Abstract
Natural products are chemical compounds or substances produced naturally by living organisms. With the development of modern technology, more and more plant extracts have been found to be useful to medical practice. Both micromolecules and macromolecules have been reported to have the ability to inhibit tumour progression, a novel weapon to fight cancer by targeting its 10 characteristic hallmarks. In this review, we focus on summarizing plant natural compounds and their derivatives with anti‐tumour properties, into categories, according to their potential therapeutic strategies against different types of human cancer. Taken together, we present a well‐grounded review of these properties, hoping to shed new light on discovery of novel anti‐tumour therapeutic drugs from known plant natural sources.
Introduction
Cancer, malignant neoplasia, one of the most deadly types of disease, encompasses a broad group of syndromes involving unregulated cell population expansion. A number of plant‐derived compounds play important roles in treatment of cancer, some of the most promising drugs such as taxol, camptothecin, combrestatin, epipodophyllotoxin and the vinca alkaloids being derived from plant sources 1, 2. In the early 19th century, morphine was first isolated from opium, opening the door to a new era in the use of natural products as medicines 3, 4. Anthracyclines, the vinca alkaloids, epipodophyllotoxin lignans, camptothecin derivatives and taxoids, launched before 1997, are still an essential part of the armamentarium for treating cancers 4. In recent years, there have been considerable advances in comprehension of natural products. In addition, anti‐cancer effects of some examples of dietary components, both in vitro and in vivo, have also been reported in different bioactive compounds from plant natural products.
Due to the diversity of natural products, anti‐cancer mechanisms are distinctive in different components. For example, some dietary compounds including curcumin, sulforaphane, soy isoflavone and resveratrol may affect cancer stem cell self‐renewal pathways. Some evidence also suggests that metabolites derived from plants may have pro‐apoptotic properties; such studies have clearly demonstrated that most chemotherapeutic agents ultimately induce cell death by activation of both extrinsic and intrinsic apoptotic pathways 5, 6. Tested on various tumour types, these could provide strategies for further cancer therapy. Chemopreventive agents of cancer stem cells (CSC), might prove to be helpful drugs to inhibit angiogenesis or induce apoptosis. Thus, according to diversity of different mechanisms, we summarize natural products that may potentially be powerful tools to cure various types of cancer.
Natural compounds and pancreatic cancer
Pancreatic cancer is a leading cause of cancer‐related death, and many genetic and environmental risk factors have been associated with it (Fig. 1). Curcumin has the potential ability to suppress development of pancreatic cancer; largely it has been shown to target a plethora of signalling pathways such as those of NF‐κB, SP1, STAT3, Notch‐1, COX‐II, ATM/Chk1 and WT1 7, 8. Curcumin treatment may also enhance IL‐8 receptors CXCR1 & CXCR2 on cell surfaces, suggesting that it inhibits proliferation of pancreatic cancer cells by inhibiting NF‐κB and IL‐8 receptor internalization 9. In addition, a further derivative of curcumin, GO‐Y030, has been found to inhibit STAT3 at low doses, where curcumin itself had little or no effect 10.
Figure 1.
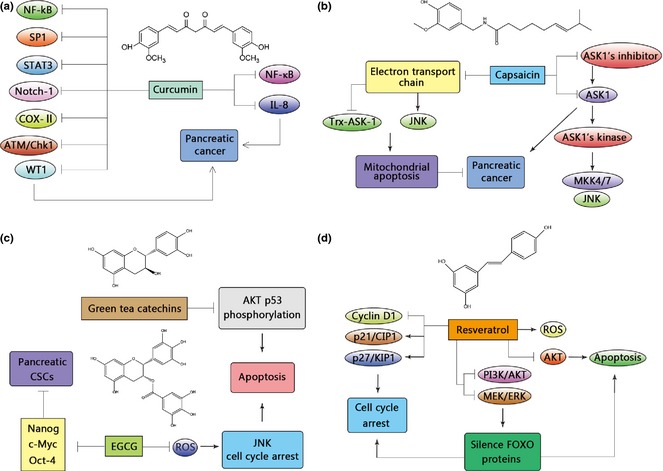
Natural compounds utilized in pancreatic cancer. Pancreatic cancer is one of the best studied cancers with treatment by natural products. Some well‐known products, such as curcumin, capsaicin, green tea catechins and resveratrol regulate it through different mechanisms.
Capsaicin, a homovanillic acid derivative, is a principle pungent constituent of chilli pepper plants, which has historically been used to treat disease. Mechanistic studies have revealed that capsaicin targets Trx‐ASK1 signalling to induce apoptosis in BxPC‐3 pancreatic cancer cells. First, it depletes levels of ASK1 endogenous inhibitor and phosphorylates ASK1 at Thr‐845. Then ASK1 kinase activity increases, leading to activation of ASK1 downstream molecules such as MKK4/7 and JNK 11. Capsaicin targets the mitochondrial electron transport chain to generate reactive oxygen species (ROS), which activate JNK and disrupt Trx‐ASK1 interaction, to induce mitochondrially induced apoptosis in pancreatic cancer 12.
Green tea catechins can inhibit growth of various types of cancer by targeting multiple signalling pathways. It inhibits phosphorylation of AKT and p53, leading to apoptosis and suppression of pancreatic tumour growth 13. ROS are involved in EGCG‐induced cell death, as EGCG dose‐dependently induces ROS generation in pancreatic cancer cells, and eventually activates JNK and cell cycle arrest, leading to apoptosis 14, 15. In addition, EGCG significantly inhibits pluripotency maintenance factors such as Nanog, c‐Myc and Oct‐4 of pancreatic CSC, thus inhibiting self‐renewal potency of pancreatic CSCs 16.
Resveratrol induces apoptosis in pancreatic cancer cells through the mitochondria‐dependent pathway, and generates moderate ROS, which are increased when resveratrol treatment is combined with ionizing radiation 17. Resveratrol induces apoptosis in INS‐1E insulinoma cells by inhibiting AKT signalling 18 and, resveratrol‐induced cell cycle arrest has been associated with up‐regulation of key cell cycle molecules such as p21/CIP1, p27/KIP1 and inhibition of cyclin D1 expression, in MIA PaCa‐2, PanC‐1 and ASPC‐1 pancreatic cancer cells 19. Resveratrol targets FOXO phosphorylation by inhibition of PI3KCI/AKT and MEK/ERK signalling in pancreatic cancer cells and eventually silences FOXO protein abrogated resveratrol‐induced cell cycle arrest and apoptosis 20.
