and stellate morphology well distinguished when stained for SMAA (a) and GFAP (b). In EA‐free medium, HSC lacked processes, were enlarged in size and expressed SMAA (c). In the perinuclear area of some of these HSC, a barely distinguishable reaction of GFAP could be seen (arrows in c, d). Double‐labelling was performed as follows: (a, c) MAb SMAA and Cy3‐conjugated anti‐mouse IgG from goat; (b, d) antiserum against GFAP and fluorescein isothiocyanate‐conjugated anti‐rabbit IgG from goat. Bar = 100 µm.
HSC of myofibroblastic phenotype
Control cultures With increasing time in culture, the population of SMAA−/GFAP+ and SMAA+/GFAP− HSC was replaced by a cell population solely expressing SMAA (Fig. 2c and d). After day 5 in EA‐free culture, the transiently semi‐activated HSC underwent changes characterized by high variability in cell morphology and size: large, flat and irregularly shaped, process‐lacking HSC resided close to small and medium‐sized SMAA‐positive cells (Fig. 2c). In some cells, only traces of GFAP with a perinuclear localization could be visualized (cf. arrows in Fig. 2c and d), whereas in other cells GFAP had completely disappeared. Most of these cells developed dynamic locomotor organelles: lamellipodia and growth cone‐like structures (Fig. 2c). In a 7‐day‐old control culture, large regions deprived of hepatocytes (Fig. 2c and d), as well as areas containing conglomerates of damaged cells non‐specifically staining with secondary antibodies, were seen. Heterogeneity in the distribution pattern of SMAA filaments was characteristic of HSC grown under control culture conditions: in some cells, sparsely distributed filaments of SMAA merged into the cell membranes strongly delineated by large amounts of polymerized SMAA (Fig. 3a); in others, they were randomly distributed throughout the cytoplasm, whereas GFAP was absent or strongly down‐regulated. By contrast, in HSC exposed to EA at a concentration of 6 µg/ml, the filamentous pattern of GFAP staining was preserved and the distribution of SMAA microfilaments was relatively dense (Fig. 3b and c, respectively).
Figure 3.
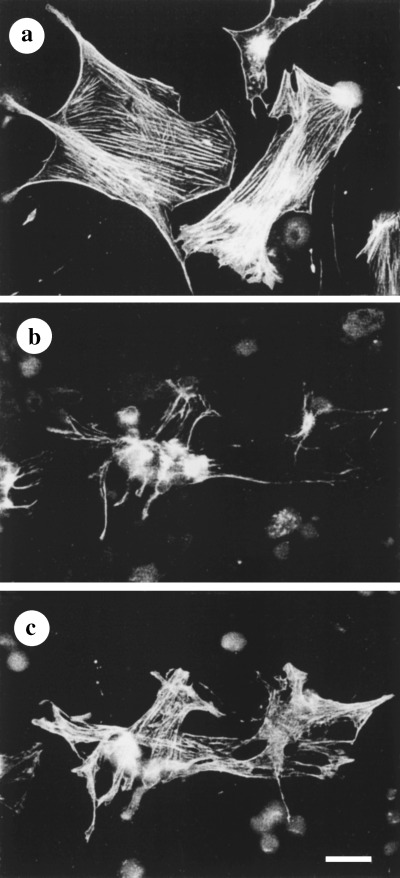
Differences in the distribution of SMAA and GFAP under high power magnification. (a) EA‐free culture. Stress fibre‐like arrangement of SMAA. (b, c) EA‐treated culture (6 µg/ml EA): localization of GFAP (b) and SMAA (c) in HSC. Some GFAP filaments are observed (b) and there is a relatively dense packing of SMAA filaments (c). Double‐labelling was performed as follows: (a, d) MAb SMAA and Cy3‐conjugated anti‐mouse IgG from goat; (b) antiserum against GFAP and fluorescein isothiocyanate‐conjugated anti‐rabbit IgG from goat. Bar = 50 µm.
