Abstract
Severe burns remain a life‐threatening local and general inflammatory condition often with serious sequelae, despite remarkable progress in their treatment over the past three decades. Cultured epidermal autografts, the first and still most up‐to‐date cell therapy for burns, plays a key role in that progress, but drawbacks to this need to be reduced by using cultured dermal–epidermal substitutes. This review focuses on what could be, in our view, the next major breakthrough in cell therapy of burns – use of mesenchymal stromal cells (MSCs). After summarizing current knowledge, including our own clinical experience with MSCs in the pioneering field of cell therapy of radiation‐induced burns, we discuss the strong rationale supporting potential interest in MSCs in treatment of thermal burns, including limited but promising pre‐clinical and clinical data in wound healing and acute inflammatory conditions other than burns. Practical options for future therapeutic applications of MSCs for burns treatment, are finally considered.
Severe burns and their treatment
Burns are traumatic destruction of the skin and sometimes underlying tissues, usually caused by a heat source, less often by electricity or chemicals and rarely by ionizing radiation. 1 Necrosis‐triggered release of inflammatory mediators and in situ formation of toxic lipid–protein complexes generate local inflammation. In the most severe cases, potentially lethal acute toxaemia with systemic inflammatory response (SIR) and organ dysfunction, with a threshold around 20–30% total body surface area burnt, and dose (burn depth and extension)‐dependent severity (1).
Severe burns evolve in three phases:
-
•
In the early shock phase, hyperinflammation causes intense plasma leakage into the interstitium, organ dysfunction and injury aggravation.
-
•
In the following hypermetabolic phase, a long‐lasting inflammatory status sustains organ dysfunctions and slows the wound‐healing process, while cell‐mediated immunity is impaired.
-
•
In the late local remodelling phase, cell/matrix interactions promote fibrotic, hypertrophic and/or retractile scarring.
Besides aggressive supportive therapy, treating burns demands that the toxic eschar be quickly removed and that structure and function of the destroyed skin be restored. This is usually achieved through surgical eschar excision and split thickness autografts from healthy skin areas of the same patient. Despite providing epidermis and also a thin layer of dermal tissue, that technique cannot restore fully functional dermis nor epidermal appendages, and its applicability is limited by available amounts of healthy skin still present. Thus, burn treatment strategies often involve a combination of mesh expanded epidermal autografts, temporary or preparative skin substitutes including skin allografts and bio‐engineered products (2), topical treatments and techniques to improve wound healing, and cell therapy in selected cases.
Cell therapy for burns: background
Cell therapy is therapeutic administration of living cells aimed at tissue regeneration, support for any defective function (such as wound healing) or modulation of pathophysiological processes (such as hyperinflammation or immune dysfunction). This definition extends to tissue engineering involving cell‐containing biomaterials.
Cultured epidermal autografts (CEAs) after Green’s technique to isolate and culture keratinocytes (3), constituted the first cell therapy for burns. These have been used for burnt patients for three decades. Although associated with dramatic survival improvement (4), CEAs have two major drawbacks: fragility and poor cosmetic quality of healed zones, mostly due to their lack of underlying dermis, which must be provided by prior skin allografts (5), and by immaturity of the resulting dermal–epidermal junction.
Dermal substitutes were the second type of cell therapy for burns. While initially they provided solely an extracellular matrix to be vascularized and repopulated with fibroblasts from the wound bed (6), in more recent approaches, matrices have been seeded with living allogeneic fibroblasts to benefit from their epidermal support and extracellular matrix remodelling ability (7).
Beyond initially disappointing clinical results when combining CEAs with dermal substitutes, dermal–epidermal bio‐engineered cultured skin substitutes are a third step in cell therapy for burns. As recently reviewed, first clinical results are highly promising but further improvements are still required (8). These will likely stem from refinements in structural components and signalling molecules included in matrices, and from use of other cell types.
Mesenchymal stromal cells (MSCs) are interesting candidates for such applications. They could bring the next major breakthrough in cell therapy for burns, as suggested by accumulating data supported by a strong rationale.
Mesenchymal stromal cells
Long suspected presence of non‐haematopoietic cells in the bone marrow was first confirmed in 1970 when Friedenstein and colleagues described such a plastic‐adherent fibroblastic population (9). In the same decade, Schofield and colleagues established that these stromal cells, along with other factors, build a micro‐environment which is essential for haematopoietic stem cells to remain undifferentiated (10), the haematopoietic niche, which has undergone intense study ever since. The cells were later shown to differentiate mainly towards mesodermal‐derived cell types (osteoblasts, chondroblasts, adipocytes) (11), supporting the concept of bone marrow mesenchymal stem cells.
