Abstract
Abstract. Objectives: The majority of solid human malignancies demonstrate DNA aneuploidy as a consequence of chromosomal instability. We wanted to investigate whether Aurora A, Aurora B, BUB1B and Mad2 were associated with the development of aneuploidy in colorectal adenocarcinomas as suggested by several in vitro studies, and if their protein levels were related to alterations at the corresponding chromosomal loci. Materials and methods: Expression levels of these spindle proteins were investigated by immunohistochemistry using tissue micro‐arrays in a series of DNA aneuploid and diploid colorectal adenocarcinomas previously examined for genomic aberrations by comparative genomic hybridization. Results: All proteins were overexpressed in malignant tissues compared to controls (P < 0.001 for all). BUB1B level was significantly reduced in aneuploid compared to diploid cancers (P = 0.001), whereas expression of the other proteins was not associated with DNA ploidy status. High levels of Aurora A (P = 0.049) and low levels of Aurora B (P = 0.031) were associated with poor prognosis, but no associations were revealed between protein expression and genomic aberration. Conclusions: A significant reduction of BUB1B level was detected in aneuploid compared to diploid colorectal cancers, consistent with earlier studies showing that loss of spindle checkpoint function may be involved in development of DNA aneuploidy. Our data also show that spindle proteins are overexpressed in colorectal cancers, and that expression of the Aurora kinases is associated with prognosis in colorectal cancer.
INTRODUCTION
A large fraction of cancers consists of cells with abnormal chromosomal content (Heim & Mitelman 1995), known as aneuploidy (Boveri 1914). Aneuploidy is associated with chromosomal instability (CIN), a condition in which cancer cells lose or gain chromosomes or chromosomal material during mitosis (Lengauer et al. 1997), and which is regarded as one of the initiating events of human carcinogenesis (Li et al. 2000; Sieber et al. 2003). Eighty‐five per cent of colorectal cancers (CRCs) are aneuploid, the remaining 15% having a diploid DNA content (Rajagopalan et al. 2003). The origins of genomic instability are still unknown (Marx 2002) but recent studies suggest that dysfunction of the Aurora kinases (Giet et al. 2005) and spindle checkpoint proteins (Kops et al. 2005) may be involved in the development of CIN and subsequently tumorigenesis. Aurora kinases play critical roles in chromosome segregation and cell division in normal proliferating cells (Meraldi et al. 2004). Overexpression of Aurora A has been shown to induce centrosome amplification and aneuploidy in vitro (Bischoff et al. 1998; Zhou et al. 1998), and in vivo studies of Aurora A show that this kinase is overexpressed in many human cancers (Tanaka et al. 1999, 2005; , Takahashi et al. 2000; Gritsko et al. 2003). Elevated levels of Aurora B also induce aneuploidy when introduced into human fibroblasts (Tatsuka et al. 1998), and levels of Aurora B are elevated in human cancer cell lines (Tatsuka et al. 1998; Adams et al. 2001; Sasai et al. 2004) and in cancers (Araki et al. 2004; Chieffi et al. 2004; Sorrentino et al. 2005). Their oncogenic potential has been demonstrated in nude mice, which after injection of cells overexpressing either Aurora A (Bischoff et al. 1998) or Aurora B (Ota et al. 2002), develop malignant tumours.
The mitotic spindle checkpoint prevents premature advance from metaphase to anaphase during mitosis, and is activated by lack of microtubule attachments to chromosomal kinetochores during metaphase (Kops et al. 2005). Mad2 (mitotic arrest deficient) and BUB1B (budding uninhibited by benzimidazole) are vital constituents of the checkpoint, and in close cooperation they initiate a ‘wait anaphase signal’ by inhibiting the anaphase promoting complex/cyclosome (APC/C) until all kinetochores are attached to corresponding microtubules (Jallepalli & Lengauer 2001). Similar to expression of Aurora A and Aurora B in malignant tissues, both Mad2 and BUB1B levels are significantly elevated in human cancers (Hernando et al. 2004; Yamamoto et al. 2007) and a recent report concerning transgenic mice overexpressing Mad2 concludes that increased levels of Mad2 promote development of aneuploidy and tumorigenesis in such animals (Sotillo et al. 2007). Our group has earlier reported an increase of BUB1B levels in progression from low to high degrees of dysplasia in patients with ulcerative colitis associated CRC (Burum‐Auensen et al. 2007b) and dysfunction of BUB1B has been shown to be related to CIN and aneuploidy (Musio et al. 2003; Hanks et al. 2004; Yamamoto et al. 2007).
The genetic region Aurora A, 20q13.2‐3, is frequently amplified in CRCs and is associated with poor prognosis (De Angelis et al. 2001) and we have recently shown that aneuploidy in CRC is preceded by 20q13.2 amplification in diploid tumour cells (De Angelis et al. 2007), consistent with Aurora A being an oncogene. Aurora B is on chromosome arm 17p13.1 and genes for the spindle checkpoint proteins Mad2 and BUB1B map to chromosome loci 4q27 and 15q14‐21, respectively. These loci are found on chromosomal arms that are frequently lost in sporadic colorectal cancers (Vogelstein et al. 1988; Al Mulla et al. 1999; De Angelis et al. 1999). The putative oncogenes and tumour suppressors localized to these frequently amplified or deleted chromosomal regions are still mostly unidentified. In the current study, we have examined protein levels of Aurora A, Aurora B, Mad2 and BUB1B in CRCs using immunohistochemistry, that have previously been analysed by DNA flow cytometry and comparative genomic hybridization (CGH), to explore possible associations between protein expression, aneuploidy and genomic aberrations.
MATERIALS AND METHODS
Patients and tissue specimens
The original cohort consisted of 67 sporadic CRCs analysed by DNA flow cytometry and CGH (De Angelis et al. 1999, 2001). Twelve of those samples were excluded due to insufficient tissue embedded in paraffin wax blocks. All 55 CRC patients were treated in the period between 1990 and 2001 at the Departments of Surgery at three Norwegian regional hospitals (Ullevål University Hospital, Asker and Bærum County Hospital and Vestfold County Hospital, Tønsberg, Norway). The cohort consisted of 30 male patients and 25 females, the average age was 68 years (range 35–88 years), mean survival time 33 months and 18 patients alive at the end of follow‐up (1 January 2007). None of the patients received irradiation or chemotherapy prior to surgery. Of the 55 specimens, 20 contained peritumoural morphologically normal mucosa that was also examined by immunohistochemistry. Normal mucosa collected from colectomy specimens without cancer from an additional 17 patients were used as normal controls. The cancers were classified as poorly – (8), moderately – (38) or highly – (6) differentiated and three showed mucinous differentiation. According to Dukes staging, 3 were classified as Dukes A, 31 were Dukes B, 13 were Dukes C and 8 were Dukes D.
