Abstract.
Objectives: Fibroadenoma is the most common benign mammary condition among women aged 35 or younger. Expression of Ki‐67 antigen has been used to compare proliferative activity of mammary fibroadenoma epithelium in the follicular and luteal phases of the menstrual cycle.
Materials and methods: Ninety eumenorrheic women were selected for tumour excision; they were assigned to either of the two groups, according to their phase of menstrual cycle. At the end of the study, 75 patients with 87 masses were evaluated by epithelial cell Ki‐67 expression, blind (no information given concerning group to which any lesion belonged).
Results: Both groups were found to be homogeneous relative to age, menarche, body mass index, previous gestation, parity, breastfeeding, number of fibroadenomas, family history of breast cancer and tabagism. Median tumour size was 2.0 cm and no relationship between proliferative activity and nodule diameter was observed. No typical pattern was observed in the expression of Ki‐67 in distinct nodules of the same patient. Average values for expression of Ki‐67 (per 1000 epithelial cells) in follicular and luteal phases were 27.88 and 37.88, respectively (P = 0.116).
Conclusion: Our findings revealed that proliferative activities in the mammary fibroadenoma epithelium did not present a statistically significant difference in the follicular and luteal phases. The present study contributes to clarifying that fibroadenoma is a neoplasm and does not undergo any change in the proliferative activity during the menstrual cycle.
Introduction
Fibroadenoma is the most common benign mammary condition among women younger than 35. Twenty‐five per cent of affected women are asymptomatic, and 13–20% of them present multiple lesions (1, 2). Fibroadenoma can be single or multiple discrete breast lump/s or lobulated and painless mass at clinical examination, occurring mainly in the upper outer quadrant of the breast. They are most frequent in the third decade of life and in black women, and present as circumscribed oval‐shaped lesions in mammography and ultrasonography. These characteristics suggest that the tumour is benign (3, 4).
It is one of the few benign breast lesions with specific cytology diagnosis by fine‐needle aspiration; it includes antler horn, monolayered groups of cohesive ductal cells, stromal tissue fragments and numerous single naked nuclei (5). The triple test (clinical examination, imaging methods and fine‐needle aspiration cytology) has a sensitivity of 99.6%; the chance for a false‐negative result (lower than 1%) increases in women with ages over 35 years (6).
In addition to its classical form, some fibroadenomas may be juvenile, giant, complex or extra‐mammary tumours, although rarely (7, 8, 9, 10), and some studies indicate that the presence of fibroadenoma is a risk factor for development of mammary carcinoma (8).
Carty et al. did not consider fibroadenoma as true neoplasia, since partial or total regression has been observed in 52% of cases (11). These authors suggest that fibroadenoma originates from the mammary lobule and has strict hormonal dependence (increases during lactation, decreases in postmenopausal patients, and does not undergo malignant change). Thus, fibroadenoma seems to be under identical hormonal influence as is observed in the mammary lobule during the menstrual cycle, however, few studies have evaluated behaviour of fibroadenoma in different phases of the menstrual cycle. Simomoto et al. have evaluated both mitotic index and average nuclear volume in fibroadenomas, and did not detect significant differences during the menstrual cycle (12).
A better understanding of behaviour of fibroadenomas relative to the sex hormones could be gained by detailed cell proliferative analysis during the menstrual cycle and immunohistochemical methods are among the most useful and commonly applied ones. Thus, we have used expression of the Ki‐67 antigen, due to its high specificity relative to cycling cells. The proliferation index is largely identified by using the Ki‐67 antibody, an immunoglobulin G‐class monoclonal mouse antibody obtained from the crude nuclear fraction of Reed‐Sternberg cells of Hodgkin's disease, derived from L‐428 cell line (13, 14, 15, 16). The antigen protein, Ki‐67, is localized to the nucleus, possibly associated either to the nucleolus or to fibrillar components, being present in the active phases of the cellular cycle (G1, S, G2 and M) and absent in G0 (14, 15, 16, 17, 18, 19). Although its functional role in the cell cycle is still unknown, it is doubtless that its expression is linked to cell proliferation. It is well established that distribution of Ki‐67 antigen is not constant, undergoing marked changes during the cycle, yet being associated to the nucleoli of proliferating cells and to chromosomes during mitosis (20, 21, 22, 23, 24).