Concentration and time‐dependent effects of EA
Low EA concentration The effects of EA on the expression of GFAP were time‐ and concentration‐dependent. These differences were seen only after day 2 in culture. After 7 days of culture in a medium supplemented with 3 µg/ml of EA, the cells had maintained their small size and process‐bearing morphology, as revealed by staining for SMAA and GFAP (Fig. 4a and b, respectively). In these cells, the intensity of GFAP staining was weaker than in the cells grown in medium containing 6 µg/ml of EA (cf. Fig. 4b with Fig. 2b).
Figure 4.
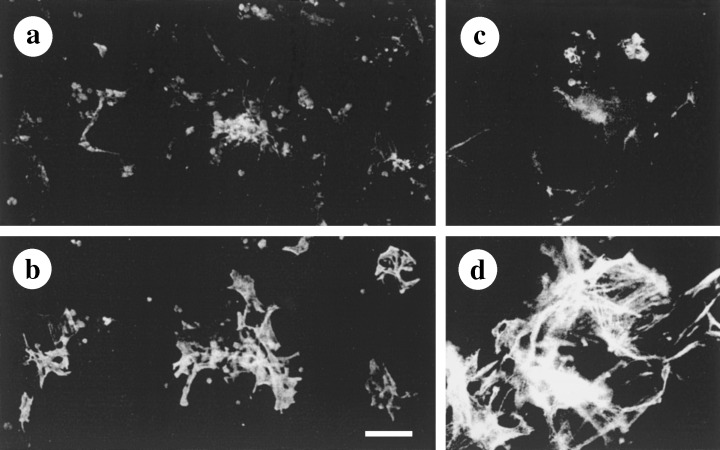
Concentration‐ and time‐dependent influence of EA on HSC activation. (a, b) Co‐expression of GFAP (a) and SMAA (b) in HSC from 7‐day‐old culture treated with a low concentration of EA (3 µg/ml). While cells preserved their stellate morphology, intensity of staining of GFAP was low. (c, d) Co‐expression of GFAP (c) and SMAA (d) in HSC from 7‐day‐old culture treated with a high concentration of EA (30 µg/ml). HSC preserved their small size and extended cellular processes that are readily seen after staining for SMAA (d), however, these cells essentially lack GFAP (c). Double‐labelling was performed as follows: (a, c) antiserum against GFAP and fluorescein isothiocyanate‐conjugated anti‐rabbit IgG from goat; (b, d) MAb SMAA and Cy3‐conjugated anti‐mouse IgG from goat. Bar = 100 µm in a and b, and 50 µm in c and d.
High EA concentrations After 4 days of exposure to 30 µg/ml of EA (Fig. 4c and d), the moderate overall activation of HSC seen at day 2 of culture had shifted to full activation (cf. Fig. 4c and d with Fig. 1h and i). The treatment of liver cell cultures with high concentrations of antioxidant resulted in an early loss of GFAP (Fig. 4d) from those HSC strongly expressing SMAA (Fig. 4c). In contrast to the shapeless and flat SMAA+/GFAP− myofibroblastic cells generated under control culture conditions, EA‐treated HSC preserved their stellate morphology and small size when stained for SMAA (cf. Fig. 4c with Fig. 3a and d). Prolonged treatment with 30 µg/ml of EA led to significant changes in the appearance of hepatocytes: in some regions of the culture, aggregates of damaged cells which were non‐specifically stained with secondary antibodies were observed (not shown). At higher concentrations, EA showed toxic effects. Two experiments conducted at an EA concentration of 100 µg/ml EA resulted in the disappearance of all of the cells from the culture dish (not shown).