Cells with similar phenotypes were then isolated from many connective tissues from the foetal stage (12) as well as from adult subjects, including adipose tissue and dermis (13). Facing the poorly consistent terminology used to describe this heterogeneous family, frequent lack of demonstration of self‐renewal, and likely heterogeneity of described cell populations in their proliferating and differentiating abilities, the International Society for Cellular Therapy proposed a unified definition for MSCs in 2006, based on a list of minimal specifications to be met by a cell population (14):
-
•
The cells adhere to plastic under standard culture conditions.
-
•
Most of them (>95%) express CD73 (5′ectonucleotidase), CD90 (Thy‐1) and CD105 (endoglin).
-
•
At least 98% of them do not express haematopoetic markers CD45, CD34 and CD14.
-
•
They can differentiate in vitro along the osteoblastic, chondrogenic and adipogenic lineages.
This differentiation potential, possibly extended to more embryologically distant cell types, has long been the rationale for use of MSCs in different models of tissue healing, further supported by homing capacity of MSCs towards injured tissues in response to various chemokines (15).
More recently, the discrepancy between consistent celerity of effects observed after in vivo MSC infusion and relatively slow proliferation and differentiation processes, clearly suggested that those effects are at least partly attributable to interactions between MSCs and neighbouring cells (16), thus extending the niche concept to tissues other than haematopoietic bone marrow. These interactions mostly occur through secretion of a broad array of factors (15, 17), summarized in Fig. 1. They can also involve cell fusion (18), mitochondrial transfer (19) or miRNA‐rich vesicular exchange (20). As a result, MSCs can modulate multiple processes such as migration, proliferation, functional activation or apoptosis, in a broad array of cells. These paracrine effects can be functionally sorted as trophic, angiogenic, immunomodulating, anti‐inflammatory, anti‐fibrotic and chemo‐attractive, although they are all intimately linked to each other (Fig. 1). All of these effects, along with differentiation ability of MSCs, are potentially interesting for burn treatment.
Figure 1.
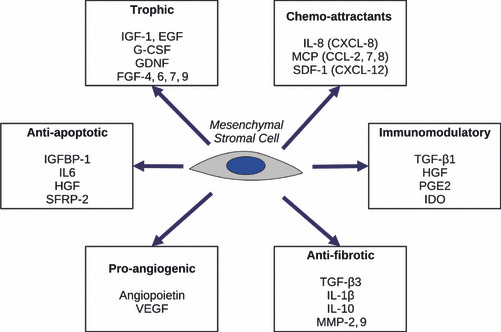
Functional effects of MSCs related to secreted proteins (14, 16). EGF, epidermal growth factor; FGF, fibroblast growth factors; GDNF, glial cell derived neurotrophic factor; G‐CSF, granulocyte colony stimulating factor; HGF, hepatocyte growth factor; IDO, indoleamine 2–3 dioxygenase; IL, interleukin; IGF, insulin‐like growth factor; IGFBP, insulin‐like growth factor binding protein; MCP, macrophage chemoattractant protein; MMP, matrix metallo‐proteinase; PGE, prostaglandin E; SDF, stromal derived factor; SFRP, secreted frizzled related protein; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
Lessons learned from radiation‐induced burns
Despite their low incidence, burns induced by overexposure to ionizing radiations such as X‐rays or γ‐rays, which define the cutaneous radiation syndrome (CRS), 2 benefited first from cell therapy with MSCs, mostly due to lack of any satisfactory alternative.
CRS pathophysiology is complex and still incompletely understood.
-
•
At the molecular level, irradiation damages DNA (plus membrane proteins and lipids at higher doses) directly or indirectly, through reactive species resulting from water radiolysis (21).
-
•
At the cellular level, irradiation can result in absence of cell alteration, DNA lesions leading to transient or permanent cycle arrest, or direct apoptosis.
-
•
At the tissue level, irradiation triggers intercellular signalling cascades, which lead to damage‐increasing inflammatory processes. For instance, cyclo‐oxygenase (COX)‐2, a key enzyme in biosynthesis of inflammatory mediators, has been found to be overexpressed after irradiation and is associated with the so‐called bystander effect, in which non‐irradiated cells may eventually suffer DNA lesions (22).