Therefore, it was our aim to verify whether proliferative events in fibroadenoma undergo changes during the menstrual cycle; from the functional point of view, such behaviour would support the hypothesis that fibroadenoma is not a true neoplasm, but a variant of normal mammary tissue. By an immunohistochemical method (expression of Ki‐67 antigen), we have analysed the proliferative activity of the mammary fibroadenoma epithelium in follicular and luteal phases of the menstrual cycle.
Materials and methods
Patients
This is a transversal, descriptive and comparative study of two groups of patients – group 1 (follicular phase) and group 2 (luteal phase) of the menstrual cycle – who attended the Federal University of São Paulo (Department of Gynecology, Division of Mastology, Section of Benign Mammary Diseases). Women (n = 90) with 116 mammary fibroadenomas (maximum two masses per patient) were initially selected. We included only patients with ages ranging from 18 to 38 years, eumenorrheic during the last 6 months, and with normal medical and Pap Smear examination.
These patients with clinically benign breast tumours (sizes ranging from 1 to 4 cm) underwent a complete breast study that included physical breast examination, fine‐needle aspiration cytology, and ultrasonography, after which excisional biopsy was performed under local anaesthaesia (1% lidocaine without vasoconstrictor). Mammography was performed in patients older than 35 years in order to exclude the possible presence of carcinoma. Patients who took hormonal medication, were lactating during the last 12 months, had endocrine diseases, were pregnant, or those taking any drug at the time of obtaining the specimen, were excluded, according to accepted protocols (12, 25, 26). All biopsies were performed between 10:00 and 11:00 h, in attempt to minimize possible interference of the circadian cycle and of hormonal release, on cell kinetics.
To better characterize the phase of the menstrual cycle, clinical and laboratory parameters were used, such as day of menstruation (before and after biopsy) and determination of serum progesterone level. Blood was collected 30 min before surgery to determine serum progesterone levels by chemiluminescent enzyme immunoassay (DPC®, Los Angeles, CA, USA), with an assay sensitivity of 0.2 ng/ml, and intra‐ and inter‐assay coefficient of variation of 6.3% and 9.1%, respectively (27). The luteal phase was determined when the values were greater than 3 ηg/ml (28).
Groups 1 (follicular phase) and 2 (luteal phase) were shown to be homogeneous relative to the age, menarche, body mass index, number of gestations, abortions, parity, breastfeeding, race, overweight, number of nodules, family history of breast cancer, and tabagism variables (1, 2). Thus, the remaining 75 patients (87 fibroadenomas) were divided in two groups, according to their respective phases of menstrual cycle. The phase was based on records concerning the last three menstrual periods and date of the subsequent one and on progesterone dosage. Then, patients were divided into two groups: group 1 (1–15th day) with 37 patients (45 masses) and group 2 (16–28th day) with 38 patients (42 masses) (26, 29).
Table 1.
Average and standard deviation (SD) values for age, age of menarche and body mass index in groups 1 (follicular phase) and 2 (luteal phase)
| Group 1 Mean ± SD | Group 2 Mean ± SD | P | |
|---|---|---|---|
| Age/year | 25.97 ± 6.37 | 26.09 ± 7.38 | 0.66 |
| Menarche/year | 12.40 ± 1.28 | 12.55 ± 1.41 | 0.65 |
| Body mass index (kg/m2) | 25.97 ± 4.48 | 24.55 ± 3.2 | 0.11 |
Mann–Whitney test.
Table 2.
Comparison between groups 1 (follicular phase) and 2 (luteal phase) and some variables.
| Variables | Group 1 n (%) | Group 2 n (%) | P |
|---|---|---|---|
| Gestation | |||
| ≥ 1 | 14 (31.8) | 13 (30.2) | 1.0 |
| 0 | 30 (68.2) | 30 (69.9) | |
| Parity | |||
| ≥ 1 | 12 (27.3) | 10 (23.3) | 0.80 |
| 0 | 32 (72.7) | 33 (76.7) | |
| Abortion | |||
| Yes | 3 (6.8) | 3 (7.0) | > 0.99 a |
| No | 41 (93.2) | 40 (93.0) | |
| Breastfeeding | |||
| Yes | 12 (27.3) | 10 (23.3) | 0.66 |
| No | 32 (72.7) | 33 (76.7) | |
| Race | |||
| Yes | 16 (36.4) | 14 (32.6) | 0.7 |
| No | 28 (63.6) | 29 (67.4) | |
| Overweight | |||
| Yes | 28 (63.6) | 21 (48.8) | 0.16 |
| No | 16 (36.4) | 22 (51.2) | |
| Number of nodules | |||
| 1 node | 21 (47.7) | 28 (65.1) | 0.13 |
| 2 nodes | 23 (52.3) | 15 (34.9) | |
| Familial history of breast cancer | |||
| Yes | 11 (25) | 4 (9.3) | 0.087 |
| No | 33 (75) | 39 (90.7) | |
| Tabagism | |||
| Yes | 9 (20.5) | 11 (25.6) | 0.61 |
| No | 35 (79.5) | 32 (74.4) | |
χ2‐test. aFisher exact test.