DISCUSSION
The result of this study was activation of HSC in rat hepatocyte primary cultures treated with EA, a naturally occurring plant polyphenol antioxidant known to inhibit the development of liver fibrosis in rats (Thresiamma & Kuttan 1996). The pathways of HSC differentiation during liver fibrosis and in culture are similar in that quiescent HSC transform to myofibroblast‐like cells. Careful analysis of the dynamics of and differences in morphology and antigenicity of differentiating HSC, i.e. of changes in the intracellular distribution and translocation of cytoskeletal proteins, leading to changes in cells’ functional capacities, revealed several stages of HSC activation: (i) semi‐activation characterized by de novo expression of SMAA, preserved GFAP cytoskeleton and process‐bearing morphology; (ii) full activation characterized by preserved process‐bearing morphology stabilized by densely packed filaments of SMAA and lacking GFAP; and (iii) activation accompanied by significant changes in morphology, absence of GFAP and sparse distribution of SMAA filaments. The features of semi‐activated HSC transiently acquired between the quiescent and activated state and reflecting their first response to changes in the environmental conditions have never before been considered as an important criterion of HSC function. However, exactly these features of HSC induced by EA appear to be functionally relevant.
In situ studies performed on cryosections of healthy rat liver (Buniatian, unpublished results) demonstrated the ability of HSC to remodel their cytoskeleton and to adapt their activities to region‐specific requirements as evidenced from: (i) direct contact of semi‐activated HSC with vessels of different calibre, (ii) accumulation of semi‐activated HSC in regions of increased blood pressure, and (iii) quiescent features of HSC adjacent to liver capillaries distributed throughout the liver parenchyma. In theory, mild activation of HSC may be of a temporary character and may be easily reversed. This might explain the decrease in number of SMAA‐positive HSC accompanying the recovery from liver fibrosis (Ramm et al. 2000; Iredale 2001) and probably associated with abating requirements for protection. The results obtained in this study support the notion (Khan et al. 2001) that the presence of SMAA in HSC cannot be used as a measure of progression of fibrosis: in the liver of patients with chronic hepatitis C infection, SMAA was expressed without an obvious correlation with the severity of liver injury. Also, the fact that SMAA is down‐regulated in cultured HSC upon exposure to high concentrations of malondialdehyde and 4‐hydroxynonenal (Olynyk et al. 2002) suggests that inactivation of HSC cannot always be considered as a factor favouring the normalization of liver function.
Analysis of current literature reveals data that provide strength to the novel view of the protective nature of HSC activation (Buniatian 2001). HSC proliferate in response to external factors of different aetiology (Davis & Kresina 1996). They transform to myofibroblast‐like cells during acute liver injury (Johnson et al. 1992; Schirmacher et al. 1993) and they respond to temporary and chronic changes in liver‐specific homeostasis by activation (for reviews, see Burt 1999; Friedman 1999; Kmiec 2001). Activated HSC regulate the contractility of blood vessels and their resistance to blood pressure (Rockey & Weisiger 1996; Thimgan & Yee 1999) and they migrate towards loci of injury. During pathological situations, activated HSC increase the production of extracellular matrix (ECM) proteins (for review see, Burt 1999) and remodel the sinusoidal wall and necrotic areas (Jin et al. 2002).
