Abstract
Objectives: Type 1 diabetes mellitus, characterized by loss of pancreatic β‐cells, can be ameliorated by islet transplantation, but this treatment is restricted by the scarcity of islet tissue and by allograft rejection.
Materials and Methods: We isolated and characterized skin‐derived precursors (SKPs) – an abundant source of autologous cells – and developed an experimental strategy to convert them into insulin‐producing cells (IPCs) in vitro within a short period of time, through extracellular factor modification and analyses of IPCs by reverse transcription–polymerase chain reaction, immunocytochemistry and enzyme‐linked immunosorbent assay.
Results: SKPs could self‐assemble to form three‐dimensional islet cell‐like clusters (dithizone‐positive) and co‐express insulin and C‐peptide. In addition, they expressed multiple genes related to pancreatic β‐cell development and function (e.g. insulin 1, insulin 2, islet‐1, Pdx‐1, NeuroD/beta2, glut‐2 and Nkx6.1), but not other pancreas‐specific hormones and enzymes (e.g. glucagon, somatostatin and amylase). Moreover, when stimulated with glucose, these cells synthesized and secreted insulin in a glucose‐regulated manner.
Conclusions: The findings of this study indicate that SKPs can differentiate into functional IPCs and can provide an abundant source of autologous cells for transplantation. This study also provides strategies to derive autologous islet‐replacement tissues from human skin stem cells.
Introduction
Type 1 (insulin‐dependent) diabetes mellitus is characterized by auto‐immune destruction of β‐cells. The common treatment for type 1 diabetes is daily injection of insulin, but this does not solve the problem of serious long‐term systemic complications (1). Two major initiatives are underway to correct β‐cells deficit: one stimulates regeneration of β‐cells in the pancreas, and the other generates β‐cells in vitro (2). Recent studies suggest that the pancreas retains a significant capacity for regeneration (3, 4), which may be masked by factors such as the autoimmune response and toxicity of high blood glucose levels (5, 6). Signals regulating β‐cell proliferation are poorly understood, yet clinical evidence indicates that type 1 diabetes can be successfully treated by simple infusion of adequate numbers of functional islets (7). Unfortunately, islet transplantation has been greatly hampered by immune rejection and scarcity of donor islets, which has prompted research groups to search for suitable substitutes for donor pancreatic islets; stem cells are a promising alternative. Embryonic stem cells have great potential to differentiate into insulin‐producing cells (IPC), however, they also have tumorigenic potential when transplanted (8). Indeed, development of a simple and reliable procedure to obtain autologous stem cells that have the ability to differentiate into functional IPCs, would provide a potentially unlimited source of islet cells for transplantation and for the avoidance immune rejection.
The skin, the body's biggest organ, maintains its homoeostasis of proliferation, differentiation and regeneration through stem cells residing in the epidermis, dermis and appendages. Recently, multipotential skin‐derived precursors (SKPs) were isolated and expanded from human and other mammalian skin; they can differentiate into both neural and mesodermal progeny, including cell types rarely found in skin, such as neurons (9, 10, 11, 12). SKPs are more easily accessible than other adult stem cells, which potentially makes them a predominant autologous donor source for stem cell therapy.
Here, we describe a method we have developed to differentiate SKPs into functional IPCs within a short period of time. Differentiated SKPs expressed transcription factors for pancreas development, such as Pdx‐1, Nkx6.1, NeuroD/beta2 and Isl‐1 and they secreted insulin in response to glucose stimulus. These findings indicate that SKPs could be used as a steady, renewable and abundant source of autologous IPCs to replace the limited β‐cell transplantation for the treatment of type 1 diabetes.
Materials and methods
Mice
Neonatal Balb/C mice were purchased from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, and treated in accordance with the institute's guidelines.
Cell isolation and culture
Cells were isolated using a previously described method (9), with modifications. Briefly, skin of neonatal mice was carefully dissected free from other tissues and cut into 1–2 mm2 pieces. These pieces were washed three times in Hank's balanced salt solution (HBSS) and digested with 0.1% trypsin for 40 min at 37 °C, followed by 0.1% DNase (Sigma, St. Louis, MO, USA) for 1 min at room temperature. Tissue pieces were washed with HBSS, and mechanically dissociated by vortexing and pipetting in 2 mls of medium. The cell suspension was poured through a 40‐µm strainer, centrifuged and resuspended in stem cell medium: Dulbecco's modified Eagle's medium (DMEM)/F12 (1 : 1) (Invitrogen, Carlsbad, CA, USA) containing antibiotics, B27 (Invitrogen), 20 ng/ml epidermal growth factor (EGF; Sigma), and 40 ng/ml basic fibroblast growth factor (bFGF; R&D Systems Europe Ltd, Abingdon, UK). Cells were cultured in 100‐mm tissue culture dishes (Falcon Labware; Becton‐Dickinson, Franklin Lakes, NJ, USA) in a 37 °C, 5% CO2 tissue‐culture incubator. Floating spheres were formed by 7–8 days of culture. To passage floating cell spheres, medium containing spheres was centrifuged and the pellet was gently dissociated using a large bore pipette. The cells were re‐seeded in fresh stem cell medium as above. Cells were then passaged every 4–6 days.
Neuron, smooth muscle and adipocyte differentiation of sphere colonies in vitro
For differentiation, spherical colonies were trypsinized and dissociated into single cells, then cultured in DMEM/F12 supplemented with B27 supplement and 1% foetal bovine serum (FBS; Hyclone, Logan, UT, USA) on poly‐D‐lysine (Sigma) and laminin (Sigma) coated 24‐well plates for 7–21 days.
In vitro induction of SKPs into IPCs
Undifferentiated SKPs (Stage 1) were cultured and expanded in medium containing 20 ng/ml EGF (Sigma), and 40 ng/ml bFGF (R&D Systems) and 1% B27; the final concentration of glucose was 17.5 mm. At Stage 2, we transferred spheres containing a total of approximately 1–2 × 105 cells to single wells in plates coated with poly‐D‐lysine and laminin. These were cultured for 2 days in a 3 : 1 mixture of low‐glucose DMEM and F12 media containing 5 mm glucose, 1 mm dibutyryl cyclic adenosine monophosphate (db‐cAMP; Sigma), 1 µm all‐trans retinoic acid (RA; Sigma), 1% B27 and 2% FBS. At Stage 3, cells were cultured in a 1 : 3 mixture of high glucose DMEM and F12 media supplemented with 10 mm nicotinamide (Sigma), 10 nm insulin‐like growth factor 1 (Sigma), 2 nm Activin‐A (R&D Systems), 1% B27 and 2% FBS for up to 1 week. Stage 3 medium included 17.3 mm glucose.
Dithizone staining
In vitro staining was performed by adding 0.1 mg/ml dithizone (Sigma) solution to culture dishes, and then incubating at 37 °C for 30 min. Then dishes were rinsed three times with HBSS, crimson‐red‐stained clusters were examined by microscopy, and numbers of dithizone‐stained cells in cultures were determined.
FACS analyses
Cells were then incubated in 1% bovine serum albumin (BSA) with CD49f antibody (1 : 100, Biolegend, San Diego, CA, USA) or CD133 antibody (1 : 100, eBioscience, San Diego, CA, USA) for 1 h at 37 °C. After they were washed three times, they were incubated in FITC‐labelled secondary antibody (1 : 200, Zhongshan Biotechnology, Beijing, China) for 1 h at 37 °C. Controls were incubated in the secondary antibody only. After the cells were washed three times with 1% BSA, they were filtered through a 70‐µm strainer and kept on ice until the flow cytometric procedure by FACS analysis commenced (Becton‐Dickinson).