Histopathological examination of the fibroadenomas was carried out independently by two pathologists, and only cases with agreeing diagnoses of simple fibroadenoma were included. Then, 15 patients whose biopsies were inconsistent with simple fibroadenoma were excluded from the study (25). The Ethics Committee of Federal University of São Paulo approved the research, and all patients gave written informed consent to participate in the study.
Histopathology and immunohistochemistry
After surgical removal, the fibroadenomas were immediately cut into two in the surgery and were fixed in 10% formaldehyde solution for a period ≤ 12 h, then were dehydrated in ethyl alcohol, diaphaned in xylene, and embedded in liquid paraffin. The set paraffin wax blocks containing the specimens were then sectioned by microtomy and 4 µm sections were mounted on glass slides. After rehydration sections were stained using haematoxylin and eosin. The diagnosis of fibroadenoma by microscopy was confirmed by two pathologists.
Tissue sections were cooled to room temperature and washed in tap water. Endogenous peroxidase was blocked using 0.3% hydrogen peroxide (in methanol) in three 5‐min washings, then sections were rinsed in distilled water and phosphate‐buffered saline (PBS). At equilibrium, sections were incubated with primary mouse anti‐human Ki‐67 monoclonal antibody (clone Ki‐S5; Dako, Carpinteria, CA, USA) diluted (1/100) in PBS, for 2 h at room temperature, and washed in PBS. This step was followed by incubation with rabbit anti‐mouse immunoglobulin G antibody (Dako ENVISION® Kit) and ABC reagent (avidin and biotinylated horseradish peroxidase) for 18 h (overnight), at 4 °C, and a final wash in PBS. The resulting complex was visulaized by incubation (in the dark) in a solution of chromogenic substrate 3‐3′diaminobenzidine (0.06 g) and hydrogen peroxide (20 vol; 1 ml) for 3–5 min at 37 °C. After washing sections in distilled water, formation of a dark brown precipitate could be observed under a light microscope (30, 31). Tissue sections were then counterstained with Harris’ haematoxylin (1 min), immersed in ammoniacal water and finally in distilled water. Thereafter, sections were dehydrated through increasing ethanol concentrations (70, 80, 95 and 100%) by immersing (1 min) sections three times in each solution. Finally, the material was diaphaned with three washes in xylene (1 min each) and slides were mounted with resin.
Immuno‐expression of the Ki‐67 antigen was then quantitatively assessed in the epithelial cells of the fibroadenomas. After exclusion of both myoepithelial and stromal cells, those with characteristic dark brown nuclei were considered to be positive. In order to perform these observations, a light microscope (Eclipse and 200, Nikon®, Augusta, GA, USA) with a lens of ×40 and final magnification of ×400 was used. Counting was performed in duplicate. The first count was manual, aided by a cell counter, in which at least 1000 cells were counted in each case, without prior knowledge of the group to which it belonged (blind study) as suggested by several studies (26, 32).The second was aided by a digital image analysis system for better recognition of superposed nuclei. A video camera (Toshiba‐CCD Color Camera model IK‐627 AT, Toshiba America Imaging Systems Division Inc., Irvine, CA, USA) for acquisition of images and an external video plate (Snappy, version 3.0) were employed, with generation of digital images transmitted to a microcomputer (Pentium III, video SAMSUNG‐SyncMaster 753DFX). Significant differences were not found between the first and final counting, thus confirming reproducibility of the method.
The cells’ proliferation index was calculated by ratio of number of nuclei stained in brown (positive nuclei for Ki‐67) per thousand epithelial cells counted, expressed as an absolute number.