Up‐regulation of type 1 collagen and fibronectin, known to facilitate cell locomotion and signal transduction in different tissues in culture (Nelson et al. 1996; Schuppan et al. 2001) seems to favour the activities of HSC and tissue repair. Interestingly, fibrillogenesis of type I and type III collagens is regulated by the pre‐existing fibronectin network and integrin receptors (Velling et al. 2002). Conversely, fibronectin fibrillogenesis in tissue culture is dependent on the synthesis of type I collagen (Dzamba et al. 1993). Activated HSC not only produce ECM proteins but also regulate their levels via synthesis and release of a number of proteases (for review, see Burt 1999). The protective nature of HSC activation has been indicated by de novo synthesis of Cu/Zn‐metallothionein I (MT), a powerful anti‐cytotoxicity system caused by oxidative stress present in astrocytes (Buniatian et al. 2001). MT is known to act on both the intra‐ and extracellular environment by regulating the levels of heavy metals and by scavenging free radicals (Hidalgo et al. 1991). In addition, activation of HSC is accompanied by de novo synthesis of glutamine synthetase (GS; Bode et al. 1998), another marker protein of astrocytes. In normal liver, GS detoxifies residual ammonia in perivenous cells by the glutamate/glutamine transformation (Watford 2000). Up‐regulation of both, MT and GS, is associated with an increase in the number of neural (Frohlich & Klessen 2000) and non‐neural (Arora et al. 1998; DeMarco et al. 1999) cells and with tissue repair. This well‐co‐ordinated system of protective mechanisms might explain the multiple cases of spontaneous reversal of liver fibrosis and cirrhosis induced by different fibrogenic factors. Irreversible damage to hepatic tissue caused by durable and massive attack of harmful agents (Maros et al. 1975) may be due to the impairment of constituents of this versatile system of protection in which activated HSC seem to play an important role.
Sparse culturing conditions affect some of the most important aspects of cell survival, i.e. cell‐to‐cell communication, signal transduction and co‐operation in cell response, which together are responsible for increased rates of cell differentiation. HSC–hepatocyte and HSC–HSC contacts are mainly based on the well‐developed processes of HSC. During liver fibrosis, the star‐shaped HSC change their morphology and acquire the features of myofibroblasts (Senoo et al. 1998). In EA‐free culture, HSC lose their processes over time. Treatment of liver cell cultures with both low and high EA concentrations preserves the processes of HSC. In accordance with this observation, EA treatment of HSC has been shown to cause an enhancement of gap junction communication (Stoner & Mukhtar 1995). In addition, EA inhibits the growth of individual HSC, which is accompanied by changes in the SMAA‐ and GFAP‐based cytoskeleton. These influences of EA on HSC morphology might play a role in slowing down or preventing liver fibrosis, during which the star‐shaped HSC change their morphology and acquire myofibroblastic features of (Senoo et al. 1998).
The results of the present study are relevant because EA, side by side with its regulatory effects exerted at moderate concentrations, may reduce the expression of GFAP in HSC at high concentrations and cause a massive detachment of cells from their substrata. These results are in accordance with the effects of dietary EA shown during the course of dimethylbenzanthracene‐induced multi‐organ carcinogenesis in rainbow trout: at low doses, dietary EA consistently suppressed stomach tumour development, whereas, at higher doses, it was toxic and showed a potential for the enhancement of tumour response in other organs (Harttig et al. 1996). Such a large spectrum of cellular responses to different concentrations of EA prohibits an arbitrary and uncontrolled usage of this antioxidant. Thus, the safety and the effectiveness of EA supplements and dosages should be carefully analysed further.
Molecular mechanism(s) underlying the behaviour of HSC and hepatocytes during changes of the environment and organ‐specific homeostasis, for example, during oxidative stress, as a result of the accumulation of reactive oxygen species and down‐regulation of antioxidants, seems to be common for both in vivo and in vitro conditions (Kim et al. 2000; Orsi & Leese 2001). The view that, in hepatic tissue, oxidative stress, accompanied by an impaired function of hepatocytes, provides an important signal for the activation of HSC (Kim et al. 2000) is also true for cell culture conditions. At certain concentrations, EA inhibits degeneration of hepatocytes and increases their survival in prolonged culture. The hepatoprotective effects of EA are based not only on its capacity to regulate liver‐specific, extracellular homeostasis by scavenging free radical species, but also on its capacity to regulate intimate intracellular mechanisms by direct interaction with double‐stranded DNA (Thulstrup et al. 1999; Festa et al. 2001). It has been shown that EA attenuates the damaging effects of H2O2 and bleomycin, strong inducers of oxygen radicals, on double‐stranded DNA (Festa et al. 2001), and EA enhancement of DNA continuity (for which type III intermediate proteins show high affinity) (Traub 1995; Tolstonog et al. 2000), may explain the maintenance of the GFAP cytoskeleton of HSC under non‐favourable, sparse culturing conditions.