Natural compounds and cervical cancer
Cervical cancer is a major threat to the health of women, perhaps representing the second most common cancer worldwide. Cervical cancers are thought to arise from lesions of lengthy persistent HPV infection, identified as the major aetiological factor in cervical carcinogenesis (Fig. 2).
Figure 2.
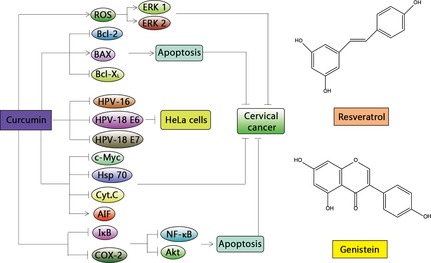
Natural compounds utilized in cervical cancer. Cervical cancer can be regulated by many natural products including curcumin, resveratrol, and genistein, and recognized mechanisms are mostly related to virus transcription, apoptosis and cell cycle arrest.
The effect of curcumin on HeLa cells derives significant changes in tumour‐related proteins linked to cell metabolism, the cell cycle and carcinogenic process – curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP‐1 translocation, and modulation of apoptosis 21. Curcumin has the ability to down‐regulate HPV‐18 transcription, selectively inhibit AP‐1 binding activity and reverse expression dynamics of c‐fos and fra‐1 in HeLa cells 22. Curcumin can reduce expression of HPV‐16, HPV‐18, E6 and E7, resulting in loss of transforming phenotype and cessation of cell population growth 23. Moreover, curcumin reduces c‐Myc transcription factor and Hsp70 chaperone protein, activates AIF, releases cytochrome c and enhances apoptosis via the mitochondrial pathway with up‐regulation of Bax and down‐regulation of Bcl‐2 and Bcl‐XL. With IR, curcumin produces increase in ROS, further leading to sustained ERK 1/2 activation 24. In addition, there is inhibitory action of curcumin on NF‐κB activation, by degradation of IκB and down‐regulation of COX‐2 in cancer cell apoptosis 25.
Resveratrol pre‐treatment inhibits cell division inducing early S‐phase arrest, suggesting a role for it in regulation of cell cycle progression in cervical cancer cells 26. In addition, COX‐1 is over‐expressed in cervical cancer cells with treatment of resveratrol, COX‐1 inhibition suppressing PGs biosynthesis, an implication of ionizing radiation, suggesting that COX‐1 inhibition by resveratrol might alter how tumour cells respond to cytotoxicity of ionizing radiation 27. A further recognized mechanism of resveratrol chemoprevention is regulation of MMP; reduced expression of MMP‐9 is exerted by it in CaSki cervical cancer cells 28. Activation of HIF‐1α and VEGF can be reverted by resveratrol by inhibition of AKT and ERK1/2 in cervical cancer cells, suggesting that resveratrol suppresses tumour angiogenic activity, at least in vitro. Furthermore, resveratrol can destabilize lysosomes, increase cytosol translocation and enzymatic activity of cathepsin L (cat L). Increased cat L activity induces cytochrome c release from mitochondria, leading to apoptotic cell death 29. Resveratrol can also be chemopreventive, chemotherapeutic and radio‐sensitizing in treatment of cervical cancers.
Genistein has demonstrated its chemopreventive activities in various cancers 30, 31. Exposure of HeLa cells to genistein has resulted in significant dose‐ and time‐dependent cell population growth inhibition, found to be mediated by apoptosis and cell cycle arrest at G2/M phase. In addition, it induces migration‐inhibition in a time‐dependent manner by modulating expression of MMP‐9 and TIMP‐1 32. Expression of MMPs and TIMPs have been shown to be correlated to migration‐inhibitory action of genistein, in HeLa cells 33. Expression of MMP‐9 has been shown to be significantly down‐regulated by 100 mm genistein in a time‐dependent manner in HeLa cells. These results are parallel with genistein‐induced suppression of MMP‐9 expression in human breast cancer 34.
Natural compounds and breast cancer
Breast cancer is a major public health concern, and therapeutic strategies have significantly improved patient prognosis 35, 36 (Fig. 3). Flavonoids, a family of polyphenolic compounds which includes flavones and isoflavones, may exert anti‐cancer activity 37. Poly‐methoxy flavones (PMFs), a novel flavonoid derived from sweet orange, has been shown to inhibit growth of human breast cancers by a Ca2+‐dependent apoptotic mechanism, in MCF‐7 cells, inducing sustained increase in concentration of intracellular Ca2+ caused by both depletion of endoplasmic reticulum Ca2+ stores and Ca2+ influx from the extracellular space 38. In addition, flavonoid 8‐prenylnaringenin (8PN), also has chemo‐preventive activity in cancer and anti‐angiogenic properties with EGF‐induced cell proliferation, by strongly inhibiting activation of the PI3KCI/AKT pathway in MCF‐7 cells.
Figure 3.
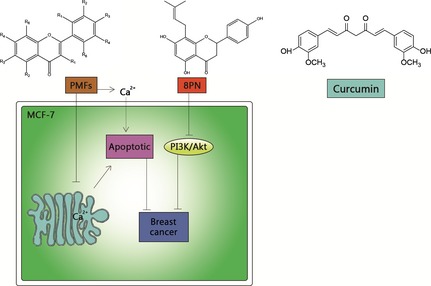
Natural compounds utilized in breast cancer. Poly‐methoxy flavones (PMFs) inhibit growth of human breast cancer via Ca2+‐dependent apoptotic mechanism in MCF‐7 cells. 8PN also has potential capacities in anti‐angiogenic properties with EGF‐induced cell proliferation by strongly inhibiting activation of the PI3K/Akt pathway. In addition, with treatment of curcumin, MCF‐7 cells have shown reduced expression of anti‐apoptotic Bcl‐2 protein and increased expression of Bax/Bcl‐2 ratio.