Statistical analysis
SPSS (Statistical Package for the Social Sciences) (SPSS Inc., Chicago, IL, USA) for Windows version 10.0 software was used for statistical analysis (33). Pearson's chi‐squared test was used to evaluate homogeneity of groups 1 (follicular phase) and 2 (luteal phase) relative to age, number of masses, menarche, parity, breast feeding, body mass index, and tabagism (34). Variables in groups 1 and 2 were compared by means of Student's t‐test for two independent samples. When non‐homogeneity was indicated by the variance values, proliferation indices were compared by both variance analysis (anova) and Mann–Whitney nonparametric test (35). P‐values < 0.05 or 5% (α ≤ 0.05) were considered significant.
Results
As expected, statistically significant difference was observed between the groups relative to serum progesterone levels and days in the menstrual cycle. Average values for size of masses found in groups 1 and 2 were 1.98 and 2.1 cm, respectively (median tumour size: 2.0 cm). Significant relationship between proliferative activity and average diameter of the tumour was not observed. Multiple fibroadenomas occurred in 17.3% (13) of patients. In 50% of the cases, the tumours were localized in the upper outer quadrant of the breast.
Concerning cell proliferation indices for Ki‐67 antigen in the epithelium of the fibroadenomas (Fig. 1, Table 3), average values for stained nuclei (per thousand counted epithelial cells) were 27.88 (SD = 27.52) in group 1 (follicular phase) and 37.88 (SD = 31.08) in group 2 (luteal phase). Although the average value found for the cell proliferation index in group 2 is higher than in group 1, the variance analysis did not show any statistically significant difference (P = 0.116) between groups.
Figure 1.
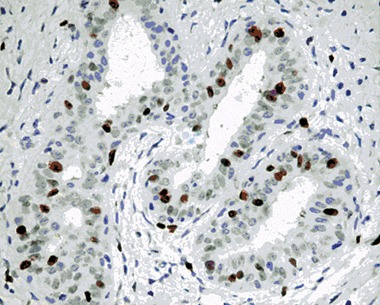
Photomicrography of histological section of fibroadenoma showing dark‐stained nucleus by Ki‐67 antibody (patient no. 41; ×400).
Table 3.
Average and standard deviations of the cell proliferation index for Ki‐67 antigen in groups 1 and 2
| Ki‐67 antigen | Group 1 | Group 2 | Total |
|---|---|---|---|
| Averages | 27.88 | 37.88 | 32.82 |
| Standard error | 4.15 | 4.47 | 3.17 |
| Standard deviation | 27.54 | 31.08 | 29.6 |
P = 0.116; anova.
Mean and median values of proliferative index (Ki‐67) for all patients were 32.82 and 24.0, respectively. When we compared the means of proliferation index in groups 1 and 2, results observed were 27.9 and 37.1, respectively (P = 0085). Median values for groups 1 and 2 were 19.5 and 35.0, respectively (P= 0.085). The percentile of follicular and luteal phases were respectively, 9.0 and 12.0 to 25th percentile; 19.5 and 35.0 to 50th percentile, and 37.7 and 50.0 to 75th percentile (Fig. 2).
Figure 2.
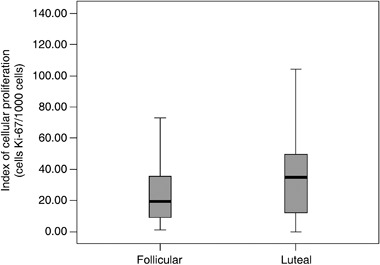
Box‐plot of the cell proliferation index for Ki‐67 antigen in the mammary fibroadenoma epithelium in groups 1 (follicular phase) and 2 (luteal phase).
Cell proliferation index for Ki‐67 antigen adjusted to age, previous gestation, breastfeeding, family history of breast cancer, overweight (BMI > 28), and tabagism was analysed, and significant differences between these values and the index mean value found previously for Ki‐67 antigen were not observed (4, 5).
Table 4.
Proliferation cell index (Ki‐67) according clinical characteristics
| Variables | Yes Median | No Median | P |
|---|---|---|---|
| Gestation | 24 | 25 | 0.73 |
| Parity | 28.5 | 21 | 0.76 |
| Breastfeeding | 21 | 29 | 0.63 |
| Number of nodules (1 = Yes; 2 = No) | 24 | 25 | 0.39 |
| Familial history of breast cancer | 45 | 22 | 0.06 |
| Tabagism | 21 | 27 | 0.55 |
| Overweight | 21 | 28 | 0.32 |
| Race (White = Yes; Not white = No) | 24 | 24 | 0.59 |
| Abortion | 18 | 24 | 0.07 |
Table 5.