ACKNOWLEDGEMENTS
The author is grateful to Prof Peter Traub and Dr Robert Shoeman for valuable remarks on the manuscript and to Ms Sabine Ebert for excellent technical assistance.
REFERENCES
- Ahmed S, Rahman A, Saleem M, Athar M, Sultana S (1999) Ellagic acid ameliorates nickel‐induced biochemical alterations: diminution of oxidative stress. Hum. Exp. Toxicol. 18, 691. [DOI] [PubMed] [Google Scholar]
- Ahn D, Putt D, Kresty L, Stoner GD, Fromm D, Hollenberg PF (1996) The effects of dietary ellagic acid on rat hepatic and esophageal mucosal cytochromes P450 and phase II enzymes. Carcinogenesis 17, 821. [DOI] [PubMed] [Google Scholar]
- Arora V, Iversen PL, Ebadi M (1998) Manipulation of metallothionein expression in the regenerating rat liver using antisense oligonucleotides. Biochem. Biophys. Res. Commun. 246, 711. [DOI] [PubMed] [Google Scholar]
- Barch DH, Rundhaugen LM, Stoner GD, Pillay NS, Rosche WA (1996)Structure‐function relationships of the dietary anti‐carcinogen ellagic acid. Carcinogenesis 17, 265. [DOI] [PubMed] [Google Scholar]
- Bode JG, Peters‐Regehr T, Gressner AM, Häussinger D (1998) De novo expression of glutamine synthetase during transformation of hepatic stellate cells into myofibroblast‐like cells. Biochem. J. 335, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buniatian GH (2001) Similar protective features of activated hepatic stellate cells and healthy astrocytes In: Wisse E, Knook DL, De Zanger R, Fraser R, eds. Cells of Hepatic Sinusoid, p. 200. The Netherlands: The Kupffer Cell Foundation. [Google Scholar]
- Buniatian G, Gebhardt R, Schrenk D, Hamprecht B (1996a) Co‐localization of three types of intermediate filament proteins in perisinusoidal stellate cells: glial fibrillary acidic protein as a new cellular marker. Eur. J. Cell Biol. 70, 23. [PubMed] [Google Scholar]
- Buniatian G, Hamprecht B, Gebhardt R (1996b) Glial fibrillary acidic protein as a marker of perisinusoidal stellate cells that can distinguish between the normal and myofibroblast‐like phenotypes. Biol. Cell 87, 65. [PubMed] [Google Scholar]
- Buniatian GH, Gebhardt R, Mecke D, Traub P, Wiesinger H (1999) Common myofibroblastic features of newborn rat astrocytes and cirrhotic rat liver stellate cells in early cultures and in vivo . Neurochem. Int. 35, 317. [DOI] [PubMed] [Google Scholar]
- Buniatian GH, Hartmann H‐J, Traub P, Weser U, Wiesinger H, Gebhardt R (2001) Acquisition of blood–tissue barrier‐supporting features by hepatic stellate cells and astrocytes of myofibroblastic phenotype. Inverse dynamics of metallothionein and glial fibrillary acidic protein expression. Neurochem. Int. 38, 373. [DOI] [PubMed] [Google Scholar]
- Buniatian GH, Hartmann H‐J, Traub P, Wiesinger H, Albinus M, Nagel W, Shoeman R, Mecke D, Weser U (2002) Glial fibrillary acidic protein‐positive cells of the kidney are capable of raising a protective biochemical barrier similar to astrocytes: expression of metallothionein in podocytes. Anat. Rec. 267, 296. [DOI] [PubMed] [Google Scholar]
- Burt AD (1999) Pathobiology of hepatic stellate cells. J. Gastroenterol. 34, 299. [DOI] [PubMed] [Google Scholar]
