Abstract
Abstract. For several millennia, the willow tree and salicin have been associated with salicylic acid, the key precursor molecule that has contributed to the discovery of acetylsalicylic acid, traded as aspirin. These molecules have been shown to possess phyto‐ and chemotherapeutic activities as analgesic drugs. In recent decades, aspirin has become the focus of extensive investigation into antiproliferative and anticancer activities. The historical steps that led to the discovery of aspirin, and its antiproliferative and anticancer potential are highlighted in this review.
INTRODUCTION
The willow tree (Salix sp.) has achieved a kind of symbolic significance associated with the discovery of aspirin, more than a century ago (Barrett et al. 1999; Lévesque & Lafont 2000; Cragg & Newman 2001; Jones 2001), although herbalists and patients have recognized the pharmacological and the clinical potential of not only the willow, but also a variety of other plants (Mayer et al. 1949; Lévesque & Lafont 2000; Sumner 2000; Dewick 2002). This comes as no surprise, because medical conditions are as old as our species, and some animals are known to preferentially consume plants with medicinal properties (Sumner 2000).
Analysis of the historical perspective of aspirin discovery starts with clinical investigation during periods before the 18th century, where willow and its extract were used to relieve different types of pain. This century was characterized by advancements in organic chemistry and particularly in pharmaceuticals. This helped to identify the active components of willow when their chemical structures were identified as salicilin, salicylic acid and acetylsalicylic acid; these were subsequently used in a variety of clinical trials. It was not until 1971, however, that the exact pharmacology of aspirin was elucidated when aspirin was shown to interfere with the synthesis of prostaglandins (PGs). Modern epidemiological studies from the 1980s have indicated that aspirin is inversely associated with the risk of developing colorectal cancer. Advances in epidemiological and biological studies have since concentrated on aspirin as a potential anticarcinogenic drug. In addition, aspirin, at relatively high doses (5–20 mm), has been shown to have cell proliferation inhibitory potential in various cancer cell populations. These doses, however, may have diverse effects, and hence limit direct anticancer potential. The main thrust of this review is to analyse the historical perspective of willow and its association with the discovery of aspirin and its use in medical/pharmacological fields.
Historical perspective of the discovery of aspirin – attributes of willow
Traditional methods of drug discovery and development have been influenced by the need to prevail over illness and people's experience in witnessing and realizing the beneficial potentials of a plant to cure ailments, perhaps by trial and error (for review see Sumner 2000). The historical account of willow (Salix sp.) goes back to early civilizations, particularly in Mesopotamia around 6000 years ago when plants were exploited for food and as a source of drugs.
Experience of both Assyrian (4000 bc) and Babylonian civilizations (605–562 bc) in Iraq has contributed to the development of medicine (Ackerknecht 1973; Sutcliffe & Dunn 1992; Chevallier 1996; Barrett et al. 1999; Burns & Fulder 2002). In ancient civilizations of that period, medicine was based on surgeons and physicians using herbal draughts to cure ailments; extracts of the willow tree being used to cure pain and inflammation. Archaeologists have found clay tablets left by the Assyrians from the Sumerian period (3500–2000) describing the use of willow leaves for such conditions (Lévesque & Lafont 2000).
The Babylonians also used willow tree extracts to treat common fever, pain and inflammation. Sumerians were the first known civilization to register medical prescriptions for pain according to a clay tablet from 4000 years ago (Wells 2003). In addition, the Code of Hammurabi (1750 bc), in Mesopotamia, sheds light on a medical history that contributed to the long chain of pharmacological achievements prompted by man's attempt to survive disease. Such notions also prompted Egyptians (1300 bc) to use willow leaves to treat inflammatory conditions. The willow is listed in the herbal remedies in the Ebers Papyrus of ancient Egypt (Nunn 1996), which describes the analgesic effects of willow leaves to ‘draw the heat out’ of inflamed wounds (Murder 1992; Jack 1997).
Chinese and Greek civilizations also exploited willow bark more than 2000 years ago to alleviate fever and pain (Riddle 1999). The Chinese used poplar tree (Populus alba L.) barks and willow (Salix babylonica L.) shoots for centuries to treat rheumatic fever, colds, haemorrhages and goitre, and as a general antiseptic for wounds and abcesses (Rainsford 1984). Furthermore, the Greeks also used willow as a form of medicine. The philosopher, Hippocrates (460–370 bc) recommended chewing willow bark to patients suffering from high temperature and pain (Gross & Greenberg 1948; Riddle 1999). He also prescribed a brew of willow leaves to ease the excruciating pains of childbirth. Around 500 years later (100 ad), a further Greek physician, Dioscorides, prescribed willow bark also to reduce the symptoms of inflammation, and the use of willow bark has continued because of its analgesic and anti‐inflammatory properties. A further Greek physician, Celsius (1 ad) who treated women with prolapsed uteri with boiled willow leaves and vinegar, is the accepted author of the famed symptoms of inflammation ‘redness and swelling with heat and pain’. The Roman Pliny speaks of corns treated with a burned willow bark paste, and Galen treated bloody wounds and ulcers with willow leaves (Wells 2003).