Extracts of the product known as P. amarus hairy root, have been demonstrated to display linear concentration‐ and time‐dependent cytotoxicity, with increase in percentage of apoptotic cells from 26 to 36%, as determined by annexin V‐FITC and propidium iodide. Observed cytotoxicity correlated well with increased levels of intracellular ROS as well as reduced mitochondrial membrane potential (MMP). This suggests an appreciable anti‐proliferative effect of hairy root extract on MCF‐7 cells, by induction of apoptosis, thereby establishing potential anti‐cancer activity of the extract 39. Curcumin, as already mentioned has been demonstrated as an effective drug against various types of cancer 40.
Natural compounds and ovarian cancer
Due to the lack of significant symptoms in its early stages and absence of effective biomarkers for early detection, ovarian cancer is often diagnosed late 41 (Fig. 4). Rauwolfia vomitoria (Rau) extract has anti‐cancer effects, which reduce cell population growth dose dependently, as shown in three tested ovarian cancer cell lines; it also completely inhibited formation of colonies in soft agar. Moreover, apoptosis was induced in a time‐ and dose‐ dependent manner and was the predominant form of Rau‐induced cell death.
Figure 4.
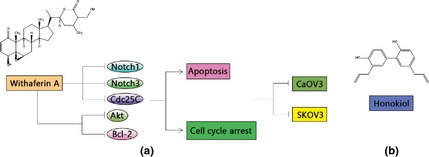
Natural compounds utilized in ovarian cancer. In ovarian cancer cells, Withaferin A can inhibit population growth and colony formation of CaOV3 and SKOV3 cells by inducing apoptosis and cell cycle arrest through down‐regulation of Notch1, Notch3, Cdc25C, total and phosphorylation of Akt, and Bcl‐2 proteins. Honokiol has many effects on ovarian tumours, such as being antioxidant, anti‐thrombotic, anti‐inflammatory, xanthine oxidase inhibitory, having an anxiolytic effect, which can significantly suppress cell proliferation.
A number of anolide natural products, including Withaferin A (WA), steroidal lactone extracted from the medicinal plant Withania somnifera, have been found to exert anti‐cancer effects in a variety of cancer cells 42. WA inhibits cell population growth and colony formation of CaOV3 and SKOV3 cells by inducing apoptosis and cell cycle arrest. These changes are correlated with down‐regulation of Notch1, Notch3, Cdc25C, total and phosphorylation of AKT, and Bcl‐2 proteins 43.
Honokiol, one of the major phenolic constituents of magnolia bark, has a number of pharmacological effects such as being antioxidant, anti‐thrombotic, anti‐inflammatory, xanthine oxidase inhibitory, and with anxiolytic effects; it has remarkable activity on ovarian tumour cells, both in vivo and in vitro 44. Honokiol also significantly suppresses cell proliferation, induces apoptosis in ovarian tumour cells, restrains tumour growth and inhibits angiogenesis in vivo 45.
Natural compounds and lung cancer
Lung cancer, the leading cause of cancer‐related death worldwide, is divided into two major types, small cell lung cancer and non‐small cell lung cancer (NSCLC). BAlthough it may lack significant symptoms at early stages, early diagnosis is still the most important key factor for improving survival of lung cancer patients (Fig. 5). Numbers of herbal plants have traditionally been frequently used to treat lung diseases, including cancer, as folk remedies and medicines. Toona sinensis leaf extract, a bioactive fraction, has been shown to have anti‐cancer effects against lung and prostate cancer cells, as well as to induce apoptotic cell morphological changes, sub‐G1 accumulation, and PARP cleavage 46. Ocimum gratissimum (OG) (Lamiaceae), an aromatic, perennial herb, activates apoptotic signalling molecules such as caspase‐3 and caspase‐9 in A549 cells 47.
Figure 5.
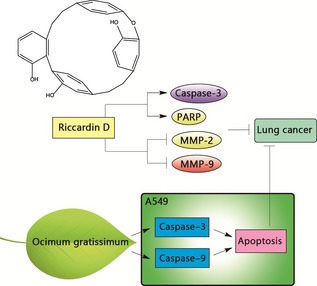
Natural compounds utilized in lung cancer. In lung cancer, Ocimum gratissimum activates apoptosis signalling molecules such as caspase‐3 and caspase‐9 in A549 cells. Riccardin D effectively inhibits proliferation and ability of invasion and migration of non‐small cell lung cancer cells, which can induce apoptosis through activating the caspase cascade signalling pathway.
In addition, ethanol and ethyl acetate extracts of Polygonum cuspidatum (Polygonaceae) show scavenging effects in A549 and H1650 cells 48. Likewise, the ethyl acetate fraction (EAF) of wampee peel exhibits high antioxidant and anti‐cancer activities in lung cancer A549 cells. EAF has revealed DPPH radical scavenging activity, reducing power and superoxide scavenging activity 21. Riccardin D is a macrocyclic bisbibenzyl compound extracted from the liverwort Dumortiera hirsute. Riccardin D effectively inhibits proliferation invasion and migration of NSCLC cells. Further, riccardin D induces apoptosis of NSCLC cells; this has been shown by increase in cells with externalization of phosphatidyl‐serine and by terminal deoxynucleotidyl transferase dUTP nick‐end labelling (TUNEL) positive in H460 xenografts, inhibiting activity of DNA topo II in H460 and A549 cells. Moreover, analysis of apoptotic proteins has shown that riccardin D activates the caspases cascade signalling pathway as demonstrated by increase in cleaved caspase‐3 and cleaved PARP in NSCLC cells in vitro. Also, inhibitory effects of riccardin D on expression of MMP‐2 and MMP‐9 have been verified in H460 xenografts in mice and reduction in vascular endothelial growth factor (VEGF) and ERK1/2 may possibly associate with inhibition of MMPs and NSCLC growth 49.