- Davis BH, Kresina TF (1996) Hepatic fibrogenesis. Clin. Laboratory Med. 16, 361. [PubMed] [Google Scholar]
- Demarco V, Dyess K, Strauss D, West CM, Neu J (1999) Inhibition of glutamine synthetase decreases proliferation of cultured rat intestinal epithelial cells. J. Nutr. 129, 57. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Wu H, Jaenisch R, Peters DM (1993) Fibronectin binding site in type I collagen regulates fibronectin fibril formation. J. Cell Biol. 121, 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa F, Aglitti T, Duranti G, Ricordy R, Perticone P, Cozzi R (2001) Strong antioxidant activity of ellagic acid in mammalian cells in vitro revealed by the comet assay. Anticancer Res. 21, 3903. [PubMed] [Google Scholar]
- Friedman SL (1999) Stellate cell activation in alcoholic fibrosis – an overview. Alcohol. Clin. Exp. Res. 23, 904. [PubMed] [Google Scholar]
- Frohlich E, Klessen C (2000) Glutamine synthetase and marker enzymes of the blood–retina barrier in fetal bovine retinal pigment epithelial cells. Graefes. Arch. Clin. Exp. Ophthalmol. 238, 500. [DOI] [PubMed] [Google Scholar]
- Gastonguay A, Boukharta M, Teel R (1998) Biodistribution of anti‐mutagenic efficacies in Salmonella typhimurium of, and inhibition of, P450 activities by ellagic acid and one analogue. Chem. Res. Toxicol. 11, 1258. [DOI] [PubMed] [Google Scholar]
- Gebhardt R, Fitzke H, Fausel M, Eisenmann‐Tappe I, Mecke D (1990) Influence of hormones and drugs on glutathione‐transferase levels in primary culture of adult rat hepatocytes. Cell Biol. Toxicol. 6, 365. [DOI] [PubMed] [Google Scholar]
- Gressner AM (2001) The up‐and‐down of hepatic stellate cells in tissue injury: apoptosis restores cellular homeostasis. Gastroenterology. 120, 1285. [DOI] [PubMed] [Google Scholar]
- Harttig U, Hendricks JD, Stoner GD, Bailey GS (1996) Organ specific, protocol dependent modulation of 7,12‐dimethylbenz[a]anthracene carcinogenesis in rainbow trout (Oncorhynchus mykiss) by dietary ellagic acid. Carcinogenesis 17, 2403. [DOI] [PubMed] [Google Scholar]
- Hidalgo J, Dingman A, Garvey JS (1991) Role of extracellular zinc and copper on metallothionein regulation in cultured rat hepatocytes. Hepatology 14, 648. [DOI] [PubMed] [Google Scholar]
- Iakovleva LV, Gerasimova OA, Karbusheva IV, Ivakhnenko AK, Buniatian ND, Sakharova TS (2001) Antioxidant properties of novel preparations – bioflavonoid derivatives and tannins. Eksp. Klin. Farmakol. 64, 55. [PubMed] [Google Scholar]
- Iredale JP (2001) Hepatic stellate cell behavior during resolution of liver injury. Semin. Liver Dis. 21, 427. [DOI] [PubMed] [Google Scholar]
- Jin YL, Enzan H, Kuroda N, Hayashi Y, Nakayama H, Zhang YH, Toi M, Miyazaki E, Hiroi M, Guo LM, Saibara T (2002) Tissue remodeling following submassive hemorrhagic necrosis in rat livers induced by an intraperitoneal injection of dimethylnitrosamine. Virchows Arch. 442, 39. [DOI] [PubMed] [Google Scholar]