Reverse transcription–polymerase chain reaction
Total RNA was prepared by using Trizol (Invitrogen). For cDNA synthesis, random sequence primers were used to prime reverse transcription (RT) reactions and synthesis was carried out using the SuperscriptTM III First‐straind System (Invitrogen). A total of 35 polymerase chain reaction (PCR) cycles was performed using Platinum Taq DNA Polymerase (Tiangen Biotech, Beijing, China). Primer sequences are listed in Table 1.
Table 1.
Primer for RT‐PCR
| Gene | Primer sequence | Length of gene (bp) | Annealing temperature (°C ) |
|---|---|---|---|
| Nestin | 5′‐GGGCCAGCACTCTTAGCTTTGATA‐3′ 5′‐TGAGCCTTCAGGGTGATCCAG‐3′ | 105 | 64 |
| Insulin I | 5′‐GTTGGTGCACTTCCTACCCCTG‐3′ 5′‐GTAGAGGGAGCAGATGCTGGTG‐3′ | 300 | 60 |
| Insulin II | 5′‐AAGCCTATCTTCCAGGTTATT‐3′ 5′‐TGGGTCCTCCACTTCACG‐3′ | 212 | 60 |
| Somatostatin | 5′‐CGGCTGAAGGAGACGCTAC‐3′ 5′‐GTGCCATTGCTGGGTTCG‐3′ | 390 | 60 |
| Glucagon | 5′‐CACTCACAGGGCACATTCACC‐3′ 5′ACCAGCCACGCAATGAATTCCTT‐3’ | 221 | 52 |
| Ngn3 | 5′‐TGGCGCCTCATCCCTTGGATG‐3′ 5′‐AGTCACCCACTTCTGCTTCG‐3′ | 157 | 60 |
| Pdx‐1 | 5′‐GACAGCAGTCTGAGGGTGA‐3′ 5′‐AGCCGCCTTTCGTTATTC‐3′ | 405 | 53 |
| Isl‐1 | 5’‐GTTTGTACGGGATCAAATGC‐3′ 5′‐ATGCTGCGTTTCTTGTCCTT‐3′ | 514 | 50 |
| Nkx2.2 | 5′‐CGGACAATGACAAGGAGACCC‐3′ 5′‐GTCGGCGTGAGACGGATGA‐3′ | 174 | 60 |
| Neurod‐1 | 5′‐CTTAGAAAGTTATTGCGTTGC‐3′ 5′‐TCCTCTTGAGTGTTATGGGT‐3′ | 168 | 52 |
| Glut‐2 | 5’‐TGTCATCGCCCTCTGCTTC‐3′ 5′‐TCCTCCTGGTCGGTTCCTC‐3′ | 270 | 60 |
| Pax4 | 5′‐AAAGAGTTTCAGCGTGGGC‐3′ 5′‐TTGAGCAATGGGTTGATGG‐3′ | 481 | 58 |
| Pax6 | 5′‐CTTGGTGGTGTCTTTGTCA‐3′ 5′‐TGGAGCCAGTCTCGTAAT‐3′ | 208 | 55 |
| Slug | 5′‐CGTCGGCAGCTCCACTCCACTCTC‐3′ 5′‐TCTTCAGGGCACCCAGGCTCACAT‐3′ | 348 | 60 |
| Twist | 5′‐CTTTCCGCCCACCCACTTCCTCTT‐3′ 5′‐GTCCACGGGCCTGTCTCGCTTTCT‐3′ | 334 | 58 |
| Snail | 5′‐CGGCGCCGTCGTCCTTCT‐3′ 5′‐GGCCTGGCACTGGTATCTCTTCAC‐3′ | 398 | 60 |
| Sox9 | 5′‐CCGCCCATCACCCGCTCGCAATAC‐3′ 5′‐GCCCCTCCTCGCTGATACTGGTG‐3′ | 544 | 60 |
| GAPDH | 5′‐GTCTTCACCACCATGGAGAAG‐3′ 5′‐GTGATGGCATGGACTGTGGTC‐3′ | 281 | 56 |
| 18S | 5′‐AATCAGGGTTCGATTCCGGA‐3′ 5′‐CCAAGATCCAACTACGAGCT‐3′ | 295 | 60 |
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde for 15 min, permeated using 0.2% Triton X‐100 for 10 min, and blocked with 5% BSA for 1 h, then incubated overnight with primary antibody at 4 °C: rabbit anti‐nestin (1 : 200, ABcam, Cambridge, MA, USA), mouse anti‐fibronectin (1 : 100, Santa Cruz Biotechnology, CA, USA), rabbit anti‐stat3 (1 : 100, NeoMarkers, Fremont, CA, USA), rabbit anti‐Oct4 (1 : 400, Millipore, Temecula, CA, USA), mouse anti‐β‐III‐tubulin (1 : 200, Beyotime Institute of Biotechnology, Haimen, China), rabbit anti‐MAP2 (Bio‐Laboratory Biotech, Beijing, China), mouse anti‐GFAP (Millipore), rabbit anti‐α‐smooth muscle actin (1 : 200, Bio‐Laboratory Biotech), mouse anti‐insulin (1 : 100, NeoMarkers), goat anti‐C‐peptide (1 : 100, Millipore), rabbit anti‐glucagon (1 : 100, NeoMarkers), rabbit anti‐somatostatin (1 : 100, NeoMarkers), rabbit anti‐amylase (1 : 200, Santa Cruz Biotechnology), rabbit anti‐Isl‐1 (Millipore), goat anti‐Nkx6.1 (Santa Cruz Biotechnology), goat anti‐Glut2 (1 : 200, Santa Cruz Biotechnology) and rabbit anti‐Pdx‐1 (1 : 200, Millipore). The following secondary antibodies were used according to the manufacturer's recommendations: FITC‐conjugated donkey anti‐mouse IgG (1 : 200), FITC‐conjugated donkey anti‐rabbit IgG (1 : 200), TRITC‐conjugated goat anti‐rabbit IgG (1 : 200), TRITC‐conjugated donkey anti‐goat IgG (1 : 200) and TRITC‐conjugated goat anti‐mouse IgG (1 : 200) (all were purchased from Proteintech Group). Stained cells were analysed by using a confocal microscope (Leica) or Nikon microscope.
The In Situ Cell Death Detection Kit (Roche Diagnostics, Germany) was used to perform an apoptosis assay. Images were captured using a Leica confocal microscope.
Bromodeoxyuridine incorporation assay
Cells were cultured for 12 or 24 h in the presence of 20 µm bromodeoxyuridine (BrdU, Roche) prior to immunostaining,then they were fixed in 70% ethanol for 20 min. After blocking with 1% BSA in phosphate‐buffered saline (PBS) for 30 min, cells were denatured with 2 m HCl for 20 min, and neutralized with 0.1 m sodium borate for 5 min at room temperature. Thereafter, anti‐BrdU antibody (1 : 200, Zhongshan Biotechnology) was applied and incubated for 1 h at 37 °C. A secondary antibody, FITC‐conjugated goat anti‐mouse IgG (1 : 200, Zhongshan Biotechnology), was then applied and incubated in a dark chamber for 1 h at 37 °C, followed by counterstaining with propidium iodide (PI) (2 µg/ml in PBS) for 15 min. After washing with PBS three times, samples were examined using a Leica confocal microscope.