The modern era of aspirin discovery began in the 18th century, when more advanced scientific information had accumulated. In 1763, the Reverend Edward Stone described the beneficial effects of willow bark, in a letter addressed to the Royal Society. Here, Reverand Stone described his results of a clinical study, treating patients suffering from ague (fever, usually taken to be malaria) with powdered willow bark in a dram of water. One hundred and thirteen years later, Thomas MacLagan, a Scottish physician, in 1876 conducted a clinical investigation to test the therapeutic efficiency of willow powder. He successfully treated himself with willow powder extract (salicin) before he applied it to a patient with acute rheumatism. His treatments resulted in a complete reduction of fever and joint inflammation (MacLagan 1876; Sneader 1997). During this period, the demand for willow trees increased because of their clinical use. Research into the willow tree in Europe became more focused, especially when supplies of Peruvian bark stopped as a result of the continental blockade imposed by Napoleon at the beginning of the 19th century (Jack 1997).
History of the chemical investigation into the benefits of aspirin
Chemical investigation into the therapeutically active substance in willow extract started in the early 1800s. Wilkinson in 1803 extracted the active substance from Cortex salicis latifoliae, which was later found to contain high levels of tannin. In the early 1820s, Brugnateli, and Fontana & Buchner obtained therapeutically active substances of a less contaminated nature and further purification was achieved by Buchner in 1828 at the University of Munich. He removed the tannins and obtained a yellowish substance, which he called salicin, the Latin name for the willow. Finally, the pure crystalline form of salicin was obtained by the French pharmacist Henri Leroux in 1829 (Leroux 1830; Rainsford 1984). This was then used for the treatment of rheumatism (Gross & Greenberg 1948; Bekemeier 1977).
A further step forward came with the description of the chemical structure of salicin. Historically, chemical investigations of salicylic acid, the precursor of aspirin, are illustrated in Fig. 1. Gay‐Lussac in 1930 indicated that salicin was a non‐nitrogenous compound, and in 1935, Raffaele Piria, the Italian chemist found through acid hydrolysis that the salicin structure was comprised of d‐glucose and salicyl alcohol moieties (Piria 1838). The salicyl alcohol was then oxidized into salicylic acid where its structure was confirmed with an authentic sample of salicylic acid, obtained from the oxidation of salicylic aldehyde (Fig. 1). Alternatively, the compound has been extracted from meadow sweet flowers (Spirea ulmaria) by the Swiss pharmacist, Pagenstecher, in early 1835, who passed it to the German chemist, Lowig, for oxidation that yielded salicylic acid (Fluckinger 1888; Rainsford 1984).
Figure 1.
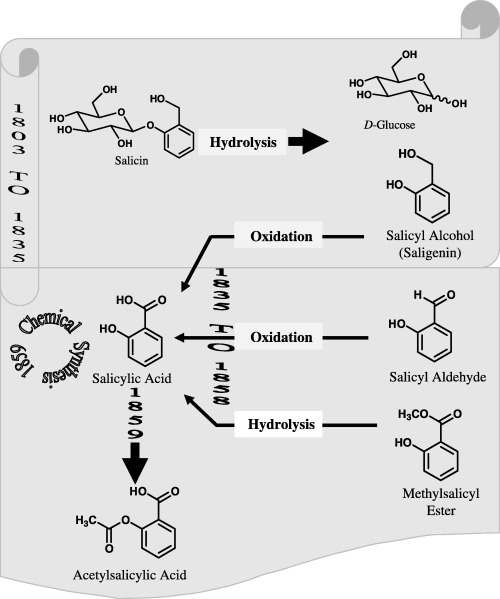
Schematic illustration of the history of aspirin discovery.
Oxidation of salicyl aldehyde also resulted in the formation of salicylic acid as illustrated. Its structure was further elucidated by the French chemist, Cohours, in 1845 and by the Scottish chemist, Couper, in 1858, who both hydrolysed the methylsalicyl ester in wintergreen (Gaultheria) oil upon treatment with phosphorous perchloride (Couper 1858; Geissler & Moller 1889). The hydroxyl radical form readily reacts with the aromatic moiety, which results in hydroxylation. Kolb & Lautemann (Kolb 1860; Kolb & Lautemann 1860) achieved the chemical synthesis of salicylic acid on a small scale. Later, they devised large‐scale chemical reactions, which led to the establishment of a commercial establishment, the Heyden Chemical Company, in Germany for the production of salicylic acid for analgesic and antipyretic purposes. However, as salicylic acid was found to be an irritant of the stomach causing bleeding at large doses, salicylic acid was preferentially chemically acetylated by Felix Hoffmann in 1897 to produce acetylsalicylic acid.