Immunohistochemistry
Tissue specimens were stored at –80 °C until thawing, and subsequently were fixed in formalin then embedded in paraffin wax. Sections were cut at 4 µm, stained with haematoxylin and eosin and were evaluated by an experienced pathologist (OPFC). The most representative areas were marked prior to tissue core sampling, using a tissue micro‐arrayer (Beecher Instruments, San Praire, NJ, USA). The diameter of punching needles was 1.0 mm and two or three tissue cores were sampled from each area of interest. Tissue cores sampled from peritumoural histologically normal mucosa were localized to less than 5 mm from the cancer margin. After being exposed to 0.5% H2O2 solution, followed by antigen retrieval with Tris‐EDTA at pH 9.0 (for all antibodies except for Aurora B, in which EDTA buffer with pH 8.0), sections were incubated for 1 h at room temperature with appropriate primary antibodies (Aurora A: 1 : 50 dilution, monoclonal antibody, Novocastra NCL‐L‐AK2, Newcastle, UK; Aurora B, BUB1B and Mad2: 1 : 200, 1 : 300 and 1 : 50 dilutions, respectively, polyclonals purchased from BD Transduction Laboratories, product ID numbers: 611083, 612503 and 610679, respectively, Franklin Lakes, NY, USA; and Ki67 monoclonal: 1 : 100 dilution, a gift from Dr. J. Gerdes, Borstel, Germany). A staining kit from DakoCytomation (EnVision, Via Real, Carpintera, CA, USA) was used for visualization of antigen–antibody interactions. Further details concerning antibody specificity and immunohistochemical analysis of spindle proteins have been described earlier (Burum‐Auensen et al. 2007a). Freshly fixed tonsil was used as positive control and primary antibodies substituted by Tris‐buffered saline (TBS) as negative control. An average of 500 randomly selected epithelial cells were counted in each tissue sample showing homogenous staining intensity, and cells were evaluated as either positive (arrow, Fig. 1) or negative (arrowhead). Number of positive cancer cells among the 500 cells evaluated was used to calculate the percentage of positive cancer cells in each sample, which is defined as protein expression in this report.
Figure 1.
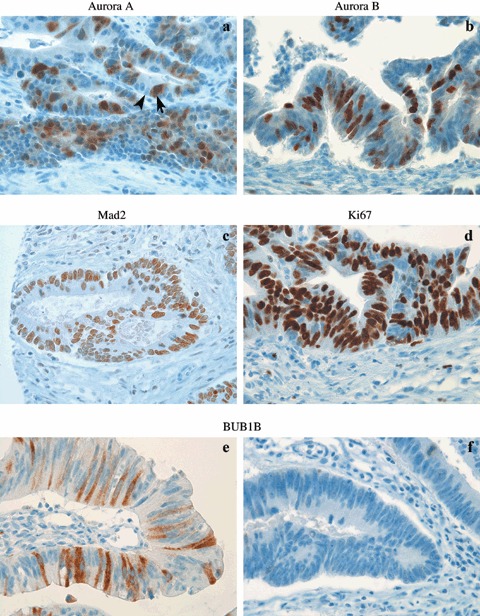
Immunohistochemical analysis of Aurora A (a), Aurora B (b), Mad2 (c), Ki67 (d) and BUB1B (e, f) in colorectal cancers. BUB1B expression was higher in diploid (e) compared to aneuploid cancers (f). Original magnification ×720.
For Western blot analysis, the antibody against Aurora B was diluted 1 : 250, the protocol for Western blot analysis having been described previously (Burum‐Auensen et al. 2007a).
DNA flow cytometry and comparative genomic hybridization
All tumours included were previously analysed by flow cytomtery and CGH, and the results have been published in earlier reports (De Angelis et al. 1999, 2001); these present a detailed methodological description of the flow cytometry and CGH procedures. Briefly, nuclear suspensions from tumour specimens were prepared using Vindelov's method (Vindelov et al. 1983) and propidium iodide staining was used for DNA content analysis, using a FACSVantage DiVA flow cytometer (BDIS, San Jose, CA, USA) with 488 nm laser excitation. For CGH analysis, fluorescein isothiocyanate‐dUTP‐labelled tumour DNA (green) and Texas Red‐dUTP‐labelled reference DNA (red) were prepared by standard nick translation procedures, mixed, and were hybridized on metaphase slides for 2–3 days at 37 °C in a humidified chamber. After formamide washings, slides were counterstained with 0.2 µm 4′,6‐diamidino‐2‐phenylindole (DAPI) in antifade solution, followed by capture of fluorescence images of DAPI, FITC and Texas Red and images were digitized using an in situ imaging system (MetaSystems, Altlussheim, Germany). Chromosomal gains and losses were scored if green : red ratio was above 1.15 and below 0.85, respectively, as described previously (De Angelis et al. 1999d). Gains were designated as high level if the green : red ratio was > 1.5.
Statistical analysis
The relationship between protein expression and histopathological features was evaluated by nonparametric methods (Mann–Whitney test, Kruskal–Wallis test and Spearman's rho correlation). Time‐to‐event analysis was performed measuring overall survival from the date of surgery to the time of last follow‐up (1 January 2007) or death. Survival was visualized with Kaplan–Meier plots. Cox proportional hazard regressions analysis was used for the survival analysis, including univariate and multiple regression as well as an optimized model selection based on Akaike's information criterion (AIC) (Akaike 1974). AIC is a weighting between model parsimony and goodness‐of‐fit to the sample data. A P‐value of = 0.05 was considered statistically significant, and P‐values obtained after multiple testing were Bonferroni corrected. Analyses were performed in SPSS 13.0 (Chicago, IL, USA) except for Cox regressions that were undertaken in R (http://www.r‐project.org/)
RESULTS
Protein expression in sporadic CRC with relation to tumour genotype and phenotype
Of the cohort, 33 (60%) cancers were aneuploid and 22 (40%) were diploid (De Angelis et al. 1999). All proteins were primarily localized to the nucleus except for BUB1B that was localized to the cytoplasm (Fig. 1). The antibody to Aurora B showed a high degree of antigen specificity (Fig. 2), indicating that Aurora B was abundantly expressed in germinal centres of normal tonsils, while epithelia localized to the basal third of colonic crypts showed only limited levels of its expression (Fig. 2). All proteins were overexpressed in cancers compared to normal colonic mucosa (P < 0.001 for all, Mann–Whitney test; Table 1). Aurora A and Aurora B expression were significantly correlated (P = 0.02, correlation coefficient 0.32) but BUB1B and Mad2 expression showed no correlation (P = 0.22). All had significantly lower expression in peritumoural mucosa compared to normal mucosal controls (Aurora A and Mad2 P < 0.001, BUB1B and Aurora B P = 0.02, Mann–Whitney test). The latter finding could be due to a negative effect of bioactive mediators released by neighbouring cancer epithelia, thus we examined the proliferative capacity of the cells by measuring Ki67 level. As for spindle proteins, the level of Ki67 was significantly reduced in the peritumoural mucosa compared to normal mucosal controls (P < 0.001, Mann–Whitney test; Fig. 3). Furthermore, expression of all spindle proteins was correlated to the Ki67 level in cancers; Aurora A P = 0.005 (correlation coefficient 0.38), Mad2 P = 0.004 (correlation coefficient 0.29), Aurora B and BUB1B P < 0.001 (correlation coefficients 0.62 and 0.53, respectively).