Average values of the cell proliferation index for Ki‐67 antigen in groups 1 and 2, adjusted to the covariables age, previous gestation, breastfeeding, family history of breast cancer, overweight and tabagism
| Ki‐67 (adjusted) | Group 1 | Group 2 |
|---|---|---|
| Averages | 27.09 | 37.69 |
| Standard error | 4.56 | 4.62 |
| 95% confidence intervals | 18.00–36.18 | 29.49–47.89 |
Age = 26.03, Gestation = 0.31, Breastfeeding = 0.40, Family history = 0.17, Tabagism = 0.22, Overweight = 25.27 (P = 0.086; anova).
Discussion
Origins of fibroadenoma are not yet established, and a controversy exists concerning promoting role of sex steroids in its genesis. Some authors have reported an increase in plasma oestradiol (36, 37). The importance of oestrogenic stimuli for origin, growth and involution of the tumour is also supported by presence of oestrogen and progesterone receptors, the number of which is variable, depending on the phase of the menstrual cycle. Kuttenn et al. (38) have studied oestrogen and progesterone receptors (RE and RP) in 46 fibroadenomas (obtained in different phases of the menstrual cycle; n = 38) from patients who use either combined oral contraceptive (n = 4) or progestagen (n = 4). Numbers of cytosol and nuclear oestrogen receptors increase during the follicular phase, reaching their maximum in the pre‐ovulatory phase, and decrease during the luteal phase. The number of RP is higher in the follicular phase, mainly in the cytosol. At the beginning of the luteal phase, the number of RP in the cytosol decreases while they increase in the nucleus; then, all RPs’ number decrease, remaining thus during the course of the luteal phase. In patients treated with oral association of oestrogen and progestagen, dosage of RP during the luteal phase shows no significant difference from those not treated. In patients treated with progesterone only, number of RP in the nucleus has been found to be significantly higher than in the cytosol. Existence of RE and RP in fibroadenoma cells, together with their variation during the menstrual cycle (or with the use of exogenous hormones), allows us a glimpse of the possible hormonal dependence of this neoplasm (38).
Some authors describe fibroadenoma as a hyperplasic lesion because of its clonality and the concept of neoplasia is supported by its unicellular or monoclonal origin. Alternatively, a reactional process is characterized by proliferation of cells from multiple origins (therefore, a polyclonal origin). Clonal analysis of fibroadenomas shows epithelial cell and polyclonal stromal components, however, some cells exhibit chromosomal abnormalities. Genetic alterations, including mutations in the p53 gene, loss of heterozygosity, microsatellite instability, and chromosomal cytogenetic aberrations, have also been described (39, 40, 41, 42). Kuijper et al. suggest that fibroadenoma may evolve either in an epithelial direction to carcinoma in situ or in a stromal one to a phyllodes tumour (43).
Noguchi et al., using a method based on restriction fragment length polymorphism, observed that fibroadenoma is polyclonal, and suggested that it is hyperplasia of a lobule (44); other authors agree with the hypothesis that fibroadenoma is a variant of mammary development (45). Thus, it is reasonable to expect that fibroadenoma is cyclic, identical to the normal breast; if this is true, a parallel could be established in order to test this hypothesis. In fact, most studies have not evaluated the influence of the hormonal profile on cell proliferative activity of fibroadenoma in such women (for example, changes in menstrual cycle, prior gestation or use of hormone therapy).
Simomoto et al. performed a morphometric analysis of mammary fibroadenoma epithelium of 20 women during the phases of their menstrual cycle (12) but calculations of both mitotic index and average nuclear volume did not show any significant difference between the follicular and luteal phases of the cycle. Other authors have studied cell proliferation in fibroadenomas by means of the Ki‐67 antigen and in evaluating 6–29 masses, 4.3–37 positive nuclei were found per 1000 epithelial cells. However, their cases were not standardized and the results were not correlated with phases of the menstrual cycle (46, 47, 48).
We have herein tried to establish a parallel with the normal breast by both studying proliferation index of fibroadenoma (defined as Ki‐67‐stained cells per thousand epithelial cells) in 75 patients, and correlating such observations with phase of the menstrual cycle. If such tumours really are a variant of normal mammary development (‘aberrations of normal development and involution’; 49), higher proliferative activity would be expected in the luteal phase. Alternatively, absence of variation in evaluating the Ki‐67 antigen would be expected as Simomoto et al. have observed by assessing mitotic index (12); however, we have used both a more specific and a reproductive marker of cell proliferation.