Some controversy exists as to who decided to acetylate salicylic acid – Felix Hoffmann or the Heyden Chemical Company. Sneader (2000), Pharmaceutical Historian at the School of Pharmacy, the University of Strathclyde, suggests that the discovery of aspirin should be attributed instead to Arthur Eichengrun, a colleague of Hoffman, who synthesized acetyl salicylic acid as early as April 1897. Eichengrun appears to have been written out of history, perhaps 50 years later by the subsequent Nazi regime who became aware of his Jewish ancestry. Whatever the case, success finally came through when the Bayer Company registered the product under the trade name Aspirin on 6 March 1899 and distributed aspirin to clinics and hospitals for the treatment of patients.
Bayer launched soluble aspirin in the form of tablets in 1900. It soon became known worldwide for safe and effective pain relief. In a few years, aspirin became readily available to doctors and the public and the name Aspirin became a drug recognized amongst professionals. It was available to the public in 1915 without prescription.
Aspirin and its hidden benefits for cancer sufferers
As publicity increased, more clinical and pharmaceutical research was conducted on aspirin, and by the 1920s, therapeutic applications became available for treating symptoms of pain related to rheumatism, lumbago and neuralgia. In 1948, the Californian general practitioner, Lawrence Craven, observed that aspirin taken regularly could dramatically reduce the risk of myocardial infarction (MI). This assumption was based on the fact that none of the 400 patients that were prescribed aspirin suffered heart attacks during the time of the investigation. Such claims have encouraged drug organizations, for example, the US Food and Drug Administration (FDA), to test aspirin as a prophylactic for MI in patients with unstable angina. The FDA also approved the use of aspirin in 1988 for the prevention of recurrent transient ischaemic attacks or mini‐strokes in men and made aspirin standard therapy for individuals with a history of strokes (Childs 2006).
Recent epidemiological analyses related to patients on long‐term aspirin therapy have indicated a decreased incidence of colorectal cancer (Rodriguez & Huerta‐Alvarez 2000; Childs 2006; Rao & Reddy 2004; Zhab et al. 2004). These studies indicate that aspirin and other non‐steroidal anti‐inflammatory drugs (NSAIDs) protect against colorectal cancer to a greater degree over protection against non‐gastrointestinal cancers. Recently, Petersen et al. (2005) have shown that a low dose of aspirin protects cells against oxidative damage and induces apoptosis. These features have been shown particularly in human lens epithelial cells, illustrating the evidence of the antioxidant potential of aspirin. Various pieces of evidence suggest that aspirin possesses antiproliferative properties mediated in different cell lines, including human colorectal tumour cells (Bellosillo et al. 1998; Din et al. 2004), gastric cancer cells (Wong et al. 1999), B‐cell chronic lymphocytic leukaemia cells (Bellosillo et al. 1998), myeloid leukaemia cell lines (Klampfer et al. 1999), vascular smooth muscle cells (Marra et al. 2000; Brooks et al. 2003), colon adenocarcinoma cells (Shiff et al. 1996) as well as many other cell types.
Generally, aspirin induces a dose‐dependent reduction in the proliferation rate of HT‐29 cells (Shiff et al. 1996). The sodium salt of aspirin also significantly inhibits cellular proliferation of human pancreatic cancer cell lines (BxPC3 and Panc‐1) in a dose‐dependent manner (Perugini et al. 2000). Goel et al. (2003) found similar effects of aspirin on the growth of three different human colon cancer cell populations (HCT116, HCT116 + chr3 and SW480) but at different rates. At 72‐h incubation with aspirin treatment, the proliferation rate of HCT116 + chr3 cells was significantly less than inhibition of HCT116 cells at 0.1 mm (14.3% versus 40.7%) and 2.5 mm (27.2% versus 47.9%). All other published investigations have concluded that the inhibitory effects of aspirin on cell population growth and cell proliferation is dose and time dependent (Qiao et al. 1998; Wong et al. 1999; Zhou et al. 2001; Kim et al. 2003). Table 1 lists effects of aspirin on the proliferation of different cell lines at different doses and incubation times. Generally, the dose inducing significant antiproliferative and pro‐apoptotic effects of aspirin in vitro is between 3 mm and 10 mm (Santini et al. 1999; Marra & Liao 2001; Zha et al. 2004), although in the literature there are reports of higher concentrations of up to 20 mm (1999, 1997; Pillinger et al. 1998) being effective.
Table 1.
The effect of aspirin on cell proliferation and cell population growth
| Dose (mm) | Cell line | Max incubation time (h) | Percentage of growth inhibition a | Reference |
|---|---|---|---|---|
| 0.4–3 | HT‐29 | 73 | 70–75 | Qiao et al. (1998) |
| 0.1–10 | AGS | 72 | 21–47 | Zhou et al. (1999) |
| 1 | AGS and MKN‐28 | 60 | 37–40 | Zhou et al. (2001) |
| 1–5 | Ishikawa human endometrial tumour cells | 96 | 21–88% | Arango et al. (2001) |
| 1–5 | OVCAR‐3 ovarian tumour cells | – | Little 68% | Drake & Becker (2002) |
| 1–3 | HeLa TG | – | 18–55 | Kim et al. (2003) |
Growth inhibition at the highest incubation time, relative to control.