Figure 2.
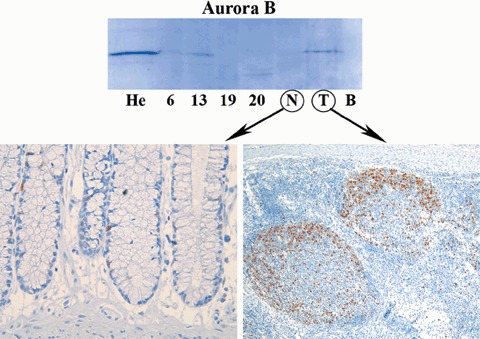
Demonstrates Western blotting and immunohistochemical analyses of Aurora B (Mw 41 kDa, antibody from BD Transduction Laboratories). He, Hela cells; #6, #13, #19, #20, colon cancers; N, normal colon; T, tonsil; B, blank. High Aurora B levels were detected in Hela cells (He) and normal tonsil (T) (positive controls), while the level of Aurora B in normal colon (N) was too low to be detected by Western blotting (negative control). Among colon cancers, levels of Aurora B were variable. Immunohistochemical analysis showed very low levels of Aurora B expression in normal colon (left), while in germinal centres of normal tonsil Aurora B was expressed abundantly (right).
Table 1.
Spindle protein expression and associations with tumour genotypes and phenotypes
| Mucosal morphology | No. of samples | Protein | Expression a (%) | Peritumoural versus normal | Cancer versus normal | Aneuploid versus diploid cancers | Protein levels versus CGH alterations | Tumour differentiation | ||
|---|---|---|---|---|---|---|---|---|---|---|
| N | P | C | ||||||||
| Normal | 17 | Aurora A | 3 | 0 | 11 | < 0.001 | < 0.001 | 0.80 | 0.53 | 0.56 |
| Tumour‐near | 20 | Aurora B | 13 | 7 | 20 | 0.002 | < 0.001 | 0.84 | 0.81 | 0.61 |
| Cancer | 55 | Mad2 | 21 | 7 | 59 | < 0.001 | < 0.001 | 0.98 | 0.40 | 0.04 |
| Total | 92 | BUB1B | 4 | 1 | 14 | 0.002 | < 0.001 | 0.001 | 0.24 | 0.70 |
Median levels; N, normal mucosal; P, peritumoural mucosa; C, cancer; CGH, comparative genomic hybridization.
Figure 3.
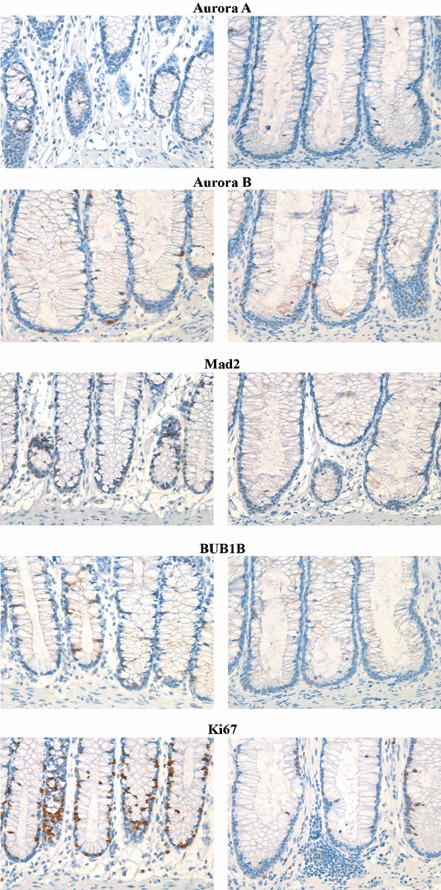
Immunohistochemical analysis of the spindle proteins and Ki67 in normal mucosal controls (left column) and peritumoural mucosa (right column). As can be seen, extent of expression was significantly reduced in the peritumoural mucosa for all proteins.
Colorectal cancers with DNA aneuploidy had significantly lower levels of BUB1B compared to diploid tumours (P = 0.001, Mann–Whitney test; Fig. 4). No association between DNA ploidy status and protein expression was found for the other proteins (Table 1).
Figure 4.
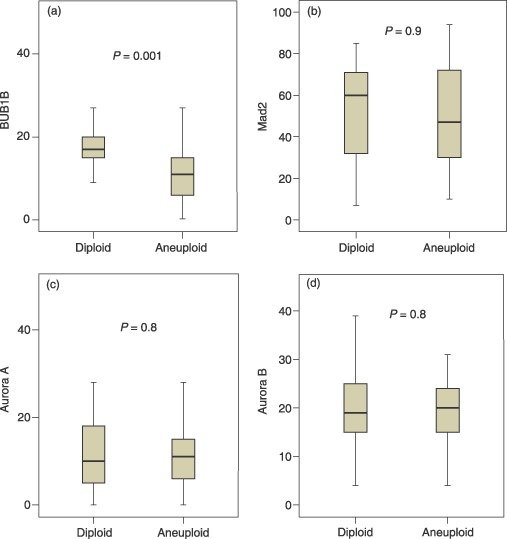
Expression of BUB1B (a), Mad2 (b), Aurora A (c) and Aurora B (d) in diploid and aneuploid colorectal cancers with indicated levels of significance. Y‐axes show percentage positive cells.