We observed that cell proliferative activity in the fibroadenomas showed no significant variation either with hormonal fluctuations or with other factors that could affect oestrogen levels, such as tabagism and body mass index. Our results show that fibroadenoma epithelium does not undergo any change in cell proliferative activity during the menstrual cycle, such as occurs in normal mammary tissue. It thus behaves as a true neoplasm since it does not suffer from influence such as oscillation of the sex steroids.
We postulate that fibroadenomas are specialized lesions of the mammary stroma that are able to stimulate adjacent epithelial cells to grow but also they maintain a kind of dependence on the epithelial component. According to the biological behavior of the neoplasm, stromal tumour could evolve to a phase of total both self‐control and independence. In some cases, stromal growth without inhibition by the adjacent epithelium could occur on the epithelium would be absent and comprised in the conjunctive neoplasm, being transformed to sarcoma (12, 43).
According to our data, cell proliferative activity in human mammary fibroadenomas, as evaluated by epithelial cell expression of the Ki‐67 antigen, did not show a statistically significant difference between follicular and luteal phases of the menstrual cycle. The results suggest that fibroadenoma is a true neoplasm, which behaves independently from fluctuations of menstrual cycle hormones.
References
- 1. Alle KM, Moss J, Venegas RJ, Khalkhali I, Klein SR (1996) Conservative management of fibroadenoma of the breast. Br. J. Surg. 83, 992–993. [DOI] [PubMed] [Google Scholar]
- 2. Greenberg R, Skornick Y, Kaplan O (1998) Management of breast fibroadenomas. J. Gen. Intern. Med. 13, 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA (1995) Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology 196, 123–134. [DOI] [PubMed] [Google Scholar]
- 4. Tabar L, Dean PB, Tot T (2001) Teaching Atlas of Mammography, 2th edn, p. 248 New York: Thieme Medical Publications. [Google Scholar]
- 5. Rosen PP, Hoda SA (2006) Fibroepithelial neoplasms In: Rosen PP, Hoda SA. ed. Breast Pathology: Diagnosis by Needle Core Biopsy, 2th edn, p. 69 Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- 6. Houssami N, Cheung MN, Dixon JM (2001) Fibroadenoma of the breast. Med. J. Aust. 174, 185–188. [DOI] [PubMed] [Google Scholar]
- 7. Dent DM, Cant PJ (1989) Fibroadenoma. World J. Surg. 13, 706–710. [DOI] [PubMed] [Google Scholar]
- 8. Dupont WD, Page DL, Parl FF, Vnencak‐Jones CL, Plummer WD Jr, Rados MS, Schuyler PA (1994) Long‐term risk of breast cancer in women with fibroadenoma. N. Engl. J. Med. 331, 10–15. [DOI] [PubMed] [Google Scholar]
- 9. Pantanowitz L, Lyle S, Tahan SR (2002) Fibroadenoma of the eyelid. Am. J. Dermatopathol. 24, 225–229. [DOI] [PubMed] [Google Scholar]
- 10. Solomon GJ, Shin SJ, Romanzi LJ (2006) A 65‐year‐old woman with a ‘hemorrhoid’: fibroadenoma of the anogenital region. Arch. Pathol. Lab. Med. 130, 30–32. [DOI] [PubMed] [Google Scholar]
- 11. Carty NJ, Carter C, Rubin C, Ravichandran D, Royle GT, Taylor I (1995) Management of fibroadenoma of the breast. Ann. R. Coll. Surg. Engl. 77, 127–130. [PMC free article] [PubMed] [Google Scholar]
- 12. Simomoto MM, Nazário AC, Gebrim LH, Simões MJ, Baracat EC, De Lima GR (1999) Morphometric analysis of the epithelium of mammary fibroadenomas during the proliferative and secretory phases of the menstrual cycle. Breast J. 5, 256–261. [DOI] [PubMed] [Google Scholar]
- 13. Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 31, 13–20. [DOI] [PubMed] [Google Scholar]
- 14. Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation‐associated human nuclear antigen defined by the monoclonal antibody Ki‐67. J. Immunol. 133, 1710–1715. [PubMed] [Google Scholar]