- Johnson SJ, Hines JE, Burt AD (1992) Phenotypic modulation of perisinusoidal cells following acute liver injury: a quantitative analysis. Int. J. Exp. Pathol. 73, 765. [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Poulos JE, Brunt EM, Li L, Solomon H, Britton RS, Bacon BR, Di Bisceglie AM (2001) Hepatic α‐smooth muscle actin expression in hepatitis C patients before and after interferon therapy. Hepatogastroenterology 48, 212. [PubMed] [Google Scholar]
- Kim KY, Choi I, Kim SS (2000) Progression of hepatic stellate cell activation is associated with the level of oxidative stress rather than cytokines during CCl4‐induced fibrogenesis. Mol. Cells 10, 289. [PubMed] [Google Scholar]
- Kim JP, Lee IK, Yun BS, Chung SH, Shim GS, Koshino H, Yoo ID (2001) Ellagic acid rhamnosides from the stem bark of Eucalyptus globulus . Phytochemistry 57, 587. [DOI] [PubMed] [Google Scholar]
- Kinjo J, Nagao S, Tanaka T, Nonaka GI, Okabe H (2001) Antiproliferative constituents in the plant 8. Seeds of Rhynchosia volubilis . Biol. Pharm. Bull. 24, 1443. [DOI] [PubMed] [Google Scholar]
- Kmiec Z (2001) Co‐operation of liver cells in health and disease. Adv. Anat. Embryol. Cell Biol. 161, 1. [DOI] [PubMed] [Google Scholar]
- Lin SS, Hung CF, Ho CC, Liu YH, Ho HC, Chung JG (2000) Effects of ellagic acid by oral administration on N‐acetylation and metabolism of 2‐aminofluorene in rat brain tissues. Neurochem. Res. 25, 1503. [DOI] [PubMed] [Google Scholar]
- Maros T, Seres‐Sturm L, Lakatos O, Seres‐Sturm MT, Blazsek V (1975) Spontaneous reversibility of advanced toxic liver cirrhosis. Acta. Morph. Acad. Sci. Hung. 23, 293. [PubMed] [Google Scholar]
- Mukhtar H, Das M, Del Tito BJ, Bickers DR (1984) Protection against 3‐methylcholanthrene‐induced skin tumorigenesis in Balb/C mice by ellagic acid. Biochem. Biophys. Res. Commun. 119, 751. [DOI] [PubMed] [Google Scholar]
- Nanni G, Majorani F, Bassi AM, Canepa C, Maloberti G, Casu A (2002) Dolichol content in isolated sinusoidal liver cells after in vivo chronic treatment with thioacetamide. Exp. Toxicol. Pathol. 54, 43. [DOI] [PubMed] [Google Scholar]
- Nelson PR, Yamamura S, Kent KC (1996) Extracellular matrix proteins are potent agonists of human smooth muscle cell migration. J. Vasc. Surg. 24, 25. [DOI] [PubMed] [Google Scholar]
- Olynyk JK, Khan NA, Ramm GA, Brown KE, O'Neill R, Britton RS, Bacon BR (2002) Aldehydic products of lipid peroxidation do not directly activate rat hepatic stellate cells. J. Gastroenterol. Hepatol. 17, 785. [DOI] [PubMed] [Google Scholar]
- Orsi NM, Leese HJ (2001) Protection against reactive oxygen species during mouse pre‐implantation embryo development: role of EDTA, oxygen tension, catalase, superoxide dismutase and pyruvate. Mol. Reprod. Dev. 59, 44. [DOI] [PubMed] [Google Scholar]
- Ramm GA, Carr SC, Bridle KR, Li L, Britton RS, Crawford DH, Vogler CA, Bacon BR, Tracy TF (2000) Morphology of liver repair following cholestatic liver injury: resolution of ductal hyperplasia, matrix deposition and regression of myofibroblasts. Liver 20, 387. [DOI] [PubMed] [Google Scholar]
- Reeves HL, Friedman SL (2002) Activation of hepatic stellate cells – a key issue in liver fibrosis. Front. Biosci. 7, 808. [DOI] [PubMed] [Google Scholar]
- Rockey DC, Weisiger RA (1996) Endothelin‐induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology 24, 233. [DOI] [PubMed] [Google Scholar]