Based on previous CGH data for these CRCs (De Angelis et al. 1999), 72% of the cancer cells showed 20q amplification; the consensus region (20q13.2) harbours the locus for AURKA gene that encodes Aurora A. The remaining cancers showed no. 20q amplification. Aurora B maps to chromosome locus 17p13.1, which was deleted in 25% of the CRCs in the present study; the remaining 75% had normal copy numbers for 17p (one cancer had 17p13 amplification; this sample was removed from the statistical analysis). Chromosomal regions containing loci for BUB1B (15q14–21) and Mad2 (4q27) were deleted in 17% and 28% of the CRCs, respectively, and the remaining cancers had normal copy numbers of these chromosomal loci. No association was revealed between protein expression and gain or loss of specific corresponding chromosomal areas (Table 1) and none of the proteins was associated with Dukes stage (data not shown). Tumours with poor differentiation had significantly higher levels of Mad2 compared to highly differentiated ones (P = 0.040, Mann–Whitney test); spindle protein expression and its relation to tumour genotypes and phenotypes are summarized in Table 1 (raw data available in supplementary table).
Survival analysis
The median level of expression (% positive cells) was used to group samples into cancers with high and low degrees of expression as depicted in Table 2. Univariate Cox regression analysis for each of the four spindle proteins did not reveal any relationship with survival (Table 2). Expanding the analysis to a multiple Cox regression, including all four proteins in the same model adjusting for the presence of other variables, resulted in a significant association between increased Aurora B levels and improved prognosis [P = 0.04, hazard ratio (HR) 0.47]. In the full multiple model, two of the proteins were clearly non‐significant (BUB1B P = 0.74 and Mad2 P = 0.59). Applying the AIC principle for model selection, these two variables were omitted and the optimized model revealed that high levels of Aurora A were associated with poor prognosis (P = 0.03, HR 2.05), and the association between elevated Aurora B and improved survival was reinforced (P = 0.03, HR 0.45). Kaplan–Meier survival curves for the Aurora kinases are shown in Fig. 5. The results remained essentially unchanged when adjusted for age at the time of surgery.
Table 2.
Spindle protein expression and survival analysis for colorectal cancers
| Tumours with high level of expression | Tumours with low level of expression | Univariate Cox regression | Multiple Cox regression | Multiple Cox regressionAIC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | CI | P‐value | HR | CI | P‐value | HR | CI | P‐value | |||
| Aurora A | 32 | 22 | 1.54 | 0.79–3.0 | 0.20 | 1.89 | 0.83–4.30 | 0.13 | 2.05 | 1.0–4.18 | 0.049 |
| Aurora B | 27 | 28 | 0.59 | 0.31–1.14 | 0.12 | 0.47 | 0.23–0.96 | 0.04 | 0.45 | 0.22–0.93 | 0.031 |
| BUB1B | 25 | 30 | 1.26 | 0.66–2.42 | 0.49 | 1.15 | 0.51–2.58 | 0.74 | |||
| Mad2 | 26 | 29 | 1.03 | 0.54–1.97 | 0.93 | 0.83 | 0.41–1.66 | 0.59 | |||
HR, hazard ratio; CI, 95% confidence interval; AIC, Akaike's information criterion (Akaike 1974).
Figure 5.
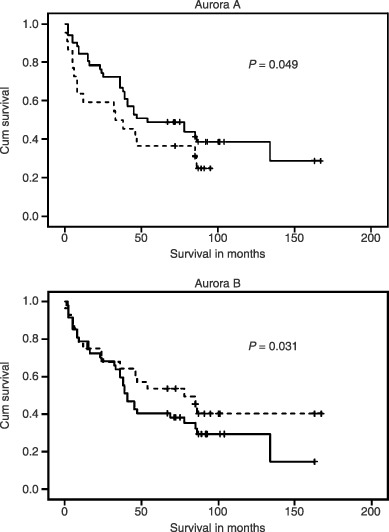
Kaplan–Meier survival curves for colorectal cancer patients stratified according to high – (dotted line) and low (full line) expression levels of Aurora A (top curve) and Aurora B (lower curve). P‐values were obtained by an optimized multiple Cox regression according to Akaike's information criterion.
DISCUSSION
DNA aneuploidy is characteristic of many types of cancer. However, the exact mechanisms behind genomic instability and the development of aneuploidy remain unknown. Since the studies of Bischoff et al. (1998), the role of the Aurora kinases in development of aneuploidy and tumorigenesis has been studied comprehensively (Katayama et al. 2003; Meraldi et al. 2004). These mitotic kinases were found to be overexpressed in different human cancers (Li & Li 2006; Qi et al. 2007), consistent with our results. These proteins are expressed primarily during mitosis, and it is well known that malignant tissues have increased mitotic activity. This could explain the increased levels, supported by significant correlations between the proteins and Ki67 expression in the present study. However, the effects of Aurora kinases probably involve critical cellular events other than mitosis and cell division. This is based on knowledge of their substrates such as TP53, BRCA1 and telomerase for Aurora A, and topoisomerase and vimentin for Aurora B (Yang et al. 2004; Li & Li 2006a). Aurora A phosphorylates and inactivates TP53 (Liu et al. 2004), allowing cells with aneuploid or tetraploid DNA to escape apoptosis. Aurora A also interacts with BRCA1 (Ouchi et al. 2004), a protein involved in DNA repair prior to mitosis, and telomerase (Yang et al. 2004) that is highly expressed in most cancers and is thought to be involved in development of genomic instability. Overexpression of Aurora A, whatever the cause, is likely to have a dramatic effect on the genomic integrity of such cells. However, our data did not show any association between Aurora A expression and aneuploidy, which is in agreement with work on testicular germ cell tumours (Mayer et al. 2003). Neither did a study of ulcerative colitis‐associated colorectal cancer (UCCRC) patients reveal any relationship between aneuploidy and Aurora A expression (Burum‐Auensen et al. 2007b), contrary to what has been reported in vitro (Bischoff et al. 1998; Zhou et al. 1998; Warner et al. 2003; Wang et al. 2006) and in animal models (Wang et al. 2006).