Western blot analysis
Different stage cells were lysed in detergent lysis buffer (SBS Genetech, Beijing, China). A total of 20‐µg protein extracts was separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis, then electro‐transferred onto a PVDF membrane. The membrane was blocked with 5% nonfat milk in tris‐buffered saline containing 0.4% Tween 20, and then incubated with primary antibody: mouse anti‐insulin (1 : 100, NeoMarkers), goat anti‐C‐peptide (1 : 100, Millipore), rabbit anti‐glucagon (1 : 100, NeoMarkers), rabbit anti‐somatostatin (1 : 100, NeoMarkers), rabbit anti‐amylase (1 : 200, Santa Cruz Biotechnology), rabbit anti‐nestin (1 : 100, ABcam), mouse anti‐fibronectin (1 : 200, Santa Cruz Biotechnology), rabbit anti‐stat3 (1 : 100, NeoMarkers) and mouse anti‐actin (1 : 300, NeoMarkers). After incubation with horseradish peroxidase‐conjugated secondary antibody, the membrane was subjected to an ECL Detection System (Santa Cruz Biotechnology).
C‐peptide quantification and in vitro insulin secretion assay
At each stage, 1 × 105 cells were washed with PBS and homogenized, then intracellular C‐peptide content was measured with Lbis(R) Mouse C‐peptide ELISA Kit (U type) (Shibayagi Co., Ltd, Japan). To estimate secreted insulin, cells were pre‐incubated for 1 h at 37 °C in Krebs Ringer bicarbonate HEPES (KRBH) buffer. For glucose‐induced insulin assay, cells were further incubated in KRBH buffer supplemented with 5 mm, 10 mm, 20 mm and 30 mm glucose for 2 h at 37 °C. Determination of secreted insulin was performed by using an ultrasensitive mouse insulin ELISA kit (Mercodia, Uppsala, Sweden), according to the manufacture's instructions. Statistical analysis was performed using Student's t‐test.
Results
Isolation of sphere colonies and differentiation into neural cells, smooth muscle cells and adipocytes
Many spheres were formed by 7–10 days after initial culture (and could be dissociated to single cells when passaged) and then proliferated to generate new spheres again (Fig. 1a–c). The cells incorporated BrdU after 24‐h exposure to it (Fig. 1h), indicating that the sphere colony cells were proliferating. Sphere colonies isolated under our conditions also expressed nestin and fibronectin (Fig. 1f,g); approximately 80% of the cells expressed these. Surprisingly, immunocytochemistry revealed that SKPs expressed Oct4 (Fig. 1d), a hallmark of embryonic stem cells, and Stat3 (Fig. 1e), a transcription factor that plays a critical role in maintenance of pluripotency of embryonic stem cells; these two markers have been reported to be expressed in porcine SKPs (12). RT‐PCR analysis revealed that SKPs of P5 and P10 expressed a subset of embryonic neural crest stem cell transcription factors such as Slug, Twist, Snail and Sox9 (Fig. 1i). SKPs, after differentiation on poly‐D‐lysine/laminin‐coated dishes for 7–21 days, were positive for NF‐M, β‐III tubulin, NSE, GFAP and α‐SMA with approximately 4–7% frequency in each case (Fig. 1j–q). Furthermore, some cells were positive using Oil Red O (adipocyte) (Fig. 1r), but the frequency was highly variable. These results are similar to those of previous studies (9, 10, 11, 13). Thus, sphere colonies isolated under our conditions retained their self‐renewal capabilities and multipotency.
Figure 1.
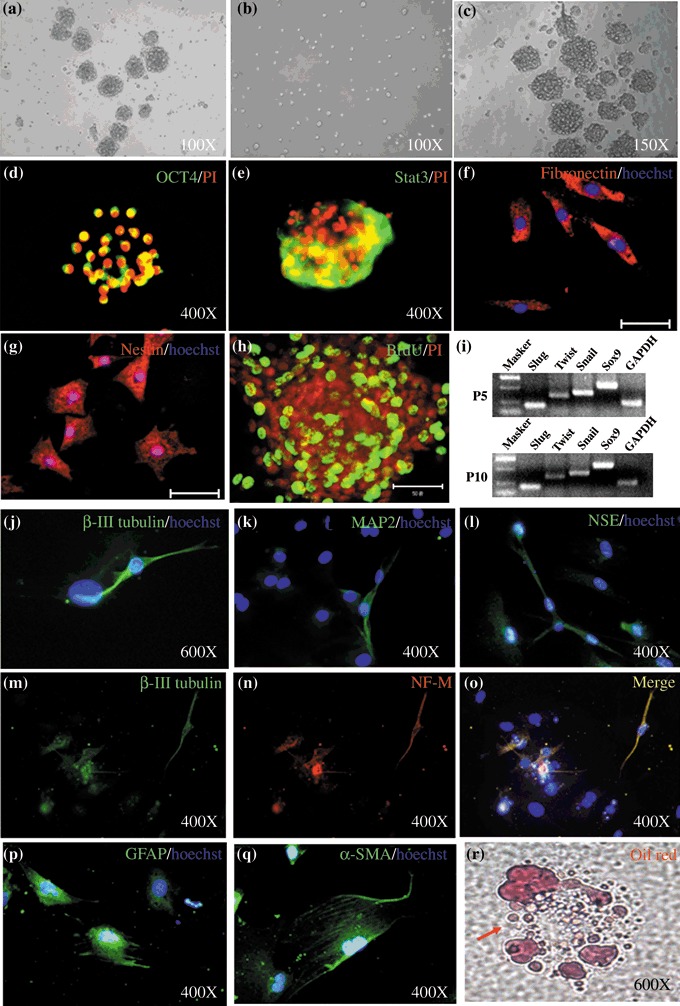
Isolation, characterization and multipotency of skin‐derived precursors (SKPs). (a–c) Following initial purification, SKPs grown as spheres in suspension could be dissociated to single cells and then would proliferate to generate further, new spheres. (d–g) Immunocytochemical analysis of cells within the sphere colonies for Oct4, Stat3, fibronectin and nestin. (h) Bromodeoxyuridine labelling of SKPs after 24 h incorporation. (i) RT‐PCR analysis for the expression of mRNAs for Slug, Twist, Snail, Sox9, and GAPDH, as a controls, in SKPs that had been passaged 5 and 10 times. (j–l, p and q) Immunocytochemical analysis of cells differentiated for β‐III tubulin, MAP2, NSE, GFAP and α‐SMA. (M‐O) Double‐labelling of β‐III tubulin and NF‐M. (r) Differentiated cells stained with Oil Red O. Scale bar: 50 µm.