The mechanism by which aspirin and related NSAIDs exert their therapeutic effects in cancer was first established based on the inhibition of PG synthesis. Two isoforms of the enzyme, cyclooxygenase or COX‐1 and COX‐2, are responsible for the synthesis of PGs in various cells, and these enzymes have been found to play an important role in a number of malignancies (Munkarah et al. 2002). PGs are signalling molecules and are responsible for regulating a number of cellular functions, including gene expression, growth and differentiation (Towndrow et al. 2000). Many cancers overproduce PGs, and this can be inhibited by aspirin. Aspirin irreversibly inhibits both of its isoforms by a cross‐acetylation process (Shimokawa & Smith 1992; Lecomte et al. 1994; Van & Botting 2003; Wu 2003). Over‐expression of COX‐2 leads to increased levels of PGs, and hence promotes cellular proliferation, resistance to apoptosis and angiogenesis, processes that contribute to the emergence of the neoplastic phenotype (Husain et al. 2001; Munkarah et al. 2002; Masmoudi et al. 2004). High levels of COX‐2 may also confer apoptosis resistance in some cancer cells by activating genes that promote cell survival (Munkarah & Ali‐Fehmi 2005; Secchiero et al. 2005). Therefore, aspirin may exert its anticarcenogenic properties via COX‐2 inhibition and subsequently inhibition of PG production, which may result in the induction of apoptosis and/or inhibition of cell proliferation (Husain et al. 2001).
In contrast, aspirin can induce antiproliferative effects by non‐PG‐dependent pathways and affect the progression of the cell cycle (Shiff et al. 1996; Arango et al. 2001; Stark et al. 2001; Goel et al. 2003; Gao et al. 2004). The effects of aspirin on various cancerous cellular regulatory molecules have been found in the inhibition of NF‐κB pathway (Yin et al. 1998), p70s6k (Law et al. 2000), the Bcl‐2 family (Redlak et al. 2005), caspases (Belloslo et al. 1998; Castano et al. 1999; Klampfer et al. 1999) and various other intracellular molecules (Pique et al. 2000). Aspirin has been shown to prevent H2O2‐induced activation of NF‐κB in a dose‐dependent manner through inhibition of phosphorylation and degradation of IκΒα and IκBβ in HeLa cells (Kutuk & Basaga 2003). On the other hand, p70s6k is an important member of the MAP kinase signalling family, important for cell cycle progression. Aspirin has been found to inhibit p70s6k activation and phosphorylation in a p38 MAPK‐independent manner (Law et al. 2000) and Goel et al. (2003) have shown that aspirin affects the expression of MMR proteins in colon cancer cell models that are deficient or proficient in a subset of specific genes (for hMLH1, hMLH2, hMLH6 and hMLHS2). These results indicate that the antiproliferative and apoptotic effects of aspirin are mediated by various mechanisms that are cell specific (Han et al. 2001).
An appraisal of the development of aspirin
Well before the 19th century, many medicinal plants like willow had become part of the pharmacopeias of allopathy, naturopathy and homeopathy, and their therapeutic basis had been investigated for contemporary approaches by many phytotherapists and physicians. Usually, when a compound is isolated and synthesized, its pharmaceutical uses are more carefully regulated; aspirin, however, has become an early exception (Desmet 1993; Desmet et al. 1997; Wells 2003). It is based on a simple molecule with multifunctional groups rendering it capable of various chemical interaction(s) with biological molecules. Hence, it possesses a range of pharmacological activities such as an anti‐inflammatory, antirheumatic antipyretic, an antidote, an analgesic, and with antiseptic properties and has become the most common over‐the‐counter drug. Derivatives and similar common NSAIDs are Ibuprofen, Ketoprofen, Diclofenac Naproxen Meloxicam and Cocodamol; however, aspirin in the last 30 years has become focus on further investigation as a cancer chemopreventive and theapeutic drug. It has been encouraged as the revival of interest in its clinical and pharmacological effects in terms of cancer treatment (Funkhouser & Sharp 1995; Wong et al. 1999; Smith et al. 2000; Hector et al. 2001; Claudia 2003).
Yet, the potential for use of aspirin as an anticancer agent is undermined by the high dose required to show the anticancer potential, and the sensitivity to it of the gastric mucosa. Using a high concentration of aspirin in vitro is not comparable with its the commonly prescribed 75 mg (0.04 mm) for human use. Xu et al. (1999) indicated that aspirin at 0.01–0.1 mm is the therapeutic concentration that is commonly achieved to selectively inhibit Cox‐2 transcription in fibroblasts, and endothelial cells. In contrast, high concentrations used in in vitro experiments exhibit nonspecific inhibition of a large number of kinases in cells. Raz (2002) and others (Frantz & O’Neill 1995; Yin et al. 1998) have questioned the use of high aspirin doses and conclude that its antitumourogenic effect in vivo is the result of the inhibition of tumour cell Cox‐2. Also, Wang & Dubois (2005) have reported that prolonged use of high doses of Cox‐2 inhibition leads to serious cardiovascular side –effects. This mechanism, though, may not have substantial relevance to the one that mediates the effects of NSAIDs in vitro, because of high concentrations habitually used in in vitro experiments (Raz 2002). Furthermore, it has been reported recently that high aspirin concentration used in cancer treatment causes complications to heart function (Patrono et al. 2004). Further investigations are therefore recommended using more physiological doses to weigh against the cost/benefit ratio of aspirin therapy in cancer (Mahdi et al. 2005).