Natural compounds and prostate cancer
Prostate cancer is a major malignancy, which tends to reveal itself in older men. At present, usual clinical treatments are far from sufficient. Hence, it is important to search for new and better drugs against it (Fig. 6). Magnolol is a lignan with phenolic hydroxyl groups, extracted from root and stem bark of the oriental herb Magnolia officinalis 50. This compound has been shown to inhibit prostate cancer cells by causing their apoptosis. It inhibits Akt enzyme activity, reduces Ser phosphorylation in pro‐apoptotic protein Bad, and significantly inhibits activity of p‐EGFR, p‐PI3K and p‐Akt. Interestingly, it does not affect viability of normal human prostate epithelial PrEC cells 50. Alkaloids are natural products containing nitrogen atoms. Many of them exhibit considerable activities against prostate cancer. Mahanine, a plant carbazole alkaloid, inhibits cell population expansion by inducing apoptosis of both androgen‐responsive LNCaP and androgen‐independent PC‐3 cells. A mechanistic study has shown that mahanine inhibits phosphorylation of PIP3‐dependent kinase 1 (PDK1), resulting in deactivation of Akt and down‐regulation of Bcl‐XL 51.
Figure 6.
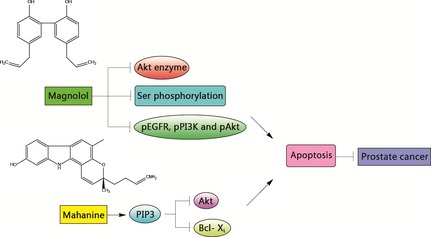
Natural compounds utilized in prostate cancer. Magnolol inhibits prostate cancer cells by induction of apoptosis through inhibiting Akt enzyme and p‐EGFR, p‐PI3K and p‐Akt activity, reducing Ser phosphorylation in pro‐apoptotic protein Bad. Mahanine inhibits cell population growth by inducing apoptosis of both androgen‐responsive LNCaP and androgen‐independent PC‐3 cells by inhibiting phosphorylation of PDK1, which results in deactivation of Akt and down‐regulation of Bcl‐XL.
Many clinical and animal studies have shown that diet‐derived flavonoids play a beneficial role in preventing or inhibiting oncogenesis. Quercetin, silibinin, protoapigenone and celastrol all inhibit prostate cancer cell proliferation. Three active tetracyclic triterpenoids have been isolated from oleogum resin of Boswellia carterii; they are 3‐oxo‐tirucallic acid, 3α‐acetoxy‐tirucallic acid and 3β‐acetoxy‐tirucallic acid 52, 53.
Natural compounds and colorectal cancer
Cancer chemoprevention using dietary factors has received much attention as an effective approach to reduce incidence of malignancy of colorectal cancer 54, 55. The flavonols quercetin and phytoalexin resveratrol are used in this kind of cancer therapy. Quercetin is the most largely studied compound in foods. In vitro, quercetin has been shown to inhibit growth and proliferation of CRC cells such as human adenocarcinoma HT‐29, COLO 201, LS‐174T, HCT‐116, SW480 and Caco‐2 cells and, to a lower extent, of non‐transformed cells such as rat intestinal epithelial cells, and human foetal colon cells (FHC) 56, 57. Several mechanisms have been proposed to explain anti‐proliferative effects of quercetin. These include: (a) cell cycle arrest in G0/G1, G2/M, and S phases; (b) inhibition of proliferation signal transduction pathway‐associated enzymes; (c) reduction in inflammatory metabolite formation by inhibition of Cox‐2 activity and expression, lipoxygenase and iNOS activities, and eicosanoid biosynthesis; (d) interaction with type II oestrogen receptors (ER); (e) down‐regulation of expression of cell cycle genes CDC6, CDK4 and cyclin D1; (f) up‐regulation of tumour‐suppressor genes for breast cancer type 2 susceptibility protein and mucin 2, and down‐regulation of Ras oncogene and survive expression; (g) down‐regulation of the β‐catenin pathway; (h) rearrangement of cytoskeleton actin microfilaments [320] and tubulin microtubules; and (i) inhibition of P‐glycoprotein, a membrane transporter that extrudes chemotherapeutic drugs 58, 59, 60, 61. In CRC cells, quercetin is also capable of reducing cell migration. Interestingly, inhibition of cell proliferation by quercetin has in some cases shown a biphasic response 62. In CRC cells, resveratrol has (a) growth‐inhibitory activity; (b) induces differentiation; (c) arrests cell proliferation and neoplastic transformation; (d) arrests the cell cycle in the S, G1/S, G2/M or, less frequently, in S/G2 phase of cell cycle; (e) induces apoptosis; and (f) presents anti‐angiogenic, anti‐invasion and anti‐metastatic characteristics; (g) capability of modulating MAPK‐transduction pathways 63, 64, 65, 66.
Natural compounds and oral cancer
Oral squamous cell carcinoma (OSCC) is the most common cancer of the oral cavity, accounting for more than 300 000 new cases annually. Based on currently available clinical assessment and treatment methods, the disease is usually only diagnosed at advanced stages, causing high morbidity and mortality. Hinokitiol is a natural component isolated from Chamacyparis taiwanensi. It has significant anti‐microbial and cytotoxic activities against oral pathogens and oral squamous cell carcinoma cells and low cytotoxic effects to normal human oral keratinocytes 67. Treatment with hinokitiol produces cytotoxic effects in murine P388 lymphocytic leukaemia cells, and also blocks androgen receptor signal transduction inhibiting growth of prostate cancer cell LNCaP 68.
Goniothalamin (GTN) is a plant bioactive styryl‐lactone with potent anti‐ tumourigenesis effects on several types of cancer. In GTN‐treated Ca9‐22 OSCC cancer cells, a number of concentration‐ or time‐dependent mechanisms seem to emerge, such as cell proliferation inhibition; sub‐G1 population and annexin‐V‐intensity significant increase; intracellular ROS level induction; mitochondrial membrane depolarization and significant increase in gamma‐H2AX intensity revealed by analysis of DNA double strand breaks 69.