Despite its similar late outcome in terms of tissue loss, the natural history of CRS is very different from that of other burns. The following clinical stages are observed (23):
-
•
Prodromal stage (24–72 h post‐irradiation) with inconstant erythema and itching, characterized by synthesis and release of pro‐inflammatory mediators by local cells.
-
•
Clinical latency period likely explained by balanced release of anti‐inflammatory mediators.
-
•
Manifestation stage (days to weeks) with reddening, blistering and ulceration. These signs correlate with histological findings of vasculitis and cutaneous infiltration by neutrophil and eosinophil polymorphonuclear cells.
-
•
Subacute stage (4–6 weeks) with persistent vasculitis, worsened by irradiated or locally recruited macrophages (24).
-
•
Chronic stage (up to 2 years) with cutaneous fibrosis, in which TGF‐β1 plays a major negative role as it increases collagen deposition by irradiated fibroblasts.
Chronic evolution of severe irradiation (locally absorbed doses above 25 Gy) is characterized by a series of inflammatory waves, which further extend initial lesions.
Current therapeutic principles for CRS mostly involve non‐specific medical and surgical interventions, delayed but quite similar to those undertaken for severe thermal burns in the early phases, and to care for further chronic non‐healing wounds at later stages (25). However, this strategy cannot tackle the specific problem of inflammatory waves, often triggered by the very surgical procedures undertaken. This leads to extension of injury damage and poor outcome, as shown in our early experience in these fields at the Percy Military Hospital. This observation, combined with fundamental data concerning trophic, immunomodulatory and anti‐inflammatory effects of MSCs, with pre‐clinical data showing that MSCs have enhanced tissue repair in a murine model of CRS (26), and with safety data from phase I/II clinical studies with MSCs in other settings, leads us to consider administering autologous MSCs as an adjuvant to surgery in CRS.
In the last 5 years, four patients have benefited from this innovative therapeutic approach combining local injection of autologous MSCs, surgical excision and epidermal autografts (27, 28). Each of them had sustained very severe localized irradiation damage (to hands, arms, or buttocks), with risk of subsequent amputation. MSCs were obtained from autologous bone marrow aspirate, and culture expanded for 15–17 days in medium containing 8% (v/v) human platelet lysate, produced by the French Military Blood Transfusion Center. After surgical excision of necrotic tissues, 150–180 × 106 MSCs were injected locally and epidermal autografts were performed. According to severity and evolution of wound kinetics, patients received two to five iterative injections of MSCs. A dramatic analgesic effect was observed in all patients as early as the day after injection. This transient result faded after a few days, which was an incentive for further injections. Faster and better epidermal engraftment was also observed, along with drop in C‐reactive protein, a systemic inflammation biomarker. Most importantly, no new inflammatory wave was observed by 5‐year follow‐up for the first patient. These observations suggest that MSCs participated in local control of inflammation, thereby allowing better epidermal engraftment and improved overall wound healing. Although formal confirmation of these effects would theoretically require a more formal randomized controlled trial, feasibility of one doesn’t appear to be realistic.
Rationale for therapy with MSCs for thermal burns
Considering the therapeutic efficacy of MSCs in radiation‐induced burns, the interest potential for them in thermal burns appears at least equivalent and maybe greater. Indeed, while study of the effects of MSCs in pre‐clinical burn models (29) has just begun, accumulated data regarding general wound repair processes provide a strong rationale for their use in that setting.
Supportive and modulatory paracrine properties of MSCs are pivotal in their effects on skin wound repair. MSCs support population growth and differentiation of keratinocytes, as shown in murine (30) and human (31) organotypic co‐cultures, even supporting formation of rete ridge‐like structures as opposed to dermal fibroblasts in the former (30). Faster healing of excision wounds with enhanced angiogenesis (associated with higher angiopoietin‐1 and VEGF expression) have also been reported in mice, locally treated with MSCs as opposed to dermal fibroblasts or sham treatment (32). Confirmation of the mostly paracrine mechanism of these effects was reported in a study yielding similar results with corresponding conditioned media, in accordance with their comparatively higher content of trophic and pro‐angiogenic factors and cytokines (Fig. 1), and lower in pro‐inflammatory ones (17). MSCs also promote migration of many cell types involved in wound healing, towards an injury site. This includes fibroblasts and keratinocytes, as illustrated in recently suggesting involvement of chemo‐attractant paracrine factors and maybe of secreted extracellular matrix proteins (collagen I and VI, fibronectin) in that process (33).