REFERENCES
- Ackerknecht EH (1973) Therapeutics: from the Primitives to the Twentieth Century. New York: Hafner Press. [Google Scholar]
- Arango HA, Icely S, Roberts WS, Cavanagh D, Becker JL (2001) Aspirin effects on endometrial cancer cell growth. Obstet. Gynecol. 97, 423–427. [DOI] [PubMed] [Google Scholar]
- Baron JA (2003) Epidemiology of non‐steroidal anti‐inflammatory drugs and cancer. Prog. Exp. Tumor Res. 37, 1–24. [DOI] [PubMed] [Google Scholar]
- Barrett B, Kiefer D, Rabago D (1999) Assessing the risks and benefits of herbal medicine: an overview of scientific evidence. Altern. Ther. Health Med. 5, 40–48. [PubMed] [Google Scholar]
- Bekemeier H (1977) In 100 Years of the Salicylic Acid as an Anti‐Rheumatic Drug. Halle, Saale: Martin‐Luther University, Halle‐Wittenberg, p. 6–13. [Google Scholar]
- Bellosillo B, Pique M, Barragan M, Castano E, Villamor N, Colomer D, Montserrat E, Pons G, Gil J (1998) Aspirin and salicylate induce apoptosis and activation of caspases in B‐cell chronic lymphocytic leukemia cells. Blood 92, 1406–1414. [PubMed] [Google Scholar]
- Brooks G, Yu XM, Wang Y, Crabbe MJ, Shattock MJ, Harper JV (2003) Non‐steroidal anti‐inflammatory drugs (NSAIDs) inhibit vascular smooth muscle cell proliferation via differential effects on the cell cycle. J. Pharm. Pharmacol. 55, 519–526. [DOI] [PubMed] [Google Scholar]
- Burns SB, Fulder S (2002) Arabic medicine: preservation and promotion: a millennium of achievement published. J. Alternative Complementary Med. 8, 407–410. [DOI] [PubMed] [Google Scholar]
- Castano E, Dalmau M, Barragan M, Pueyo G, Bartrons R, Gil J (1999) Aspirin induces cell death and caspase‐dependent phosphatidylserine externalization in HT‐29 human colon adenocarcinoma cells. Br. J. Cancer 81, 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier A (1996) The Encyclopedia of Medicinal Plants. London: Dorling Kindersley; 96 , 128. [Google Scholar]
- Childs PE (2006) The centenary of Aspirin: wonder drug of the twentieth century [WWW document]. URL http://www.ul.ie~childsp/CinA/Issue59/TOC43_Aspirin.htm[accessed February 2006].
- Claudia O (2003) Aspirin protects against cancer of the upper aerodigestive tract. Lancet Oncol. 4, 200. [Google Scholar]
- Couper AS (1858) On a New Chemical Theory and Resonance on Salicylic Acid. Alembic Club Reprints No. 21. Edinburgh: E. S. Livingstone. [Google Scholar]
- Cragg GM, Newman DJ (2001) Natural product drug discovery in the next millennium. Pharm. Biol. 39, 8–17. [DOI] [PubMed] [Google Scholar]
- Desmet PA (1993) An introduction to herbal pharmacoepi‐demiology. J. Ethnopharmacol. 38, 197–208. [DOI] [PubMed] [Google Scholar]
- Desmet PA, Van den Eertwegh AJ, Lesterhui W, Stricker BH (1997) Hepatotoxicity associated with herbal tablets. Br. Med. J. 313, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewick PM (2002) Medicinal Natural Products: A Biosynthetic Approach. John Wiley and Sons, Sussex. [Google Scholar]
- Din FV, Dunlop MG, Stark LA (2004) Evidence for colorectal cancer cell specificity of aspirin effects on NF kappa B signaling and apoptosis. Br. J. Cancer 91, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JG, Becker JL (2002) Aspirin‐induced inhibition of ovarian tumor cell growth Obstet. Gynecol. 100, 677–682. [DOI] [PubMed] [Google Scholar]
- Fluckinger FA (1888) Pharmazeutische Chemie. Berlin: R Gaertner's Verlag. [Google Scholar]
- Frantz B, O'Neill EA (1995) The effect of sodium salicylate and aspirin on NF‐κB. Science 270, 2017–2018. [DOI] [PubMed] [Google Scholar]
- Funkhouser EM, Sharp GB (1995) Aspirin and reduced risk of esophageal carcinoma. Cancer 76, 1116–1119. [DOI] [PubMed] [Google Scholar]