As with the Aurora kinases in the present study, BUB1B and Mad2 were also highly expressed in CRCs compared to their normal counterparts, in agreement with other reports (Tanaka et al. 2001; Li et al. 2003a; Li & Zhang 2004; Yamamoto et al. 2007). In UCCRC, BUB1B expression increased during the progressive morphological changes from dysplasia towards cancer, with highest expression in malignant specimens (Burum‐Auensen et al. 2007b). However, this is in contrast to a further immunohistochemical study of BUB1B in sporadic CRC, in which levels of BUB1B in cancer were reduced compared to normal controls (Shin et al. 2003). Our results revealed significantly reduced levels of BUB1B in aneuploid compared to diploid CRCs, suggesting that this relative decrease of BUB1B might reflect a compromised spindle checkpoint in aneuploid cancers. In urothelial bladder cancer, increased levels of BUB1B have been shown to be associated with chromosomal instability (Yamamoto et al. 2007). For Mad2, the other spindle checkpoint protein, a similar tendency of reduced protein level in aneuploid cancers was seen; however, this was not significant (Fig. 4). Similar to Aurora A expression, BUB1B expression was not associated with aneuploidy in UCCRC.
Duesberg and collaborators suggest that DNA aneuploidy is an initial event in carcinogenesis, and is required for malignant tissue growth (Li et al. 2000). However, it is well known and shown in our report that some cancers have a diploid/near‐diploid genome, and still have malignant behaviour, demonstrating that aneuploidy is not a prerequisite. Others emphasize the role of polyploid cells to be important intermediaries on the way to aneuploidy and cancer (Storchova & Pellman 2004), because such cells are known to be genomically unstable. Walter Giaretti (1994) proposed a model for the colorectal adenoma–carcinoma sequence, which is initiated by appearance of near‐diploid subclones in precancerous adenomas, followed by development of aneuploid (hypotetraploid) cells in advanced cancers. Aneuploid colon cancer cells with reduced levels of BUB1B, shown in our report, could have arisen from such a subclone of diploid cancer cells that acquired a new malignant phenotype characterized by decreased levels of BUB1B (and to some extent Mad2) during cancer evolution. Such cells would generate new aneuploid daughters caused by failure of the spindle checkpoint to detect spindle errors during metaphase, allowing a new dominant subclone of cancer cells. Data supporting this hypothesis include BUB1B gene mutations shown in an autosomal recessive condition characterized by aneuploidy and childhood cancer (Hanks & Rahman 2005), and that CIN is associated with loss of spindle checkpoint function in colon cancer cell lines (Cahill et al. 1998). However, another study reports that aneuploid colon cancer cells do have a robust spindle checkpoint (Tighe et al. 2001), based on the observation that colon cancer cells with CIN undergo transient mitotic arrest in response to spindle damage. Thus, despite significant reduction of BUB1B in aneuploid CRCs, the role of the spindle checkpoint in the development of CIN and aneuploidy still needs further investigation.
Mad2 expression was significantly higher in poorly differentiated tumours compared to highly differentiated ones, in agreement with a study of CRCs (Li & Zhang 2004). In contrast, an opposite expression pattern was revealed in gastric cancer in which high levels of Mad2 were associated with increased degree of differentiation (Tanaka et al. 2001). Some of the contradictory results concerning expression of spindle proteins assessed by immunohistochemistry may be caused by differences in methodology and analysis of immunohistochemical staining (Tanaka et al. 1999, 2005; Gritsko et al. 2003; Royce et al. 2004; Tong et al. 2004; Burum‐Auensen et al. 2007a), unfortunately a common feature in the field of immunohistochemistry (Mcshane et al. 2006).
Previous investigations have revealed aberrations of chromosome arm 20q13 in many cancers (Tanner et al. 1994; Kallioniemi et al. 1995; Mahlamaki et al. 1997), and in our cohort of CRCs 75% showed 20q13 amplification (De Angelis et al. 1999). Furthermore, recent data suggest that 20q13 amplification is a critical region for development of CIN, and that aneuploidy in CRC is preceded by gain of this region (De Angelis et al. 2007). Increased copies of genes here would presumably increase Aurora A transcripts and lead to higher levels of protein. However, our results did not reveal any association between the level of Aurora A and CRC with or without 20q13 amplification. In adenocarcinomas of the pancreas, Aurora A levels have been found to be higher when measured by immunohistochemistry, Western and Northern blot analyses, but accompanying gain of the 20q13.2 region was rarely seen (Li et al. 2003). Thus, even though target genes involved in the development of CIN and aneuploidy are likely to be found at 20q13, post‐transcriptional and post‐translational modifications are likely to have a substantial effect on protein level, which may explain the lack of correlation between Aurora A levels and 20q13 amplification. Furthermore, loss of genetic regions encoding for Aurora B, Mad2 or BUB1B had no significant influence on the protein levels, in agreement with a study of BUB1B in bladder cancer (Yamamoto et al. 2007).
One explanation for the lack of relationship between protein levels and genetic aberrations may be that CGH fails to detect minor genetic changes. CGH allows for detection of gross chromosomal aberrations, but its resolution is limited to 3–5 million base pairs (Shaffer & Bejjani 2006). The length of genes encoding spindle proteins ranges from 1253 to 3670 base pairs (Aurora B and BUB1B, respectively); thus, minor unbalanced genetic alterations can not be detectable by this method although they may have a considerable effect on protein expression. Minor genomic errors might be detected by array CGH, a high‐resolution analysis of DNA copy number variation with the ability to detect all genomic imbalances, including deletion, duplication, amplification and DNA aneuploidy (Shaffer & Bejjani 2006).
Based on the concept of field cancerization (Slaughter et al. 1953), and that tissues adjacent to tumours harbour some genetic alterations similar to those within that are thought to increase proliferative ability (Tabor et al. 2003), reduced levels of Ki67 as well as of the spindle proteins in near‐tumour mucosa were surprising. Studies of preneoplastic fields surrounding malignant tissue often report elevated Ki67 levels (Braakhuis et al. 2002). However, in remodelling (landscaping) the tumour microenvironment (Kinzler & Vogelstein 1998), extracellular matrix surrounding the cancer is degraded by enzymes and proteases (Bogenrieder & Herlyn 2003) with likely a negative effect on neighbouring epithelia. Together with elevated levels of cytotoxic cytokines such as tumour necrosis factor and tumour growth factor‐β (Galliher et al. 2006) that normally induce cell‐cycle arrest in epithelial cells, this may explain reduced cell proliferation in near‐tumour areas demonstrated in this report.