Derivation of IPCs from SKPs by combination of RA, cAMP, and other factors
Recent studies have found that skin stem cells share many features with pancreatic stem cells (14). In addition, we found that SKPs weakly expressed CD49f (4%) and CD133 (3%) (Fig. 2j), which are conserved markers of islet progenitor cells (15). Therefore, we speculated that SKPs would have the potential to differentiate into IPCs. Next, we developed an effective method with RA, cAMP, Activin‐A and other factors to differentiate SPKs into functional IPCs; the induction process is illustrated in Fig. 2(a). Undifferentiated SKPs were termed Stage 1; as spheres differentiated to become Stage 2, cells migrated out of the sphere to form a flat monolayer (Fig. 2c). After removal from Stage 2, cells cultured to become Stage 3 formed islet‐like clusters at day 7 (Fig. 2d). The number of clusters was 10–20/well in 24‐well culture plates. The conserved markers of pancreatic progenitor cells (CD49f and CD133) were not expressed after initiation of differentiation (Fig. 2j). Moreover, expression of stem cell markers (nestin, fibronectin and Stat3) gradually decreased from Stage 1 to Stage 3 cells (Fig. 2i). Pancreatic β‐cells package insulin by forming the 2‐Zn‐insulin hexamer. The islet‐like clusters were distinctly crimson red after staining with the zinc‐chelating reagent dithizone (Fig. 2g). Undifferentiated SKPs and differentiated cells at Stage 2 did not stain (Fig. 2e,f). These results suggested that SKPs gradually lost stem cell markers during differentiation and cells in Stage 3 possibly attained the pancreatic endocrine cell fate by chemical induction.
Figure 2.
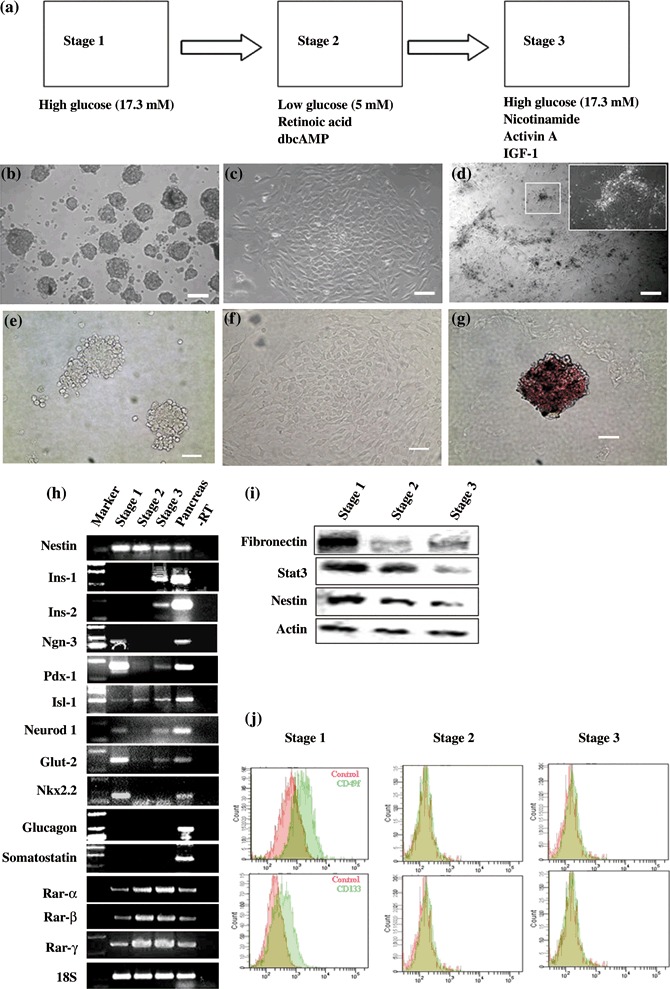
Developments of cells expressing islet markers from skin‐derived precursors ( SKPs). (a) Sketch of SKP differentiation protocol. (b–d) Stage‐specific cell cluster morphology; back arrow shows islet‐like cluster. (e–g) Dithizone staining of Stages 1–3 cells. Islet‐like clusters in Stage 3 were stained distinct crimson red by using dithizone (g). Cell in Stages 1 and 2 are not stained (e and f). (h). RT‐PCR analysis of gene expression during Stages 1–3 of pancreatic and endodermal markers. Pancreas RNA served as a positive control. (i) Western blot analysis of stem cell markers during Stage 1–3. (j) Examination of CD133 and CD49f, conserved marker of pancreatic progenitor, by flow cytometric analysis during Stage 1–3. Scale bar: 100 µm.
Gene expression in differentiated IPCs
Pancreatic development and gene expression are regulated by specialized transcription factors. To detect whether endocrine‐specific transcription factors and pancreatic specific genes were expressed in the differentiated cells, RT‐PCR analysis was performed at different stages (Fig. 2h). Transcripts for Ngn3, Pdx‐1, Nkx2.2, NeuroD/beta2 and Isl‐1, which are known to be expressed in pancreatic endocrine progenitor cells (16), were expressed in undifferentiated SKPs. As cells differentiated in Stage 2, Ngn3 disappeared and Isl‐1 was enhanced. After removal from Stage 2 to Stage 3, islet‐like clusters were formed and expressed the insulin genes (insulin 1 and insulin 2), pancreatic endocrine‐specific transcription factors (Pdx‐1, NeuroD/beta2 and Isl‐1) and an essential regulator of glucose response (Glut‐2), but not other islet endocrine hormones (glucagon, somatostatin), also, we did not detect Ngn3 expression in Stage 3 cells. Ngn3 is crucial for development of the pancreas, but expression of Ngn3 is inactive in mature pancreatic endocrine β‐cells (17). Thus, the gene expression analysis demonstrated that SKP‐derived IPCs were similar to pancreatic insulin‐producing β‐cells. Interestingly, the transcripts Ngn3, Pdx‐1, NeuroD/beta2 and Nkx2.2 disappeared after RA and cAMP treatment, and then Pdx‐1 and NeuroD/beta2 appeared again in Stage 3 (islet‐like cluster formation). Therefore, are RA and cAMP essential to induce SKPs to IPCs differentiation?
RA and cAMP are essential to differentiate SKPs towards IPCs development
To detect whether RA and cAMP are essential for differentiation of SKPs into IPCs, cells were cultured in the absence of the Stage 2 environment. We found no islet‐like cluster formation, and failed to detect insulin 1, insulin 2, Pdx‐1, Glut‐2 and NeuroD/beta2 expression. Other pancreatic hormones (glucagon and somatostatin) also were not found (Fig. 3a). Several studies suggest that RA regulates pattern formation of the vertebrate neural plate (18, 19, 20) and promotes differentiation of embryonic carcinoma cells into neurons (21). However, RA can also induce development of primitive endodermal cells from a subset of embryonic carcinoma cells (22), and is an endogenous signal that directs development of posterior organs, such as the pancreas, from embryonic endoderm (23). Here, we found that SKPs expressed RA receptors including RAR‐α, RAR‐β and RAR‐γ (Fig. 2h), indicating that they have the competence to detect RA signals. After treatment by RA, SKPs increased expression of Pax4 and Pax6 (Fig. 3b); these two transcription factor were expressed in neurons and insulin‐producing β‐cells (24). Expression of Pax6 has been also shown to be mediated by RA during embryonic stem cell differentiation (25). However, when exposed to RA for 2 days and further cultured in neuron differentiation medium for 7 days, β‐III tubulin‐positive cells did not increase in number (Fig. 3c,d), implying that the RA signal did not enhance neuron differentiation in our culture system. In addition, several studies have shown that treatment with cAMP can inhibit embryonic carcinoma cell proliferation and direct both embryonic carcinoma and embryonic stem cells towards endoderm formation (26, 27). We found by BrdU incorporation analysis that treatment with cAMP decreased cell proliferation (Fig. 3e,f). In the light of these discoveries we suggest that RA and cAMP were essential to promote SKP differentiation into IPCs by inhibiting cell proliferation and promoting the differentiation direction towards endocrine cells, not to the neural lineage.