In some popular folk medicine, Terminalia catappa leaves (TCE) are believed to possess anti‐cancer activities. Treatment by ethanolic extracts of TCE on SCC‐4 oral cancer cells, has been analysed, revealing that TCE treatment inhibited cell migration/invasion capacities and protein levels of MMP‐2, MMP‐9 and u‐PA. Further studies also indicated that TCE inhibited phosphorylation of ERK1/2, JNK1/2 and Akt, while expression of nuclear protein NF‐kB, c‐Jun and c‐Fos were inhibited 70.
Natural compounds and hepatocellular carcinoma
Hepatocellular carcinoma (HCC) accounts for 70–85% of the total liver cancer burden, and is another of the leading causes of cancer‐related death worldwide. Sadly, treatment available for advanced HCC remains disappointing 11. Epigallocatechin gallate (EGCG), also known as epigallocatechin 3‐gallate, is a well‐studied poly‐phenolic in relation to treatment for HCC. EGCG induces apoptosis and cell cycle arrest, and exhibits anti‐angiogenic and anti‐metastatic potential to HCC cells. Paradoxically, EGCG seems to exert its cytostatic effects against cancer cells by pro‐oxidant activity, although it has strong antioxidant properties 71. In addition, a number of animal studies have shown that EGCG prevents chemical‐induced HCC.
Quercetin is one of the best‐studied polyphenols, it is found in onions, apples, berries, tea and red wine. It seems to exert its chemopreventive potential by inhibition and induction of survival and death signalling pathways, respectively, in liver cancer cells. Moreover, in animal studies, quercetin has been shown to protect from DEN‐ or AFB‐induced liver carcinogenesis, mainly due to its strong antioxidant activity and consequent prevention of ROS‐induced DNA mutation in genes critical to cell cycle control, such as p53 72. Curcumin is the principal curcuminoid of the spice turmeric, one of the most well‐studied plant polyphenols regarding its effects on HCC. Its suppression of HCC cells is largely due to inhibition of abnormal cell proliferation and apoptosis by modulation of relevant signalling pathways. Moreover, studies both in vitro and in vivo have demonstrated anti‐angiogenic and anti‐metastatic properties of curcumin against hepatocarcinogenesis 73.
Conclusions and perspectives
Despite years of effort at fighting cancer, it remains the one of the htree most deadly diseases of world, recognised as possessing 10 characteristic hallmarks 74. Interestingly, growing evidence supports key roles for plant natural products against cancer in its typical hallmarks. Paclitaxel, a microtubule‐stabilizing compound, can induce expression of inflammatory genes in monocytes and tumour cells, corresponding to the hallmark of tumour‐promoting inflammation 75. Also metabolites derived from plants may possess pro‐apoptotic properties and have great potential for possible applications in cancer prevention, such as extracts of Solanum muricatum (Pepino) 76. Meliacine (MA), a substance present in leaf extracts of Melia azedarach L., reduces viral load and abolishes inflammatory reactions and neovascularization, displays anti‐herpetic and immunomodulatory activities, impedes VEGF transcription, and therefore interferes with the angiogenic process 77. Methanolic extract of Rhizophora apiculata has been evaluated by using B16F‐10 melanoma‐induced lung metastasis model in C57BL/6 mice, indicating that natural products can affect the metastatic process 78. Moreover, crude extract, ethyl acetate fraction and flavonoids, isolated from leaves of Scutia buxifolia, protect against chromosome damage induced by H2O2 in human lymphocytes, by analysing cell population growth rate, cell viability, mitotic index and chromosomal instability 79.
Future targeted therapy would be used some synthetic molecules to target specific proteins in tumour growth and progression 80. However, the vast majority of common tumours have been found to be dependent on multiple signalling pathway redundancies and adaptive mechanisms rather than a single ‘targetable’ oncogenic activation, either rendering tumours primarily resistant to targeted drugs or facilitating acquired resistance to cell signalling inhibition after only a few months treatment. These results bring the re‐emergence of natural products in oncology 81. Recently, some studies have demonstrated that natural products statically tend to target proteins with a high number of protein–protein interactions that are particularly essential to an organism 81; this can prove that using natural products may be a further potential therapeutic strategy in cancer.
Natural compounds derived from plant organisms provide an inestimable source of chemical structures with a variety of different anti‐cancer effects; some typical plant products and their mechanisms for being used as anti‐tumour agents have been summerised. It is hoped that more efficient and effective application of natural products will improve the drug discovery process. With increasing applications of molecular biological techniques for availability of novel compounds and combinatorial chemistry approaches, there will be further developments in the use of novel natural products and chemical libraries based on natural products for future drug discovery.
Acknowledgements
We thank Prof./Dr. Yan Cheng for her critical review of this paper. This work was supported by grants from the National 973 Basic Research Program of China (no. 2010CB529900), the Key Projects of the National Science and Technology Pillar Program (no. 2012BAI30B02), National Natural Science Foundation of China (nos U1170302, 81160543, 81260628, 81303270 and 81172374), West China Hospital‐Chengdu Science and Technology Department Translational Medicine Innovation Foundation (no. ZH13039), and Shenyang Pharmaceutical University Scientific Research Fund (no. ZCJJ2013407).