- Gao J, Niwa K, Sun W, Takemura M, Lian Z, Onogi K, Seishima M, Mori H, Tamaya T (2004) Non‐steroidal anti‐inflammatory drugs inhibit cellular proliferation and upregulate cyclooxygenase‐2 protein expression in endometrial cancer cells. Cancer Sci. 95, 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E, Moller J (1889) Real Encyclopadie der Gesamten Pharmcie. Leipzing: Urban und Schwarzenberg. [Google Scholar]
- Goel A, Chang DK, Ricciardiello L, Gasche C, Boland CR (2003) A novel mechanism for aspirin‐mediated growth inhibition of human colon cancer cells. Clin. Cancer Res. 9, 383–390. [PubMed] [Google Scholar]
- Gross M, Greenberg LA (1948) The Salicylates. A Critical Bibliographic Review. New Haven, CT: Hillhouse Press. [Google Scholar]
- Han Z, Pantazis P, Wyche JH, Kouttab N, Kidd VJ, Hendrickson EA (2001) A fas‐associated death domain protein‐dependent mechanism mediates the apoptotic action of non‐steroidal anti‐inflammatory drugs in the human leukemic Jurkat Cell line. J. Biol. Chem. 276, 38748–38754. [DOI] [PubMed] [Google Scholar]
- Hector AA, Suzane I, Williams SR, Denis C, Jeanne LB (2001) Aspirin effects on endometrial Cancer Cell Growth. Obstet. Gynecol. 97, 423–427. [DOI] [PubMed] [Google Scholar]
- Husain SS, Szabo IL, Pai R, Soreghan B, Jones MK (2001) Tarnawski AS MAPK (ERK2) kinase – a key target for NSAIDs‐induced inhibition of gastric cancer cell proliferation and growth. Life Sci. 69, 3045–3054. [DOI] [PubMed] [Google Scholar]
- Jack DB (1997) One hundred years of aspirin. Lancet 350 (9075), 437–439. [DOI] [PubMed] [Google Scholar]
- Jones R (2001) Nonsteroidal anti‐inflammatory drug prescribing: past, present, and future. Am. J. Med. 110 (Suppl. 1, 1), S4–S7. [DOI] [PubMed] [Google Scholar]
- Kim KY, Seol JY, Jeon GJ, Nam MJ (2003) The combined treatment of aspirin and induces apoptosis by the regulation of bcl‐2 and caspase‐3 in human cervical cancer cell. Cancer Lett. 189, 157–166. [DOI] [PubMed] [Google Scholar]
- Klampfer L, Cammenga J, Wisniewski HG, Nimer SD (1999) Sodium salicylate activates caspases and induces apoptosis of myeloid leukemia cell lines. Blood. 93, 2386–2394. [PubMed] [Google Scholar]
- Kolbe H (1860) Über Synthese der Salicylsäure. Liebigs Ann. Chim. 113, 125–127. [Google Scholar]
- Kolbe H, Lautemann E (1860) Über die Constitution und Basicitat der Salicylsäure. Liebigs Ann. Chem. 115, 157–206. [Google Scholar]
- Kutuk O, Basaga H (2003) Aspirin prevents apoptosis and NF‐kappaB activation induced by H2O2 in hela cells. Free Radic. Res. 37, 1267–1276. [DOI] [PubMed] [Google Scholar]
- Law BK, Waltner‐Law ME, Entingh AJ, Chytil A, Aakre ME, Norgaard P, Moses HL (2000) Salicylate‐induced growth arrest is associated with inhibition of p70s6k and down‐regulation of c‐myc, cyclin D1, cyclin A, and proliferating cell nuclear antigen. J. Biol. Chem. 275, 38261–3827. [DOI] [PubMed] [Google Scholar]
- Lecomte M, Leneuville O, Ji C, Dewitt DL, Smith WL (1994) Acetylation of human prostaglandin endoperoxide synthase‐2 (cyclooxygenase‐2) by aspirin. J. Biol. Chem. 269, 13207–13215. [PubMed] [Google Scholar]
- Leroux H (1830) Discovery of salicine. J. Chim. Med. 6, 340–432. [Google Scholar]
- Lévesque H, Lafont O (2000) Aspirin throughout the ages: an historical review. L’aspirine à travers les siècles: rappel historique. Rev. Med. Interne 21 (Suppl. 1), 8s–17s. [DOI] [PubMed] [Google Scholar]
- MacLagan TJ (1876) The treatment of rheumatism by salicin and salicylic acid. Lancet I, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi JG, Al Karaawi M, Pepper CJ, Bowen ID (2005) Sub‐millimolar concentrations of the novel phenolic compound JMC‐18 induces apoptosis in the human HT‐1080 fibrosarcoma cell line, European Cell Proliferation Society 27th Meeting, Queen Mary, University of London, 7th−10th September 2005.