Figure 3.
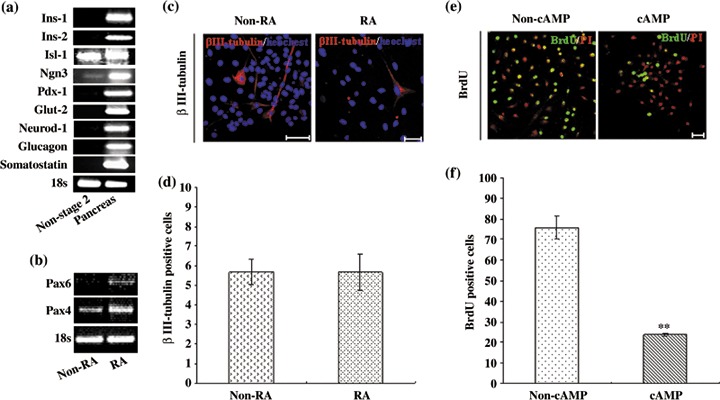
Retinoic acid (RA) and cyclic adenosine monophosphate (cAMP) are essential for insulin‐producing cell development. (a) RT‐PCR analysis of gene expression of differentiated cells without Stage 2 induction. (b) RT‐PCR analysis of gene expression of differentiated cells by RA treatment for 48 h. (c–d) Immunocytochemical analysis of β‐III tubulin and statistics of β‐III tubulin‐positive cells by RA and non‐RA treatment (P > 0.05). (e–f) After 48 h cAMP and non‐cAMP treatment, 12 h bromodeoxyuridine (BrdU) incorporation assay and statistics of BrdU‐positive cells. Scale bar: 50 µm. Statistical significance tested by Student's t‐test: **P < 0.01.
Insulin expression by differentiated cells
To determine whether the differentiated cells in islet‐like cluster actually synthesized insulin protein, IPCs were stained with anti‐insulin antibody and visualized under a confocal microscope (Fig. 4a). As shown in Fig. 4(b), data collected from three independent cultures indicated that there were about 50% insulin‐positive cells in one islet‐like cluster. To exclude the possibility that apoptotic cells might take up insulin from the medium (28), we analysed whether IPCs were apoptotic using a TUNEL assay. We found no C‐peptide‐positive cells were costained with TUNEL (Fig. 4c–i), showing that IPCs were not apoptotic. The cells co‐stained with insulin and C‐peptide, but not for glucagon, somatostatin or amylase (Fig. 5a). Furthermore, we used Western blot analysis to confirm the immunostaining results (Fig. 5b). In addition, most of IPCs were costained with Nkx6.1, Pdx‐1, Isl‐1 and Glut‐2, suggesting that differentiated cells might be functional IPCs (Fig. 5c).
Figure 4.

Percentage of insulin‐positive cells as in the order of 50% and C‐peptide‐positive cells in the final induction stage were TUNEL‐negative. (a) Immunocytochemical analysis of insulin. (b) Statistics of insulin‐positive cells in the final induction stage. (c–e) As a positive control, differentiated skin‐derived precursors were treated with DNase to induce apoptosis and stained with TUNEL. (f–i) Double staining C‐peptide and TUNEL of insulin‐producing cells in Stage 3. Scale bar: 50 µm.
Figure 5.
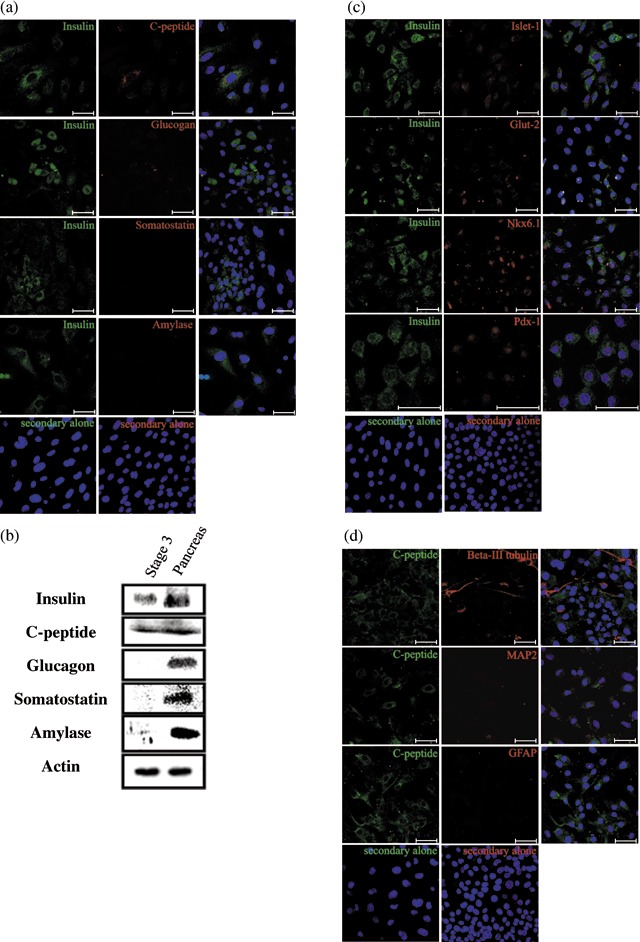
Insulin‐producing cells (IPCs) in Stage 3 express characteristic pancreatic β‐cell markers and did not express markers of differentiated neural cells. (a) Co‐staining of Stage 3 IPCs with antibodies against insulin and other pancreatic endocrine hormones showing that insulin‐positive cells also expressed C‐peptide but not glucagon, somatostatin or amylase. (b) Western blot analysis to confirm the expression of endocrine hormones. (c) Co‐expression of insulin and the indicated marker genes specific for the endocrine β‐cells (Isl‐1, Nkx6.1, Glut‐2 and Pdx‐1) in IPCs. (d) No co‐staining of C‐peptide or neural lineage markers (β‐III tubulin, MAP2 and GFAP) was found in Stage 3 IPCs. Scale bar: 50 µm.
Skin‐derived precursors can spontaneously differentiate into neural lineages after withdrawal of growth factor (EGF and bFGF). Of the differentiated cells in Stage 3, about 6% were β‐III tubulin positive, none of which expressed C‐peptide (Fig. 5d). C‐peptide‐positive cells showed no co‐expression of GFAP or MAP2 (Fig. 5d), which are markers of neural lineage. These data suggest that nearly all the insulin‐positive cells produced in Stage 3 were non‐neural.
IPCs are glucose respondent
To quantify insulin expression in IPCs, we used a mouse C‐peptide ELISA kit. C‐peptide levels at Stages 1 and 2 were undetectable (Fig. 6a), consistent with the lack of insulin transcript in the cells at those points as previously shown by RT‐PCR. In Stage 3, C‐peptide levels were approximately 46 pg/105 cells (Fig. 6a), and the average percentage of insulin‐positive cells was 50%. Thus, we estimate 92 pg/105 cells. A single β‐cell contains approximately 2.16 pg C‐peptide (29), hence, we calculate that insulin C‐peptide content in one IPC is approximately 0.4% of the level in native β‐cells.
Figure 6.