Anti‐fibrotic and local anti‐inflammatory effects of MSCs also appear promising, although they are slightly less consistently documented. Animal studies have reported locally injected MSCs help regenerate normal dermal architecture with very fine scars (34) and increased tensile strength (35) after incision wounds. However, others have argued that MSCs could be involved in keloid formation (36). Clinical applications have thus far been scarce. One study has demonstrated feasibility of spraying MSCs suspended in fibrin on to acute and chronic wounds, and suggested benefit achieved in that setting (37). In a further investigation, allogeneic MSCs in a collagen sponge applied to chronic non‐healing wounds, reportedly allowed wound closure in 18 out of 20 patients (38).
Harnessing differentiation potential of MSCs in skin wound repair is another interesting, although less advertised, approach, especially when taking into account their ability to differentiate towards not only mesodermal (basically fibroblasts in that setting) but also non‐mesodermal lineages. Several reports have indeed suggested that MSCs could transdifferentiate into keratinocyte‐like cells both in vivo after homing, towards the skin in irradiated mice (39) or towards injured sites after biopsy wounds (40), and in vitro, provided that they are placed in an optimized skin‐like micro‐environment (41). Endothelial transdifferentiation of MSCs has been reported in vivo in animal models of mechanical wounds (32, 40) and in cutaneous ischaemia (42), but its net effect on angiogenesis was hardly distinguishable from that of paracrine pro‐angiogenic effects (32). The same is probably true for relative presence of paracrine secretions versus differentiation for epidermal support or anti‐fibrotic properties of MSCs.
In vitro transdifferentiation of MSCs into sweat gland cells has also been reported by a single group, who claimed successful in vivo structural and functional reconstitution of functional sweat glands in anhydrotic scars from those transdifferentiated cells; unfortunately this investigation had serious methodological limitations (43). Although beginning to address the highly relevant problem of lack of skin appendages in burn sequelae, these findings yet need to be confirmed in better‐designed studies.
MSCs in thermal burns have thus far been evaluated in only one well‐conducted animal examination, to our knowledge. 3 MSCs in a collagen and glycosaminoglycan matrix compared to matrix alone and no matrix were applied to standardized split thickness burns of pigs, and yielded improved wound healing with better keratinization, less contraction, histologically less inflammation and less proliferative connective tissue (29). Further pre‐clinical and clinical studies are thus warranted.
Practical considerations and future challenges
Paracrine mechanisms, rather than post‐engraftment differentiation and proliferation, seemingly predominate in therapeutic effects of MSCs. Combined with their low immunogenicity (35), this fact hints at the potential interest of allogeneic cell therapy with MSCs in burns, an already‐tested strategy in other settings (45). Both in thermal and radiation‐induced burns, cell dysfunction related to extent of the burns, associated treatments, or associated medullary irradiation would likely hamper therapeutic potential of MSCs. In a recent ex vivo study, for instance, proliferation and osteoblastic differentiation of MSCs were impaired after irradiation, with doses as low as 2 Gy (46).
Furthermore, a delay in treatment of at least 2 weeks is required to obtain clinically relevant (although not yet optimized in a dose–effect study) numbers of cultured cells from harvested ones; this is another argument in favour of allogeneic cell therapy. MSCs could indeed be beneficial in early lesions, as they have been shown to modulate cell survival, inhibit neutrophil oxidative burst (47) and modulate macrophage activation (48). In such indications, immediately available cryopreserved allogeneic MSCs could offer an interesting alternative to cultured autologous ones.
As suggested earlier, one could alternatively use mere solutions of secretion products (conditioned media) rather than whole cells. Indeed, although potential therapeutic effects of MSCs cannot be reduced to their paracrine secretions, this approach would allow treatment as early as with allogeneic cultured cells, but with the benefit of strict absence of immunogenicity.
This approach would also contribute to addressing one major potential drawback of MSCs, risk of induced malignancy. MSCs have been shown to promote malignancy in animal models by at least two mechanisms: they have been suspected of providing a micro‐environment capable of sustaining tumour growth (49), and their own malignant transformation has also been reported, apparently promoted by culturing them, ex vivo (50). Because of the relatively low number of patients thus far treated with cultured MSCs in various therapeutic settings, induced malignancies cannot be formally excluded. Validating culture conditions which limit genetic alterations, and appropriate tests for genetic integrity of cultured cells, and properly minimizing residual heterogeneity of obtained cell populations, are therefore prerequisites to their safe clinical use. Finally, more thorough understanding of the signals involved in differentiation of MSCs is also highly desirable, not only to control their differentiation towards skin‐specific cells, but also to help prevent their malignant transformation.