- Marra DE, Liao JK (2001) Salicylates and vascular smooth muscle cells proliferation: molecular mechanism for cell cycle arrest. Trends Cardiovasc. Med. 11, 339–344. [DOI] [PubMed] [Google Scholar]
- Marra DE, Simoncini T, Liao JK (2000) Inhibition of vascular smooth muscle cell proliferation by sodium salicylate mediated by upregulation of p21Waf1 and p27KiP1 . Circulation 102, 2124–2130. [DOI] [PubMed] [Google Scholar]
- Masmoudi Chevalier TL, Sabatier L, Soria JC (2004) Cyclooxygenase 2 inhibitors and cancer chemoprevention. Bull. Cancer. 91 (Suppl. 2), S77–S84. [PubMed] [Google Scholar]
- Mayer R, Mayer M, Mayer R, Mayer M (1949) Biological salicyl therapy with cortex salicus [Weidenrinde]. Pharmazie 4, 77–81. [PubMed] [Google Scholar]
- Munkarah A, Ali‐Fehmi R (2005. ) COX‐2: a protein with an active role in gynecological cancers. Curr. Opin. Obstet. Gynecol. 17, 49–53. [DOI] [PubMed] [Google Scholar]
- Munkarah AR, Morris R, Baumann P, Deppe G, Malone J, Diamond MP, Saed GM (2002) Effects of prostaglandin E(2) on proliferation and apoptosis of epithelial ovarian cancer cells. J. Soc. Gynecol. Investig. 9, 168–173. [PubMed] [Google Scholar]
- Murder MJ (1992) Magic and Medicine. Oxford: Oxford University Press. [Google Scholar]
- Nunn JF (1996) Ancient Egyptian Medicine. London: British Museum Press, p. 158. [PubMed] [Google Scholar]
- Patrono C, Coller B, Fitzgerald GA, Hirsh J, Roth G (2004) Platelet‐active drugs: the relationships among dose, effectiveness, and side effects. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126, 234S–264S. [DOI] [PubMed] [Google Scholar]
- Perugini RA, McDade TB, Vittimberga FJ, Duffy AJ, Callery MP (2000) Sodium salicylate inhibits proliferation and induces G1 cell cycle arrest in human pancreatic cancer cell lines. J. Gastrointest. Surg. 4, 24–33. [DOI] [PubMed] [Google Scholar]
- Petersen A, Zetterberg M, Sjostrand J, Palsson AZ, Karlsson JO (2005) Potential protective effects of NSAIDs/ASA in oxidatively stressed human lens epithelial cells and intact mouse lenses in culture. Ophthalmic Res. 37, 318–327. [DOI] [PubMed] [Google Scholar]
- Pillinger MH, Capodici C, Rosenthal P, Kheterpal N, Hanft S, Philips MR, Weissmann G (1998) Modes of action of aspirin‐like drugs: salicylates inhibit erk activation and integrin‐dependent neutrophil adhesion. Proc. Natl Acad. Sci. USA 95, 14540–14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique M, Barragan M, Dalmau M, Bellosillo B, Pons G, Gil J (2000) Aspirin induces apoptosis through mitochondrial cytochrome c release. FEBS Lett. 480, 193–196. [DOI] [PubMed] [Google Scholar]
- Piria R (1838) Comptes Rendues de l’Academie des Sciences. Paris 6, 338. [Google Scholar]
- Qiao L, Hanif R, Sphicas E, Shiff SJ, Rifas B (1998) Effect of aspirin on induction of apoptosis in HT‐29 human colon adenocarcinoma cells. Biochem. Pharmacol 553–564. [DOI] [PubMed]
- Rainsford KD (1984) Aspirin and the Salicylates. London: Butterworths. [Google Scholar]
- Rao CV, Reddy BS (2004) NSAIDs and chemoprevention. Curr. Cancer Drug Targets 4, 29–42. [DOI] [PubMed] [Google Scholar]
- Raz A (2002) Is inhibition of cyclooxygenase required for the anti‐tumorigenic effects of nonsterolidal, anti‐inflammatory drugs (NSAIDs)? In vitro versus in vivo results and the relevance for the prevention and treatment of cancer. Biochem. Pharmacol. 63, 343–347. [DOI] [PubMed] [Google Scholar]
- Redlak MJ, Power JJ, Miller TA (2005) Role of mitochondria in aspirin‐induced apoptosis in human gastric epithelial cells. 1. Am. J Physiol. Gastrointest. Liver Physiol. 289, G731–G738. [DOI] [PubMed] [Google Scholar]
- Riddle JM (1999) Historical Data as an Aid in Pharmaceutical Prospecting and Drug Safety Determination. J. Altern. Complement. Med. 5, 195–201. [DOI] [PubMed] [Google Scholar]
- Rodriguez LAG, Huerta‐Alvarez C (2000) Reduced incidence of colorectal adenoma among long‐term users of nonsteroidal antiinflammatory drugs: a pooled analysis of published studies and a new population‐based study. Epidemiology 11, 376–381. [DOI] [PubMed] [Google Scholar]
- Santini G, Sciulli MG, Marinacci R, Fusco O, Spoletini L, Pace A, Ricciardulli A, Natoli C, Procopio A, Maclouf J, Patrignani P (1999) Cyclooxygenase‐independent induction of p21WAF‐1/cip1, apoptosis and differentiation by L‐745 337, a selective PGH synthase‐2 inhibitor, and salicylate in HT‐29 cells. Apoptosis 4, 151–162. [DOI] [PubMed] [Google Scholar]