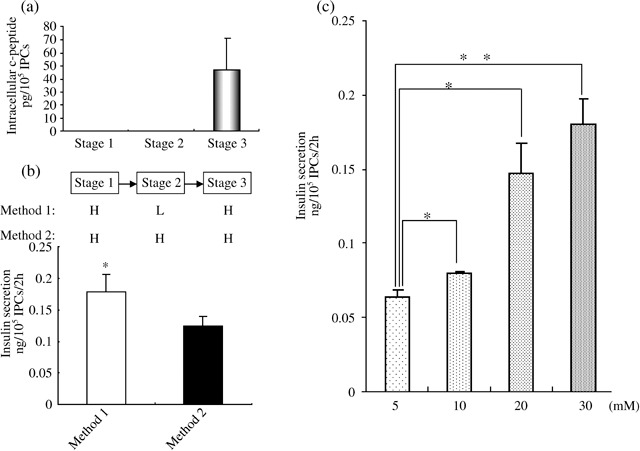
Intracellular C‐peptide content and insulin secretion analysis. (a) Intracellular C‐peptide content of cells during Stages 1–3. (b) ELISA assay of insulin secretion from insulin‐producing cells (IPCs) in Stage 3 exposed to 30 mm glucose after culture in Stage 2 with different glucose concentration. High glucose is designated ‘H’; low glucose designated ‘L’. (c) ELISA assay of insulin secretion from IPCs exposed to 5, 10, 20, 30 mm glucose for 2 h. Statistical significance tested by Student's t‐test: *P < 0.05, **P < 0.01.
Glucose transporter (Glut‐2) is an essential regulator of glucose responses in pancreatic β‐cells. Glut‐2 was expressed in Stage 3 suggested that IPCs could sense and respond to glucose. To analyse whether insulin secretion from IPCs was regulated by glucose, cells were washed three times by KRBH buffer containing 0.5% BSA and further incubated for 1 h, then stimulated by addition of 5 mm, 10 mm, 20 mm and 30 mm glucose to the medium for 2 h in individual experiments. After this, insulin release in KRBH buffer was examined by ELISA. Differentiated IPCs secreted insulin in a glucose concentration‐dependent manner (Fig. 6c), but undifferentiated SKPs did not show no glucose dependency (data not shown). Overall, these data demonstrate that islet‐like clusters derived from SKPs can secrete insulin in a glucose‐regulated manner under appropriate conditions.
Prolonged exposure to high glucose levels can severely reduce insulin expression in pancreatic β‐cells (30). High glucose also inhibits differentiation of neural stem cells into IPCs, but glucose reduction in differentiated culture medium blunts neuronegenic programmes of neural stem cells and directs them to develop into pancreatic cells (31). In our study, we found that when cultured to all three differentiated stages with the same high glucose concentration, differentiated cells reduced insulin production as shown by ELISA assay (Fig. 6b), indicating that glucose reduction in Stage 2 could increase insulin expression. Therefore, we switched SKP culture conditions from high glucose (17.5 mm, ‘Stage 1’) to low glucose (5 mm, ‘Stage 2’; see Fig. 2a) to increase insulin expression.
Discussion
There is widespread interest in developing tissue replacement strategies for the treatment of diseases, such as diabetes mellitus, cardiac infarction and Parkinson disease. Much attention has now been focused on the promising field of embryonic stem cells, but recent studies suggest that SKPs have an unusually broad differentiation potential. For example, SKPs isolated from human and other mammals could differentiate into ectodermal lineage (neurons and glia), mesoderm (such as adipocyte and smooth muscle) and germ cells (9, 10, 11, 32). Here, we show that SKPs have a similarly broad differentiation potential, and that specific in vitro culture conditions can induce SKPs to differentiate into endodermal cells (endocrine cells or IPCs).
Here, we demonstrated that SKPs have the potential to differentiate into IPCs. These results might seem unexpected at first thought, because the pancreas and skin normally derive from endoderm and ectoderm, respectively, which are distinct embryonic germ layers. But deliberation on several recent studies suggests that it is reasonable. First, it is well known that pancreatic islets share a set of developmental regulators with neurons during development (24). Moreover, pancreatic progenitor cells can form neuron‐like cells (33, 34), and neural stem cells have successfully been differentiated into IPCs in vitro (31), which further confirmed their relationship. Secondly, SKPs could spontaneously differentiate into neural lineage after removal of growth factors (bFGF and EGF) from the culture medium, implying that SKPs are also neurogenic. Based on the similarity of neural lineage and the pancreas, the relationship between SKPs and neural lineages made us curious about the correlation of SKPs and pancreatic cells. As expected, a recent study found that skin‐derived adult stem cells share many features with pancreatic stem cells, which lends strong support to our study (14). Based on the similarities between neurons and the pancreas and between skin and pancreatic stem cells, it is not very surprising that SKPs have the potential to differentiate into pancreatic endocrine cells.
Many previous studies have devoted efforts into converting embryonic stem cells to IPCs (35, 36, 37, 38, 39, 40). Among these, one study selected Nestin‐positive cells derived from embryiod body (EB) and utilized nicotinamide to produce IPCs (36); other studies adopted induction protocols that are losely parallel to the development process (35, 40). Compared to these studies, the induction process in our work, for SKPs to differentiate into IPCs is very different. Although SKPs are Nestin positive and can differentiate into neural cells spontaneously, SKPs may not be at the same development stage with Nestin‐positive cells derived from embryonic stem cells. Consistent with previous observations, our study demonstrates that SKPs could differentiate to many cells (including neurons, muscle and adipocytes) spontaneously, but a previous study indicates that these Nestin‐positive cells derived from embryonic stem cells seem to be limited to neural cells (41). Moreover, our induction protocol is very different from previous methods parallel to the development process (35, 40). In our three‐stage induction proctocol, cells cultured in Stage 2 did not express insulin 1, insulin 2, Pdx‐1, Neurod‐1, Glut‐2 and Ngn3. After treatment with a combination of nicotinamide, insulin‐like growth factor 1 and Activin‐A in Stage 3, cells could self‐assemble to form three‐dimensional islet‐like clusters and express pancreatic β‐cell‐specific gene. Unexpectedly, only insulin‐positive cells (and not other exocrine cells) were observed at Stage 3, suggesting that this induction process is not the same as the development process in vivo where the Ngn3‐positive cells are the common progenitors for all exocrine cells (17). After treatment with RA in Stage 2 (Fig. 3b), cells expressed Pax6 whose mutation results in deficiency of all pancreatic endocrine cells (42). Pax6 is necessary for endocrine cell development, but is not restricted to any particular endocrine cell fate. However, in mature islets, Nkx6.1 expression is restricted to β‐cells where it has been suggested to act as a transcriptional repressor of glucagon promoter activity, while Nkx2.2 marks the α‐, β‐, and PP‐cells (43). We detected expression of Nkx6.1 (Fig. 5) but not of Nkx2.2 in Stage 3 cells (Fig. 2). These may well imply that SKPs might be induced to the direction of the insulin‐producing endocrine cell lineage, but not to glucagon nor somatostatin, in our experimental system.