Conclusion
Considering current evidence concerning paracrine properties and differentiation ability of MSCs in the wound repair setting, along with our clinical experience with MSCs in irradiation situations, we are convinced that MSCs will have a central place in future cell therapies for burns. In our view, already foreseeable applications of MSCs encompass their uses:
-
•
in vitro, alone or in combination, to replace current murine feeder cells (3T3 fibroblasts) used to culture human keratinocytes after Green’s technique;
-
•
directly in vivo, to promote healing of superficial or split thickness burns, as well as that of epidermal autograft donor sites;
-
•
in vivo in association with plain or cultured epidermal autografts, to enhance engraftment, as we have observed in locally irradiated patients;
-
•
integrated in extracellular matrix‐like biomaterials as part of bio‐engineering skin substitutes.
Ongoing research efforts in the field include isolation of MSCs from other tissues, comparative analysis of effects of MSCs and of their secretary products, orientation of the secretary cell phenotype after stimulation, development of new ways of delivery and new skin substitutes.
Potential interest in MSCs for burns is not limited to skin wound repair. Indeed, because of their mostly paracrine anti‐inflammatory properties, one could contemplate using MSCs to modulate early SIR in severe burns and/or their frequent septic complications, as suggested in an animal model of severe abdominal sepsis, basically a septic SIR (48). In the near future, MSCs could be used to control inflammatory mechanisms involved in acute lung injury/respiratory distress syndrome (ALI/ARDS), a life‐threatening respiratory condition often encountered in burn patients with smoke inhalation. Promising pre‐clinical results have been already reported in mice (51) and in ex vivo‐perfused human lung (52); clinical trials are awaited.
Finally, while this review deliberately focused on MSCs, we do not override the many other research avenues in cell therapy for burns, including ongoing development of skin substitutes, understanding and control of cell/matrix interactions, or future prospects with other cell types such as induced pluripotent stem cells or immunomodulatory Tγδ cells (53). While CEAs help fight the survival battle after burns, cell therapy with MSCs and such complementary approaches will hopefully help win the next battle, that of quality of life.
Disclosure of potential conflicts of interest
None relevant to the present work.
Pathophysiological peculiarities of the latter are described in the corresponding separate section of this review.
Footnotes
These penetrating radiations can actually damage more than the sole cutaneous tissue.
One group reported studies in rats and even claimed clinical benefit in one patient, but poor documentation precludes any firm conclusion (44).
References
- 1. Allgöwer M, Schoenenberger GA, Sparkes BG (2008) Pernicious effectors in burns. Burns 34(Suppl. 1), S1–S55. [DOI] [PubMed] [Google Scholar]
- 2. Bargues L, Prat M, Leclerc T, Bey E, Lataillade J (2010) Present and future of cell therapy in burns (in french). Pathol. Biol. (in press) doi: 10.1016/j.patbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 3. Rheinwald JG, Green H (1975) Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343. [DOI] [PubMed] [Google Scholar]
- 4. Carsin H, Ainaud P, Le Bever H, Rives J, Lakhel A, Stephanazzi J et al. (2000) Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single‐center experience with 30 patients. Burns 26, 379–387. [DOI] [PubMed] [Google Scholar]
- 5. Cuono C, Langdon R, McGuire J (1986) Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet 8490, 1123–1124. [DOI] [PubMed] [Google Scholar]
- 6. Burke JF, Yannas IV, Quinby WCJ, Bondoc CC, Jung WK (1981) Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 194, 413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coulomb B, Friteau L, Baruch J, Guilbaud J, Chretien‐Marquet B, Glicenstein J et al. (1998) Advantage of the presence of living dermal fibroblasts within in vitro reconstructed skin for grafting in humans. Plast. Reconstr. Surg. 101, 1891–1903. [DOI] [PubMed] [Google Scholar]
- 8. Brusselaers N, Pirayesh A, Hoeksema H, Richters CD, Verbelen J, Beele H et al. (2010) Skin replacement in burn wounds. J. Trauma 68, 490–501. [DOI] [PubMed] [Google Scholar]