High levels of Aurora A expression were found to be associated with poor prognosis, while prognosis was improved with increased levels of Aurora B. Patients with aneuploid tumours have previously been shown to have worse prognosis compared to patients with diploid ones (Devita et al. 1997), and Aurora A induces aneuploidy in vitro (Zhou et al. 1998). However, there was no association shown in our study between Aurora A expression and aneuploidy, and the poor prognosis associated with high levels of Aurora A must be caused by factors other than aneuploidy. At present, there exist no agreed cut‐off values to separate tumours with high levels of spindle proteins from ones with low degrees of expression. Thus, there exists a possibility for biased statistical analyses due to inappropriate cut‐off values. Overexpression of Aurora A is known to cause centrosome amplification and spindle abnormalities during mitosis. This is probably followed by an increase of Aurora B due to increased number of unattached kinetochores during mitosis, activating the spindle checkpoint, which prevents further mitotic progression. This was not observed in our study; on the contrary, reduced Aurora B expression was associated with increase of Aurora A. Thus, further studies are needed to explore the relationship between Aurora A and Aurora B, because increased levels of Aurora B alone have been shown to induce aneuploidy (Tatsuka et al. 1998) and tumorigenesis (Ota et al. 2002).
Our group has shown that Aurora A, Aurora B, BUB1B and Mad2 are overexpressed in CRC, and that reduced levels of BUB1B are associated with DNA aneuploidy. We suggest that aneuploid CRCs might evolve from a subclone of diploid cancer cells, and the transition from diploidy to aneuploidy might be caused by spindle checkpoint dysfunction, itself due to reduced levels of BUB1B. Increased expression of Aurora A and Aurora B were associated with poor and good prognosis in CRC, respectively, and high levels of Mad2 were associated with poor tumour differentiation. The expression of individual proteins was not influenced by gain or loss of the corresponding chromosomal loci assessed by CGH.
Supporting information
Table S1. The table summarizes the spindle protein expression in 55 sporadic CRCs including staging, differentiation, DNA ploidy and genomic aberrations at their corresponding genomic loci.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
ACKNOWLEDGEMENTS
This work was supported by the Norwegian Cancer Society. The authors thank Head Biomedical Scientist Anne Karin Amundgaard at the Department of Medical Informatics, Radiumhospitalet, for excellent technical support. All investigations of the study were in accordance with the Helsinki Declaration and were approved by the regional ethical committee for scientific studies on human tissues (S‐060629).
REFERENCES
- Adams RR, Eckley DM, Vagnarelli P, Wheatley SP, Gerloff DL, Mackay AM, Svingen PA, Kaufmann SH, Earnshaw WC (2001) Human INCENP colocalizes with the Aurora‐B/AIRK2 kinase on chromosomes and is overexpressed in tumour cells. Chromosoma 110, 65–74. [DOI] [PubMed] [Google Scholar]
- Akaike H (1974) A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19, 716–723. [Google Scholar]
- Al Mulla F, Keith WN, Pickford IR, Going JJ, Birnie GD (1999) Comparative genomic hybridization analysis of primary colorectal carcinomas and their synchronous metastases. Genes Chromosomes Cancer 24, 306–314. [DOI] [PubMed] [Google Scholar]
- Araki K, Nozaki K, Ueba T, Tatsuka M, Hashimoto N (2004) High expression of Aurora‐B/Aurora and Ipll‐like midbody‐associated protein (AIM‐1) in astrocytomas. J. Neurooncol. 67, 53–64. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD (1998) A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17, 3052–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenrieder T, Herlyn M (2003) Axis of evil: molecular mechanisms of cancer metastasis. Oncogene 22, 6524–6536. [DOI] [PubMed] [Google Scholar]
- Boveri T (1914) Zur Frage der Entstehung Maligner Tumoren. Jena, Germany: Verlag von Gustav Fischer. [Google Scholar]
- Braakhuis BJ, Tabor MP, Van Leemans CR, Dw I, Snow GB, Brakenhoff RH (2002) Second primary tumors and field cancerization in oral and oropharyngeal cancer: molecular techniques provide new insights and definitions. Head Neck 24, 198–206. [DOI] [PubMed] [Google Scholar]
- Burum‐Auensen E, De Angelis PM, Schjolberg AR, Kravik KL, Aure M, Clausen OP (2007a) Subcellular localization of the spindle proteins Aurora a, Mad2, and BUBR1 assessed by immunohistochemistry. J Histochem Cytochem. 55, 477–486. [DOI] [PubMed] [Google Scholar]
- Burum‐Auensen E, De Angelis PM, Schjolberg AR, Roislien J, Andersen SN, Clausen OP (2007b) Spindle proteins Aurora A and BUB1B, but not Mad2, are aberrantly expressed in dysplastic mucosa of patients with longstanding ulcerative colitis. J. Clin. Pathol. 60, 1403–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B (1998) Mutations of mitotic checkpoint genes in human cancers. Nature 392, 300–303. [DOI] [PubMed] [Google Scholar]
- Chieffi P, Troncone G, Caleo A, Libertini S, Linardopoulos S, Tramontano D, Portella G (2004) Aurora B expression in normal testis and seminomas. J. Endocrinol. 181, 263–270. [DOI] [PubMed] [Google Scholar]
- De Angelis PM, Clausen OP, Schjolberg A, Stokke T (1999) Chromosomal gains and losses in primary colorectal carcinomas detected by CGH and their associations with tumour DNA ploidy, genotypes and phenotypes. Br. J. Cancer 80, 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis PM, Stokke T, Beigi M, Flatberg G, Enger M, Haug K, Aass HC, Schjolberg A, Andresen PA, Ariansen S, Bo AS, Mjaland O, Clausen OP (2007) Chromosomal 20q gain in the DNA diploid component of aneuploid colorectal carcinomas. Int. J. Cancer 120, 2734–2738. [DOI] [PubMed] [Google Scholar]
- De Angelis PM, Stokke T, Beigi M, Mjaland O, Clausen OP (2001) Prognostic significance of recurrent chromosomal aberrations detected by comparative genomic hybridization in sporadic colorectal cancer. Int. J. Colorectal Dis. 16, 38–45. [DOI] [PubMed] [Google Scholar]