References
- 1. Cirla A, Mann J (2003) Combretastatins, from natural products to drug discovery. Nat. Prod. Rep. 20, 558–564. [DOI] [PubMed] [Google Scholar]
- 2. Canel C, Moraes RM, Dayan FE, Ferreira D (2000) Podophyllotoxin. Phytochemistry 54, 115–120. [DOI] [PubMed] [Google Scholar]
- 3. Clardy J, Walsh C (2004) Lessons from natural molecules. Nature 432, 829–837. [DOI] [PubMed] [Google Scholar]
- 4. Balunas MJ, Kinghorn AD (2005) Drug discovery from medicinal plants. Life Sci. 78, 431–441. [DOI] [PubMed] [Google Scholar]
- 5. Liu B, Min MW, Bao JK (2009) Induction of apoptosis by Concanavalin A and its molecular mechanisms in cancer cells. Autophagy 5, 432–433. [DOI] [PubMed] [Google Scholar]
- 6. Radhika NK, Sreejith P, Asha V (2010) Cytotoxic and apoptotic activity of Cheilanthes farinosa (Forsk.) Kaulf. against human hepatoma, Hep3B cells. J. Ethnopharmacol. 128, 166–171. [DOI] [PubMed] [Google Scholar]
- 7. Glienke W, Maute L, Wicht J, Bergmann L (2009) Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and down regulation of survivin/BIRC5 gene expression. Cancer Invest. 28, 166–171. [DOI] [PubMed] [Google Scholar]
- 8. Sahu R, Batra S, Srivastava S (2009) Activation of ATM/Chk1 by Curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br. J. Cancer 100, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hidaka H, Ishiko T, Furuhashi T, Kamohara H, Suzuki S, Miyazaki M, et al (2002) Curcumin inhibits interleukin 8 production and enhances interleukin 8 receptor expression on the cell surface. Cancer 95, 1206–1214. [DOI] [PubMed] [Google Scholar]
- 10. Hutzen B, Friedman L, Sobo M, Lin L, Cen L, De Angelis S, et al (2009) Curcumin analogue GO‐Y030 inhibits STAT3 activity and cell growth in breast and pancreatic carcinomas. Int. J. Oncol. 35, 867–872. [DOI] [PubMed] [Google Scholar]
- 11. Yang CS, Wang X (2010) Green tea and cancer prevention. Nutr. Cancer 62, 931–937. [DOI] [PubMed] [Google Scholar]
- 12. Pramanik KC, Srivastava SK (2012) Apoptosis signal‐regulating kinase 1‐thioredoxin complex dissociation by capsaicin causes pancreatic tumor growth suppression by inducing apoptosis. Antioxid. Redox Signal. 17, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang L, Pang E, Loo RRO, Rao J, Go VLW, Loo JA, et al (2011) Concomitant inhibition of HSP90, its mitochondrial localized homologue TRAP1 and HSP27 by green tea in pancreatic cancer HPAF‐II cells. Proteomics 11, 4638–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qanungo S, Das M, Haldar S, Basu A (2005) Epigallocatechin‐3‐gallate induces mitochondrial membrane depolarization and caspase‐dependent apoptosis in pancreatic cancer cells. Carcinogenesis 26, 958–967. [DOI] [PubMed] [Google Scholar]
- 15. Hariharan D, Saied A, Kocher HM (2008) Analysis of mortality rates for pancreatic cancer across the world. HPB 10, 58–62. doi: 10.1080/13651820701883148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang SN, Fu J, Nall D, Rodova M, Shankar S, Srivastava RK (2012) Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int. J. Cancer 131, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shankar S, Suthakar G, Srivastava RK (2007) Epigallocatechin‐3‐gallate inhibits cell cycle and induces apoptosis in pancreatic cancer. Front. Biosci. 12, 5039–5051. [DOI] [PubMed] [Google Scholar]
- 18. Sun W, Wang W, Kim J, Keng P, Yang S, Zhang H, et al (2008) Anti‐cancer effect of resveratrol is associated with induction of apoptosis via a mitochondrial pathway alignment. Adv. Exp. Med. Biol. 614, 179–186. [DOI] [PubMed] [Google Scholar]
- 19. Mo W, Xu X, Xu L, Wang F, Ke A, Wang X, et al (2011) Resveratrol inhibits proliferation and induces apoptosis through the hedgehog signaling pathway in pancreatic cancer cell. Pancreatology. 11, 601–609. [DOI] [PubMed] [Google Scholar]
- 20. Roy SK, Chen Q, Fu J, Shankar S, Srivastava RK (2011) Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS One 6, e25166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Q, Ganapathy S, Singh KP, Shankar S, Srivastava RK (2010) Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS One 5, e15288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Divya CS, Pillai MR (2006) Antitumor action of Curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP‐1 translocation, and modulation of apoptosis. Mol. Carcinog. 45, 320–332. [DOI] [PubMed] [Google Scholar]
- 23. Prusty BK, Das BC (2005) Constitutive activation of transcription factor AP‐1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP‐1 activity in HeLa cells by Curcumin. Int. J. Cancer 113, 951–960. [DOI] [PubMed] [Google Scholar]
- 24. Singh M, Singh N (2011) Curcumin counteracts the proliferative effect of estradiol and induces apoptosis in cervical cancer cells. Mol. Cell. Biochem. 347, 1–11. [DOI] [PubMed] [Google Scholar]
- 25. Javvadi P, Segan AT, Tuttle SW, Koumenis C (2008) The chemopreventive agent Curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen‐activated protein kinase pathway. Mol. Pharmacol. 73, 1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sreekanth C, Bava S, Sreekumar E, Anto R (2011) Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene 30, 3139–3152. [DOI] [PubMed] [Google Scholar]
- 27. Zoberi I, Bradbury CM, Curry HA, Bisht KS, Goswami PC, Roti Roti JL, et al (2002) Radiosensitizing and anti‐proliferative effects of resveratrol in two human cervical tumor cell lines. Cancer Lett. 175, 165–173. [DOI] [PubMed] [Google Scholar]
- 28. Kramer MP, Węsierska‐Gądek J (2009) Monitoring of long‐term effects of resveratrol on cell cycle progression of human HeLa cells after administration of a single dose. Ann. N. Y. Acad. Sci. 1171, 257–263. [DOI] [PubMed] [Google Scholar]