- Schwenger P, Alpert D, Skolnik EY, Vilcek J (1999) Cell‐type‐specific activation of c‐Jun N‐terminal kinase by salicylates. J. Cell Physiol. 179, 109–114. [DOI] [PubMed] [Google Scholar]
- Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik EY, Vilcek J (1997) Sodium salicylate induces apoptosis via p38 mitogen‐activated protein kinase but inhibits tumor necrosis factor‐induced c‐Jun N‐terminal kinase/stress‐activated protein kinase activation. Proc. Natl Acad. Sci. USA 94, 2869–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchiero P, Barbarotto E, Gonelli A, Tiribelli M, Zerbinati C, Celeghini C, Agostinelli C, Pileri SA, Zauli G (2005) Potential pathogenetic implications of cyclooxygenase‐2 overexpression in B chronic lymphoid leukemia cells. Am. J. Pathol 167, 1599–15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiff SJ, Koutsos MI, Qiao L, Rigas B (1996) Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: effects on cell cycle and apoptosis. Exp. Cell Res. 222, 179–188. [DOI] [PubMed] [Google Scholar]
- Shimokawa T, Smith WL (1992) Prostaglandin endoperoxide synthase. The aspirin acetlyation region. J. Biol. Chem. 267, 12387–12392. [PubMed] [Google Scholar]
- Smith ML, Hawcroft G, Hull MA (2000) The effect of non‐steroidal anti‐inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur. J. Cancer 36, 664–674. [DOI] [PubMed] [Google Scholar]
- Sneader W (1997) The discovery of aspirin. Pharm. J. 259, 614–617. [Google Scholar]
- Sneader W (2000) the discovery of aspirin: a reappraisal. BMJ 321, 1591–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LA, Din FV, Zwacka RM, Dunlop MG (2001) Aspirin‐induced activation of the NF‐kappaB signaling pathway: a novel mechanism for aspirin‐mediated apoptosis in colon cancer cells. FASEB J. 15, 1273–1275. [PubMed] [Google Scholar]
- Sumner J (2000) The Natural History of Medicinal Plants. Oregon: Timber Press. [Google Scholar]
- Sutcliffe J, Dunn N (1992) A History of Medicine. New York: Barnes and Noble. [Google Scholar]
- Towndrow KM, Mertens JJ, Jeong JK, Weber TJ, Monks TJ, Lau SS (2000) Stress‐ and growth‐related gene expression are independent of chemical‐induced prostaglandin E2 Synthesis in renal epithelial cells. Chem. Res. Toxicol. 13, 111–117. [DOI] [PubMed] [Google Scholar]
- Vane JR, Botting RM (2003) The mechanism of action of aspirin. Thromb. Res. 110, 255–258. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN (2005) Prostaglandins and cancer; Gut. August 23. http://gut.bmjjournals.com. [DOI] [PMC free article] [PubMed]
- Wells JCD (2003) Poppy juice and willow bark: advances in their use for the 21st century. The Pain Web for health professionals [WWW document]. URL http://www.thepainwebcom/doclib/topics/000009.htm[accessed August 2004].
- Wong BCY, Zhu GH, Lam SK (1999) Aspirin induced apoptosis in gastric cancer cells. Biomed. Pharmacother. 53, 315–318. [DOI] [PubMed] [Google Scholar]
- Wu KK (2003) Aspirin and other cyclooxygenase inhibitors: new therapeutic insights. Semin. Vasc. Med. 3, 107–1012. [DOI] [PubMed] [Google Scholar]
- Xu X‐M, Sansores‐Garcia L, Cheen X‐M, Matijevic‐Alekic NM, Wu KK (1999) Suppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylate. Proc. Natl Acta Sci. USA 96, 5292–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin MJ, Yamamoto Y, Gaynor RB (1998) The antiinflammatory agents aspirin and salicylate inhibit the activity of I (kappa) B kinase‐beta. Nature 396, 77–80. [DOI] [PubMed] [Google Scholar]
- Zhab S, Yegnasubramanianc V, Nelsona WH, Isaacsb WB, De Marzo AM (2004) Cyclooxygenases in cancer: progress and perspective. Cancer Lett. 215, 1–20. [DOI] [PubMed] [Google Scholar]
- Zhou XM, Chun B, Wong Y, Fan XM, Zhang HB, Kung MCM, Fan DM, Lam SK (2001) Non‐steroidal anti‐inflammatory drugs induce apoptosis in gastric cancer cells through up‐regulation of bax and bak. Carcinogenesis 22, 1393–1397. [DOI] [PubMed] [Google Scholar]
- Zhou XM, Wong BCY, Fan XM, Zhang HB, Lin MCM, Kung HF, Fan DM, Lam SK (1999) Non‐steroidal anti‐inflammatory drug‐induced apoptosis in gastric cancer cells in blocked by protein kinase C activation through inhibition of c‐myc. Br. J. Cancer 79, 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]