- 9. Friedenstein AJ, Chailakhjan RK, Lalykina KS (1970) The development of fibroblast colonies in monolayer cultures of guinea‐pig bone marrow and spleen cells. Cell Tissue Kinet. 3, 393–403. [DOI] [PubMed] [Google Scholar]
- 10. Schofield R (1978) The relationship between the spleen colony‐forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25. [PubMed] [Google Scholar]
- 11. Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084. [DOI] [PubMed] [Google Scholar]
- 12. Romanov YA, Svintsitskaya VA, Smirnov VN (2003) Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC‐like cells from umbilical cord. Stem Cells 21, 105–110. [DOI] [PubMed] [Google Scholar]
- 13. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T (2005) Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 52, 2521–2529. [DOI] [PubMed] [Google Scholar]
- 14. Dominici M, Le Blanc K, Mueller I, Slaper‐Cortenbach I, Marini F, Krause D et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8, 315–317. [DOI] [PubMed] [Google Scholar]
- 15. Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25, 2739–2749. [DOI] [PubMed] [Google Scholar]
- 16. Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H et al. (2005) Paracrine action accounts for marked protection of ischemic heart by Akt‐modified mesenchymal stem cells. Nat. Med. 11, 367–368. [DOI] [PubMed] [Google Scholar]
- 17. Chen L, Tredget EE, Wu PYG, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3, e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A et al. (2003) Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc. Natl. Acad. Sci. USA 100, 2397–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spees JL, Olson SD, Whitney MJ, Prockop DJ (2006) Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. USA 103, 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen TS, Lai RC, Lee MM, Choo ABH, Lee CN, Lim SK (2010) Mesenchymal stem cell secretes microparticles enriched in pre‐micrornas. Nucleic Acids Res. 38, 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeggo P, Lavin MF (2009) Cellular radiosensitivity: how much better do we understand it?. Int. J. Radiat. Biol. 85, 1061–1081. [DOI] [PubMed] [Google Scholar]
- 22. Hei TK, Ballas LK, Brenner DJ, Geard CR (2009) Advances in radiobiological studies using a microbeam. J. Radiat. Res. 50(Suppl. A), A7–A12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peter RU (2005) Cutaneous radiation syndrome in multi‐organ failure. BJR Suppl. 27, 180–184. [Google Scholar]
- 24. Stewart FA, Dörr W (2009) Milestones in normal tissue radiation biology over the past 50 years: from clonogenic cell survival to cytokine networks and back to stem cell recovery. Int. J. Radiat. Biol. 85, 574–586. [DOI] [PubMed] [Google Scholar]
- 25. Friesecke I, Beyrer K, Fliedner TM (2001) How to cope with radiation accidents: the medical management. Br. J. Radiol. 74, 121–122. [DOI] [PubMed] [Google Scholar]
- 26. François S, Mouiseddine M, Mathieu N, Semont A, Monti P, Dudoignon N et al. (2007) Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Ann. Hematol. 86, 1–8. [DOI] [PubMed] [Google Scholar]
- 27. Bey E, Prat M, Duhamel P, Benderitter M, Brachet M, Trompier F et al. (2010) Emerging therapy for improving wound repair of severe radiation burns using local bone marrow‐derived stem cell administrations. Wound Repair Regen. 18, 50–58. [DOI] [PubMed] [Google Scholar]
- 28. Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I et al. (2007) New approach to radiation burn treatment by dosimetry‐guided surgery combined with autologous mesenchymal stem cell therapy. Regen. Med. 2, 785–794. [DOI] [PubMed] [Google Scholar]
- 29. Liu P, Deng Z, Han S, Liu T, Wen N, Lu W et al. (2008) Tissue‐engineered skin containing mesenchymal stem cells improves burn wounds. Artif. Organs 32, 925–931. [DOI] [PubMed] [Google Scholar]
- 30. Aoki S, Toda S, Ando T, Sugihara H (2004) Bone marrow stromal cells, preadipocytes, and dermal fibroblasts promote epidermal regeneration in their distinctive fashions. Mol. Biol. Cell 15, 4647–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laco F, Kun M, Weber HJ, Ramakrishna S, Chan CK (2009) The dose effect of human bone marrow‐derived mesenchymal stem cells on epidermal development in organotypic co‐culture. J. Dermatol. Sci. 55, 150–160. [DOI] [PubMed] [Google Scholar]
- 32. Wu Y, Chen L, Scott PG, Tredget EE (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25, 2648–2659. [DOI] [PubMed] [Google Scholar]