- 29. Woo J‐H, Lim JH, Kim Y‐H, Suh S‐I, Min DS, Chang J‐S, et al (2003) Resveratrol inhibits phorbol myristate acetate‐induced matrix metalloproteinase‐9 expression by inhibiting JNK and PKC δ signal transduction. Oncogene 23, 1845–1853. [DOI] [PubMed] [Google Scholar]
- 30. Hsu K‐F, Wu C‐L, Huang S‐C, Wu C‐M, Hsiao J‐R, Yo Y‐T, et al (2009) Cathepsin L mediates resveratrol‐induced autophagy and apoptotic cell death in cervical cancer cells. Autophagy 5, 451–460. [DOI] [PubMed] [Google Scholar]
- 31. Liu B, Cheng Y, Bian H‐J, Bao J‐K (2009) Molecular mechanisms of Polygonatum cyrtonema lectin induced apoptosis and autophagy in cancer cells. Autophagy 5, 253–255. [DOI] [PubMed] [Google Scholar]
- 32. Chodon D, Ramamurty N, Sakthisekaran D (2007) Preliminary studies on induction of apoptosis by genistein on HepG2 cell line. Toxicol. In Vitro 21, 887–891. [DOI] [PubMed] [Google Scholar]
- 33. Osborne CK, Yochmowitz MG, Knight WA, McGuire WL (1980) The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer 46, 2884–2888. [DOI] [PubMed] [Google Scholar]
- 34. Pavese JM, Farmer RL, Bergan RC (2010) Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 29, 465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, Bhuiyan M, Sarkar F (1999) Induction of apoptosis and inhibition of c‐erbB‐2 in MDA‐MB‐435 cells by genistein. Int. J. Oncol. 15, 525–558. [DOI] [PubMed] [Google Scholar]
- 36. DeSantis C, Siegel R, Bandi P, Jemal A (2011) Breast cancer statistics. CA Cancer J. Clin. 61, 409–418. [DOI] [PubMed] [Google Scholar]
- 37. Findlay M, Von Minckwitz G, Wardley A (2008) Effective oral chemotherapy for breast cancer, pillars of strength. Ann. Oncol. 19, 212–222. [DOI] [PubMed] [Google Scholar]
- 38. Cornwell T, Cohick W, Raskin I (2004) Dietary phytoestrogens and health. Phytochemistry 65, 995–1016. [DOI] [PubMed] [Google Scholar]
- 39. Sergeev IN, Li S, Colby J, Ho C‐T, Dushenkov S (2006) Polymethoxylated flavones induce Ca(2+)‐mediated apoptosis in breast cancer cells. Life Sci. 80, 245–253. [DOI] [PubMed] [Google Scholar]
- 40. Abhyankar G, Suprasanna P, Pandey B, Mishra K, Rao K, Reddy V (2010) Hairy root extract of Phyllanthus amarus induces apoptotic cell death in human breast cancer cells. Innov. Food Sci. Emerg. 11, 526–532. [Google Scholar]
- 41. Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL (2012) The flavonoid quercetin in disease prevention and therapy, facts and fancies. Biochem. Pharmacol. 83, 6–15. [DOI] [PubMed] [Google Scholar]
- 42. Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al (2001) Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 7, 1894–1900. [PubMed] [Google Scholar]
- 43. Buys SS, Partridge E, Greene MH, Prorok PC, Reding D, Riley TL, et al (2005) Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial, findings from the initial screen of a randomized trial. Am. J. Obstet. Gynecol. 193, 1630–1639. [DOI] [PubMed] [Google Scholar]
- 44. Yu Y, Hamza A, Zhang T, Gu M, Zou P, Newman B, et al (2010) Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem. Pharmacol. 79, 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang X, Samadi AK, Roby KF, Timmermann B, Cohen MS (2012) Inhibition of cell growth and induction of apoptosis in ovarian carcinoma cell lines CaOV3 and SKOV3 by natural withanolide Withaferin A. Gynecol. Oncol. 124, 606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuribara H, Stavinoha WB, Maruyama Y (1998) Behavioural pharmacological characteristics of honokiol, an anxiolytic agent present in extracts of Magnolia bark, evaluated by an elevated plus‐maze test in mice. J. Pharm. Pharmacol. 50, 819–826. [DOI] [PubMed] [Google Scholar]
- 47. Li Z, Liu Y, Zhao X, Pan X, Yin R, Huang C, et al (2008) Honokiol, a natural therapeutic candidate, induces apoptosis and inhibits angiogenesis of ovarian tumor cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 140, 95–102. [DOI] [PubMed] [Google Scholar]
- 48. Yang C‐J, Huang Y‐J, Wang C‐Y, Wang C‐S, Wang P‐H, Hung J‐Y, et al (2010) Antiproliferative and antitumorigenic activity of Toona sinensis leaf extracts in lung adenocarcinoma. J. Med. Food 13, 54–61. [DOI] [PubMed] [Google Scholar]
- 49. Lin Y‐W, Yang F‐J, Chen C‐L, Lee W‐T, Chen R‐S (2010) Free radical scavenging activity and antiproliferative potential of Polygonum cuspidatum root extracts. J. Nat. Med. 64, 146–152. [DOI] [PubMed] [Google Scholar]
- 50. Lee DH, Szczepanski MJ, Lee YJ (2009) Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt signaling pathway in human prostate cancer cells. J. Cell. Biochem. 106, 1113–1122. [DOI] [PubMed] [Google Scholar]
- 51. Sinha S, Pal BC, Jagadeesh S, Banerjee PP, Bandyopadhaya A, Bhattacharya S (2006) Mahanine inhibits growth and induces apoptosis in prostate cancer cells through the deactivation of Akt and activation of caspases. Prostate 66, 1257–1265. [DOI] [PubMed] [Google Scholar]
- 52. Estrada AC, Syrovets T, Pitterle K, Lunov O, Büchele B, Schimana‐Pfeifer J, et al (2010) Tirucallic acids are novel pleckstrin homology domain‐dependent Akt inhibitors inducing apoptosis in prostate cancer cells. Mol. Pharmacol. 77, 378–387. [DOI] [PubMed] [Google Scholar]
- 53. Fu L‐L, Zhou C‐C, Yao S, Yu J‐Y, Liu B, Bao J‐K (2011) Plant lectins: targeting programmed cell death pathways as anti‐tumor agents. Int. J. Biochem. Cell Biol. 43, 1442–1449. [DOI] [PubMed] [Google Scholar]