- 15. Brown DC, Gatter KC (1990) Monoclonal antibody Ki‐67; its use in histopathology. Histopathology 17, 489–503. [DOI] [PubMed] [Google Scholar]
- 16. Goodson WH, Moore DH, Ljung BM, Chew K, Florendo C, Mayall B, Smith HS, Waldman FM (1998) The functional relationship between in vivo bromodeoxyuridine labeling index and Ki‐67 proliferation index in human breast cancer. Breast Cancer Res. Treat. 49, 155–164. [DOI] [PubMed] [Google Scholar]
- 17. Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J (1992) Monoclonal antibodies against recombinant parts of the Ki‐67 antigen (MIB1 and MIB3) detect proliferating cells in microwave‐processed formalin‐fixed parafin sections. J. Pathol. 168, 357–363. [DOI] [PubMed] [Google Scholar]
- 18. Parrado C, Falkmer UG, Hoog A, Falkmer S, Ahrens O, Rius F, Grimelius L (1996) A technique for automatic/interactive assessment of the proliferating fraction of neoplastic cells in solid tumors. A methodological study on the Ki‐67 immunoreactive cells in human mammary carcinomas, including a comparison with the results of conventional S‐phase fraction assessments by means of DNA cytometry. Gen. Diagn. Pathol. 141, 215–217. [PubMed] [Google Scholar]
- 19. Jansen RLH, Hupperets PS, Arends JW, Joosten‐Achjanie SR, Volovics A, Schouten HC, Hillen HF (1998) MIB‐1 labelling index is an independent prognostic marker in primary breast cancer. Br. J. Cancer 78, 460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Dierendonck JH, Keijzer R, Van De Velde CJ, Cornelisse CJ (1989) Nuclear distribution of the Ki‐67 antigen during the cell cycle: comparison with growth fraction in human breast cancer cells. Cancer Res. 49, 2999–3006. [PubMed] [Google Scholar]
- 21. Verheijen R, Kuijpers HJ, Van Driel R, Beck JL, Van Dierendonck JH, Brakenhoff GJ, Ramaekers FC (1989) Ki‐67 detects a nuclear matrix‐associated proliferation‐related antigen. II. Localization in mitotic cells and association with chromosomes. J. Cell Sci. 92, 531–540. [DOI] [PubMed] [Google Scholar]
- 22. Kill IR (1996) Localisation of the Ki‐67 antigen within the nucleolus. Evidence for a fibrillarin‐deficient region of the dense fibrillar component. J. Cell Sci. 109, 1253–1263. [DOI] [PubMed] [Google Scholar]
- 23. Starborg M, Gell K, Brundell E, Hoog C (1996) The murine Ki‐67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J. Cell Sci. 109, 143–153. [DOI] [PubMed] [Google Scholar]
- 24. Bridger JM, Kill IR, Lichter P (1998) Association of pKi‐67 with satellite DNA of the human genome in early G1 cells. Chromosome Res. 6, 13–24. [DOI] [PubMed] [Google Scholar]
- 25. Nazαrio ACP, De Lima GR, Simões MJ, Novo NF (1995) Cell kinetics of the human mammary lobule during the proliferative and secretory phase of the menstrual cycle. Bull. Assoc. Anat. 79, 23–27. [PubMed] [Google Scholar]
- 26. Navarrete MA, Maier CM, Falzoni R, Quadros LG, Lima GR, Baracat EC, Nazario AC (2005) Assessment of the proliferative, apoptotic and cellular renovation indices of the human mammary epithelium during the follicular and luteal phases of the menstrual cycle. Breast Cancer Res. 7, 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Israel R, Mishell DR Jr, Stone SC, Thorneycroft IH, Moyer DL (1972) Single luteal phase serum progesterone assay as an indicator of ovulation. Am. J. Obstet. Gynecol. 112, 1043–1046. [DOI] [PubMed] [Google Scholar]
- 28. Speroff L, Fritz MA (2004) Clinical Gynecologic Endocrinology and Infertility, 7th edn. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- 29. Cericatto R, Pozzobon A, Morsch DM, Menke CH, Brum IS, Spritzer PM (2005) Estrogen receptor‐α, bcl‐2 and c‐myc gene expression in fibroadenomas and adjacent normal breast: association with nodule size, hormonal and reproductive features. Steroids 70, 153–160. [DOI] [PubMed] [Google Scholar]