Recent studies have demonstrated the feasibility of generating IPCs from progenitor cells of various sources, including the pancreas, liver, intestinal epithelium, bone marrow and brain, as well as embryonic stem cells of mouse and human origin (31, 35, 36, 44, 45, 46, 47, 48, 49). However, some obstacles such as immunity against newly formed β‐cells derived from heterogenous embryonic and adult stem cells still remain, and it is also difficult to obtain enough autologous adult stem cells from these organs. To overcome these limitations, we explored SKPs, which are easily accessible, as a source for differentiation into IPCs in vitro. In the present study, we isolated and characterized SKPs. We also generated functional IPCs from SKPs under an in vitro differentiation procedure and confirmed the presence of insulin production by RT‐PCR, Western blot analysis and immunofluorescence. Furthermore, we tested the functionality of the in vitro‐generated IPCs from mouse skin by measuring insulin release in response to glucose challenge. However, there are some limitations to our findings that should be noted. On the one hand, our molecular analysis of differentiated IPCs demonstrated that they were still distinct from mature pancreatic islet β‐cells, implying that IPCs derived by our methods are not mature β‐cells. The RT‐PCR result confirmed that IPCs derived from SKPs expressed a combination of gene products that were nearly similar to developing pancreatic islet cells, but the temporal sequence of expression of the genes did not precisely recapitulate that of embryonic pancreas development. Also, Nkx2.2 that regulates β‐cell function in mature islet (50) was undetectable in the differentiated ones (Fig. 2h). Further experiments to elucidate these differences may reveal the extent of SKP development towards an endocrine cell fate. Recent studies have demonstrated that there were numerous similarities between neural and pancreatic endocrine cells during their development; SKPs also have a potential for neural differentiation. Based on these similarities, we speculated that manipulated expression of β‐cell transcript factors, like Nkx2.2, in differentiated cells would permit IPC differentiation that would better mimic pancreatic β‐cells. On the other hand, although insulin released by IPCs was glucose sensitive in vitro, the cells were not similar to pancreatic β‐cells. We found that IPCs secrete nearly 40% insulin content per half hour incubation with 30 mm glucose (Table S1); this is a very high percentage of insulin secretion compared to the physiological level. Previous studies suggest that only unusual conditions, such as fatty acid overexposure, will induce high percentage of insulin secretion and 30–50% decrease of insulin content (51). Hence, the secretion pattern of IPCs is constitutive and not well regulated by glucose; moreover, insulin synthesis by IPCs is stimulated by glucose. We found that after incubation in high glucose for 2 h (Fig. 6), the amount of insulin secreted at that time was around twice the total insulin in cells at half an hour (Table S1). This result indicates that IPCs in our study increased insulin synthesis after glucose stimulation. This characteristic shows that our IPCs are consistent with β‐cells, since previous studies demonstrate that high glucose stimulates insulin synthesis (52, 53); but our IPCs were still immature and far from therapeutic requirement. We also failed to ameliorate glucose regulation (data not shown) when we attempted to transplant IPCs to diabetic animals. Prior studies suggest that insulin production of not lower than 10% of normal level may be required to improve glucose regulation in diabetic patients and animal models (54). Future work should seek to explain whether insulin release can be stimulated by other stimulators, such as amino acids or sulphonylureas, and to improve insulin expression and secretion by more effective procedures..
Nevertheless, compared to IPCs derived from other adult stem cell sources, we developed a simple and accessible way to generate functional IPCs from an abundant autologous stem cell source, SKPs, this hopefully would solve the major problems of availability of donor cells and immunogenic rejection. This study provides strategies to derive autologous islet‐replacement tissues from human skin stem cells and other types of multipotent stem cell.
Supporting information
Table S1. Glucose‐induced insulin release data correspond to the amount of insulin secreted within 30 min after 30 mM glucose stimulation. The results were reproduced in three independent experiments
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgements
We thank Dr Julang Li (Department of Animal and Poultry Science, University of Guelph) for providing SKPs isolation protocol. We also thank Dr Aaron J. W. Hsueh (Stanford University School of Medicine) for helpful discussion. This work was supported by the National Basic Program of China 2007CB947401, 2006CB944006) and Chinese National Manned Space Program (Project 921‐2).
References
- 1. Nathan DM (1993) Long‐term complications of diabetes mellitus. N. Engl. J. Med. 328, 1676–1685. [DOI] [PubMed] [Google Scholar]
- 2. Bonner‐Weir S, Weir GC (2005) New sources of pancreatic β cells. Nat. Biotechnol. 23, 857–861. [DOI] [PubMed] [Google Scholar]
- 3. Brennand K, Huangfu D, Melton D (2007) All β‐cells contribute equally to islet growth and maintenance. PLoS Biol. 5: e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teta M, Rankin MM, Long SY, Stein GM, Kushner JA (2007) Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell 12, 817–826. [DOI] [PubMed] [Google Scholar]
- 5. Kodama S, Kühtreiber W, Fujimura S, Dale EA, Faustman DL (2003) Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science 302, 1223–1227. [DOI] [PubMed] [Google Scholar]
- 6. Pelengaris S, Khan M, Evan GI (2002) Suppression of Myc‐induced apoptosis in β cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109, 321–334. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid‐free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238. [DOI] [PubMed] [Google Scholar]
- 8. Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE (2005) Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell‐derived insulin‐producing cells. Am. J. Pathol. 166, 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toma JG, Akhavan M, Fernandes KJ, Barnabé‐Heider F, Sadikot A, Kaplan DR, Miller FD (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 3, 778–784. [DOI] [PubMed] [Google Scholar]
- 10. Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S (2004) Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin‐derived stem cells. Lancet 364, 172–178. [DOI] [PubMed] [Google Scholar]
- 11. Toma JG, McKenzie IA, Bagli D, Miller FD (2005) Isolation and characterization of multipotent skin‐derived precursors from human skin. Stem Cells 23, 727–737. [DOI] [PubMed] [Google Scholar]
- 12. Dyce PW, Zhu H, Craig J, Li J (2004) Stem cells with multilineage potential derived from porcine skin. Biochem. Biophys. Res. Commun. 316, 651–658. [DOI] [PubMed] [Google Scholar]
- 13. Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe‐Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, Kaplan DR, Labosky PA, Rafuse V, Hui CC, Miller FD (2004) A dermal niche for multipotent adult skin‐derived precursor cells. Nat. Cell Biol. 6, 1082–1093. [DOI] [PubMed] [Google Scholar]
- 14. Kajahn J, Gorjup E, Von Tiede SBH, Paus R, Kruse C, Danner S (2007) Skin‐derived human adult stem cells surprisingly share many features with human pancreatic stem cells. Eur. J. Cell Biol. 87, 39–46. [DOI] [PubMed] [Google Scholar]
- 15. Sugiyama T, Rodriguez. RT , McLean GW, Kim SK (2007) Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc. Natl. Acad. Sci. USA 104, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gangaram‐Panday ST, Faas MM, De VP (2007) Towards stem‐cell therapy in the endocrine pancreas. Trends Mol. Med. 13, 164–173. [DOI] [PubMed] [Google Scholar]