- Schirmacher P, Geerts A, Jung W, Pietrangelo A, Rogler CE, Dienes HP (1993) The role of Ito cells in the biosynthesis of HGF‐SF in the liver. EXS 65, 285. [PubMed] [Google Scholar]
- Schuppan D, Ruehl M, Somasundaram R, Hahn EG (2001) Matrix as a modulator of hepatic fibrogenesis. Semin. Liver. Dis. 21, 351. [DOI] [PubMed] [Google Scholar]
- Seglen PO (1976) Preparation of isolated rat liver cells. Meth. Cell Biol. 13, 29. [DOI] [PubMed] [Google Scholar]
- Senoo H, Imai K, Matano Y, Sato M (1998) Molecular mechanisms in the reversible regulation of morphology, proliferation and collagen metabolism in hepatic stellate cells by the three‐dimensional structure of the extracellular matrix. J. Gastroenterol. Hepatol. 13, S19. [PubMed] [Google Scholar]
- Singh K, Khanna AK, Chander R (1999a) Hepatoprotective activity of ellagic acid against carbon tetrachloride induced hepatotoxicity in rats. Indian J. Exp. Biol. 37, 1025. [PubMed] [Google Scholar]
- Singh K, Khanna AK, Visen PK, Chander R (1999b) Protective effect of ellagic acid on t‐butyl hydroperoxide induced lipid peroxidation in isolated rat hepatocytes. Indian. J. Exp. Biol. 37, 939. [PubMed] [Google Scholar]
- Solon S, Lopes L, Teixeira De Sousa P, Schmeda‐Hirschmann G (2000) Free radical scavenging activity of Lafoensia pacari . J. Ethnopharmacol. 72, 173. [DOI] [PubMed] [Google Scholar]
- Stich HF, Tsang SS, Palcic B (1990) The effect of retinoids, carotenoids and phenolics on chromosomal instability of bovine papillomavirus DNA‐carrying cells. Mutat. Res. 241, 387. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Mukhtar H (1995) Polyphenols as cancer chemopreventive agents. J. Cell Biochem. (Suppl.) 22, 169. [DOI] [PubMed] [Google Scholar]
- Thimgan MS, Yee HF Jr (1999) Quantitation of rat hepatic stellate cell contraction: stellate cells’ contribution to sinusoidal resistance. Am. J. Physiol. 277, G137. [DOI] [PubMed] [Google Scholar]
- Thresiamma KC, Kuttan R (1996) Inhibition of liver fibrosis by ellagic acid 1996. Indian J. Physiol. Pharmacol. 40, 363. [PubMed] [Google Scholar]
- Thulstrup PW, Thormann T, Spanget‐Larsen J, Bisgaard HC (1999) Interaction between ellagic acid and calf thymus DNA studied with flow linear dichroism UV‐VIS spectroscopy. Biochem. Biophys. Ries. Commun. 265, 416. [DOI] [PubMed] [Google Scholar]
- Tolstonog GV, Wang X, Shoeman R, Traub P (2000) Intermediate filaments reconstituted from vimentin, desmin, and glial fibrillary acidic protein selectively bind repetitive and mobile DNA sequences from a mixture of mouse genomic DNA fragments. DNA Cell Biol. 19, 647. [DOI] [PubMed] [Google Scholar]
- Traub P (1995) Intermediate filaments and gene regulation. Physiol. Chem. Phys. Med. NMR 27, 377. [PubMed] [Google Scholar]
- Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S (2002) Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins α 11β 1 and α 2β 1. J. Biol. Chem. 277, 37377. [DOI] [PubMed] [Google Scholar]
- Watford M (2000) Glutamine and glutamate metabolism across the liver sinusoid. J. Nutr. 130, 983S. [DOI] [PubMed] [Google Scholar]