- 30. Farr AG, Nakane PK (1981) Immunohistochemistry with enzyme labeled antibodies: a brief review. J. Imunnol. Methods 47, 129–144. [DOI] [PubMed] [Google Scholar]
- 31. Elias JM (1982) Principles and Techniques in Diagnostic Histopathology: Developments in Imunohistochemistry and Enzyme Histochemistry. New York: Noyes Publications, EUA. [Google Scholar]
- 32. Eigèliené N, Härkönen P, Erkkola R (2006) Effects of estradiol and medroxyprogesterone acetate on morphology, proliferation and apoptosis of human breast tissue inorgan cultures. BMC Cancer 6, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nie NH et al (1975) Statistical Pachage for the Social Sciences. New York: McGraw‐Hill Education. [Google Scholar]
- 34. Agresti A (2002) Categorical Data Analysis, 2th edn. New York: Wiley‐Interscience. [Google Scholar]
- 35. Conover WJ (1980) Practical Nonparametric Statistics, 2th edn. New York: John Wiley & Sons. [Google Scholar]
- 36. De Boever J, Vandekerckhove D (1982) Benign breast disease: steroid concentrations. J. Steroid Biochem. 17, 338. [Google Scholar]
- 37. Wang DY, Fentiman IS (1985) Epidemiology and endocrinology of benign breast disease. Breast Cancer Res. Treat. 6, 5–36. [DOI] [PubMed] [Google Scholar]
- 38. Kuttenn F, Fournier S, Durand JC, Mauvais‐Jarvis P (1981) Estradiol and progesterone receptors in human breast fibroadenomas. J. Clin. Endocrinol. Metab. 52, 1225–1229. [DOI] [PubMed] [Google Scholar]
- 39. Noguchi S, Motomura K, Inaji H, Imaoka S, Koyama H (1993) Clonal analysis of fibroadenoma and phyllodes tumor of the breast. Cancer Res. 53, 4071–4074. [PubMed] [Google Scholar]
- 40. Dietrich CU, Pandis N, Teixeira MR, Bardi G, Gerdes AM, Andersen JA, Heim S (1995) Chromosome abnormalities in benign hyperproliferative disorders of epithelial and stromal breast tissue. Int. J. Cancer 60, 49–53. [DOI] [PubMed] [Google Scholar]
- 41. Franco N, Picard SF, Mege F, Arnould L, Lizard‐Nacol S (2001) Absence of genetic abnormalities in fibroadenomas of the breast determinated at p53 gene mutations ant microsatellite alterations. Cancer Res. 61, 7955–7958. [PubMed] [Google Scholar]
- 42. Franco N, Arnould L, Mege F, Picard SF, Arveux P, Lizard‐Nacol S (2003) Comparative analysis of molecular alterations in fibroadenomas associated or not with breast cancer. Arch. Surg. 138, 291–295. [DOI] [PubMed] [Google Scholar]
- 43. Kuijper A, Buerger H, Simon R, Schaefer KL, Croonen A, Boecker W, Van Der Wall E, Van Diest JP (2002) Analysis of the progression of fibroepithelial tumours of the breast by PCR‐based clonality assay. J. Pathol. 197, 575–581. [DOI] [PubMed] [Google Scholar]
- 44. Noguchi S, Aihara T, Koyama H, Motomura K, Inaji H, Imaoka S (1995) Clonal analysis of benign and malignant human breast tumors by means of polymerase chain reaction. Cancer Lett. 90, 57–63. [DOI] [PubMed] [Google Scholar]
- 45. Dixon JM, Mansel RE (1994) ABC of breast diseases. Congenital problems and aberrations of normal breast development and involution. BMJ 309, 797–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lellé RJ, Heidenreich W, Stauch G, Wecke I, Gerdes J (1987) Determination of growth fractions in benign breast disease (BBD) with monoclonal antibody Ki‐67. J. Cancer Res. Clin. Oncol. 113, 73–77. [DOI] [PubMed] [Google Scholar]
- 47. Maiorano E, Albrizio M (1995) Tubular adenoma of the breast: an immunohistochemical study of ten cases. Pathol. Res. Praxt. 191, 1222–1230. [DOI] [PubMed] [Google Scholar]
- 48. Kaya R, Pestereli HE, Erdogan G, Gülkesen KH, Karaveli S (2001) Proliferating activity in differential diagnosis of benign phyllodes tumor and cellular fibroadenomas: is it helpful? Pathol. Oncol. Res. 7, 213–216. [DOI] [PubMed] [Google Scholar]
- 49. Dixon JM (1991) Cystic disease and fibroadenoma of the breast: natural history and relation to breast cancer risk. Br. Med. Bull. 47, 258–271. [DOI] [PubMed] [Google Scholar]