- 17. Gu G, Dubauskaite J, Melton DA (2002) Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457. [DOI] [PubMed] [Google Scholar]
- 18. Durston AJ, Timmermans JP, Hage WJ, Hendriks HF, De VN, Heideveld M, Nieuwkoop PD (1989) Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature 340, 140–144. [DOI] [PubMed] [Google Scholar]
- 19. Wagner M, Thaller C, Jessell T, Eichele G (1990) Polarizing activity and retinoid synthesis in the floor plate of the neural tube. Nature 345, 819–822. [DOI] [PubMed] [Google Scholar]
- 20. Guthrie S (1996) Patterning the hindbrain. Curr. Opin. Neurobiol. 6, 41–48. [DOI] [PubMed] [Google Scholar]
- 21. Bain G, Ray WJ, Yao M, Gottlieb DI (1994) From embryonal carcinoma cells to neurons: the P19 pathway. BioEssays 16, 343–348. [DOI] [PubMed] [Google Scholar]
- 22. Tulachan SS, Doi R, Kawaguchi Y, Tsuji S, Nakajima S, Masui T, Koizumi M, Toyoda E, Mori T, Ito D, Kami K, Fujimoto K, Imamura M (2003) All‐trans retinoic acid induces differentiation of ducts and endocrine cells by mesenchymal/epithelial interactions in embryonic pancreas. Diabetes 52, 76–84. [DOI] [PubMed] [Google Scholar]
- 23. Stafford D, Prince VE (2002) Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr. Biol. 12, 1215–1220. [DOI] [PubMed] [Google Scholar]
- 24. Edlund H (2002) Pancreatic organogenesis: developmental mechanisms and implications for therapy. Nat. Rev. Genet. 3, 524–532. [DOI] [PubMed] [Google Scholar]
- 25. GajoviÇ S, St‐Onge L, Yokota Y, Gruss P (1997) Retinoic acid mediates Pax6 expression during in vitro differentiation of embryonic stem cells. Differentiation 62, 187–192. [DOI] [PubMed] [Google Scholar]
- 26. Grover A, Adamson ED (1986) Evidence for the existence of an early common biochemical pathway in the differentiation of F9 cells into visceral or parietal endoderm: modulation by cyclic AMP. Dev. Biol. 114, 492–503. [DOI] [PubMed] [Google Scholar]
- 27. Globus RK, Strewler GJ, Nissenson RA (1994) Up‐regulation of adenylyl cyclase signaling and Gsa expression during differentiation of embryonic stem cells. Endocr. J. 2, 419–427. [Google Scholar]
- 28. Rajagopal J, Anderson WJ, Kume S, Martinez. OI , Melton DA (2003) Insulin staining of ES cell progeny from insulin uptake. Science 299, 363. [DOI] [PubMed] [Google Scholar]
- 29. Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S (2000) The cell physiology of biphasic insulin secretion. News Physiol. Sci. 15, 72–77. [DOI] [PubMed] [Google Scholar]
- 30. Leahy JL, Cooper HE, Deal DA, Weir GC (1986) Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J. Clin. Invest. 77, 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hori Y, Gu X, Xie X, Kim SK (2005) Differentiation of insulin‐producing cells from human neural progenitor cells. PLoS Med. 2, e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dyce PW, Wen L, Li J (2006) In vitro germline potential of stem cells derived from fetal porcine skin. Nat. Cell Biol. 8, 384–390. [DOI] [PubMed] [Google Scholar]
- 33. Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, Van Der Kooy D (2004) Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 22, 1115–1124. [DOI] [PubMed] [Google Scholar]
- 34. Choi Y, Ta M, Atouf F, Lumelsky N (2004) Adult pancreas generates multipotent stem cells and pancreatic and nonpancreatic progeny. Stem Cells 22, 1070–1084. [DOI] [PubMed] [Google Scholar]
- 35. D’Amour AK, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE (2006) Production of pancreatic hormone‐expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401. [DOI] [PubMed] [Google Scholar]
- 36. Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. (2001) Differentiation of embryonic stem cells to insulin‐secreting structures similar to pancreatic islets. Science 292, 1389–1394. [DOI] [PubMed] [Google Scholar]
- 37. Soria B, Roche E, Berná G, León‐Quinto T, Reig JA, Martín F (2000) Insulin‐secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin‐induced diabetic mice. Diabetes 49, 157–162. [DOI] [PubMed] [Google Scholar]
- 38. Assady S, Maor G, Amit M, Itskovitz‐Eldor J, Skorecki KL, Tzukerman M (2001) Insulin production by human embryonic stem cells. Diabetes 50, 1691–1697. [DOI] [PubMed] [Google Scholar]
- 39. Bai L, Meredith G, Tuch BE (2005) Glucagon‐like peptide‐1 enhances production of insulin in insulin‐producing cells derived from mouse embryonic stem cells. J. Endocrinol. 186, 343–352. [DOI] [PubMed] [Google Scholar]
- 40. Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’Amour KA, Carpenter MK, Baetge EE (2008) Pancreatic endoderm derived from human embryonic stem cells generates glucose‐responsive insulin‐secreting cells in vivo . Nat. Biotechnol. 26, 443–452. [DOI] [PubMed] [Google Scholar]
- 41. Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD (2000) Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat. Biotechnol. 18, 675–679. [DOI] [PubMed] [Google Scholar]
- 42. Sander M, Neubüser A, Kalamaras J, Ee HC, Martin GR, German MS (1997) Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 11, 1662–1673. [DOI] [PubMed] [Google Scholar]
- 43. Jørgensen MC, Ahnfelt‐Rønne J, Hald J, Madsen OD, Serup P, Hecksher‐Sørensen J (2007) An illustrated review of early pancreas development in the mouse. Endocr. Rev. 28, 685–705. [DOI] [PubMed] [Google Scholar]
- 44. Ramiya VK, Maraist M, Arfors KE, Schatz. DA , Peck AB, Cornelius JG (2000) Reversal of insulin‐dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat. Med. 6, 278–282. [DOI] [PubMed] [Google Scholar]
- 45. Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB (2002) In vitro trans‐differentiation of adult hepatic stem cells into pancreatic endocrine hormone‐producing cells. Proc. Natl. Acad. Sci. USA 99, 8078–8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zalzman M, Anker‐Kitai L, Efrat S (2005) Differentiation of human liver‐derived, insulin‐producing cells toward the β‐cell phenotype. Diabetes 54, 2568–2575. [DOI] [PubMed] [Google Scholar]
- 47. Suzuki A, Nakauchi H, Taniguchi H (2003) Glucagon‐like peptide 1 (1–37) converts intestinal epithelial cells into insulin‐producing cells. Proc. Natl. Acad. Sci. USA 100, 5034–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ianus A, Holz. GG , Theise ND, Hussain MA (2003) In vivo derivation of glucose‐competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J. Clin. Invest. 111, 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ (2004) In vivo and in vitro characterization of insulin‐producing cells obtained from murine bone marrow. Diabetes 53, 1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Doyle MJ, Sussel L (2007) Nkx2.2 regulates β‐cell function in the mature islet. Diabetes 56, 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ (1998) Chronic exposure to free fatty acid reduces pancreatic β cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J. Clin. Invest. 101, 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ling Z, Heimberg H, Foriers A, Schuit F, Pipeleers D (1998) Differential expression of rat insulin I and II messenger ribonucleic acid after prolonged exposure of islet β‐cells to elevated glucose levels. Endocrinology 139, 491–495. [DOI] [PubMed] [Google Scholar]
- 53. Schuit F, Flamez. D , De VA, Pipeleers D (2002) Glucose‐regulated gene expression maintaining the glucose‐responsive state of β‐cells. Diabetes 51 (Suppl. 3), 326–332. [DOI] [PubMed] [Google Scholar]
- 54. Gaber AO, Fraga DW, Callicutt CS, Gerling IC, Sabek OM, Kotb MY (2001) Improved in vivo pancreatic islet function after prolonged in vitro islet culture. Transplantation 72, 1730–1736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Glucose‐induced insulin release data correspond to the amount of insulin secreted within 30 min after 30 mM glucose stimulation. The results were reproduced in three independent experiments
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