- 33. Walter MNM, Wright KT, Fuller HR, MacNeil S, Johnson WEB (2010) Mesenchymal stem cell‐conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp. Cell Res. 316, 1271–1281. [DOI] [PubMed] [Google Scholar]
- 34. Satoh H, Kishi K, Tanaka T, Kubota Y, Nakajima T, Akasaka Y et al. (2004) Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds. Cell Transplant. 13, 405–412. [DOI] [PubMed] [Google Scholar]
- 35. Stoff A, Rivera AA, Sanjib Banerjee N, Moore ST, Michael Numnum T, Espinosa‐de‐Los‐Monteros A et al. (2009) Promotion of incisional wound repair by human mesenchymal stem cell transplantation. Exp. Dermatol. 18, 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akino K, Akita S, Yakabe A, Mineda T, Hayashi T, Hirano A (2008) Human mesenchymal stem cells may be involved in keloid pathogenesis. Int. J. Dermatol. 47, 1112–1117. [DOI] [PubMed] [Google Scholar]
- 37. Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N et al. (2007) Autologous bone marrow‐derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 13, 1299–1312. [DOI] [PubMed] [Google Scholar]
- 38. Yoshikawa T, Mitsuno H, Nonaka I, Sen Y, Kawanishi K, Inada Y et al. (2008) Wound therapy by marrow mesenchymal cell transplantation. Plast. Reconstr. Surg. 121, 860–877. [DOI] [PubMed] [Google Scholar]
- 39. Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z et al. (2005) Engrafted bone marrow‐derived flk‐(1+) mesenchymal stem cells regenerate skin tissue. Tissue Eng. 11, 110–119. [DOI] [PubMed] [Google Scholar]
- 40. Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H (2008) Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 180, 2581–2587. [DOI] [PubMed] [Google Scholar]
- 41. Ma K, Laco F, Ramakrishna S, Liao S, Chan CK (2009) Differentiation of bone marrow‐derived mesenchymal stem cells into multi‐layered epidermis‐like cells in 3d organotypic coculture. Biomaterials 30, 3251–3258. [DOI] [PubMed] [Google Scholar]
- 42. Hamou C, Callaghan MJ, Thangarajah H, Chang E, Chang EI, Grogan RH et al. (2009) Mesenchymal stem cells can participate in ischemic neovascularization. Plast. Reconstr. Surg. 123, 45S–55S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sheng Z, Fu X, Cai S, Lei Y, Sun T, Bai X et al. (2009) Regeneration of functional sweat gland‐like structures by transplanted differentiated bone marrow mesenchymal stem cells. Wound Repair Regen. 17, 427–435. [DOI] [PubMed] [Google Scholar]
- 44. Rasulov MF, Vasilchenkov AV, Onishchenko NA, Krasheninnikov ME, Kravchenko VI, Gorshenin TL et al. (2005) First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull. Exp. Biol. Med. 139, 141–144. [DOI] [PubMed] [Google Scholar]
- 45. Le Blanc K, Ringdén O (2005) Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 11, 321–334. [DOI] [PubMed] [Google Scholar]
- 46. Li J, Kwong DLW, Chan GC (2007) The effects of various irradiation doses on the growth and differentiation of marrow‐derived human mesenchymal stromal cells. Pediatr. Transplant. 11, 379–387. [DOI] [PubMed] [Google Scholar]
- 47. Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F et al. (2008) Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 26, 151–162. [DOI] [PubMed] [Google Scholar]
- 48. Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K et al. (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin e(2)‐dependent reprogramming of host macrophages to increase their interleukin‐10 production. Nat. Med. 15, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F (2007) Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia 21, 304–310. [DOI] [PubMed] [Google Scholar]
- 50. Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S et al. (2007) Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 25, 371–379. [DOI] [PubMed] [Google Scholar]
- 51. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA (2007) Intrapulmonary delivery of bone marrow‐derived mesenchymal stem cells improves survival and attenuates endotoxin‐induced acute lung injury in mice. J. Immunol. 179, 1855–1863. [DOI] [PubMed] [Google Scholar]
- 52. Lee JW, Fang X, Gupta N, Serikov V, Matthay MA (2009) Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin‐induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA 106, 16357–16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwacha MG (2009) Gammadelta T‐cells: potential regulators of the post‐burn inflammatory response. Burns 35, 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]