- Devita V, Hellman S, Rosenberg S (1997) Cancer‐Principles and Practice. New York: Lipincott‐Raven. [Google Scholar]
- Galliher AJ, Neil JR, Schiemann WP (2006) Role of transforming growth factor‐beta in cancer progression. Future Oncol. 2, 743–763. [DOI] [PubMed] [Google Scholar]
- Giaretti W (1994) A model of DNA aneuploidization and evolution in colorectal cancer. Lab. Invest. 71, 904–910. [PubMed] [Google Scholar]
- Giet R, Petretti C, Prigent C (2005) Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 15, 241–250. [DOI] [PubMed] [Google Scholar]
- Gritsko TM, Coppola D, Paciga JE, Yang L, Sun M, Shelley SA, Fiorica JV, Nicosia SV, Cheng JQ (2003) Activation and overexpression of centrosome kinase BTAK/Aurora‐A in human ovarian cancer. Clin. Cancer Res. 9, 1420–1426. [PubMed] [Google Scholar]
- Hanks S, Coleman K, Reid S, Plaja A, Firth H, Fitzpatrick D, Kidd A, Mehes K, Nash R, Robin N, Shannon N, Tolmie J, Swansbury J, Irrthum A, Douglas J, Rahman N (2004) Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 36, 1159–1161. [DOI] [PubMed] [Google Scholar]
- Hanks S, Rahman N (2005) Aneuploidy–cancer predisposition syndromes: a new link between the mitotic spindle checkpoint and cancer. Cell Cycle 4, 225–227. [PubMed] [Google Scholar]
- Heim S, Mitelman F (1995) Cancer Cytogenetics. New York: Wiley‐Liss Inc. [Google Scholar]
- Hernando E, Nahle Z, Juan G, Diaz‐Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald W, Benezra R, Lowe SW, Cordon‐Cardo C (2004) Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature 430, 797–802. [DOI] [PubMed] [Google Scholar]
- Jallepalli PV, Lengauer C (2001) Chromosome segregation and cancer: cutting through the mystery. Nat. Rev. Cancer 1, 109–117. [DOI] [PubMed] [Google Scholar]
- Kallioniemi A, Kallioniemi OP, Citro G, Sauter G, Devries S, Kerschmann R, Caroll P, Waldman F (1995) Identification of gains and losses of DNA sequences in primary bladder cancer by comparative genomic hybridization. Genes Chromosomes Cancer 12, 213–219. [DOI] [PubMed] [Google Scholar]
- Katayama H, Brinkley WR, Sen S (2003) The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 22, 451–464. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B (1998) Landscaping the cancer terrain. Science 280, 1036–1037. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Weaver BA, Cleveland DW (2005) On the road to cancer: aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer 5, 773–785. [DOI] [PubMed] [Google Scholar]
- Lengauer C, Kinzler KW, Vogelstein B (1997) Genetic instability in colorectal cancers. Nature 386, 623–627. [DOI] [PubMed] [Google Scholar]
- Li JJ, Li SA (2006) Mitotic kinases: the key to duplication, segregation, and cytokinesis errors, chromosomal instability, and oncogenesis. Pharmacol. Ther. 111, 974–984. [DOI] [PubMed] [Google Scholar]
- Li GQ, Zhang HF (2004) Mad2 and p27 expression profiles in colorectal cancer and its clinical significance. World J. Gastroenterol. 10, 3218–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Sonik A, Stindl R, Rasnick D, Duesberg P (2000) Aneuploidy vs. gene mutation hypothesis of cancer: recent study claims mutation but is found to support aneuploidy. Proc. Natl. Acad. Sci. USA 97, 3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GQ, Li H, Zhang HF (2003a) Mad2 and p53 expression profiles in colorectal cancer and its clinical significance. World J. Gastroenterol. 9, 1972–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, Friess H, Sen S (2003b) Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin. Cancer Res. 9, 991–997. [PubMed] [Google Scholar]
- Liu Q, Kaneko S, Yang L, Feldman RI, Nicosia SV, Chen J, Cheng JQ (2004) Aurora‐A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J. Biol. Chem. 279, 52175–52182. [DOI] [PubMed] [Google Scholar]
- Mahlamaki EH, Hoglund M, Gorunova L, Karhu R, Dawiskiba S, Andren‐Sandberg A, Kallioniemi OP, Johansson B (1997) Comparative genomic hybridization reveals frequent gains of 20q, 8q, 11q, 12p, and 17q, and losses of 18q, 9p, and 15q in pancreatic cancer. Genes Chromosomes Cancer 20, 383–391. [DOI] [PubMed] [Google Scholar]
- Marx J (2002) Debate surges over the origins of genomic defects in cancer. Science 297, 544–546. [DOI] [PubMed] [Google Scholar]
- Mayer F, Stoop H, Sen S, Bokemeyer C, Oosterhuis JW, Looijenga LH (2003) Aneuploidy of human testicular germ cell tumors is associated with amplification of centrosomes. Oncogene 22, 3859–3866. [DOI] [PubMed] [Google Scholar]
- Mcshane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) Reporting recommendations for tumor marker prognostic studies (remark). Exp. Oncol. 28, 99–105. [PubMed] [Google Scholar]
- Meraldi P, Honda R, Nigg EA (2004) Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr. Opin. Genet. Dev. 14, 29–36. [DOI] [PubMed] [Google Scholar]
- Musio A, Montagna C, Zambroni D, Indino E, Barbieri O, Citti L, Villa A, Ried T, Vezzoni P (2003) Inhibition of BUB1 results in genomic instability and anchorage‐independent growth of normal human fibroblasts. Cancer Res. 63, 2855–2863. [PubMed] [Google Scholar]
- Ota T, Suto S, Katayama H, Han ZB, Suzuki F, Maeda M, Tanino M, Terada Y, Tatsuka M (2002) Increased mitotic phosphorylation of histone H3 attributable to AIM‐1/Aurora‐B overexpression contributes to chromosome number instability. Cancer Res. 62, 5168–5177. [PubMed] [Google Scholar]
- Ouchi M, Fujiuchi N, Sasai K, Katayama H, Minamishima YA, Ongusaha PP, Deng C, Sen S, Lee SW, Ouchi T (2004) BRCA1 phosphorylation by Aurora‐A in the regulation of G2 to M transition. J. Biol. Chem. 279, 19643–19648. [DOI] [PubMed] [Google Scholar]
- Qi G, Ogawa I, Kudo Y, Miyauchi M, Siriwardena BS, Shimamoto F, Tatsuka M, Takata T (2007) Aurora B expression and its correlation with cell proliferation and metastasis in oral cancer. Virchows Arch. 450, 297–302. [DOI] [PubMed] [Google Scholar]