- 54. Prasad KN, Hao J, Yi C, Zhang D, Qiu S, Jiang Y, et al (2009) Antioxidant and anticancer activities of wampee (Clausena lansium (Lour.) Skeels) peel. J. Biomed. Biotechnol. 2009, 612805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xue X, Sun D‐F, Sun C‐C, Liu H‐P, Yue B, Zhao C‐R, et al (2012) Inhibitory effect of riccardin D on growth of human non‐small cell lung cancer: in vitro and in vivo studies. Lung Cancer 76, 300–308. [DOI] [PubMed] [Google Scholar]
- 56. Siegel R, Naishadham D, Jemal A (2012) Cancer statistics. CA Cancer J. Clin. 62, 10–29. [DOI] [PubMed] [Google Scholar]
- 57. Gonçalves P, Araújo JR, Pinho MJ, Martel F (2011) In vitro studies on the inhibition of colon cancer by butyrate and polyphenolic compounds. Nutr. Cancer 63, 282–294. [DOI] [PubMed] [Google Scholar]
- 58. Kim WK, Bang MH, Kim ES, Kang NE, Jung KC, et al (2005) Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT‐29 human colon cancer cells. J. Nutr. Biochem. 16, 155–162. [DOI] [PubMed] [Google Scholar]
- 59. Psahoulia FH, Moumtzi S, Roberts ML, Sasazuki T, Shirasawa S, Pintzas A (2007) Quercetin mediates preferential degradation of oncogenic Ras and causes autophagy in Ha‐RAS‐transformed human colon cells. Carcinogenesis 28, 1021–1031. [DOI] [PubMed] [Google Scholar]
- 60. Mertens‐Talcott SU, Percival SS (2005) Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 218, 141–151. [DOI] [PubMed] [Google Scholar]
- 61. Gee JM, Hara H, Johnson IT (2002) Suppression of intestinal crypt cell proliferation and aberrant crypt foci by dietary quercetin in rats. Nutr. Cancer 43, 193–201. [DOI] [PubMed] [Google Scholar]
- 62. Johnson IT (2007) Phytochemicals and cancer. Proc. Nutr. Soc. 66, 207–215. [DOI] [PubMed] [Google Scholar]
- 63. Nair HK, Rao KV, Aalinkeel R, Mahajan S, Chawda R, Schwartz SA (2004) Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes. Clin. Diagn. Lab. Immunol. 11, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL (2009) Multiple molecular targets of resveratrol, anti‐carcinogenic mechanisms. Arch. Biochem. Biophys. 486, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB (2002) Resveratrol induces growth inhibition, S‐phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 8, 893–903. [PubMed] [Google Scholar]
- 66. Juan MEl, Wenzel U, Daniel H, Planas JM (2008) Resveratrol induces apoptosis through ROS‐dependent mitochondria pathway in HT‐29 human colorectal carcinoma cells. J. Agric. Food Chem. 56, 4813–4818. [DOI] [PubMed] [Google Scholar]
- 67. Prasanna R, Chinnakonda Chandramoorthy H, Ramaiyapillai P, Sakthisekaran D (2011) In vitro evaluation of anticancer effect of Cassia auriculata leaf extract and Curcumin through induction of apoptosis in human breast and larynx cancer cell lines. Biomed. Prevent. Nutr. 1, 153–160. [Google Scholar]
- 68. Shih Y‐H, Chang K‐W, Hsia S‐M, Yu C‐C, Fuh L‐J, Chi T‐Y, et al (2013) In vitro antimicrobial and anticancer potential of hinokitiol against oral pathogens and oral cancer cell lines. Microbiol. Res. 12, 254–262. [DOI] [PubMed] [Google Scholar]
- 69. Liu S, Yamauchi H (2006) Hinokitiol, a metal chelator derived from natural plants, suppresses cell growth and disrupts androgen receptor signaling in prostate carcinoma cell lines. Biochem. Biophys. Res. Commun. 351, 26–32. [DOI] [PubMed] [Google Scholar]
- 70. Yen C‐Y, Chiu C‐C, Haung R‐W, Yeh C‐C, Huang K‐J, Chang K‐F, et al (2012) Antiproliferative effects of goniothalamin on Ca9‐22 oral cancer cells through apoptosis, DNA damage and ROS induction. Mutat. Res. 747, 253–258. [DOI] [PubMed] [Google Scholar]
- 71. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J. Clin. 61, 69–90. [DOI] [PubMed] [Google Scholar]
- 72. Li Y, Martin RC (2011) Herbal medicine and hepatocellular carcinoma, applications and challenges. Evid. Based Complement. Alternat. Med. 2011, 541209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brückner M, Westphal S, Domschke W, Kucharzik T, Lügering A (2012) Green tea polyphenol epigallocatechin‐3‐gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohns Colitis. 6, 226–235. [DOI] [PubMed] [Google Scholar]
- 74. Hanahan D, Weinberg RA (2011) Hallmarks of cancer, the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- 75. Napoleone E, Zurlo F, Latella MC, Amore C, Di Santo A, et al (2009) Paclitaxel down‐regulates tissue factor in cancer and host tumour‐associated cells. Eur. J. Cancer 45, 470–477. [DOI] [PubMed] [Google Scholar]
- 76. Ren W, Tang DG (1999) Extract of Solanum muricatum (Pepino/CSG) inhibits tumor growth by inducing apoptosis. Anticancer Res. 19, 403–408. [PubMed] [Google Scholar]
- 77. Bueno CA, Lombardi MG, Sales ME, Alché LE (2012) A natural antiviral and immunomodulatory compound with antiangiogenic properties. Microvasc. Res. 84, 235–241. [DOI] [PubMed] [Google Scholar]
- 78. Prabhu VV1, Guruvayoorappan C (2013) Inhibition of metastatic lung cancer in C57BL/6 mice by marine mangrove Rhizophora apiculata . Asian Pac. J. Cancer Prev. 14, 1833–1840. [DOI] [PubMed] [Google Scholar]
- 79. Boligon AA, Sagrillo MR, Machado LF, de Souza Filho O, Machado MM, da Cruz IB et al (2012) Protective effects of extracts and flavonoids isolated from Scutia buxifolia Reissek against chromosome damage in human lymphocytes exposed to hydrogen peroxide. Molecules 17, 5757–5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Armstrong EP (2010) Prophylaxis of cervical cancer and related cervical disease: a review of the cost‐effectiveness of vaccination against oncogenic HPV types. J. Managed Care Phar. 16, 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yu J, Ma Y, Drisko J, Chen Q (2013) Antitumor activities of rauwolfia vomitoria extract and potentiation of carboplatin effects against ovarian cancer. Curr. Ther. Res. Clin. Exp. 75, 8–14. doi: 10.1016/j.curtheres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