- Rajagopalan H, Nowak MA, Vogelstein B, Lengauer C (2003) The significance of unstable chromosomes in colorectal cancer. Nat. Rev. Cancer 3, 695–701. [DOI] [PubMed] [Google Scholar]
- Royce ME, Xia W, Sahin AA, Katayama H, Johnston DA, Hortobagyi G, Sen S, Hung MC (2004) STK15/Aurora A expression in primary breast tumors is correlated with nuclear grade but not with prognosis. Cancer 100, 12–19. [DOI] [PubMed] [Google Scholar]
- Sasai K, Katayama H, Stenoien DL, Fujii S, Honda R, Kimura M, Okano Y, Tatsuka M, Suzuki F, Nigg EA, Earnshaw WC, Brinkley WR, Sen S (2004) Aurora C kinase is a novel chromosomal passenger protein that can complement Aurora‐B kinase function in mitotic cells. Cell Motil. Cytoskeleton 59, 249–263. [DOI] [PubMed] [Google Scholar]
- Shaffer LG, Bejjani BA (2006) Medical applications of array CGH and the transformation of clinical cytogenetics. Cytogenet. Genome Res. 115, 303–309. [DOI] [PubMed] [Google Scholar]
- Shin HJ, Baek KH, Jeon AH, Park MT, Lee SJ, Kang CM, Lee HS, Yoo SH, Chung DH, Sung YC, Mckeon F, Lee CW (2003) Dual roles of human BubR1, a mitotic checkpoint kinase, in the monitoring of chromosomal instability. Cancer Cell 4, 483–497. [DOI] [PubMed] [Google Scholar]
- Sieber OM, Heinimann K, Tomlinson IP (2003) Genomic instability – the engine of tumorigenesis? Nat. Rev. Cancer 3, 701–708. [DOI] [PubMed] [Google Scholar]
- Slaughter DP, Soutwick HW, Smejkal W (1953) Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6, 963–968. [DOI] [PubMed] [Google Scholar]
- Sorrentino R, Libertini S, Pallante PL, Troncone G, Palombini L, Bavetsias V, Spalletti‐Cernia D, Laccetti P, Linardopoulos S, Chieffi P, Fusco A, Portella G (2005) Aurora B overexpression associates with the thyroid carcinoma undifferentiated phenotype and is required for thyroid carcinoma cell proliferation. J. Clin. Endocrinol. Metab. 90, 928–935. [DOI] [PubMed] [Google Scholar]
- Sotillo R, Hernando E, Diaz‐Rodriguez E, Teruya‐Feldstein J, Cordon‐Cardo C, Lowe SW, Benezra R (2007) Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 11, 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchova Z, Pellman D (2004) From polyploidy to aneuploidy, genome instability and cancer. Nat. Rev. Mol. Cell Biol. 5, 45–54. [DOI] [PubMed] [Google Scholar]
- Tabor MP, Braakhuis BJ, Van Der Wal JE, Van Diest PJ, Leemans CR, Brakenhoff RH, Kummer JA (2003) Comparative molecular and histological grading of epithelial dysplasia of the oral cavity and the oropharynx. J. Pathol. 199, 354–360. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Futamura M, Yoshimi N, Sano J, Katada M, Takagi Y, Kimura M, Yoshioka T, Okano Y, Saji S (2000) Centrosomal kinases, HsAIRK1 and HsAIRK3, are overexpressed in primary colorectal cancers. Jpn. J. Cancer Res. 91, 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Hashimoto Y, Ito T, Okumura T, Kan T, Watanabe G, Imamura M, Inazawa J, Shimada Y (2005) The clinical significance of Aurora A/STK15/BTAK expression in human esophageal squamous cell carcinoma. Clin. Cancer Res. 11, 1827–1834. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kimura M, Matsunaga K, Fukada D, Mori H, Okano Y (1999) Centrosomal kinase AIK1 is overexpressed in invasive ductal carcinoma of the breast. Cancer Res. 59, 2041–2044. [PubMed] [Google Scholar]
- Tanaka K, Nishioka J, Kato K, Nakamura A, Mouri T, Miki C, Kusunoki M, Nobori T (2001) Mitotic checkpoint protein hsMAD2 as a marker predicting liver metastasis of human gastric cancers. Jpn. J. Cancer Res. 92, 952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner MM, Tirkkonen M, Kallioniemi A, Collins C, Stokke T, Karhu R, Kowbel D, Shadravan F, Hintz M, Kuo WL (1994) Increased copy number at 20q13 in breast cancer: defining the critical region and exclusion of candidate genes. Cancer Res. 54, 4257–4260. [PubMed] [Google Scholar]
- Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, Suzuki F, Terada Y (1998) Multinuclearity and increased ploidy caused by overexpression of the Aurora‐ and Ipl1‐like midbody‐associated protein mitotic kinase in human cancer cells. Cancer Res. 58, 4811–4816. [PubMed] [Google Scholar]
- Tighe A, Johnson VL, Albertella M, Taylor SS (2001) Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep 2, 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong T, Zhong Y, Kong J, Dong L, Song Y, Fu M, Liu Z, Wang M, Guo L, Lu S, Wu M, Zhan Q (2004) Overexpression of Aurora A contributes to malignant development of human esophageal squamous cell carcinoma. Clin. Cancer Res. 10, 7304–7310. [DOI] [PubMed] [Google Scholar]
- Vindelov LL, Christensen IJ, Nissen NI (1983) A detergent‐trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry 3, 323–327. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL (1988) Genetic alterations during colorectal‐tumor development. N. Engl. J. Med. 319, 525–532. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhou YX, Qiao W, Tominaga Y, Ouchi M, Ouchi T, Deng CX (2006) Overexpression of Aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene 25, 7148–7158. [DOI] [PubMed] [Google Scholar]
- Warner SL, Bearss DJ, Han H, Von Hoff DD (2003) Targeting Aurora‐2 kinase in cancer. Mol. Cancer Ther. 2, 589–595. [PubMed] [Google Scholar]
- Yamamoto Y, Matsuyama H, Chochi Y, Okuda M, Kawauchi S, Inoue R, Furuya T, Oga A, Naito K, Sasaki K (2007) Overexpression of BUBR1 is associated with chromosomal instability in bladder cancer. Cancer Genet. Cytogenet. 174, 42–47. [DOI] [PubMed] [Google Scholar]
- Yang H, Ou CC, Feldman RI, Nicosia SV, Kruk PA, Cheng JQ (2004) Aurora A kinase regulates telomerase activity through c‐Myc in human ovarian and breast epithelial cells. Cancer Res. 64, 463–467. [DOI] [PubMed] [Google Scholar]
- Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S (1998) Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 20, 189–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The table summarizes the spindle protein expression in 55 sporadic CRCs including staging, differentiation, DNA ploidy and genomic aberrations at their corresponding genomic loci.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


